Breeding for Enhancing Legumovirus Resistance in Mungbean: Current Understanding and Future Directions
Abstract
1. Introduction
2. Genome Organization of YMD-Causing Legumovirus
3. Host–Virus–Vector Interaction
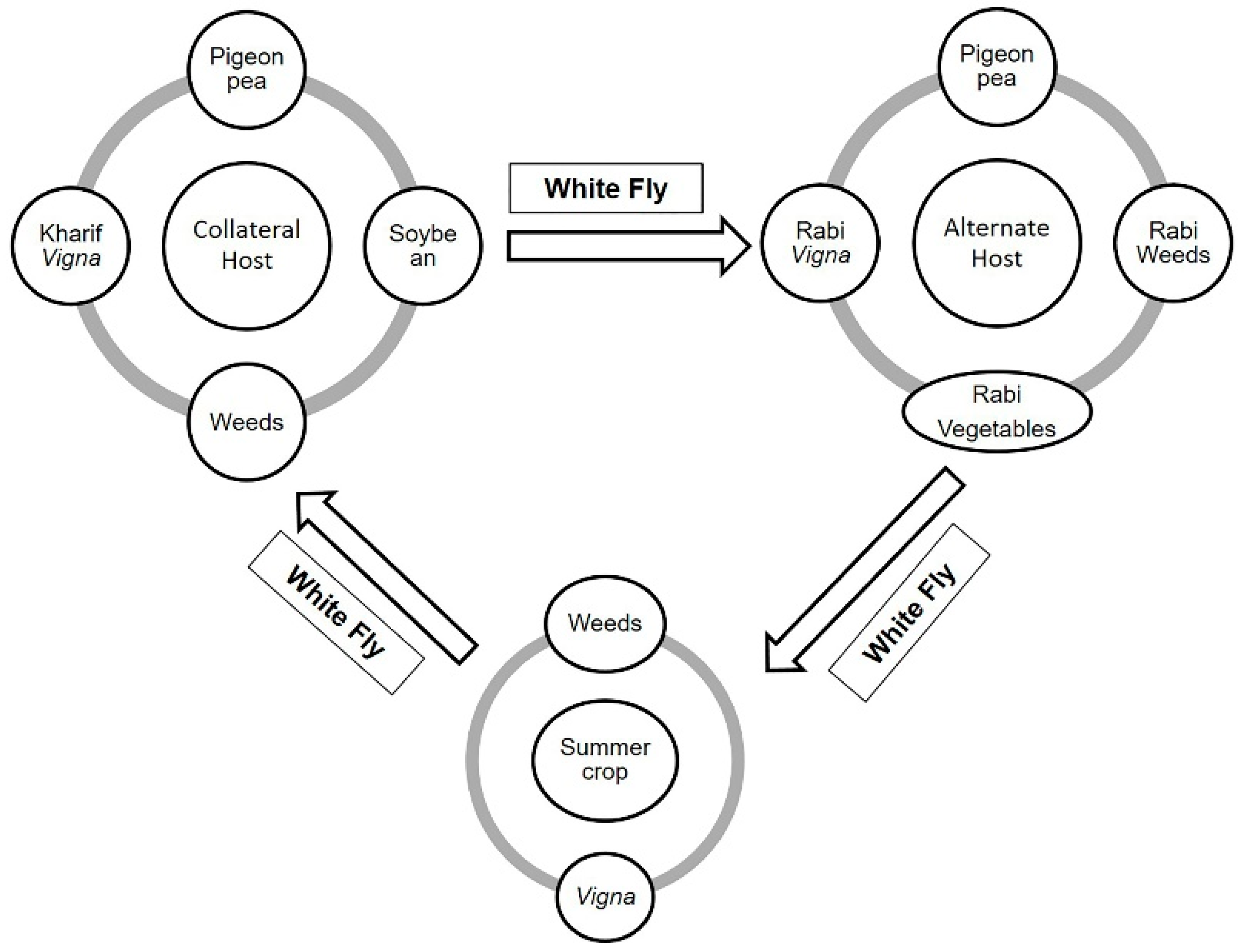
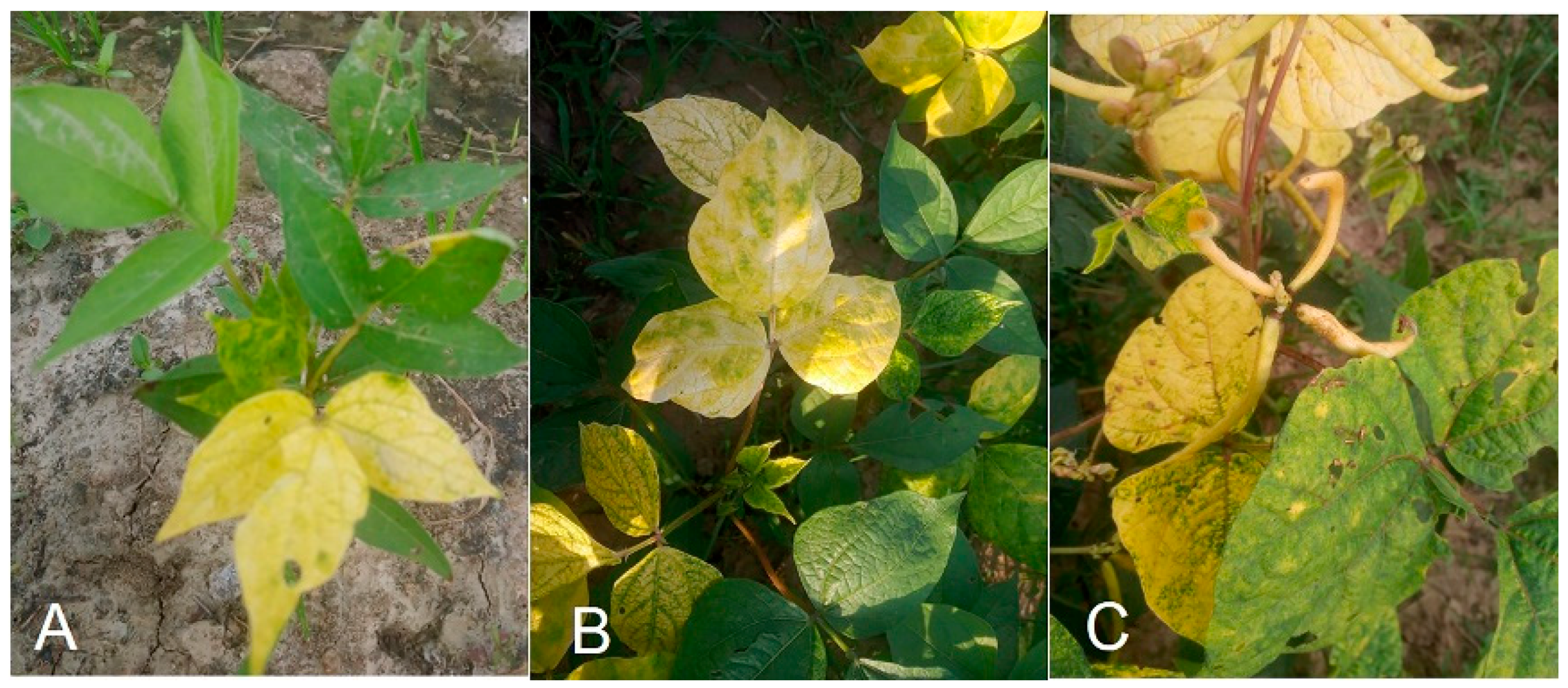
4. Host Range and Disease Symptoms
5. Host-Pathogen Resistance
5.1. Physical Basis of Resistance
5.2. Biochemical Basis of Resistance
5.3. Genetic Basis of Resistance
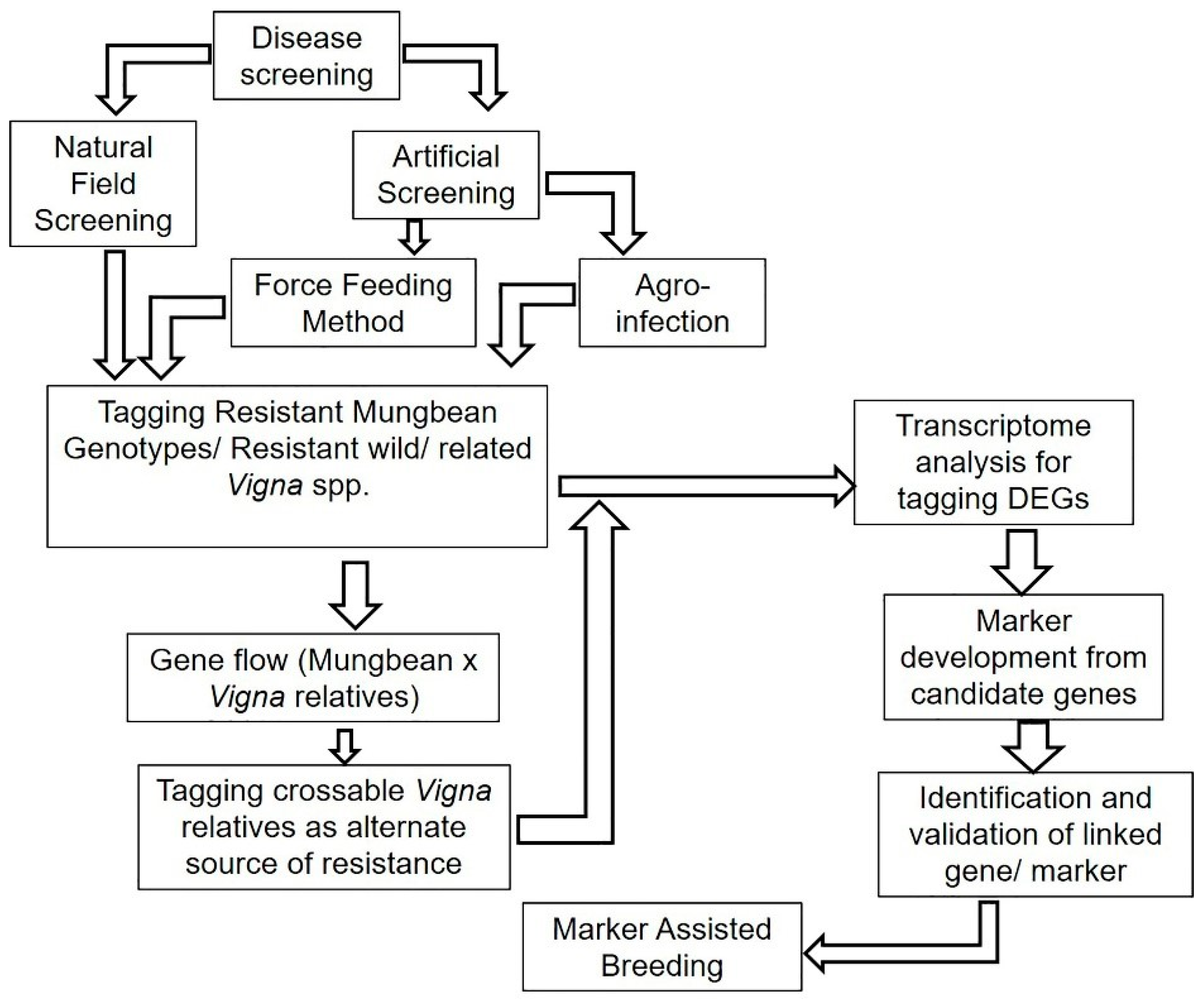
6. Breeding Strategies for Enhancing LYMV Resistance
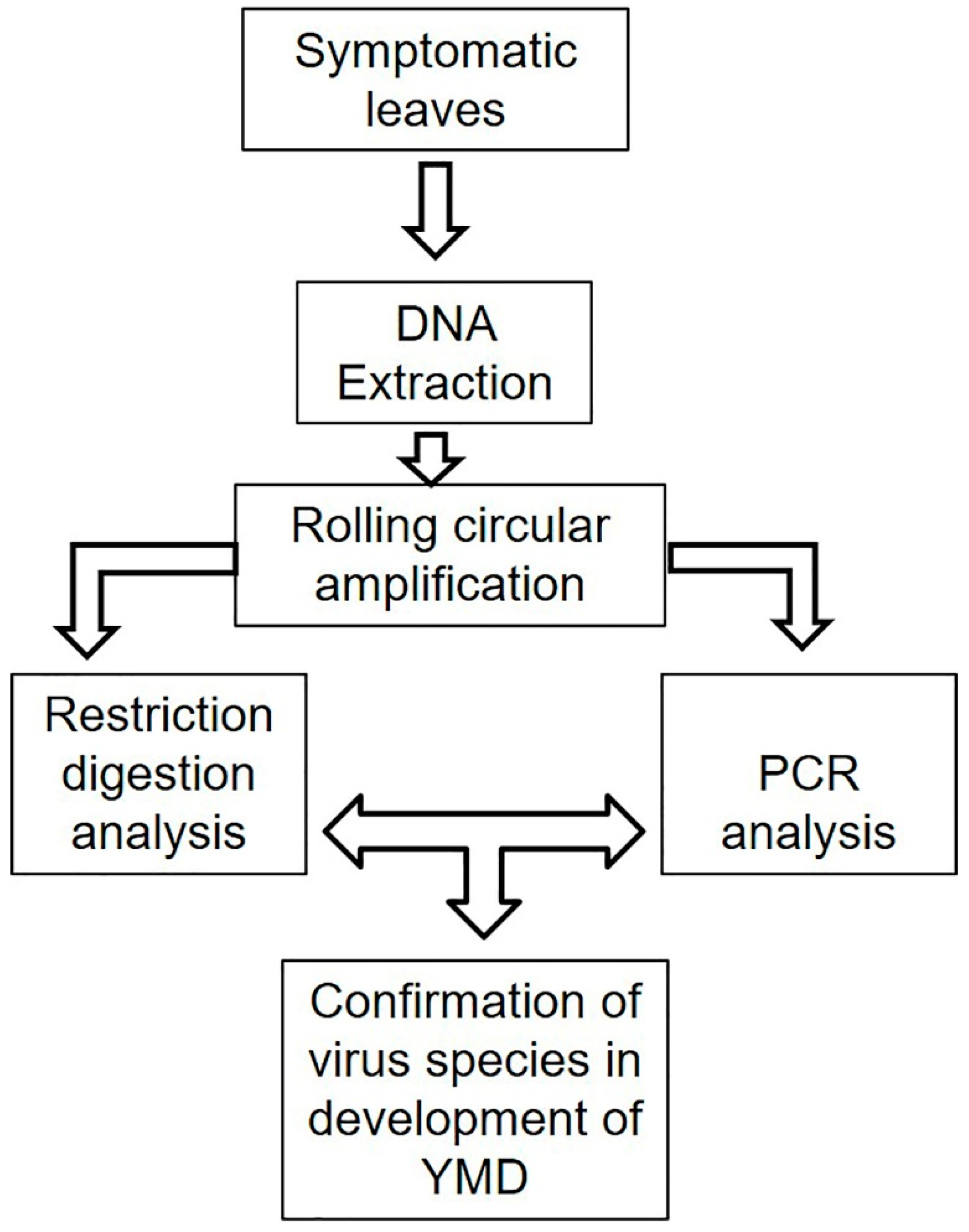
6.1. Pathogen Identification
6.2. Effective Phenotyping Procedure
6.3. Durable Sources of LYMV Resistance
6.4. Exploiting LYMV-Linked Markers and QTLs
6.5. Transcriptomics Approaches
6.6. Genome Editing Approaches
7. Concluding Remarks and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, D.P.; Singh, B.B.; Pratap, A. Genetic improvement of mungbean and urdbean and their role in enhancing pulse production in India. Indian J. Genet. Plant Breed. 2016, 76, 550–567. [Google Scholar] [CrossRef]
- Jat, S.; Shivay, Y.; Parihar, C.M.; Meena, H. Evaluation of summer legumes for their economic feasibility, nutrient accumulation and soil fertility. J. Food Legum. 2012, 25, 239–242. [Google Scholar]
- Kang, Y.J.; Kim, S.K.; Kim, M.Y.; Lestari, P.; Kim, K.H.; Ha, B.-K.; Jun, T.H.; Hwang, W.J.; Lee, T.; Lee, J.; et al. Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 2014, 5, 5443. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Z.; Khan, M.A.A.; Karim, M.M.; Ahmed, M.; Ahmed, F. Field performance of some mungbean varieties against Mungbean Yellow Mosaic Virus and Cercospora leaf spot diseases. J. Exp. Biosci. 2010, 1, 11–16. [Google Scholar]
- Ali, M.; Kumar, S. Advances in Mungbean and Urdbean; Kanpur Report; Indian Institute of Pulses Research: Kanpur, India, 2006; pp. 1–19. [Google Scholar]
- Honda, Y. Mechanical transmission, purification, and some properties of whitefly-borne mung bean Yellow Mosaic Virus in Thailand. Plant Dis. 1983, 67, 801–804. [Google Scholar] [CrossRef]
- Verma, A.; Dhar, A.K.; Mandal, B. MYMV transmission and control in India. In Proceedings of the Mungbean Yellow Mosaic Disease: Proceedings of an International Workshop, Taipei, Taiwan, 2–3 July 1991; pp. 8–27. [Google Scholar]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Haq, Q.M.I.; Rouhibakhsh, A.; Ali, A.; Malathi, V.G. Infectivity analysis of a blackgram isolate of mungbean yellow mosaic virus and genetic assortment with MYMIV in selective hosts. Virus Genes 2011, 42, 429–439. [Google Scholar] [CrossRef]
- Capoor, S.P.; Varma, P.M. Yellow mosaic of Phaseolus lunatus L. Curr. Sci. 1948, 17, 152–153. [Google Scholar]
- Capoor, S.P.; Varma, P.M. A new virus disease of Dolichos lablab. Curr. Sci. 1950, 19, 248–249. [Google Scholar]
- Suruthi, V.; Nakkeeran, S.; Renukadevi, P.; Malathi, V.G.; Rajasree, V. Evidence of seed transmission of Dolichos Yellow Mosaic Virus, a begomovirus infecting lablab-bean in India. Virus Dis. 2018, 29, 506–512. [Google Scholar] [CrossRef]
- Nariani, T.K. Yellow mosaic of mung (Phaseolus aureus L.). Indian Phytopathol. 1960, 13, 24–29. [Google Scholar]
- Maiti, S.; Basak, J.; Kundagrami, S.; Kundu, A.; Pal, A. Molecular marker-assisted genotyping of Mungbean Yellow Mosaic India Virus resistant germplasms of mungbean and urdbean. Mol. Biotechnol. 2011, 47, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.; Karthikeyan, A.; Nagarajan, P.; Raveendran, M.; Senthil, N.; Pandiyan, M.; Angappan, K.; Ramalingam, J.; Bharathi, M.; Rabindran, R. Screening of mungbean (Vigna radiata) germplasm for resistance to mungbean yellow mosaic virus using agroinoculation. Can. J. Plant Pathol. 2013, 35, 424–430. [Google Scholar] [CrossRef]
- Khattak, G.; Haq, M.A.; Ashraf, M.; Elahi, T. Genetics of Mungbean Yellow Mosaic Virus (MYMV) in mungbean (Vigna radiata L.) wilczek. J. Genet. Breed. 2000, 54, 237–243. [Google Scholar]
- Kitsanachandee, R.; Somta, P.; Chatchawankanphanich, O.; Akhtar, K.P.; Shah, T.M.; Nair, R.M.; Bains, T.S.; Sirari, A.; Kaur, L.; Srinives, P. Detection of quantitative trait loci for Mungbean Yellow Mosaic India Virus (MYMIV) resistance in mungbean (Vigna radiata (L.) Wilczek) in India and Pakistan. Breed. Sci. 2013, 63, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, K.K.; Baisakh, B.; Mohanty, A. Recent developments on Yellow Mosaic Virus (YMV) and Mung bean Yellow Mosaic India Virus (MYMIV) resistance linked DNA markers in Vigna species—A review. Environ. Ecol. 2013, 32, 372–376. [Google Scholar]
- Naimuddin, M.A.; Singh, N.P. Yellow mosaic of mungbean and urdbean: Current status and future strategies. J. Food Legum. 2016, 29, 77–93. [Google Scholar]
- Briddon, R.; Patil, B.; Bagewadi, B.; Nawaz-ul-Rehman, M.; Fauquet, C. Distinct evolutionary histories of DNA-A and DNA-B components of bipartite begomoviruses. BMC Evol. Biol. 2010, 10, 97. [Google Scholar] [CrossRef]
- Qazi, J.; Ilyas, M.; Mansoor, S.; Briddon, R.O.B.W. Legume Yellow Mosaic Viruses: Genetically isolated begomoviruses. Mol. Plant Pathol. 2007, 8, 343–348. [Google Scholar] [CrossRef]
- Malathi, V.G.; John, P. Geminiviruses infecting legumes. In Characterization, Diagnosis and Management of Plant Viruses; Rao, G.P., Lava, K.P., Holguin-Pena, R.J., Eds.; Studium Press: Houston, TX, USA, 2008; pp. 97–123. [Google Scholar]
- Fauquet, C.M.; Stanley, J. Geminivirus classification and nomenclature: Progress and problems. Ann. Appl. Biol. 2003, 142, 165–189. [Google Scholar] [CrossRef]
- Maruthi, M.N.; Rekha, A.R.; Govindappa, M.R.; Colvin, J.; Muniyappa, V. A distinct begomovirus causes Indian Dolichos Yellow Mosaic Disease. Plant Pathol. 2006, 55, 290. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.-D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Pant, V.; Gupta, D.; Choudhury, N.R.; Malathi, V.G.; Varma, A.; Mukherjee, S.K. Molecular characterization of the Rep protein of the blackgram isolate of Indian Mungbean Yellow Mosaic Virus. J. Gen. Virol. 2001, 82, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.K.; Dasgupta, I. Begomovirus research in India: A critical appraisal and the way ahead. J. Biosci. 2012, 37, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C. Geminivirus DNA replication. Cell. Mol. Life Sci. 1999, 56, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Hanley-Bowdoin, L.; Settlage, S.B.; Orozco, B.M.; Nagar, S.; Robertson, D. Geminiviruses: Models for plant DNA replication, transcription, and cell cycle regulation. CRC. Crit. Rev. Plant Sci. 1999, 18, 71–106. [Google Scholar] [CrossRef]
- Raghavan, V.; Malik, P.S.; Choudhury, N.R.; Mukherjee, S.K. The DNA-A component of a plant geminivirus (Indian Mung bean Yellow Mosaic Virus) replicates in budding yeast cells. J. Virol. 2004, 78, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.R.; Malik, P.S.; Singh, D.K.; Islam, M.N.; Kaliappan, K.; Mukherjee, S.K. The oligomeric Rep protein of Mungbean Yellow Mosaic India Virus (MYMIV) is a likely replicative helicase. Nucleic Acids Res. 2006, 34, 6362–6377. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Karjee, S.; Malik, P.; Islam, M.; Mukherjee, S. DNA replication and pathogenecity of MYMIV. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Formatex: Badajoz, Spain, 2007; pp. 155–162. [Google Scholar]
- Akram, M.; Naimuddin; Agnihotri, A.K.; Gupta, S.; Singh, N.P. Characterisation of full genome of Dolichos Yellow Mosaic Virus based on sequence comparison, genetic recombination and phylogenetic relationship. Ann. Appl. Biol. 2015, 167, 354–363. [Google Scholar] [CrossRef]
- Brown, J.K.; Zerbini, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.C.F.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A.; et al. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef]
- Naimuddin; Akram, M.; Sanjeev, G. Identification of Mungbean Yellow Mosaic India Virus infecting Vigna mungo var. silvestris L. Phytopathol. Mediterr. 2011, 50, 94–100. [Google Scholar]
- Naimuddin; Akram, M.; Pratap, A. First report of natural infection of Mungbean Yellow Mosaic India Virus in two wild species of Vigna. New Dis. Rep. 2011, 23, 21. [Google Scholar] [CrossRef]
- Naimuddin; Akram, M.; Gupta, S.; Agnihotri, A.K. Ageratum conyzoides harbours Mungbean Yellow Mosaic India Virus. Plant Pathol. J. 2014, 13, 59–64. [Google Scholar]
- Biswas, M.; Maqani, N.; Rai, R.; Kumaran, S.P.; Iyer, K.R.; Sendinc, E.; Smith, J.S.; Laloraya, S. Limiting the extent of the RDN1 heterochromatin domain by a silencing barrier and Sir2 protein levels in Saccharomyces cerevisiae. Mol. Cell. Biol. 2009, 29, 2889–2898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Islam, M.; Sony, S.; Borna, R. Molecular characterization of mungbean yellow mosaic disease and coat protein gene in mungbean varieties of Bangladesh. Plant Tissue Cult. Biotechnol. 2012, 22, 73–81. [Google Scholar] [CrossRef]
- Kaur, L.; Srihari, J.M.; Natesan, S.; Bharthi, N.; Malathi, V.G.; Nagarajan, P. Molecular cloning and characterization of from mung bean from northern region of Tamil Nadu indicates association of Mungbean Yellow Mosaic India Virus DNA A with a recombinant DNA B. J. Mycol. Plant Pathol. 2015, 45, 173–181. [Google Scholar]
- Nair, R.M.; Götz, M.; Winter, S.; Giri, R.R.; Boddepalli, V.N.; Sirari, A.; Bains, T.S.; Taggar, G.K.; Dikshit, H.K.; Aski, M.; et al. Identification of mungbean lines with tolerance or resistance to yellow mosaic in fields in india where different begomovirus species and different Bemisia tabaci cryptic species predominate. Eur. J. Plant Pathol. 2017, 149, 349–365. [Google Scholar] [CrossRef]
- Ahmad, M.; Harwood, R.F. Studies on a whitefly-transmitted yellow mosaic of urd bean (Phaseolus mungo). Plant Dis. Rep. 1973, 57, 800–802. [Google Scholar]
- Bashir, M.; Zubair, M.; Malik, B.A. Disease resistance sources and utilization in breeding improved mungbean in Pakistan. In Proceedings of the Second International Symposium, Bangkok, Thailand, 16–20 November 1987; pp. 623–630. [Google Scholar]
- Wei, J.; Zhao, J.-J.; Zhang, T.; Li, F.-F.; Ghanim, M.; Zhou, X.-P.; Ye, G.-Y.; Liu, S.-S.; Wang, X.-W. Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J. Virol. 2014, 88, 13460–13468. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.G. Studies on the Yellow Mosaic of Blackgram Caused by Mungbean Yellow Mosaic Virus. Ph.D. Thesis, G.B. Pant University of Agriculture and Technology, Pantnagar, India, 1971. [Google Scholar]
- Rathi, Y.P.S. Mungbean Yellow Mosaic Virus: Host Range and Relationship with the Vector, Bemisia Tabaci Genn. Ph.D. Thesis, Pant University of Agriculture & Technology, Pantnagar, India, 1972. [Google Scholar]
- Murugesan, S.; Chelliah, S. Influence of sowing time on the incidence of the vector Bemisia tabaci (Genn.) and the Yellow Mosaic Disease of greengram. Madras Agric. J. 1977, 64, 128–130. [Google Scholar]
- Stanley, J.; Markham, P.G.; Callis, R.J.; Pinner, M.S. The nucleotide sequence of an infectious clone of the geminivirus Beet Curly Top Virus. EMBO J. 1986, 5, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, H.K.; Devamani Jayappa, B.D. Host range of Yellow Mosaic Virus and influence of age of seedlings on transmission of MYMV in mungbean. Res. J. Agric. Sci. 2017, 8, 1235–1237. [Google Scholar]
- Deepa, H.; Govindappa, M.R.; Kenganal, M.; Kulkarni, S.A.; Biradar, S.A. Screening of greengram genotypes against Mungbean Yellow Mosaic Virus diseases under field condition. Int. J. Pure Appl. Biosci. 2017, 5, 1049–1056. [Google Scholar]
- Sai, C.B.; Nagarajan, P.; Raveendran, M.; Rabindran, R.; Senthil, N. Understanding the inheritance of Mungbean Yellow Mosaic Virus (MYMV) resistance in mungbean (Vigna radiata L. Wilczek). Mol. Breed. 2017, 37, 63. [Google Scholar] [CrossRef]
- Usharani, K.S.; Surendranath, B.; Haq, Q.M.R.; Malathi, V.G. Yellow Mosaic Virus infecting soybean in northern India is distinct from the species infecting soybean in southern and western India. Curr. Sci. 2004, 86, 845–850. [Google Scholar]
- Shahid, M.S.; Pudashini, B.J.; Khatri-Chhetri, G.B.; Ikegami, M.; Natsuaki, K.T. First report of Mungbean Yellow Mosaic India Virus on kidney bean in Nepal. New Dis. Rep. 2012, 25, 30. [Google Scholar] [CrossRef]
- Biswas, K.K.; Malathi, V.G.; Varma, A. Diagnosis of symptomless yellow mosaic begomovirus infection in pigeonpea by using cloned Mungbean Yellow Mosaic India Virus as probe. J. Plant Biochem. Biotechnol. 2008, 17, 9–14. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, V.; Rathi, P.; Shukla, S. Linkage mapping of Mungbean Yellow Mosaic India Virus (MYMIV) resistance gene in soybean. Breed. Sci. 2017, 67, 95–100. [Google Scholar] [CrossRef]
- Marabi, R.; Sagare, D.; Sagare, S.; Das, S.B.; Bhowmick, A.K.; Noda, H. Molecular detection of Mungbean Yellow Mosaic India Virus (MYMIV) infecting soybean in Madhya Pradesh. Biosci. Biotechnol. Res. Asia 2017, 14, 315–318. [Google Scholar] [CrossRef]
- Marabi, R.; Das, S.B.; Tripathi, N.; Bhowmick, A.K.; Pachori, R.; Vibha, V. Molecular identification of Mungbean Yellow Mosaic India Virus (MYMIV) from whitefly and soybean in Jabalpur district of Madhya Pradesh, Central India. Int. J. Chem. Stud. 2018, 6, 894–896. [Google Scholar]
- Bhaskara Reddy, B.V.; Obaiah, S.; Prasanthi, L.; Sivaprasad, Y.; Sujitha, A.; Giridhara Krishna, T. Mungbean Yellow Mosaic India Virus is associated with yellow mosaic disease of blackgram (Vigna mungo L.) in Andhra Pradesh, India. Arch. Phytopathol. Plant Prot. 2015, 48, 345–353. [Google Scholar] [CrossRef]
- Chakraborty, N.; Basak, J. Comparative transcriptome profiling of a resistant vs. susceptible Vigna mungo cultivar in response to Mungbean Yellow Mosaic India Virus infection reveals new insight into MYMIV resistance. Curr. Plant Biol. 2018, 15, 8–24. [Google Scholar] [CrossRef]
- Marabi, R.; Sagare, D. Molecular identification of Mungbean Yellow Mosaic India Virus (MYMIV) from alternate weed and crop hosts. Ann. Plant Prot. Sci. 2017, 25, 152–155. [Google Scholar]
- Shahid, M.; Al-Mahmooli, I.; Al-Sadi, A.; Briddon, R. Identification of Mungbean Yellow Mosaic India Virus infecting cucumber in Oman. Plant Dis. 2018, 102, 465. [Google Scholar] [CrossRef]
- Fauquet, C.M.; Briddon, R.W.; Brown, J.K.; Moriones, E.; Stanley, J.; Zerbini, M.; Zhou, X. Geminivirus strain demarcation and nomenclature. Arch. Virol. 2008, 153, 783–821. [Google Scholar] [CrossRef] [PubMed]
- Prema, G.U.; Rangaswamy, K.T. Field evaluation of horse gram germplasm/genotypes against Horse gram Yellow Mosaic Virus (HgYMV) disease and biological transmission of Horse gram Yellow Mosaic Virus to different leguminous hosts through white flies. Int. J. Agric. Sci. 2017, 9, 4934–4939. [Google Scholar]
- Poehlman, J.M. The Mungbean; Oxford & IBH Pub.: New Delhi, India, 1991; pp. 169–274. ISBN 0813313783. [Google Scholar]
- Aliyu, B.; Ng, N.Q.; Fawole, I. Inheritance of pubescences in crosses between Cowpea (Vigna unquiculata (L)) WAIP) and V. Rhomboidea Burtt. Davy. Niger. J. Genet. 2000, 15, 9–14. [Google Scholar] [CrossRef]
- Mohammed, M.S.; Russom, Z.; Abdul, S.D. Inheritance of hairiness and pod shattering, heritability and correlation studies in crosses between cultivated cowpea (Vigna unguiculata (L.) Walp.) and its wild (var. pubescens) relative. Euphytica 2010, 171, 397–407. [Google Scholar] [CrossRef]
- Fatokun, C.A.; Singh, B.B. Improving Cowpea-Cereals Systems in the Dry Savannas; Annual Report; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2001; p. 79. [Google Scholar]
- Dwivedi, S.; Singh, D.P. Inheritance of pod pubscence and seed coat colour in urdbean. Crop Improv. 1986, 13, 54–57. [Google Scholar]
- Elden, T.C.; Elgin, J.H., Jr.; Soper, J.F. Inheritance of pubescence in selected clones from two alfalfa populations and relationship to potato leafhopper resistance. Crop Sci. 1986, 26, 1143–1146. [Google Scholar] [CrossRef]
- Gunasinghe, U.B.; Irwin, M.E.; Kampmeier, G.E. Soybean leaf pubescence affects aphid vector transmission and field spread of Soybean Mosaic Virus. Ann. Appl. Biol. 1988, 112, 259–272. [Google Scholar] [CrossRef]
- Singh, C.M.; Mishra, S.B.; Pandey, A.; Arya, M. Multivariate analysis in mungbean [Vigna radiata (L.) Wilczek] to identify the genetic donors for pubescence and agro-morphological traits. Legum. Res. 2015, 38, 767–771. [Google Scholar] [CrossRef]
- Inbar, M.; Gerling, D. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu. Rev. Entomol. 2007, 53, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campos, S.; Martínez-Ayala, A.; Márquez-Martín, B.; Aragón-Caballero, L.; Navas-Castillo, J.; Moriones, E. Fulfilling Koch’s postulates confirms the monopartite nature of tomato leaf deformation virus: A begomovirus native to the new world. Virus Res. 2013, 173, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.L.D.; Picanço, M.; Guedes, R.N.C.; Moreira, M.D. Factors affecting attack rate of whitefly on the eggplant. Pesqui. Agropecuária Bras. 2003, 38, 545–549. [Google Scholar] [CrossRef][Green Version]
- Pramanik, P.; Singha, S.; Chatterjee, M.L. Varietal preference of whitefly, Bemisia tabaci (Gennadius) among pumpkin cultivars. Pest Manag. Econ. Zool. 2004, 12, 105–107. [Google Scholar]
- Navon, A.; Melamed-Madjar, V.; Zur, M.; Ben-Moshe, E. Effects of cotton cultivars on feeding of Heliothis armigera and Spodoptera littoralis larvae and on oviposition of Bemisia tabaci. Agric. Ecosyst. Environ. 1991, 35, 73–80. [Google Scholar] [CrossRef]
- Ozgur, A.F.; Sekeroglu, E. Population development of Bemisia tabaci (Homoptera: Aleurodidae) on various cotton cultivars in Cukurova, Turkey. Agric. Ecosyst. Environ. 1986, 17, 83–88. [Google Scholar] [CrossRef]
- Lakshminarayan, S.; Singh, P.S.; Mishra, D.S. Relationship between whitefly population, YMV disease and morphological parameters of green gram germplasm. Environ. Ecol. 2008, 26, 978–982. [Google Scholar]
- Taggar, G.K.; Gill, R.S. Preference of whitefly, Bemisia tabaci, towards black gram genotypes: Role of morphological leaf characteristics. Phytoparasitica 2012, 40, 461–474. [Google Scholar] [CrossRef]
- Ashraf, M.; Zafar, Z.U. Some physiological characteristics in resistant and susceptible cotton cultivars infected with Cotton Leaf Curl Virus. Biol. Plant. 1999, 42, 615–620. [Google Scholar] [CrossRef]
- Chu, C.-C.; Freeman, T.P.; Buckner, J.S.; Henneberry, T.J.; Nelson, D.R.; Natwick, E.T. Susceptibility of upland cotton cultivars to Bemisia tabaci biotype B (Homoptera: Aleyrodidae) in relation to leaf age and trichome density. Ann. Entomol. Soc. Am. 2001, 94, 743–749. [Google Scholar] [CrossRef]
- Hasanuzzaman, A.T.M.; Islam, M.N.; Zhang, Y.; Zhang, C.-Y.; Liu, T.-X. Leaf morphological characters can be a factor for intra-varietal preference of whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) among eggplant varieties. PLoS ONE 2016, 11, e0153880. [Google Scholar] [CrossRef] [PubMed]
- Wynd, F.L. Metabolic phenomena associated with virus infection in plants. Bot. Rev. 1943, 9, 395. [Google Scholar] [CrossRef]
- Selman, I.W.; Brierley, M.R.; Pegg, G.F.; Hill, T.A. Changes in the free amino acids and amides in tomato plants inoculated with Tomato Spotted Wilt Virus. Ann. Appl. Biol. 1961, 49, 601–615. [Google Scholar] [CrossRef]
- Bozarth, R.F.; Diener, T.O. Changes in concentration of free amino acids and amides induced in tobacco plants by potato virus X and potato virus Y. Virology 1963, 21, 188–193. [Google Scholar] [CrossRef]
- Chhabra, K.S.; Kooner, B.S.; Saxena, A.K.; Sharma, A.K. Effect of biochemical components on the incidence of insect pest complex and Yellow Mosaic Virus in mungbean. Crop Improv. 1981, 8, 56–59. [Google Scholar]
- Chhabra, K.S.; Kooner, B.S.; Saxena, A.K.; Sharma, A.K. Influence of biochemical components on the incidence of insect pests and Yellow Mosaic Virus in blackgram. Indian J. Entomol. 1984, 46, 148–156. [Google Scholar]
- Chhabra, K.S.; Kooner, B.S. Sources of resistance in mungbean against major insectpests and Yellow Mosaic Virus. Legum. Res. 1992, 14, 175–184. [Google Scholar]
- Thind, S.K.; Monga, P.K.; Kaur, N.; Cheema, S.S. Analysis of some biochemical and micro-nutrient constituents of Yellow Mosaic Virus infected moong. Indian J. Virol. 1996, 12, 157–159. [Google Scholar]
- Dantre, R.K.; Keshwal, R.L.; Khare, M.N. Biochemical changes induced by yellow mosaic virus in the resistant and susceptible cultivar of soybean. Indian J. Virol. 1996, 12, 47–49. [Google Scholar]
- Sultana, N.; Kasem, M.A.; Hossain, M.D.; Alam, M.S. Biochemical changes of some promising lines of yard long bean due to the infection of Yellow Mosaic Virus. Thai J. Agric. Sci. 1998, 31, 322–327. [Google Scholar]
- Mali, P.C.; Uday, B.; Satish, L. Effect of planting dates and development of Yellow Mosaic Virus on biochemical constituents of moth bean genotypes. Indian Phytopathol. 2000, 53, 379–383. [Google Scholar]
- Chand, P.; Varma, J.P. Some characteristics of mungbean and urdbean varieties resistant and susceptible to Yellow Mosaic Virus. Indian Phytopathol. 1980, 33, 48–53. [Google Scholar]
- Sinha, A.; Srivastava, M. Biochemical changes in mungbean plants infected by Mungbean Yellow Mosaic Virus. Int. J. Virol. 2010, 6, 150–157. [Google Scholar] [CrossRef][Green Version]
- Ram, H.H.; Singh, K.; Verma, V.D. Breeding for resistance to Yellow Mosaic Virus through interspecific hybridization in soybean [Glycine formosana]. Soybean Genet. Newsl. 1984, 11, 46–48. [Google Scholar]
- Gill, C.K.; Labh, S. Biochemical changes in mungbean cultivar, ML-267 infected with Yellow Mosaic Virus. Insect Environ. 2000, 6, 86–87. [Google Scholar]
- Hemida, S.K. Effect of Bean Yellow Mosaic Virus on physiological parameters of Vicia faba and Phaseolus vulgaris. Int. J. Agric. Biol. 2005, 7, 154–157. [Google Scholar]
- Gunnar Fossdal, C.; Sharma, P.; Lönneborg, A. Isolation of the first putative peroxidase cDNA from a conifer and the local and systemic accumulation of related proteins upon pathogen infection. Plant Mol. Biol. 2001, 47, 423–435. [Google Scholar] [CrossRef]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Perveen, S.S.; Qaisrani, T.M.; Bhutta, S.; Perveen, R.; Naqvi, S.H.M. HPLC analysis of cotton phenols and their contribution in bollworm resistance. Online J. Biol. Sci. 2001, 1, 587–590. [Google Scholar]
- Singh, D.; Patel, P.N. Studies on resistance in crops to bacterial diseases in India. III. Investigations on inheritance of reactions to bacterial leaf spot and yellow mosaic diseases and linkage, if any, with other characters in mungbean. Indian Phytopathol. 1977, 30, 202–206. [Google Scholar]
- Malik, I.A.; Ali, Y.; Saleem, M. Incorporation of tolerance to Mungbean Yellow Mosaic Virus from local germplasm into exotic large-seeded mungbean. In Mungbean. Proceedings of the Second International Symposium in Mungbean; Shanmugasundaram, S., Ed.; AVRDC: Tainan, Taiwan, 1988; pp. 297–307. [Google Scholar]
- Saleem, M.; Haris, W.A.A.; Malik, I.A. Inheritance of Yellow Mosaic Virus in mungbean (Vigna radiata L. Wilczek). Pak. J. Phytopathol. 1998, 10, 30–32. [Google Scholar]
- Reddy, K.R.; Singh, D.P. Inheritance of resistance to Mungbean Yellow Mosaic Virus. Madras Agric. J. 1995, 88, 199–201. [Google Scholar]
- Dhole, V.J.; Reddy, K.S. Development of a SCAR marker linked with a MYMV resistance gene in mungbean (Vigna radiata L. Wilczek). Plant Breed. 2013, 132, 127–132. [Google Scholar] [CrossRef]
- Pal, S.S.; Dhaliwal, H.S.; Bains, S.S. Inheritance of resistance to Yellow Mosaic Virus in some Vigna species. Plant Breed. 1991, 106, 168–171. [Google Scholar] [CrossRef]
- Singh, D.P. Inheritance of resistance to Yellow Mosaic Virus in blackgram (Vigna mungo (L.) Hepper). Theor. Appl. Genet. 1980, 57, 233–235. [Google Scholar] [CrossRef]
- Verma, R.P.S.; Singh, D.P. The allelic relationship of genes giving resistance to Mungbean Yellow Mosaic Virus in blackgram. Theor. Appl. Genet. 1986, 72, 737–738. [Google Scholar] [CrossRef]
- Shukla, G.P.; Pandya, B.P. Resistance to yellow mosaic in greengram. SABRAO J. Breed. Genet. 1985, 17, 165–171. [Google Scholar]
- Solanki, I.S.; Dahiya, B.S.; Waldia, R.S. Resistance to Mungbean Yellow Mosaic Virus in blackgram. Indian J. Genet. Plant Breed. 1982, 42, 240–242. [Google Scholar]
- Murugan, E.; Nadarajan, N. Genetic studies on differential expression of Mungbean Yellow Mosaic Virus resistance related to trichome density in urd bean (Vigna mungo (L.) Hepper). Indian J. Plant Genet. Resour. 2012, 25, 135–138. [Google Scholar]
- Alam, A.K.M.M.; Somta, P.; Srinives, P.; Mahbubul Alam, A.K.M.; Somta, P.; Srinives, P. Identification and confirmation of quantitative trait loci controlling resistance to mungbean yellow mosaic disease in mungbean [Vigna radiata (L.) Wilczek]. Mol. Breed. 2014, 34, 1497–1506. [Google Scholar] [CrossRef]
- Selvi, R.; Muthiah, A.R.; Manivannan, N.; Raveendran, T.S.; Manickam, A.; Samiyappan, R. Tagging of RAPD marker for MYMV resistance in mungbean (Vigna radiata (L.) Wilczek). Asian J. Plant Sci. 2006, 5, 277–280. [Google Scholar]
- Akbar, W.; Aslam, M.; Maqbool, M.A.; Ali, M.; Arshad, M. Inheritance pattern of mungbean yellow mosaic disease resistance and gene action for different traits in mungbean (Vigna radiata (L.) Wilczek) under protected and unprotected field conditions. Plant Breed. 2018, 137, 763–772. [Google Scholar] [CrossRef]
- Khan, M.G.; Ahmad, W.; Khattak, G.S.S.; Siraj-ud-Din; Ahmad, A. Mode of inheritance of resistance to Mungbean Yellow Mosaic Virus (MYMV) in mungbean (Vigna radiata (l.) Wilczek). Sarhad J. Agric. 2007, 23, 1071–1074. [Google Scholar]
- Khattak, G.S.S.; Saeed, I.; Shah, S.A. Breeding high yielding and disease resistant mungbean (Vigna radiata (L.) Wilczek) genotypes. Pak. J. Bot. 2008, 40, 1411–1417. [Google Scholar]
- Singh, A.; Dikshit, H.K.; Jain, N.; Singh, D.; Yadav, R.N. Efficiency of SSR, ISSR and RAPD markers in molecular characterization of mungbean and other Vigna species. Indian J. Biotechnol. 2014, 13, 81–88. [Google Scholar]
- Gupta, S.; Gupta, D.S.; Anjum, T.K.; Pratap, A.; Kumar, J. Inheritance and molecular tagging of MYMIV resistance gene in blackgram (Vigna mungo L. Hepper). Euphytica 2013, 193, 27–37. [Google Scholar] [CrossRef]
- Kumar, B.; Talukdar, A.; Verma, K.; Bala, I.; Harish, G.D.; Gowda, S.; Lal, S.K.; Sapra, R.L.; Singh, K.P. Mapping of Yellow Mosaic Virus (YMV) resistance in soybean (Glycine max L. Merr.) through association mapping approach. Genetica 2015, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, T.S.; Brar, J.S.; Sandhu, S.S.; Verma, M.M. Inheritance of resistance to Mungbean Yellow Mosaic Virus in green gram. J. Res. Punjab Agric. Univ. 1985, 22, 607–611. [Google Scholar]
- Malik, I.A.; Sarwar, G.; Ali, Y. Inheritance of tolerance to Mungbean Yellow Mosaic Virus and some morphological characters. Pakistan J. Bot. 1986, 18, 189–198. [Google Scholar]
- Malik, I.A. Breeding for resistance to MYMV and its vector in Pakistan. In Proceedings of the International Workshop on Mungbean Yellow Mosaic Disease, Tainan, Taiwan, 2–3 July 1991; p. 79. [Google Scholar]
- Ammavasai, S.; Phogat, D.S.; Solanki, I.S. Inheritance of resistance to mungbean yellow mosaic virus (MYMV) in green gram [Vigna radiata (L.) Wilczek]. Indian J. Genet. Plant Breed. 2004, 64, 146. [Google Scholar]
- Dhole, V.J.; Reddy, K.S. Genetic analysis of resistance to Mungbean Yellow Mosaic Virus in mungbean (Vigna radiata). Plant Breed. 2012, 131, 414–417. [Google Scholar] [CrossRef]
- Kothandaraman, S.V.; Devadason, A.; Ganesan, M.V. Seed-borne nature of a begomovirus, Mung bean Yellow Mosaic Virus in black gram. Appl. Microbiol. Biotechnol. 2016, 100, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Bhanu, S.H.; Jayalakshmidevi, R.S.; Reddy, B.V.B.; Prasanthi, L. Host range studies for Yellow Mosaic Virus (YMV) infecting pulses. Int. J. Trop. Agric. 2015, 33, 1173–1185. [Google Scholar]
- Suman, S.; Sharma, V.; Kumar, H.; Shahi, V.K. Screening of mungbean [Vigna radiata (L.) Wilczek] genotypes for resistance to Mungbean Yellow Mosaic Virus (MYMV). Environ. Ecol. 2015, 33, 855–859. [Google Scholar]
- Khaliq, N.; Kaul, V.; Shankar, U.; Ganai, S.; Sharma, S.; Norboo, T. Screening of mungbean (Vigna radiata (L.) Wilczek) varieties against whitefly (Bemisia tabaci Genn.) and Mungbean Yellow Mosaic Virus (MYMV). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 129–132. [Google Scholar] [CrossRef]
- Singh, P.K.; Rai, N.; Singh, D.V.; Singh, A.P. Incidence of Dolichos Yellow Mosaic Virus (DYMV) and yield potential in indian bean (Lablal purpureus) F1’S. J. Agric. Technol. 2012, 8, 1469–1474. [Google Scholar]
- Taggar, G.; Gill, R.S.; Sandhu, J.S. Evaluation of black gram [Vigna mungo (L.) Hepper] genotypes against whitefly, Bemisia tabaci (Gennadius) under screen-house conditions. Acta Phytopathol. Entomol. Hungarica 2013, 48, 53–62. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Shobhana, V.G.; Sudha, M.; Raveendran, M.; Senthil, N.; Pandiyan, M.; Nagarajan, P. Mungbean Yellow Mosaic Virus (MYMV): A threat to green gram (Vigna radiata) production in Asia. Int. J. Pest Manag. 2014, 60, 314–324. [Google Scholar] [CrossRef]
- Nath, P. Effect of sowing time on the incidence of Yellow Mosaic Virus disease and whitefly population on greengram. Ann. Agric. Res. 1994, 15, 174–177. [Google Scholar]
- Ilyas, M.; Qazi, J.; Mansoor, S.; Briddon, R.W. Genetic diversity and phylogeography of begomoviruses infecting legumes in Pakistan. J. Gen. Virol. 2010, 91, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Ariyo, O.; Koerbler, M.; Dixon, A.; Atiri, G.; Winter, S. Development of an efficient virus transmission technique to screen cassava genotypes for resistance to cassava mosaic disease. In Proceedings of the Conference on International Agricultural Research for Development, Göttingen, Germany, 8–10 October 2003; pp. 1–11. [Google Scholar]
- Jamsari, L.S.; Utami, H.P.; Herberg, F.; Nellen, W.; Ferita, I. Injection technique could as a new promising method for artificial infection of geminivirus particles in chili pepper (Capsicum annuum L.). Asian J. Agric. Res. 2015, 9, 23–32. [Google Scholar]
- Palauqui, J.C.; Elmayan, T.; Pollien, J.M.; Vaucheret, H. Systemic acquired silencing: Transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 1997, 16, 4738–4745. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Kobayashi, K.; Yamaoka, N.; Ishikawa, M.; Nishiguchi, M. Graft transmission of RNA silencing to non-transgenic scions for conferring virus resistance in tobacco. PLoS ONE 2013, 8, e63257. [Google Scholar]
- Mayo, M.; Ryabov, E.; Fraser, G.; Taliansky, M. Mechanical transmission of Potato leafroll virus. J. Gen. Virol. 2000, 81, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.M.; Ziegler-Graff, V.; Reutenauer, A.; Herrbach, E.; Lemaire, O.; Guilley, H.; Richards, K.; Jonard, G. Agroinfection as an alternative to insects for infecting plants with Beet Western Yellows Luteovirus. Proc. Natl. Acad. Sci. USA 1992, 89, 9136–9140. [Google Scholar] [CrossRef]
- Mutterer, J.D.; Ziegler-Graff, V.; Richards, K.E. Agro-infection as a means of transmitting luteoviruses to host plants for study of gene expression. In The Luteoviridae; Smith, H.G., Barker, H., Eds.; CABI Publishing: New York, NY, USA, 1999; pp. 43–67. [Google Scholar]
- Lapidot, M.; Polston, J.E. Biology and epidemiology of Bemisia-vectored viruses. In Bionomics and Management of a Global Pest; Springer: Dordrecht, The Netherlands, 2010; pp. 227–231. [Google Scholar]
- Mandal, B.; Varma, A.; Malathi, V.G. Systemic infection of Vigna mungo using the cloned DNAs of the blackgram isolate of Mungbean Yellow Mosaic Geminivirus through agroinoculation and transmission of the progeny virus by whiteflies. J. Phytopathol. 1997, 145, 505–510. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Sudha, M.; Nagarajan, P.; Pandiyan, M.; Muthurajan, R.; Natesan, S.; Kathithachalam, A. Using SSR marker to identify the MYMV resistance gene in mungbean [Vigna radiata (L.) Wilczek]. Rom. J. Biol.—Plant Biol. 2012, 57, 105–113. [Google Scholar]
- Karthikeyan, A.; Sudha, M.; Natesan, S.; Pandiyan, M.; Muthurajan, R.; Nagrajan, P. Screening and identification of random amplified polymorphic DNA (RAPD) markers linked to Mungbean Yellow Mosaic Virus (MYMV) resistance in mungbean (Vigna radiata (L.) Wilczek). Arch. Phytopathol. Plant Prot. 2012, 45, 712–716. [Google Scholar] [CrossRef]
- Bashir, M.; Zubair, M. Studies on Viral Disease of Major Pulse Crops and Identification of Resistant Sources; Technical Annual Report (April 2004 to June 2005) of APL Project; Crop Sciences Institute, National Agricultural Research Centre: Islamabad, Pakistan, 2005; p. 169. [Google Scholar]
- Rogers, S.G.; Bisaro, D.M.; Horsch, R.B.; Fraley, R.T.; Hoffmann, N.L.; Brand, L.; Elmer, J.S.; Lloyd, A.M. Tomato golden mosaic virus A component DNA replicates autonomously in transgenic plants. Cell 1986, 45, 593–600. [Google Scholar] [CrossRef]
- Jacob, S.S.; Vanitharani, R.; Karthikeyan, A.S.; Chinchore, Y.; Thillaichidambaram, P.; Veluthambi, K. Mungbean Yellow Mosaic Virus-Vi agroinfection by codelivery of DNA A and DNA B from one agrobacterium strain. Plant Dis. 2003, 87, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Sudha, M.; Pandiyan, M.; Natesan, S.; Shobhana, V.; Nagarajan, P. Screening of MYMV resistant mungbean (Vigna radiata L. Wilczek) progenies through agroinoculation. Int. J. Plant Pathol. 2011, 2, 115–125. [Google Scholar] [CrossRef][Green Version]
- Malathi, V.G.; Surendranath, B.; Naghma, A.; Roy, A. Adaptation to new hosts shown by the cloned components of Mungbean Yellow Mosaic India Virus causing cowpea golden mosaic in northern India. Can. J. Plant Pathol. 2010, 27, 439–447. [Google Scholar] [CrossRef]
- Kumar, J.; Gupta, D.S.; Gupta, S.; Dubey, S.; Gupta, P.; Kumar, S. Quantitative trait loci from identification to exploitation for crop improvement. Plant Cell Rep. 2017, 36, 1187–1213. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Chattopadhyay, D. Differential soybean gene expression during early phase of infection with Mungbean Yellow Mosaic India Virus. Mol. Biol. Rep. 2014, 41, 5123–5134. [Google Scholar] [CrossRef] [PubMed]
- Rouhibakhsh, A.; Malathi, V.G. Infectivity of blackgram isolate of Mungbean Yellow Mosaic India Virus on cowpea. Indian J. Virol. 2008, 19, 191–195. [Google Scholar]
- Singh, C.M.; Kumar, R.; Mishra, S.B.; Pandey, A.; Arya, M. Characterization of mungbean genotypes against Mungbean Yellow Mosaic Virus and cercospora leaf spot diseases under north east plain zone. Int. J. Agric. Environ. Biotechnol. 2015, 8, 119–125. [Google Scholar] [CrossRef]
- Parihar, A.K.; Basandrai, A.K.; Sirari, A.; Dinakaran, D.; Singh, D.; Kannan, K.; Kushawaha, K.P.S.; Adinarayan, M.; Akram, M.; Latha, T.K.S.; et al. Assessment of mungbean genotypes for durable resistance to yellow mosaic disease: Genotype × environment interactions. Plant Breed. 2017, 136, 94–100. [Google Scholar] [CrossRef]
- Ghafoor, A.; Zubair, M.; Malik, B.A.; Iqbal, S.M. Evaluation of selected germplasm of mungbean (Vigna radiata (L.) Wilczek). Pakistan J. Bot. 1992, 24, 112–118. [Google Scholar]
- Anjum, N.A.; Umar, S.; Chan, M.-T.; Reumann, S.; Corpas, F.J. Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–53. ISBN 978-90-481-9404-9. [Google Scholar]
- Dikshit, H.K.; Sharma, T.R.; Singh, B.B.; Kumari, J. Molecular and morphological characterization of fixed lines from diverse cross in mung bean (Vigna radiata (L.) Wilczek). J. Genet. 2009, 88, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.B.; Ajeigbe, H.A.; Tarawali, S.A.; Fernandez-Rivera, S.; Abubakar, M. Improving the production and utilization of cowpea as food and fodder. Field Crops Res. 2003, 84, 169–177. [Google Scholar] [CrossRef]
- Singh, B.V.; Ahuja, M.R. Phaseolus sublobatus Roxb.: A source of resistance to Yellow Mosaic Virus for cultivated mung. Indian J. Genet. Plant Breed. 1977, 37, 130–132. [Google Scholar]
- Pandiyan, M.; Ramamoorthi, N.; Ganesh, S.K.; Jebaraj, S.; Pagarajan, P.; Ponnuswami, B. Broadening the genetic base and introgression of MYMV resistance and yield improvement through unexplored genes from wild relatives in mungbean. Plant Mutat. Rep. 2008, 2, 33–38. [Google Scholar]
- Isemura, T.; Kaga, A.; Tabata, S.; Somta, P.; Srinives, P.; Shimizu, T.; Jo, U.; Vaughan, D.A.; Tomooka, N. Construction of a Genetic Linkage Map and Genetic Analysis of Domestication Related Traits in Mungbean (Vigna radiata). PLoS ONE 2012, 7, e41304. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A. Use of EST database markers from M. truncatula in the transferability to other forage legumes. J. Environ. Biol. 2011, 32, 347–354. [Google Scholar] [PubMed]
- Pratap, A.; Gupta, S.; Tomar, R.; Malviya, N.; Maurya, R.; Pandey, V.R.; Mehandi, S.; Singh, N.P. Cross-genera amplification of informative microsatellite markers from common bean and scarlet runner bean for assessment of genetic diversity in mungbean (Vigna radiata). Plant Breed. 2016, 135, 499–505. [Google Scholar] [CrossRef]
- Basak, J.; Kundagrami, S.; Ghose, T.K.; Pal, A. Development of Yellow Mosaic Virus (YMV) resistance linked DNA marker in Vigna mungo from populations segregating for YMV-reaction. Mol. Breed. 2005, 14, 375–383. [Google Scholar] [CrossRef]
- Souframanien, J.; Gopalakrishna, T. ISSR and SCAR markers linked to the Mungbean Yellow Mosaic Virus (MYMV) resistance gene in blackgram [Vigna mungo (L.) Hepper]. Plant Breed. 2006, 125, 619–622. [Google Scholar] [CrossRef]
- Binyamin, R.; Khan, M.A.; Khan, N.A.; Khan, A.I. Application of SCAR markers linked with Mungbean Yellow Mosaic Virus disease-resistance gene in Pakistan mungbean germplasm. Genet. Mol. Res. 2015, 14, 2825–2830. [Google Scholar] [CrossRef]
- Kundu, A.; Patel, A.; Paul, S.; Pal, A. Transcript dynamics at early stages of molecular interactions of MYMIV with resistant and susceptible genotypes of the leguminous host, Vigna mungo. PLoS ONE 2015, 10, e0124687. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Paul, S.; Pal, A. Isolation, characterization, and structure analysis of a Non-TIR-NBS-LRR encoding candidate gene from MYMIV-resistant Vigna mungo. Mol. Biotechnol. 2012, 52, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.B.; Bhareti, P.; Muthamilarasan, M.; Mukherjee, M.; Khan, Y.; Rathi, P.; Prasad, M. Genome-wide SNP identification and characterization in two soybean cultivars with contrasting Mungbean Yellow Mosaic India Virus disease resistance traits. PLoS ONE 2015, 10, e0123897. [Google Scholar] [CrossRef] [PubMed]
- Naresh, P.; Krishna Reddy, M.; Reddy, A.C.; Lavanya, B.; Lakshmana Reddy, D.C.; Madhavi Reddy, K. Isolation, characterization and genetic diversity of NBS-LRR class disease-resistant gene analogs in multiple virus resistant line of chilli (Capsicum annuum L.). 3 Biotech 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Dey, N.; Chaudhuri, S.; Pal, A. Molecular and biochemical characterization of a Vigna mungo MAP kinase associated with Mungbean Yellow Mosaic India Virus infection and deciphering its role in restricting the virus multiplication. Plant Sci. 2017, 262, 127–140. [Google Scholar] [CrossRef]
- Biswas, K.; Biswas, K.; Tarafdar, A. Multiple and mixed infections with yellow mosaic, leaf crinkle and bud necrosis disease complex in mungbean: A threat to cultivation of mungbean in India. Legum. Res. 2015, 38, 382–388. [Google Scholar] [CrossRef]
- Pratap, A.; Gupta, S.; Nair, M.R.; Gupta, K.S.; Schafleitner, R.; Basu, S.P.; Singh, M.C.; Prajapati, U.; Gupta, K.A.; Nayyar, H.; et al. Using plant phenomics to exploit the gains of genomics. Agronomy 2019, 9, 126. [Google Scholar] [CrossRef]
- Ferro, M.M.M.; Ramos-Sobrinho, R.; Xavier, C.A.D.; Zerbini, F.M.; Lima, G.S.A.; Nagata, T.; Assunção, I.P. New approach for the construction of infectious clones of a circular DNA plant virus using Gibson Assembly. J. Virol. Methods 2019, 263, 20–23. [Google Scholar] [CrossRef]
- Sehrawat, N.; Yadav, M.; Bhat, K.V.; Sairam, R.K.; Jaiwal, P.K. Introgression of Mungbean Yellow Mosaic Virus resistance in Vigna mungo (L.) Hepper and purity testing of F1 hybrids using SSRs. Turkish J. Agric. For. 2016, 40, 95–100. [Google Scholar] [CrossRef]
- Chaisan, T.; Somta, P.; Srinives, P.; Chanprame, S.; Kaveeta, R.; Dumrongkittikule, S. Development of tetraploid plants from an interspecific hybrid between mungbean (Vigna radiata) and rice bean (Vigna umbellata). J. Crop Sci. Biotechnol. 2013, 16, 45–51. [Google Scholar] [CrossRef]
- Bhanu, A.N.; Kumar, P.; Singh, M.N.; Srivastava, K.; Hemantaranjan, A. Assessment of genetic purity of inter-specific F1 hybrids involving Vigna radiata and Vigna umbellata. J. Exp. Biol. 2017, 5, 636–643. [Google Scholar] [CrossRef]
- Mathivathana, M.K.; Murukarthick, J.; Karthikeyan, A.; Jang, W.; Dhasarathan, M.; Jagadeeshselvam, N.; Sudha, M.; Vanniarajan, C.; Karthikeyan, G.; Yang, T.-J.; et al. Detection of QTLs associated with Mungbean Yellow Mosaic Virus (MYMV) resistance using the interspecific cross of Vigna radiata × Vigna umbellata. J. Appl. Genet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.; Anusuya, P.; Mahadev, N.G.; Karthikeyan, A.; Nagarajan, P.; Raveendran, M.; Senthil, N.; Pandiyan, M.; Angappan, K.; Balasubramanian, P. Molecular studies on mungbean (Vigna radiata (L.) Wilczek) and ricebean (Vigna umbellata (Thunb.)) interspecific hybridisation for Mungbean Yellow Mosaic Virus resistance and development of species-specific SCAR marker for ricebean. Arch. Phytopathol. Plant Prot. 2013, 46, 503–517. [Google Scholar] [CrossRef]
- Sudha, M.; Karthikeyan, A.; Shobhana, V.G.; Nagarajan, P.; Raveendran, M.; Senthil, N.; Pandiyan, M.; Angappan, K.; Balasubramanian, P.; Rabindran, R. Search for Vigna species conferring resistance to Mungbean Yellow Mosaic Virus in mungbean. Plant Genet. Resour. 2015, 13, 162–167. [Google Scholar] [CrossRef]
- Chen, H.-M.; Ku, H.-M.; Schafleitner, R.; Bains, T.S.; George Kuo, C.; Liu, C.-A.; Nair, R.M. The major quantitative trait locus for Mungbean Yellow Mosaic Indian Virus resistance is tightly linked in repulsion phase to the major bruchid resistance locus in a cross between mungbean [Vigna radiata (L.) Wilczek] and its wild relative Vigna radiata ssp. sublobata. Euphytica 2013, 192, 205–216. [Google Scholar] [CrossRef]
- Pandiyan, M.; Natesan, S.; Ramamoorthi, N.; Muthiah, A.; Tomooka, N. Interspecific hybridization of Vigna radiata x 13 wild Vigna species for developing MYMV donar. Electron. J. Plant Breed. 2010, 1, 600–610. [Google Scholar]
- Liu, M.-S.; Kuo, T.C.-Y.; Ko, C.-Y.; Wu, D.-C.; Li, K.-Y.; Lin, W.-J.; Lin, C.-P.; Wang, Y.-W.; Schafleitner, R.; Lo, H.-F.; et al. Genomic and transcriptomic comparison of nucleotide variations for insights into bruchid resistance of mungbean (Vigna radiata [L.] R. Wilczek). BMC Plant Biol. 2016, 16, 46. [Google Scholar] [CrossRef]
| Viruses | Hosts | References |
|---|---|---|
| MYMV | Pigeon pea (Cajanus cajan) | [49,50,51] |
| Urdbean (V. mungo) | [49,51] | |
| Soybean (Glycine max) | [49,51,52] | |
| Common bean (Phaseolus vulgaris) | [49,51] | |
| Horse gram (Macrotyloma uniflorum) | [49,50] | |
| Cowpea (Vigna unguiculata) | [50] | |
| Nicotiana benthamiana | [50] | |
| Croton bonplandianum | [50] | |
| Euphorbia geniculata | [50] | |
| Parthenium hysterophorus | [50] | |
| Malvestrum cormandelianum | [50] | |
| Acalypha indica | [50] | |
| Alternanthera sessilis | [50] | |
| MYMIV | Common bean (P. vulgaris) | [53] |
| Lima bean (P. lunatus) | [53] | |
| Pigeon pea (C. cajan) | [54] | |
| Soybean (G. max) | [55,56,57] | |
| Urdbean (V. mungo) | [58,59,60] | |
| Wild mungbean (V. radiata var. sublobata) | [36] | |
| Wild urdbean (V. mungo var. silvestris) | [35] | |
| Cucumber (Cucumis sativus) | [61] | |
| Ageratum conizoides | [37,60,62] | |
| Corchorus olitorius | [37,60,62] | |
| A. sessilis | [37,60,62] | |
| HgYMV | Common bean (P. vulgaris) | [63] |
| Pole bean (Phaseolus coccineus) | [63] | |
| Soybean (G. max) | [63] | |
| Lima bean (P. lunatus) | [63] | |
| Rice bean (Vigna umbellata) | [63] | |
| Moth bean (Vigna aconitifolia) | [21,63] |
| Disease | Cross Combinations | Population Type | Population Size | Inheritance Pattern | References |
|---|---|---|---|---|---|
| Yellow Mosaic Disease (YMD) | NM 1-32-1 × NM 6-68-2 | F2 | 150 | Two major genes with additive effects | [114] |
| MYMIV Disease | KPS2 × NM10-12-1 | RILs | 122 | QTL | [17] |
| BM1 × BM6 | F2 | – | QTL | [112] | |
| MYMV Disease | NM92 × VC2272 | F2 | 300 | Single recessive gene | [115] |
| 6601 × VC2272 | F2 | 240 | Single recessive gene | [115] | |
| 6601 × Pusa Baisakhi | F2 | 240 | Single recessive gene | [115] | |
| VC3902A × NM92 | F2 | 340 | Single recessive gene | [115] | |
| VC3902A × ML-5 | F2 | 360 | Single recessive gene | [115] | |
| NM92 × Pusa Baisaki | F2 | 220 | Single recessive gene | [115] | |
| VC 1560D × 6601 | F2 | 400 | Single recessive gene | [115] | |
| VC 1560D × NM92 | F2 | 400 | Single recessive gene | [115] | |
| NM 92 × NM 98 | F2 | − | Single recessive gene | [116] | |
| VBN(Gg)2 × KMG189 | F2/RILs | − | Single recessive gene | [51] |
| Primer Name | Accession Number | Forward-Sequence | Reverse-Sequence | Product Size (bps) | Detected DNA | References |
|---|---|---|---|---|---|---|
| NM 1 (AV1P) F/NM 2 (AV1P) R | FJ821189 | GTA TTT GCA KCA WGT TCA AGA | AGG DGT CAT TAG CTT AGC | 1000 | MYMIV/DNA A | [35,37] |
| AC1P | FJ663015 | AGT TGA TAT GGA TGT AATAGC3 | ACA AAA ACG ACT TCA AATATG CCA A | 1100 | MYMIV/DNA A | [35,37] |
| MYMIV-MP | FJ663015 | ATG GAA AAT TAT TCA GGT GCA | CTA CAA CGC TTT GTT CAC ATT | 900 | MYMIV/DNA B | [35,37] |
| AC2P | FJ663015 | AGC TAA TGA CCC CTA AAT TAT | GAG TAC TTG GAT GAA GAG AAC | 480 | MYMIV/DNA A | [35,37] |
| AC3P | FJ663015 | TTA TGA TTC GAT ATT GAA TTA ATA | CTG AAG TGTGGG TGT AGC TAT | 450 | MYMIV/DNA A | [35,37] |
| AC4P | FJ663015 | CAA ATT ACAATT TAA GTT ATG | ACT TCT AGCCTT GTC AAC ACC AG | 390 | MYMIV/DNA A | [35,37] |
| MYMV-Coat protein (CP) | AY271896 | ATG GG (T/G) TCC GTT GTA TGC TTG | GGC GTC ATT AGC ATA GGC AAT | 1000 | MYMV/DNA A | [35,37] |
| MYMV-MP | AY271896 | ATG GAG AAT TAT TCA GGC GCA | TTA CAA CGC TTT GTT CAC ATT | 900 | MYMV/DNA B | [35,37] |
| HYMV-CP | NC_005635 | ATG CTT GCA ATT AAG TAC TTG CA | TAG GCG TCA TTA GCA TAG GCA | 1050 | HgYMV/DNA A | [35,37] |
| HYMV-MP | NC_005635 | ATG GAG CAT TAT TCC GGT GCA | TTA CA(G/A) GGT TTT GTT TAC AGT | 900 | HgYMV/DNA B | [35,37] |
| DoYMV-CP | AY309241 | CTG TGA AAT TTG TGC AGG | TAC GCG GTT GCG AAT ATG TAT | 900 | DoYMV/DNA A | [35,37] |
| Rep VI | AF361431.1 | AATGTAAAAGGCGACTCATA | GAGAATTCACCGGTCGCGGGGCA | 566 | MYMIV | [38] |
| CP | AY271896 | ACACGAGCTCCTCTACCCCGATATCGAATG | ACACGGATCCGTTGCATACACAGGATTTG | 750 | MYMV/DNA-A | [39] |
| Deng-F/R | − | TAATATTACC(GT)G(AT)(GT)G(AGC)CC(GC)C | TAATATTACC(GT)G(AT)(GT)G(AGC)CC(GC)CCCTTCACA | 530 | MYMIV/partial DNA A | [40] |
| PAL1v1978/PAR 1c496 | − | GCATCTGCAGGCCCACATYGTCTTYCCNGT | AATACTGCAGGGCTTYCTRTACATRGG | 1.5 kbp | MYMIV/partial DNA A | [40] |
| AV 494/AC 1048 | FJ821189 | GCC(C/T)AT(G/A)TA(T/C)AG(A/G)AAGCC(A/C)AG | GG(A/G)TT(A/G/T)GA(G/A)GCATG(T/A/C)GTACATG | 500–600 bp | MYMIV/partial DNA A | [40] |
| AC-abut/AV-abut | MF818048.1 | GTAAAGCTTTACGCATAATG | AAAGCTTACATCCTCCAC | 2.7 kbp | MYMIV Full length DNA A | [40] |
| BV-abut/BC-abut | MF818046.1 | CCAGGATCCAATGATGCCT | ATTGGATCCTGGAGATTCA | 2.7 kbp | MYMIV Full length DNA B | [40] |
| RHA-F/AC-abut | − | TCAAGCTCCCGGTGCATGTTGCA | GTAAAGCTTTACGCATAATG | 920 bp | MYMIV Right half of DNA-A | [40] |
| PAR1v772/1c1960 | − | GGNAARATHTGGATGGA | ACNGGNAARACNATGTGGGC | 1.1 kb | MYMIV/DNA A | [40] |
| Viruses | Legume Crop | References |
|---|---|---|
| MYMV | Mungbean | [15,51,131,146,147,148] |
| Urdbean | [142] | |
| MYMIV | Mungbean | [9,149,150,151] |
| Urdbean | [9,58,149] | |
| Cowpea | [9,149,150,152] | |
| Pigeon pea | [54] | |
| HgYMV | Moth bean | [21] |
| Sl. No. | Candidate Genes Associated with LYMVs | Function of Gene | Expression in MYMIV Resistant Genotypes at Different Time Intervals | Expression in MYMIV Susceptible Genotypes at Different Time Intervals | References |
|---|---|---|---|---|---|
| 1 | Allene oxide cyclase (AOC: XM_014661012.1), Allene oxide synthase (AOS: XM_017565246.1), Acyl activating enzyme 1 (AAE1: XM_014663780.1) | Jasmonic acid biosynthesis pathway | Upregulated | Downregulated | [59,167] |
| 2 | Glycine-rich protein (GRP: XM_017558252.1), Proline-rich protein encoding gene (PRP: PRP-U72769.1), Hydroxy-proline-rich glycoprotein encoding gene (HRGP: XM_017559232.1) | Cell wall synthesis | Upregulated | Downregulated | [59,167] |
| 3 | Lipid transfer protein (LTPs: XM_014636461.1), PR proteins such as PR1 (JZ168313), PR2, PR3, PR4, PR5 (JZ168398) | Pathogenesis-related proteins | Upregulated | Downregulated | [59,167] |
| 4 | Pyridoxin (PDX1: XM_014661423.1), Glutathione-S-transferases (GST: XM_014648972.2), Peroxiredoxins (JK086508.1), Cu/Zn superoxide dismutase (JZ168376.1), and Ferredoxin-like protein such as PRX (JZ168355.1), SOD, and TRX | Reactive oxygen scavengers | PDX1 up and early expression of ROS gene | PDX1 Down but in the late expression of ROS genes | [59,167] |
| 5 | RUBISCO (XM_014661061.1), PEPC, ribose-5-phosphate isomerase and aldehyde dehydrogenase (JZ168331.1) | Metabolic pathways genes | Upregulated | Downregulated | [59,167] |
| 6 | WRKY transcription factor | Transcription factor | Upregulated | Downregulated | [59,167] |
| 7 | MAP kinase (JZ168283.1) | Protein kinases | Upregulated | Downregulated | [59,167,171] |
| 8 | Drought responsive ESTs (CPRD2 and CPRD14: JZ168282.1) | Drought responsive | Upregulated | Downregulated | [59,167] |
| 9 | Ubiquitin ligase | Ubiquitin proteasome system | Upregulated | Downregulated | [59,167] |
| 10 | Ca2+ responsive calreticulins (CRTs) and calmodulins (CAM) (JZ168377) | Ca2+ response | Upregulated | Downregulated | [59,167] |
| 11 | Phenylpropanoid pathway (PAL: JZ168300.1) | Biosynthesis of lignin | Upregulated | Downregulated | [59,167] |
| 12 | R gene NBS-LRR (JZ168271.1), HSP90 (JZ168383) | Plant disease resistance against pathogens | Upregulated | Downregulated | [59,167] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, C.M.; Singh, P.; Pratap, A.; Pandey, R.; Purwar, S.; Vibha; Douglas, C.A.; Baek, K.-H.; Mishra, A.K. Breeding for Enhancing Legumovirus Resistance in Mungbean: Current Understanding and Future Directions. Agronomy 2019, 9, 622. https://doi.org/10.3390/agronomy9100622
Singh CM, Singh P, Pratap A, Pandey R, Purwar S, Vibha, Douglas CA, Baek K-H, Mishra AK. Breeding for Enhancing Legumovirus Resistance in Mungbean: Current Understanding and Future Directions. Agronomy. 2019; 9(10):622. https://doi.org/10.3390/agronomy9100622
Chicago/Turabian StyleSingh, Chandra Mohan, Poornima Singh, Aditya Pratap, Rakesh Pandey, Shalini Purwar, Vibha, Colin Andrew Douglas, Kwang-Hyun Baek, and Awdhesh Kumar Mishra. 2019. "Breeding for Enhancing Legumovirus Resistance in Mungbean: Current Understanding and Future Directions" Agronomy 9, no. 10: 622. https://doi.org/10.3390/agronomy9100622
APA StyleSingh, C. M., Singh, P., Pratap, A., Pandey, R., Purwar, S., Vibha, Douglas, C. A., Baek, K.-H., & Mishra, A. K. (2019). Breeding for Enhancing Legumovirus Resistance in Mungbean: Current Understanding and Future Directions. Agronomy, 9(10), 622. https://doi.org/10.3390/agronomy9100622







