Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Method
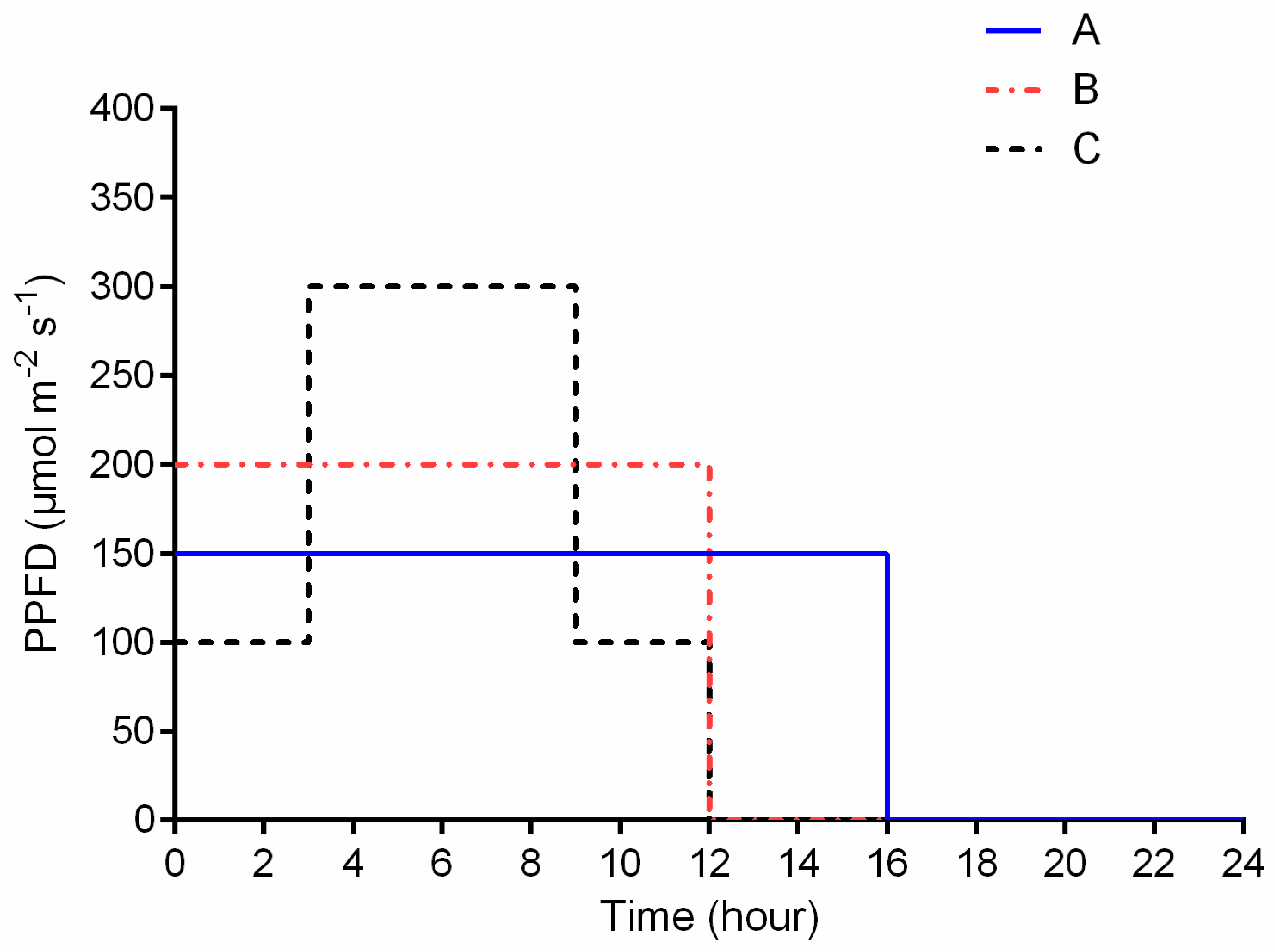
2.2. Treatments
2.3. Measurements
2.3.1. Growth Parameters and Chlorophyll Content Measurements
2.3.2. Gas-Exchange Parameter
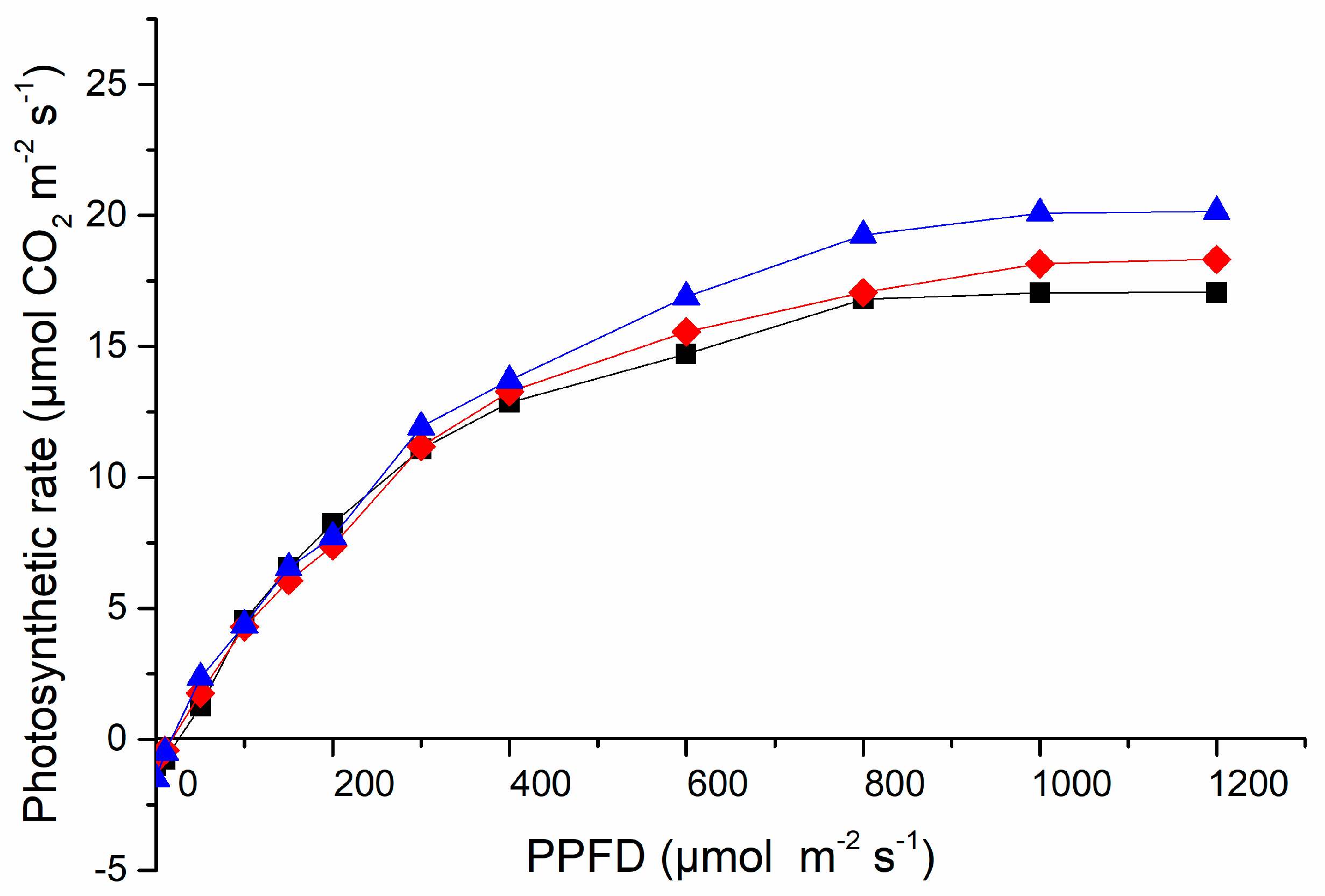
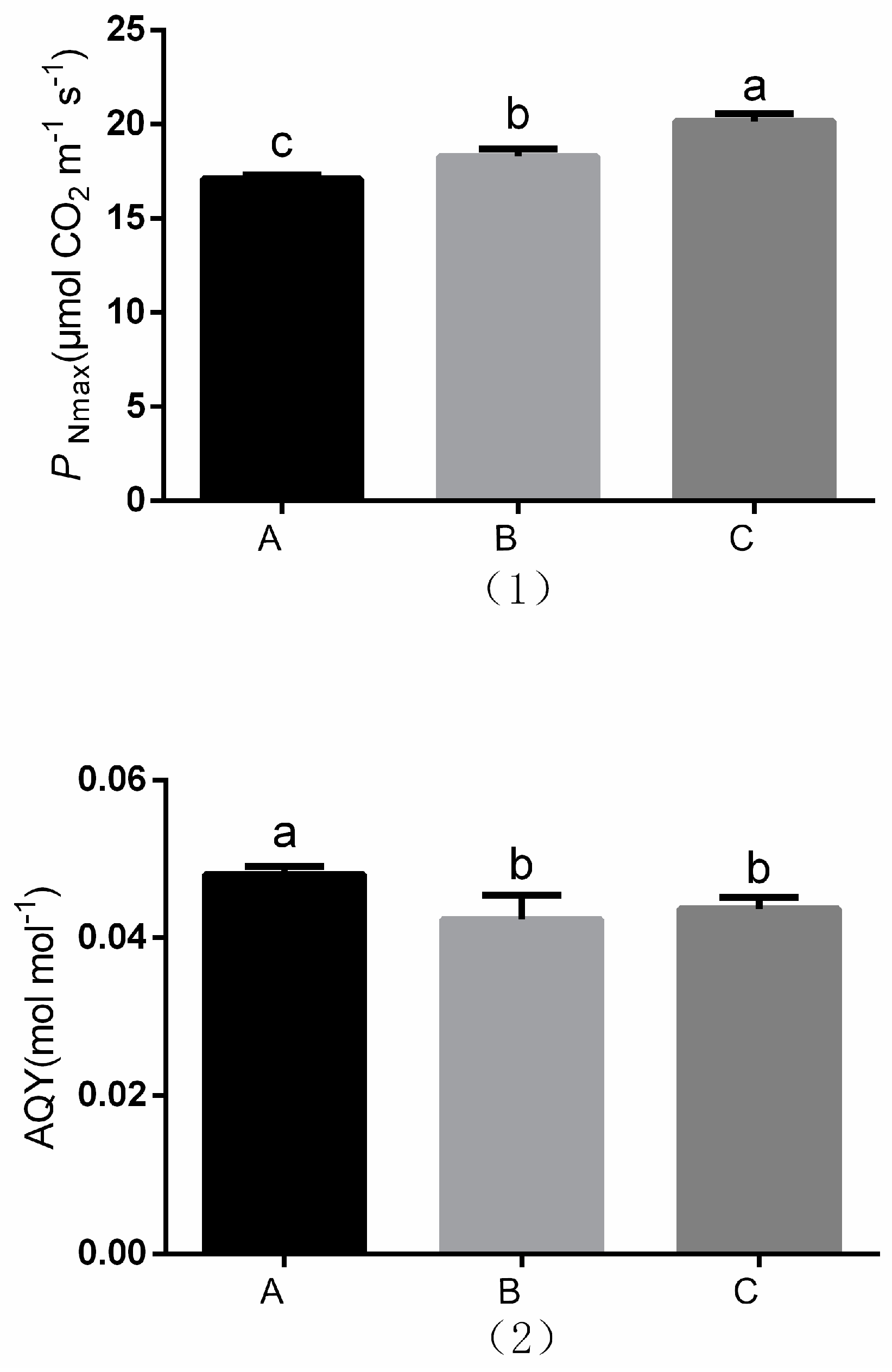
2.3.3. Light Response Curve Measurements
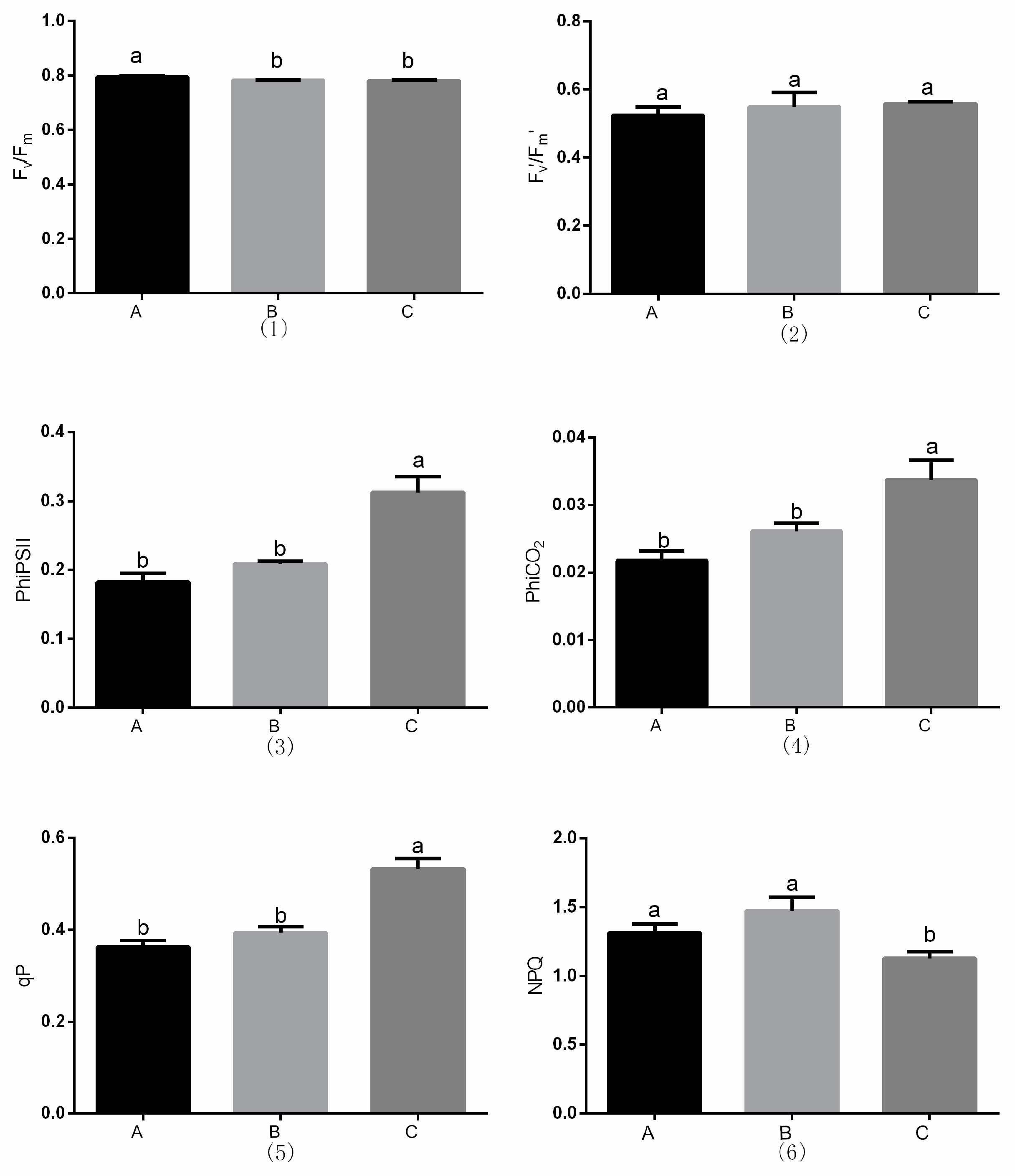
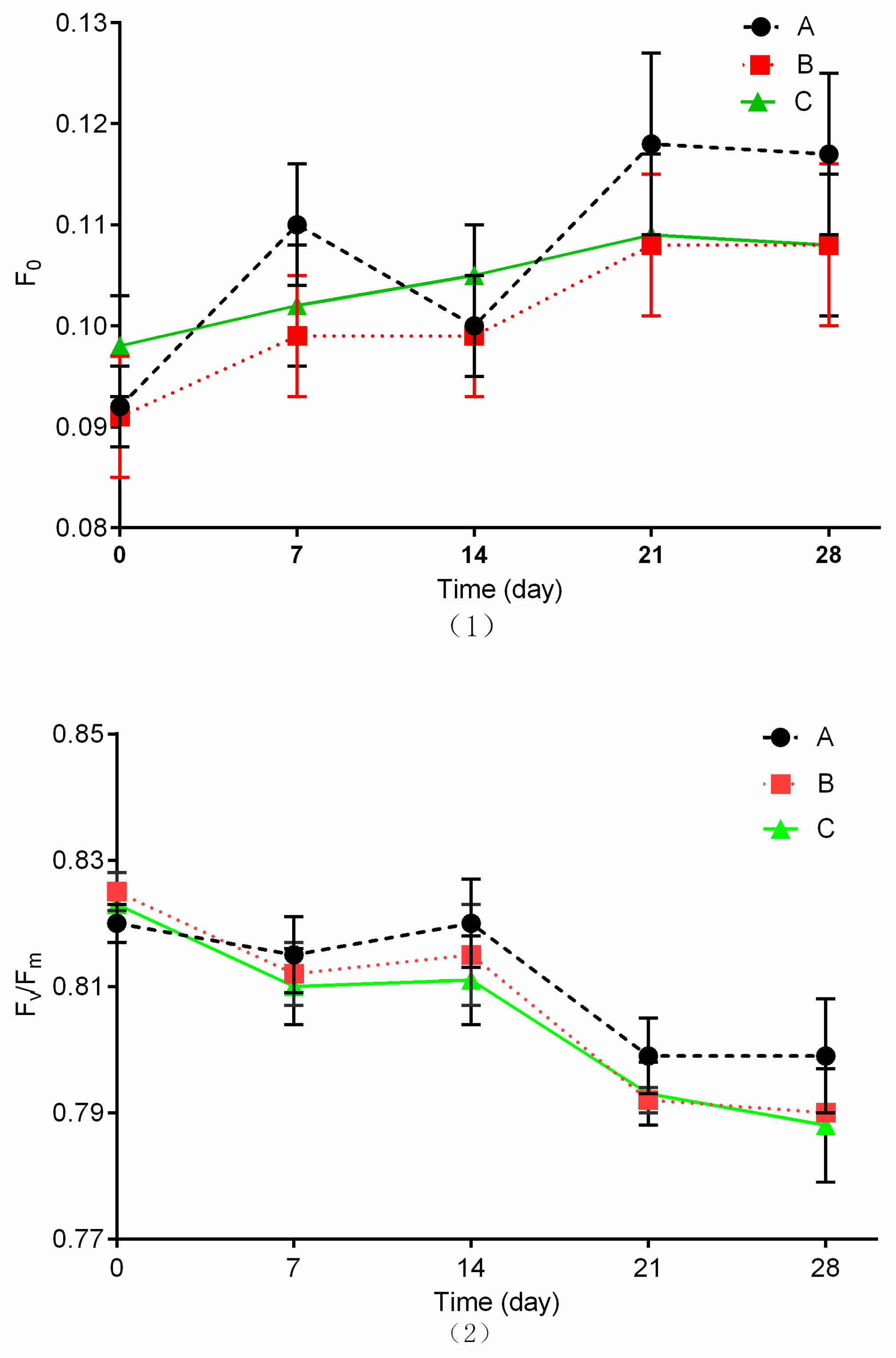
2.3.4. Chlorophyll Fluorescence Parameter Measurements
2.4. Statistical Analysis
3. Results
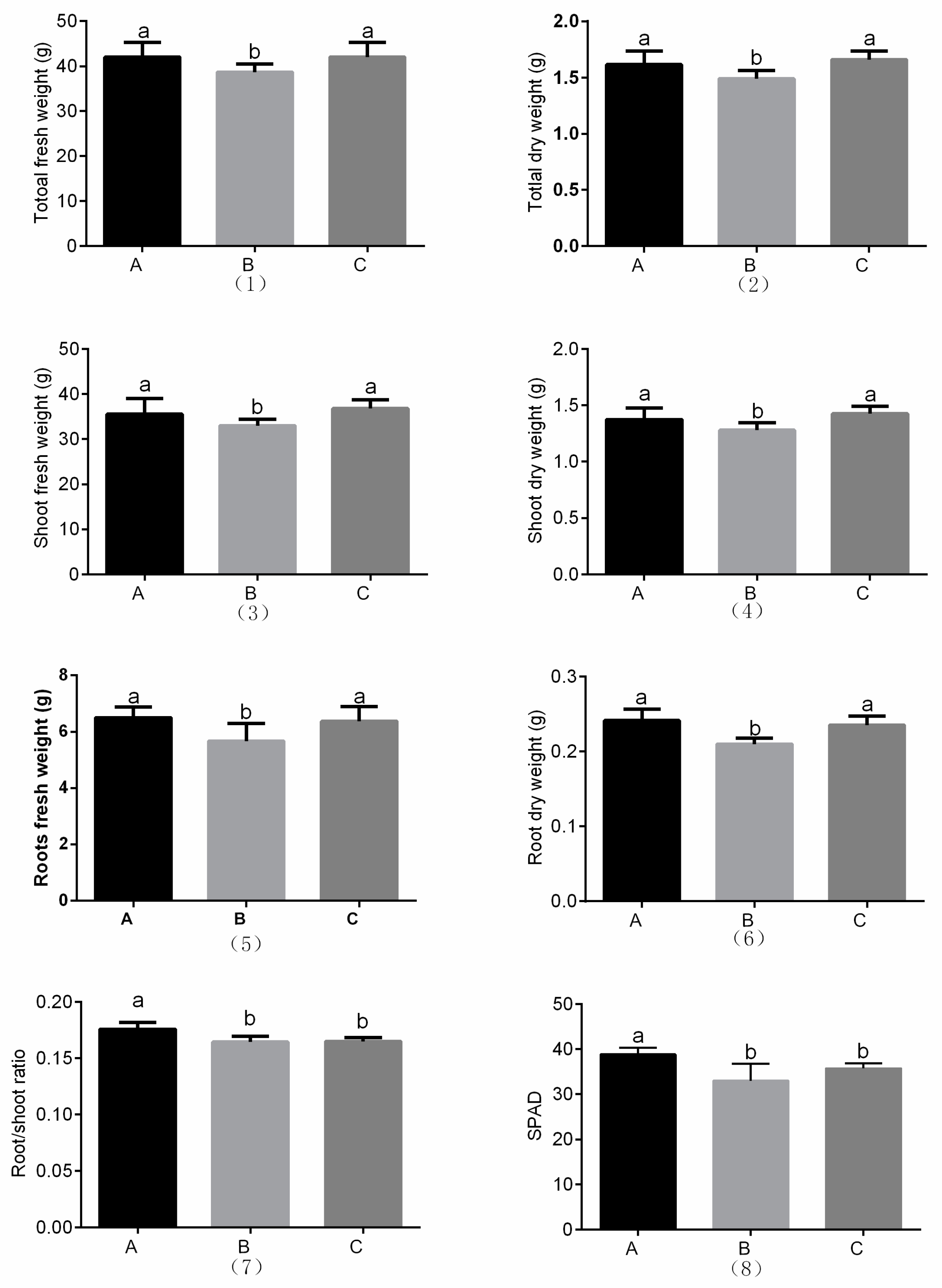
3.1. Plant Growth and Chlorophyll Content
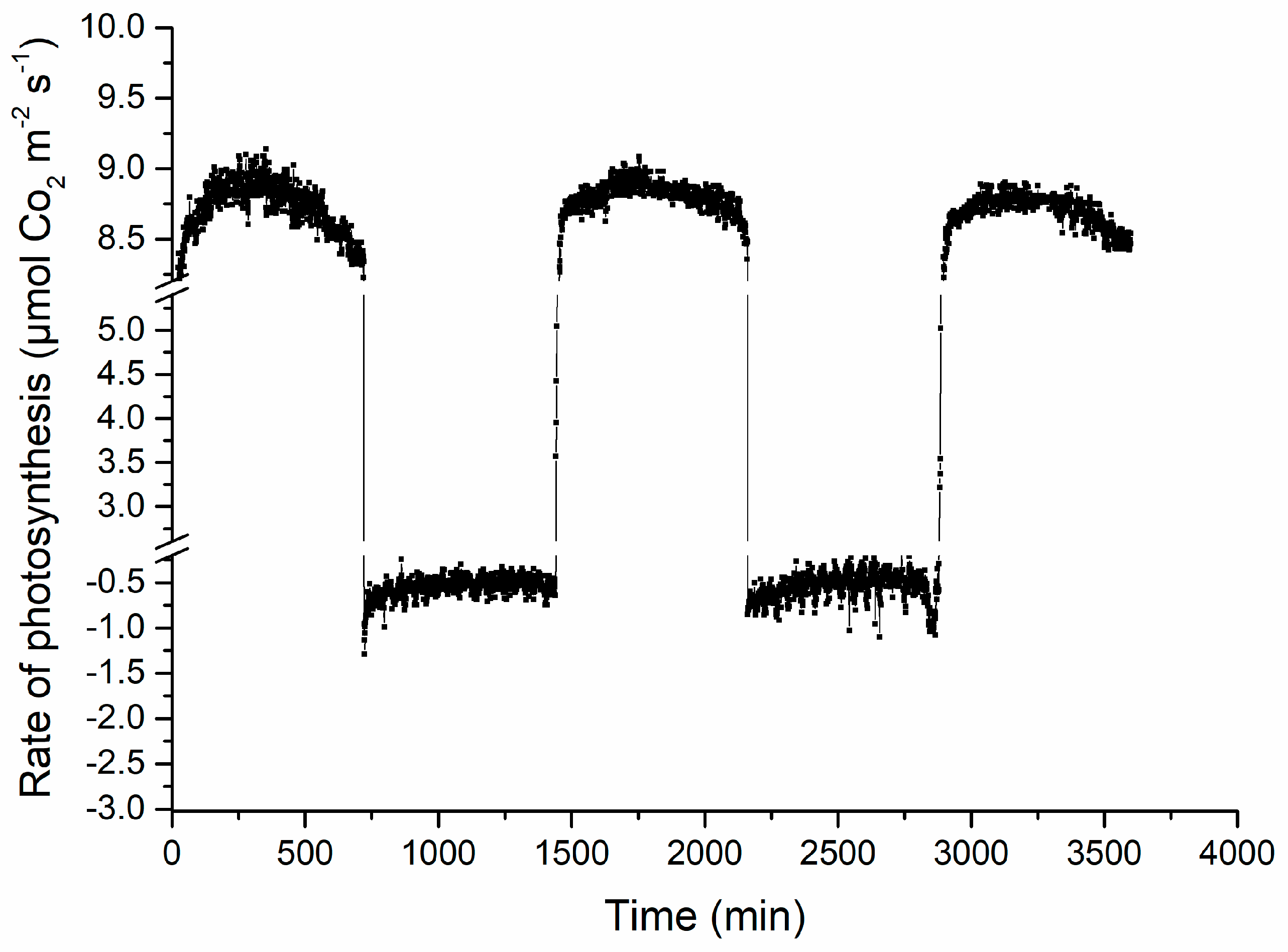
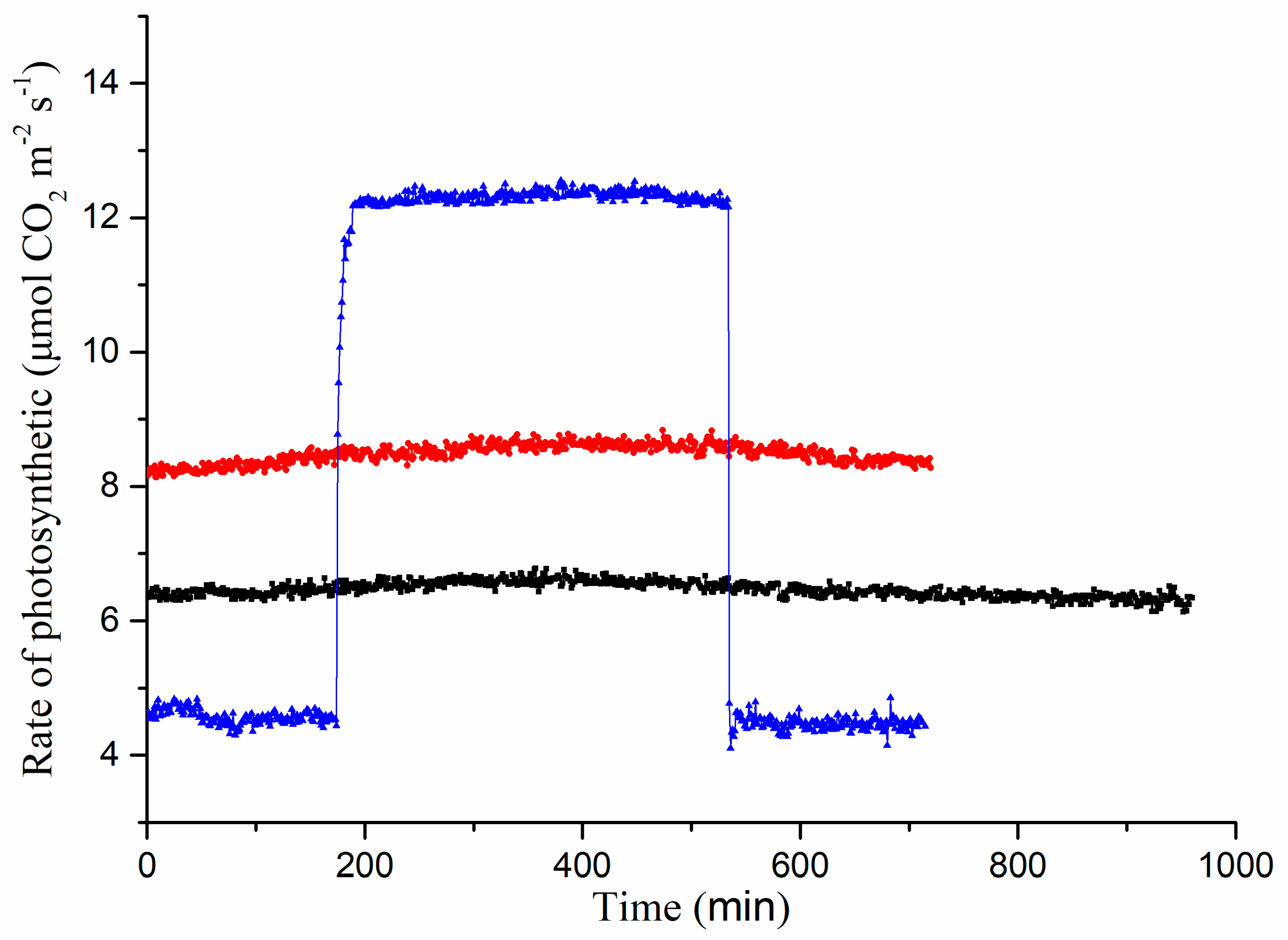
3.2. Mreasurements of Photosynthetic Parameters
3.3. Light Response Curve Measurements
3.4. Chlorophyll Fluorescence Parameter Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Kozai, T. Resource Use Efficiency of Closed Plant Production System with Artificial Light: Concept, Estimation and Application to Plant Factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T. Sustainable Plant Factory: Closed Plant Production Systems With Artificial Light For High Resource Use Efficiencies And Quality Produce. Acta Horticulturae 2013, 1004, 27–40. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf Size and Angle Vary Widely across Species: What Consequences for Light Interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Gaudreau, L.; Charbonneau, J.; Vézina, L.-P.; Gosselin, A. Photoperiod and photosynthetic photon flux influence growth and quality of greenhouse-grown lettuce. HortScience 1994, 29, 1285–1289. [Google Scholar] [CrossRef]
- Kitaya, Y.; Niu, G.; Kozai, T.; Ohashi, M. Photosynthetic photon flux, photoperiod, and CO2 concentration affect growth and morphology of lettuce plug transplants. HortScience 1998, 33, 988–991. [Google Scholar] [CrossRef]
- Ikead, A.; Nakayama, S.; Kitaya, Y.; Yabukyi, K. Basic study on material production in plant factory (1). Environ. Control Biol. 1988, 26, 107–112. [Google Scholar] [CrossRef][Green Version]
- Vlahos, J.; Heuvelink, E.; Martakis, G. A growth analysis study of three Achimenes cultivars grown under three light regimes. Sci. Hortic. 1991, 46, 275–282. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Shevela, D.; Björn, L.O. Photosynthesis: Solar Energy for Life; World Scientific Publishing: Singapore, 2018. [Google Scholar]
- Chen, X.-L.; Yang, Q.-C.; Song, W.-P.; Wang, L.-C.; Guo, W.-Z.; Xue, X.-Z. Growth and nutritional properties of lettuce affected by different alternating intervals of red and blue LED irradiation. Sci. Hortic. 2017, 223, 44–52. [Google Scholar] [CrossRef]
- Abidi, F.; Girault, T.; Douillet, O.; Guillemain, G.; Sintes, G.; Laffaire, M.; Ahmed, H.B.; Smiti, S.; Huché-Thélier, L.; Leduc, N. Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol. 2013, 15, 67–74. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Takaki, M.; Azevedo, R.A. Plant pigments: The many faces of light perception. Acta Physiol. Plant. 2011, 33, 241–248. [Google Scholar] [CrossRef]
- Yanagi, T.; Okamoto, K.; Takita, S. Effects of Blue, Red, and Blue/Red Lights of Two Different PPF Levels on Growth and Morphogenesis of Lettuce Plants; International Society for Horticultural Science: Narita, Japan, 1996; Volume 440. [Google Scholar]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and Accumulation of Secondary Metabolites in Perilla as Affected by Photosynthetic Photon Flux Density and Electrical Conductivity of the Nutrient Solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- McClung, C.R. Plant circadian rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Knight, M.R.; Kondo, T.; Masson, P.; Sedbrook, J.; Haley, A.; Trewavas, A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 1995, 269, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Tingay, S.; Wang, Z.-Y.; Tobin, E.M. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 2002, 129, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Son, K.-H.; Lee, J.-H.; Oh, Y.; Kim, D.; Oh, M.-M.; In, B.-C. Growth and Bioactive Compound Synthesis in Cultivated Lettuce Subject to Light-quality Changes. HortScience 2017, 52, 584–591. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of Photosynthetic Photon Flux Density and Root-zone Temperature for Enhancing Secondary Metabolite Accumulation and Production of Coriander in Plant Factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef]
- Shimokawa, A.; Tonooka, Y.; Matsumoto, M.; Ara, H.; Suzuki, H.; Yamauchi, N.; Shigyo, M. Effect of alternating red and blue light irradiation generated by light emitting diodes on the growth of leaf lettuce. bioRxiv 2014, 003103. [Google Scholar]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Bassman, J.H.; Zwier, J.C. Gas exchange characteristics of Populus trichocarpa, Populus deltoides and Populus trichocarpa× P. deltoides clones. Tree Physiol. 1991, 8, 145–159. [Google Scholar] [CrossRef]
- Sun, J.; Lu, N.; Xu, H.; Maruo, T.; Guo, S. Root zone cooling and exogenous spermidine root-pretreatment promoting Lactuca sativa L. Growth and photosynthesis in the high-temperature season. Front. Plant Sci. 2016, 7, 368. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Govindjee, J. Chlorophyll a fluorescence. In Light-Harvest. Antennas Photosynth; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 43–63. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z.; Merzlyak, M. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J. Appl. Phycol. 2008, 20, 245–251. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Mozafar, A.; Schreiber, P.; Oertli, J. Photoperiod and root-zone temperature: Interacting effects on growth and mineral nutrients of maize. Plant Soil 1993, 153, 71–78. [Google Scholar] [CrossRef]
- Liang, W.; Nie, D.; Wu, S.; Bai, W.; Shen, S.; Hunan, P.F.B.G. Effects of shading on the growth and photosynthesis of Macropanax rosthornii seedlings. Chin. J. Ecol. 2015, 34, 413–419. [Google Scholar]
- Wang, Q.; Zhang, Q.; Fan, D.; Lu, C. Photosynthetic light and CO2 utilization and C4 traits of two novel super-rice hybrids. J. Plant Physiol. 2006, 163, 529–537. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Garab, G.; Adams, W., III; Govindjee, U. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Berlin, Germany, 2014; Volume 40. [Google Scholar]
- Papageorgiou, G.C. The non-photochemical quenching of the electronically excited state of chlorophyll a in plants: Definitions, timelines, viewpoints, open questions. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Berlin, Germany, 2014; pp. 1–44. [Google Scholar]
- Ögren, E.; Sjöström, M. Estimation of the effect of photoinhibition on the carbon gain in leaves of a willow canopy. Planta 1990, 181, 560–567. [Google Scholar] [CrossRef]
- Groom, Q.J.; Baker, N.R. Analysis of light-induced depressions of photosynthesis in leaves of a wheat crop during the winter. Plant Physiol. 1992, 100, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-M.; Yu, X.-P.; Cheng, J. The application of chlorophyll fluorescence kinetics in the study of physiological responses of plants to environmental stresses. Acta Agric. Zhejiangensis 2006, 18, 51. [Google Scholar]
- Shevela, D.; Ananyev, G.; Vatland, A.K.; Arnold, J.; Mamedov, F.; Eichacker, L.A.; Dismukes, G.C.; Messinger, J. ‘Birth defects’ of photosystem II make it highly susceptible to photodamage during chloroplast biogenesis. Physiol. Plant. 2019, 166, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis. Agronomy 2019, 9, 857. https://doi.org/10.3390/agronomy9120857
Mao H, Hang T, Zhang X, Lu N. Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis. Agronomy. 2019; 9(12):857. https://doi.org/10.3390/agronomy9120857
Chicago/Turabian StyleMao, Hanping, Teng Hang, Xiaodong Zhang, and Na Lu. 2019. "Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis" Agronomy 9, no. 12: 857. https://doi.org/10.3390/agronomy9120857
APA StyleMao, H., Hang, T., Zhang, X., & Lu, N. (2019). Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis. Agronomy, 9(12), 857. https://doi.org/10.3390/agronomy9120857



