Prohexadione-Calcium Application during Vegetative Growth Affects Growth of Mother Plants, Runners, and Runner Plants of Maehyang Strawberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Application Methods and Concentration of Pro–Ca
2.3. Measurements of Plant Growth Characteristics
2.4. Measurement of Chlorophyll Fluorescence (Fv/Fm)
2.5. Statistical Analysis
3. Results and Discussion
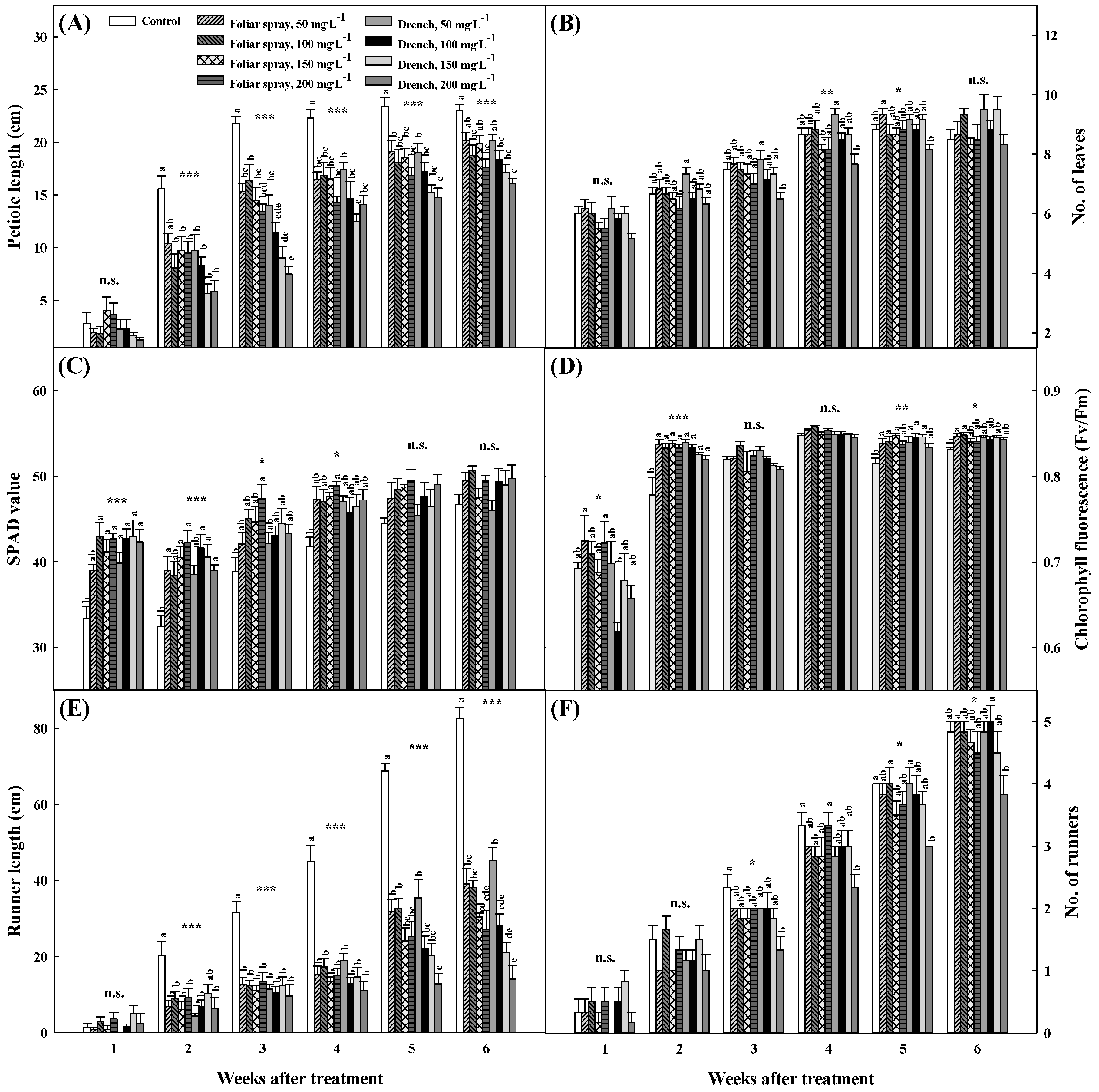
3.1. Growth Characteristics of Mother Plants and Runners after Pro–Ca Treatment for Six Weeks
3.2. Growth Characteristics of Mother Plants at 15 Weeks after Treatment with Pro–Ca
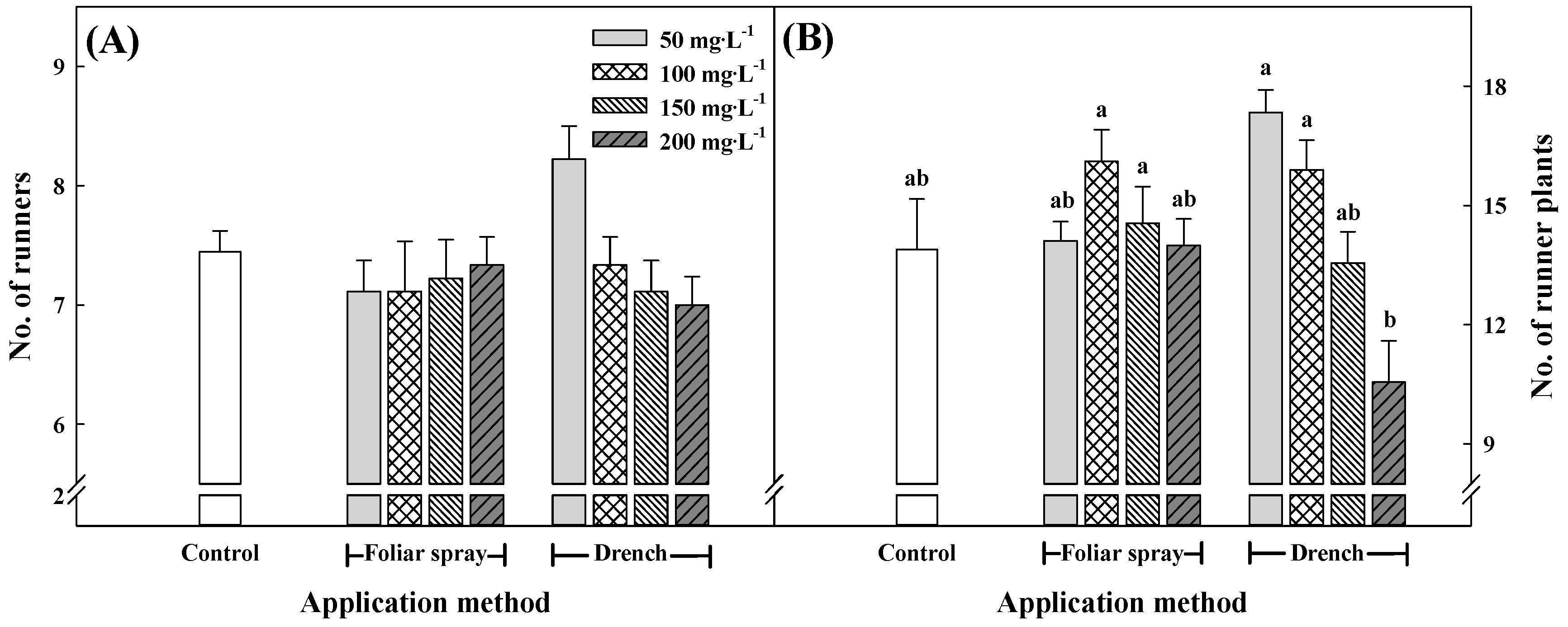
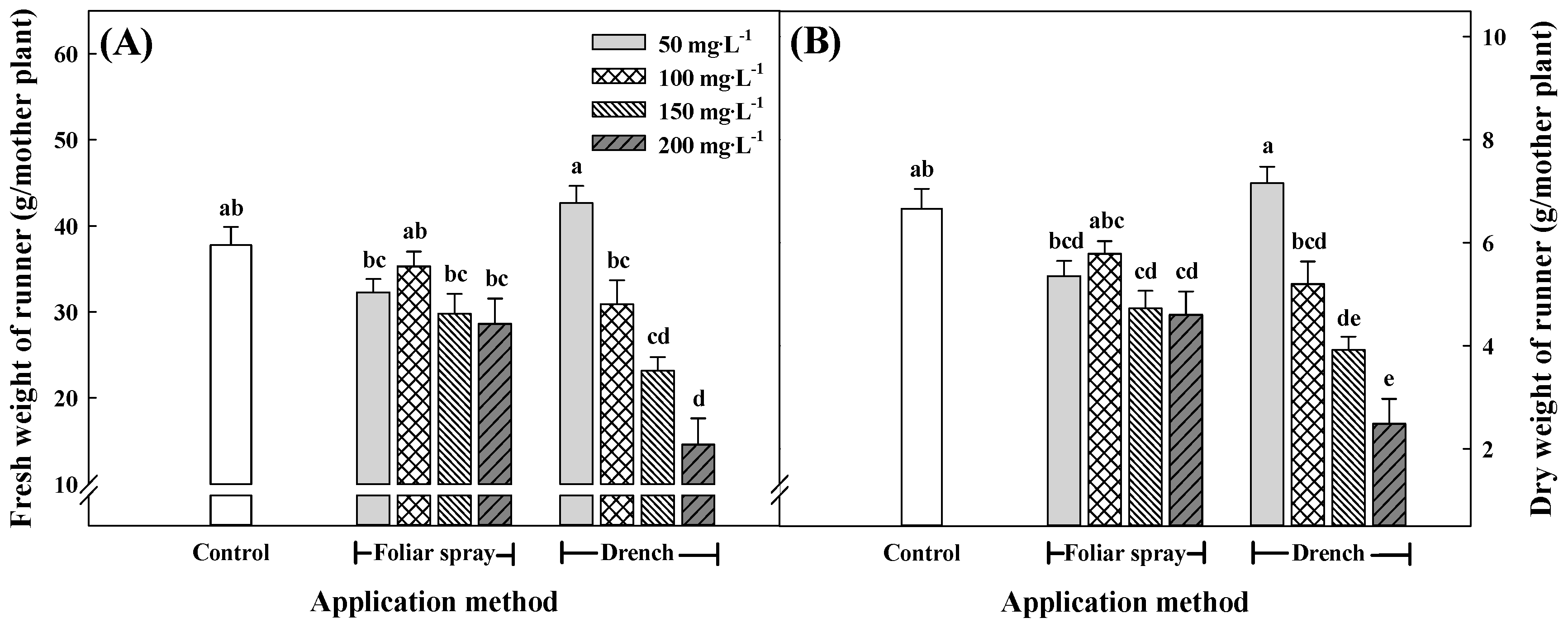
3.3. Growth Characteristics of Runners and Runner Plants at 15 Weeks after Treatment with Pro–Ca
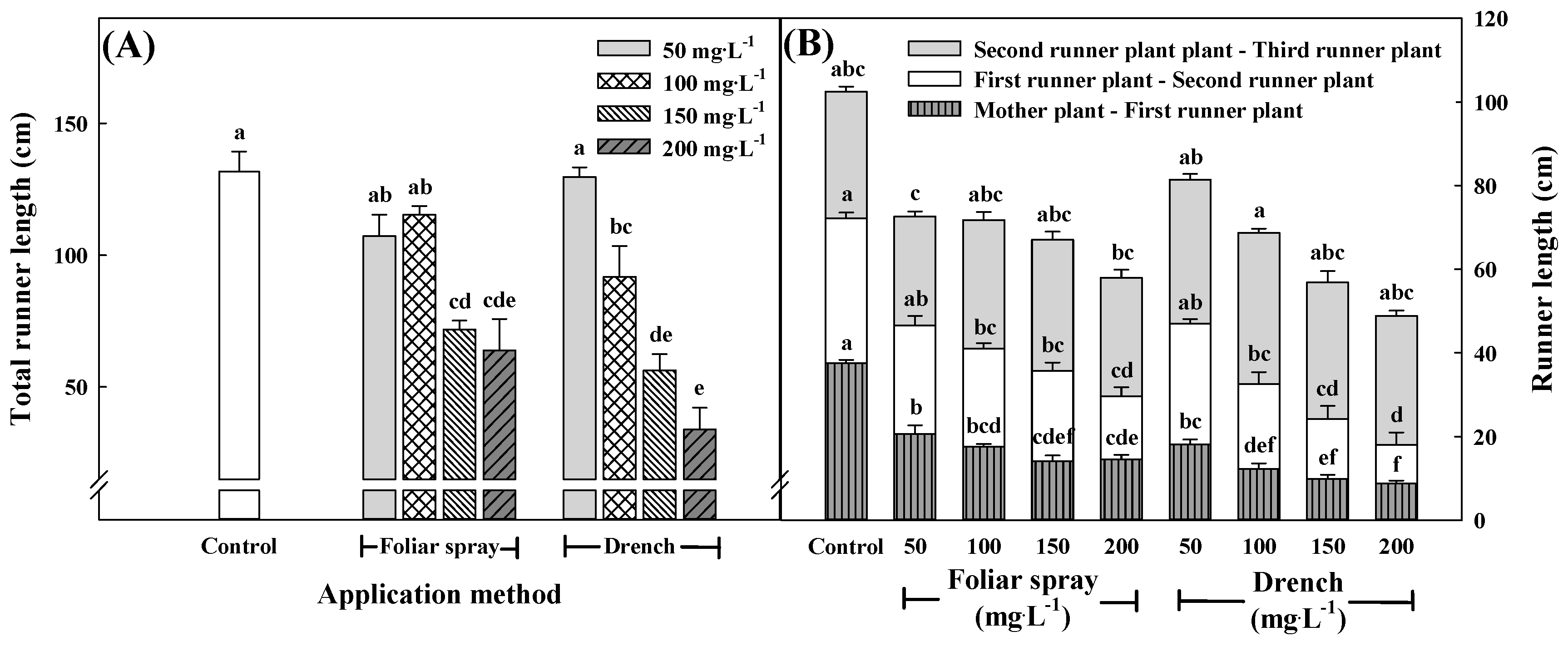
3.4. Growth Characteristics of the First, Second, and Third Runner Plants at 15 Weeks after Treatment with Pro–Ca
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, S.M.; Kim, J.T.; Hamayun, M.; Hwang, I.C.; Khan, A.L.; Kim, Y.H.; Lee, J.H.; Lee, I.J. Influence of prohexadione-calcium on growth and gibberellins content of Chinese cabbage grown in alpine region of South Korea. Sci. Hortic. 2010, 125, 88–92. [Google Scholar] [CrossRef]
- Altintas, S. Effects of chlormequat chloride and different rates of prohexadione-calcium on seedling growth, flowering, fruit development and yield of tomato. Afr. J. Biotechnol. 2011, 10, 17160–17169. [Google Scholar]
- Latimer, J.G. Growth retardants affect landscape performance of Zinnia, Impatiens, and marigold. HortScience 1991, 26, 557–560. [Google Scholar] [CrossRef]
- Yoon, T.M.; Sagong, D.H. Growth control of ‘Fuji’ apple trees by use of prohexadione-calcium. Korean J. Hortic. Sci. Technol. 2005, 23, 269–274. [Google Scholar]
- Hwang, I.C.; Lee, I.J.; Cho, T.K.; Kim, J.T.; Yoon, C.S. Development of labor-saving plant growth regulator, prohexadione-calcium, for growth inhibition. Korean J. Weed Sci. 2009, 29, 1–8. [Google Scholar]
- Kim, H.C.; Cho, Y.H.; Ku, Y.G.; Hwang, S.J.; Bae, J.H. Growth characteristics of grafted tomato seedlings following treatment with various concentrations of diniconazole during the summer growth season. Korean J. Hortic. Sci. Technol. 2016, 34, 249–256. [Google Scholar]
- Ilias, I.; Ouzounidou, G.; Giannakoula, A.; Papadopoulou, P. Effects of gibberellic acid and prohexadione-calcium on growth, chlorophyll fluorescence and quality of okra plant. Biol. Plant 2007, 51, 575–578. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, I.J.; Hamayun, M.; Kim, J.T.; Won, J.G.; Hwang, I.C.; Kim, K.U. Effect of prohexadione-calcium on growth components and endogenous gibberellins contents of rice (Oryza sativa L.). J. Agron. Crop Sci. 2007, 193, 445–451. [Google Scholar] [CrossRef]
- Medjdoub, R.; Val, J.; Blanco, A. Prohexadione-Ca inhibits vegetative growth of ‘Smoothee Golden Delicious’ apple trees. Sci. Hortic. 2004, 101, 243–253. [Google Scholar] [CrossRef]
- Byers, R.E.; Yoder, K.S. Prohexadione-calcium inhibits apple, but not peach, tree growth, but has little influence on apple fruit thinning or quality. HortScience 1999, 34, 1205–1209. [Google Scholar] [CrossRef]
- Brown, R.G.S.; Kawaide, H.; Yang, Y.Y.; Rademacher, W.; Kamiya, Y. Daminozide and prohexadione have similar modes of action as inhibitors of the late stages of gibberellin metabolism. Physiol. Plant 1997, 101, 309–313. [Google Scholar] [CrossRef]
- Evans, J.R.; Evans, R.R.; Regusci, C.L.; Rademacher, W. Mode of action, metabolism, and uptake of BAS 125W, prohexadione-calcium. HortScience 1999, 34, 1200–1201. [Google Scholar] [CrossRef]
- Ito, A.; Sakamoto, D.; Itai, A.; Nishijima, T.; Oyama-Okudo, N.; Nakamura, Y.; Moriguchi, T.; Nakajima, I. Effects of GA3+4 and GA4+7 application either alone or combined with prohexadione-ca on fruit development of Japanese pear ‘Kosui’. Hortic. J. 2016, 85, 201–208. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Hamayun, M.; Kim, J.T.; Lee, J.H.; Hwang, I.C.; Yoon, C.S.; Lee, I.J. Effects of prohexadione calcium on growth and gibberellins contents of Chrysanthemum morifolium R. cv Monalisa White. Sci. Hortic. 2010, 123, 423–427. [Google Scholar] [CrossRef]
- Ilias, I.; Rajapakse, N. Prohexadione-calcium affects growth and flowering of petunia and impatiens grown under photoselective films. Sci. Hortic. 2005, 106, 190–202. [Google Scholar] [CrossRef]
- Hytönen, T.; Elomaa, P.; Moritz, T.; Junttila, O. Gibberellin mediates daylength-controlled differentiation of vegetative meristems in strawberry (Fragaria × ananassa Duch). BMC Plant Biol. 2009, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Reekie, J.Y.; Hicklenton, P.R.; Struik, P.C. Prohexadione-calcium modifies growth and increases photosynthesis in strawberry nursery plants. Can. J. Plant Sci. 2005, 85, 671–677. [Google Scholar] [CrossRef]
- Reekie, J.Y.; Struik, P.C.; Hicklenton, P.R.; Duval, J.R. Dry matter partitioning in a nursery and a plasticulture fruit field of strawberry cultivars ‘Sweet Charlie’ and ‘Camarosa’ as affected by prohexadione-calcium and partial leaf removal. Eur. J. Hortic. Sci. 2007, 72, 122–129. [Google Scholar]
- Jun, H.J.; Jeon, E.H.; Kang, S.I.; Bae, G.H. Optimum nutrient solution strength for Korean strawberry cultivar ‘Daewang’ during seedling period. Korean J. Hortic. Sci. Technol. 2014, 32, 812–818. [Google Scholar] [CrossRef]
- Na, Y.W.; Jeong, H.J.; Lee, S.Y.; Choi, H.G.; Kim, S.H.; Rho, I.R. Chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hortic. Environ. Biotechnol. 2014, 55, 280–286. [Google Scholar] [CrossRef]
- Park, G.S.; Choi, J.M. Medium depths and fixation dates of ‘Seolhyang’ strawberry runner plantlets in nursery field influence the seedling quality and early growth after transplanting. Korean J. Hortic. Sci. Technol. 2015, 33, 518–524. [Google Scholar] [CrossRef]
- Choi, J.M.; Nam, M.H.; Lee, H.S.; Kim, D.Y.; Yoon, M.K.; Ko, K.D. Influence of Ca fertilization on the growth and appearance of physiological disorders in mother plants and occurrence of daughter plants in propagation of ‘Seolhyang’ strawberry through soil cultivation. Korean J. Hortic. Sci. Technol. 2012, 30, 657–663. [Google Scholar] [CrossRef]
- Choi, J.M.; Latigui, A.; Lee, C.W. Visual symptom and tissue nutrient contents in dry matter and petiole sap for diagnostic criteria of phosphorus nutrition for ‘Seolhyang’ strawberry cultivation. Hortic. Environ. Biotechnol. 2013, 54, 52–57. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, J.M.; Kim, T.I.; Kim, H.S.; Lee, I.H. Influence of bicarbonate concentrations in nutrient solution on the growth, occurrence of daughter plants and nutrient uptake in vegetative propagation of ‘Seolhyang’ strawberry. Korean J. Hortic. Sci. Technol. 2014, 32, 149–156. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, I.S.; Choi, J.M. Influence of sulfur concentration on bicarbonate injury reduction during vegetative growth in ‘Seolhyang’ strawberry. Hortic. Sci. Technol. 2018, 36, 362–369. [Google Scholar]
- Caruso, G.; Villari, G.; Melchionna, G.; Conti, S. Effects of cultural cycles and nutrient solutions on plant growth, yield and fruit quality of alpine strawberry (Fragaria vesca L.) grown in hydroponics. Sci. Hortic. 2011, 129, 479–485. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, H.M.; Jeong, H.W.; Lee, H.R.; Jeong, B.R.; Kang, N.J.; Hwang, S.J. Growth of mother plants and occurrence of daughter plants of ‘Maehyang’ strawberry as affected by different EC levels of nutrient solution during nursery period. Protected Hortic. Plant Fac. 2018, 27, 185–190. [Google Scholar] [CrossRef]
- Narváez-Ortiz, W.A.; Lieth, J.H.; Grattan, S.R.; Benavides-Mendoza, A.; Evans, R.Y.; Preciado-Rangel, P.; Valenzuela-García, J.R.; Gonzalez-Fuentes, J.A. Implications of physiological integration of stolon interconnected plants for salinity management in soilless strawberry production. Sci. Hortic. 2018, 241, 124–130. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Won, E.J.; Jeong, B.R. Effect of plant growth retardants on the growth characteristics of potted Spathiphyllum in ebb and flow system. Korean J. Hortic. Sci. Technol. 2007, 25, 443–450. [Google Scholar]
- Sun, E.S.; Kang, H.M.; Kim, Y.S.; Kim, I.S. Effects of seed soaking treatment of diniconazol on the inhibition of stretching of tomato and cucumber seedlings. J. Bio-Environ. Control 2007, 19, 55–62. [Google Scholar]
- Sagong, D.H.; Song, Y.Y.; Park, M.Y.; Kweon, H.J.; Kim, M.J.; Yoon, T.M. Photosynthesis, shoot growth and fruit quality in ‘Fuji’/M.9 mature apple trees in response to prohexadione-calcium treatments. Korean J. Hortic. Sci. Technol. 2014, 32, 762–770. [Google Scholar] [CrossRef]
- Agehara, S.; Leskovar, D.I. Growth suppression by exogenous abscisic acid and uniconazole for prolonged marketability of tomato transplants in commercial conditions. HortScience 2017, 52, 606–611. [Google Scholar] [CrossRef]
- Kofidis, G.; Giannakoula, A.; Ilias, I.F. Growth, anatomy and chlorophyll fluorescence of coriander plants (Coriandrum sativum L.) treated with prohexadione-calcium and daminozide. Acta Biol. Crac. Ser. Bot. 2008, 50, 55–62. [Google Scholar]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Evans, R.R.; Evans, J.R.; Rademacher, W. Prohexadione-calcium for suppression of vegetative growth in eastern apples. Acta Hortic. 1997, 451, 663–666. [Google Scholar] [CrossRef]
- Schupp, J.R.; Robinson, T.L.; Cowgill, W.P.J.; Compton, J.M. Effect of water conditioner and surfactants on vegetative growth control and fruit cracking of ‘Empire’ apple caused by prohexadione-calcium. HortScience 2003, 36, 1205–1209. [Google Scholar] [CrossRef]
- Lee, M.Y. Suppression of Stretchiness in Pot Kalanchoe by Various Applications of Plant Growth Retardants. Master’s Thesis, Gyeongsang National University, Jinju, Korea, 2003. [Google Scholar]
- Kender, W.J.; Carpenter, S.; Braun, J.W. Runner formation in ever-bearing strawberry as influenced by growth-promoting and inhibiting substances. Ann. Bot. 1971, 35, 1045–1052. [Google Scholar] [CrossRef]
- Singh, J.P.; Randhawa, G.S.; Jain, N.L. Response of strawberry to gibberellic acid. Indian J. Hortic. Sci. 1960, 17, 21–30. [Google Scholar]
- Momenpour, A.; Taghavi, T.S.; Manochehr, S. Effects of benzyladenine and gibberellin on runner production and some vegetative traits of three strawberry cultivars. Afr. J. Agr. Res. 2011, 6, 4357–4361. [Google Scholar]
- Barreto, C.F.; Ferreira, L.V.; Costa, S.I.; Schiavon, A.V.; Becker, T.B.; Vignolo, G.K.; Antunes, L.E.C. Concentration and periods of application of prohexadione-calcium in the growth of strawberry seedlings. Semina: Ciências Agrárias 2018, 39, 1937–1944. [Google Scholar] [CrossRef]
- Pipattanawong, N.; Fujishige, N.; Yamane, K.; Ijiro, Y.; Ogata, R. Effects of growth regulators and fertilizer on runner production, flowering, and growth in day-neutral strawberries. Jpn. J. Trop. Agric. 1996, 40, 101–105. [Google Scholar]
- Savini, G.; Giorgi, V.; Scarano, E.; Neri, D. Strawberry plant relationship through the stolon. Physiol. Plant 2008, 134, 421–429. [Google Scholar] [CrossRef]
- Kim, T.I.; Kim, W.S.; Choi, J.H.; Jang, W.S.; Seo, K.S. Comparison of runner production and growth characteristics among strawberry cultivars. Korean J. Hortic. Sci. Technol. 1999, 17, 111–114. [Google Scholar]
- Sabatini, E.; Noferini, M.; Fiori, G.; Grappadelli, L.C.; Costa, G. Prohexadione-ca positively affects gas exchanges and chlorophyll content of apple and pear trees. Eur. J. Hortic. Sci. 2003, 68, 123–128. [Google Scholar]
- Pereira, I.D.S.; Goncalves, M.A.; Picolotto, L.; Vignolo, G.K.; Antunes, L.E.C. Prohexadione-calcium growth control of ‘Camarosa’ strawberry seedlings cultivated in commercial substrate. Amazon. J. Agric. Environ. Sci. 2016, 59, 93–98. [Google Scholar]
- Hofius, D.; Börnke, F.A.J. Photosynthesis, carbohydrate metabolism and source-sink relations. In Potato Biology and Biotechnology; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 257–285. [Google Scholar]
- Barrett, J.E.; Bartuska, C.A.; Nell, T.A. Application techniques alter uniconazole efficacy on chrysanthemums. HortScience 1994, 29, 893–895. [Google Scholar] [CrossRef]
- Hwang, S.J.; Lee, M.Y.; Park, Y.H.; Sivanesan, I.; Jeong, B.R. Suppression of stem growth in pot kalanchoe ‘Gold Strike’ by recycled subirrigational supply of plant growth retardants. Afr. J. Biotechnol. 2008, 7, 1487–1493. [Google Scholar]
- Shin, W.G.; Hwang, S.J.; Sivanesan, I.; Jeong, B.R. Height suppression of tomato plug seedlings by an environment friendly seed treatment of plant growth retardants. Afr. J. Biotechnol. 2009, 8, 4100–4107. [Google Scholar]
| Experiment Factor | Petiole Length (cm) | Crown Diameter (mm) | Fresh Weight (g/plant) | Dry Weight (g/plant) | Leaf Area (cm2/plant) | ||
|---|---|---|---|---|---|---|---|
| Leaves + Petioles | Crown | Leaves + Petioles | Crown | ||||
| Application method | |||||||
| Foliar spray | 18.6 | 16.5 | 72.6 | 6.9 | 17.2 | 1.4 | 1561.1 |
| Drench | 16.4 | 16.8 | 67.3 | 7.2 | 15.6 | 1.5 | 1449.3 |
| * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Concentration (mg·L−1) | |||||||
| Control (0) | 23.9 a | 17.0 | 87.2 a | 6.8 | 26.9 a | 1.4 | 1867.3 a |
| 50 | 19.3 b | 16.3 | 75.5 ab | 7.4 | 18.0 b | 1.5 | 1538.7 b |
| 100 | 18.0 bc | 16.4 | 71.5 b | 7.3 | 16.3 b | 1.5 | 1541.0 b |
| 150 | 17.4 bc | 16.8 | 66.1 b | 6.3 | 15.6 b | 1.3 | 1557.7 b |
| 200 | 15.5 c | 17.1 | 66.8 b | 7.2 | 15.6 b | 1.5 | 1383.4 b |
| n.s. | n.s. | n.s. | |||||
| Experiment Factor | Fresh Weight (g/plant) | Dry Weight (g/plant) | ||||
|---|---|---|---|---|---|---|
| First Runner Plant | Second Runner Plant | Third Runner Plant | First Runner Plant | Second Runner Plant | Third Runner Plant | |
| Application method | ||||||
| Foliar spray | 19.2 | 13.4 | 4.5 | 3.9 | 2.7 | 0.78 |
| Drench | 17.2 | 11.2 | 3.3 | 3.7 | 2.4 | 0.67 |
| n.s. | * | * | n.s. | n.s. | n.s. | |
| Concentration (mg·L−1) | ||||||
| Control (0) | 17.5 | 8.7 b | 3.5 b | 4.0 | 1.9 b | 0.72 abc |
| 50 | 18.5 | 13.3 a | 5.3 a | 3.9 | 2.9 a | 1.02 a |
| 100 | 19.3 | 13.7 a | 4.4 ab | 4.1 | 2.8 a | 0.82 ab |
| 150 | 17.3 | 10.4 ab | 2.9 b | 3.5 | 2.1 b | 0.50 c |
| 200 | 17.7 | 11.7 ab | 3.1 b | 3.6 | 2.3 ab | 0.57 bc |
| n.s. | n.s. | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Lee, H.R.; Kang, J.H.; Hwang, S.J. Prohexadione-Calcium Application during Vegetative Growth Affects Growth of Mother Plants, Runners, and Runner Plants of Maehyang Strawberry. Agronomy 2019, 9, 155. https://doi.org/10.3390/agronomy9030155
Kim HM, Lee HR, Kang JH, Hwang SJ. Prohexadione-Calcium Application during Vegetative Growth Affects Growth of Mother Plants, Runners, and Runner Plants of Maehyang Strawberry. Agronomy. 2019; 9(3):155. https://doi.org/10.3390/agronomy9030155
Chicago/Turabian StyleKim, Hyeon Min, Hye Ri Lee, Jae Hyeon Kang, and Seung Jae Hwang. 2019. "Prohexadione-Calcium Application during Vegetative Growth Affects Growth of Mother Plants, Runners, and Runner Plants of Maehyang Strawberry" Agronomy 9, no. 3: 155. https://doi.org/10.3390/agronomy9030155
APA StyleKim, H. M., Lee, H. R., Kang, J. H., & Hwang, S. J. (2019). Prohexadione-Calcium Application during Vegetative Growth Affects Growth of Mother Plants, Runners, and Runner Plants of Maehyang Strawberry. Agronomy, 9(3), 155. https://doi.org/10.3390/agronomy9030155





