Prospects of Bioenergy Cropping Systems for A More Social-Ecologically Sound Bioeconomy
Abstract
1. Introduction
2. Potential Contribution of Bioenergy Crop Cultivation in a Changing World
- (1)
- (2)
- (3)
- (4)
- These BCS should foster rural development and support the vast number of small-scale family farmers, managing about 80% of the global agricultural land and natural resources [84].
- (5)
- Accordingly, bioenergy crop cultivation must be planned and implemented systematically, and with the adoption of holistic approaches.
2.1. The Potential Social-Ecological Contribution of Bioenergy Crop Cultivation
2.1.1. Bioenergy Crop Cultivation and Biodiversity
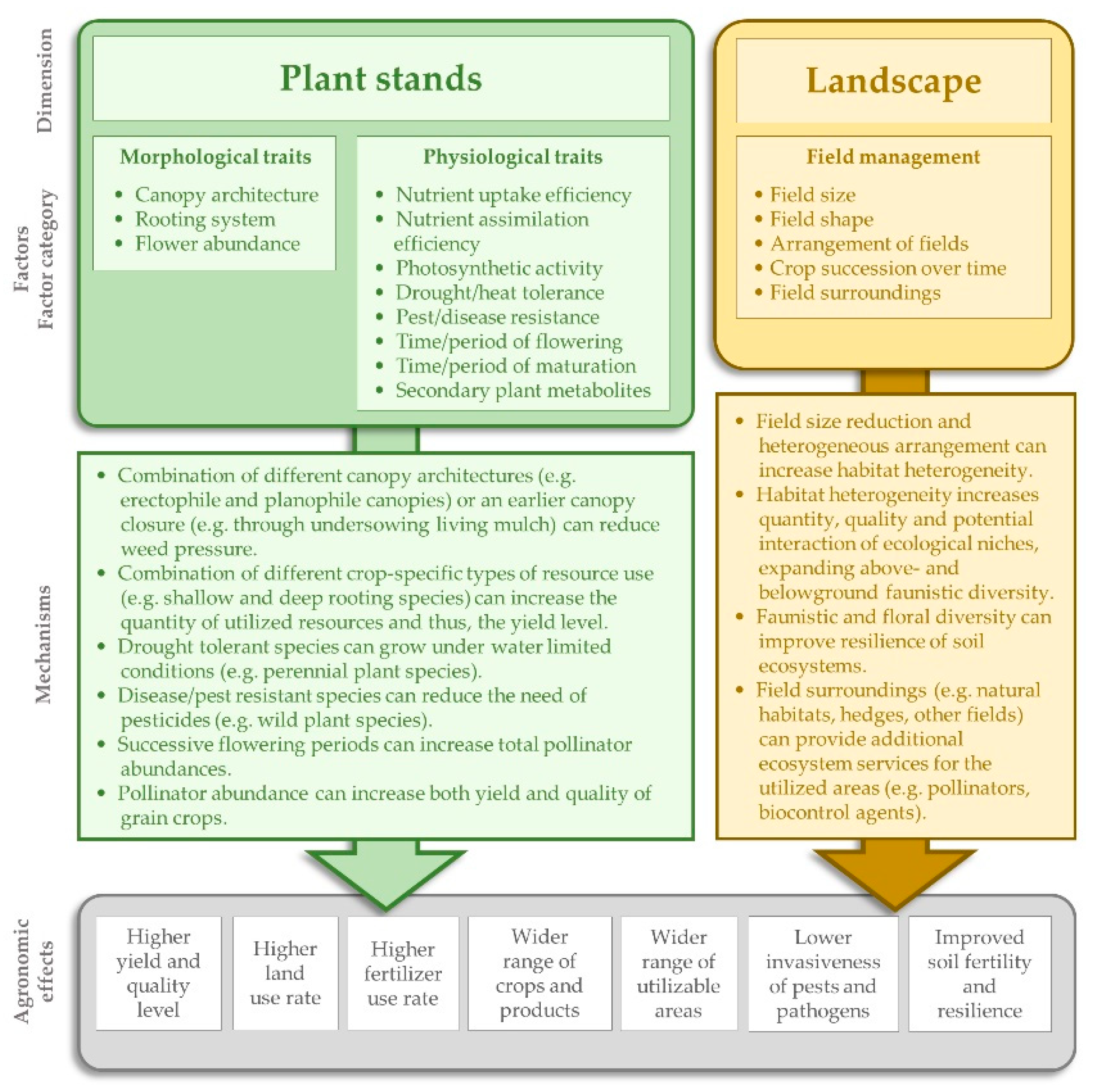
2.1.2. Spatial and Temporal Diversification of BCS
- (1)
- (2)
- (3)
- (4)
2.1.3. Low-Input Agriculture, GHG Mitigation Potential and the Role of Legumes
2.1.4. Groundwater Protection and Nutrient Recycling
2.1.5. Soil Erosion Mitigation under Steep Slope Conditions
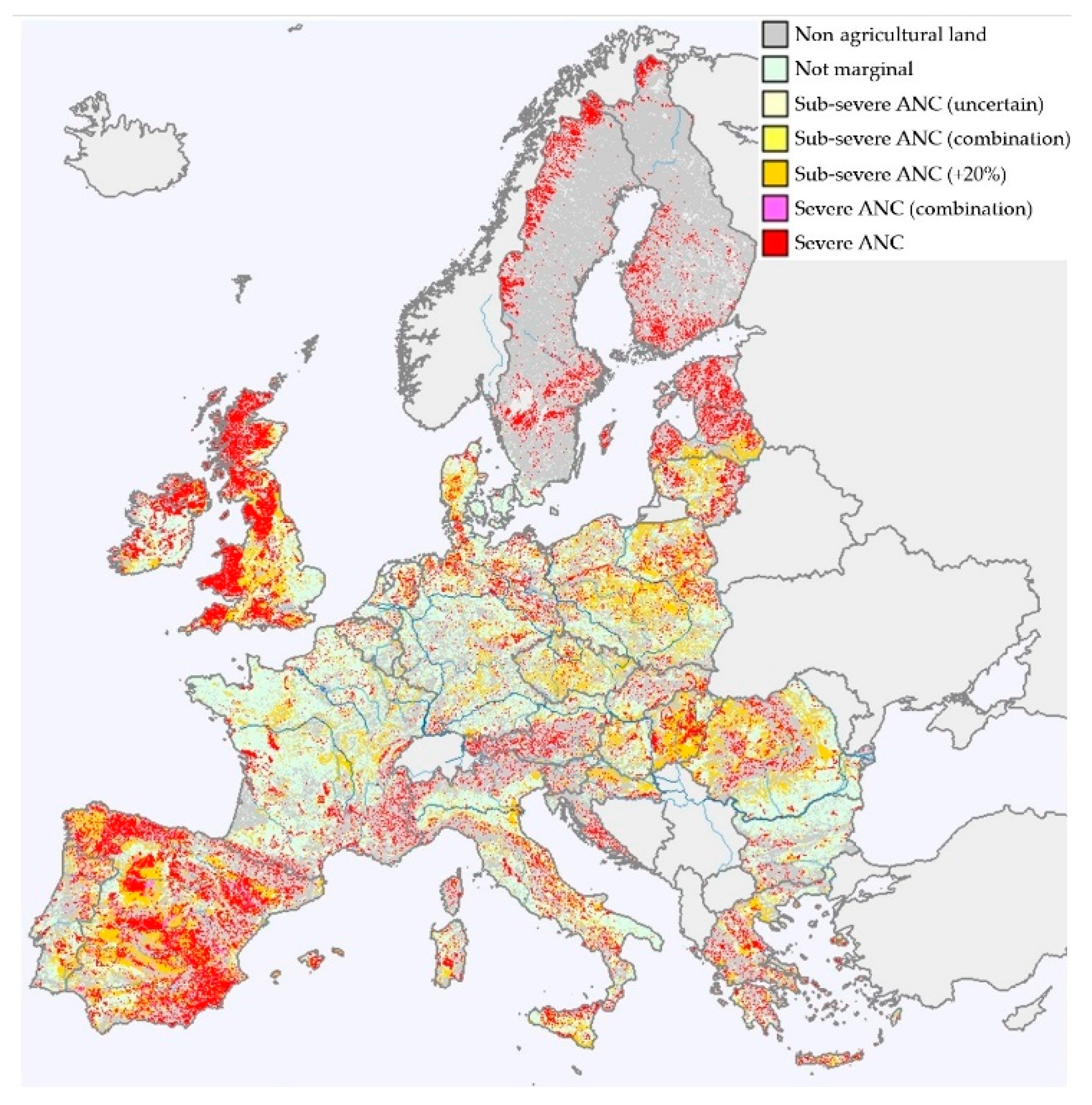
2.2. The Potential Growth Suitability of Bioenergy Crops on Marginal Agricultural Lands
2.3. Climate Change Effects on Agriculture and Adaptation Strategies for Bioenergy Cropping Systems
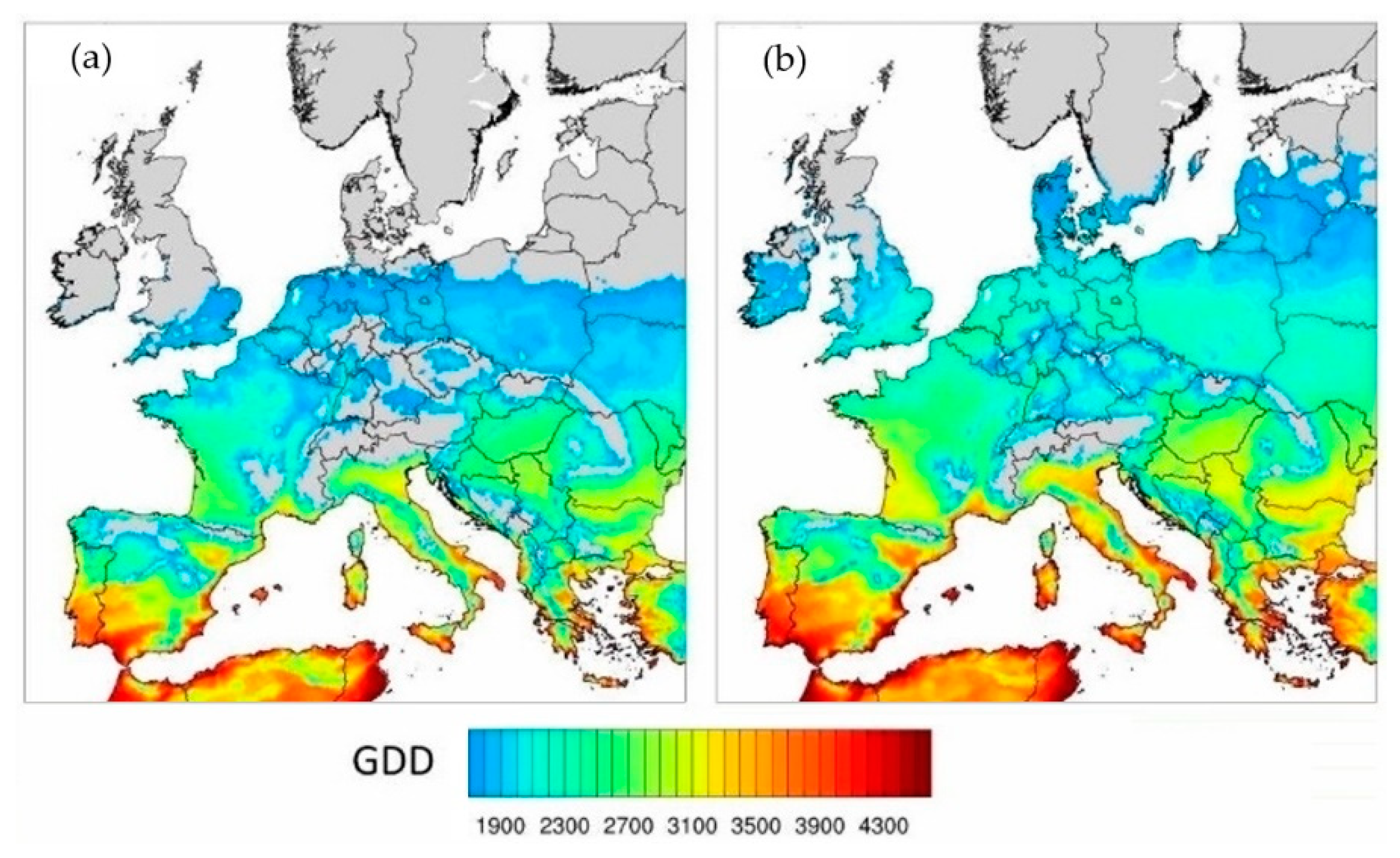
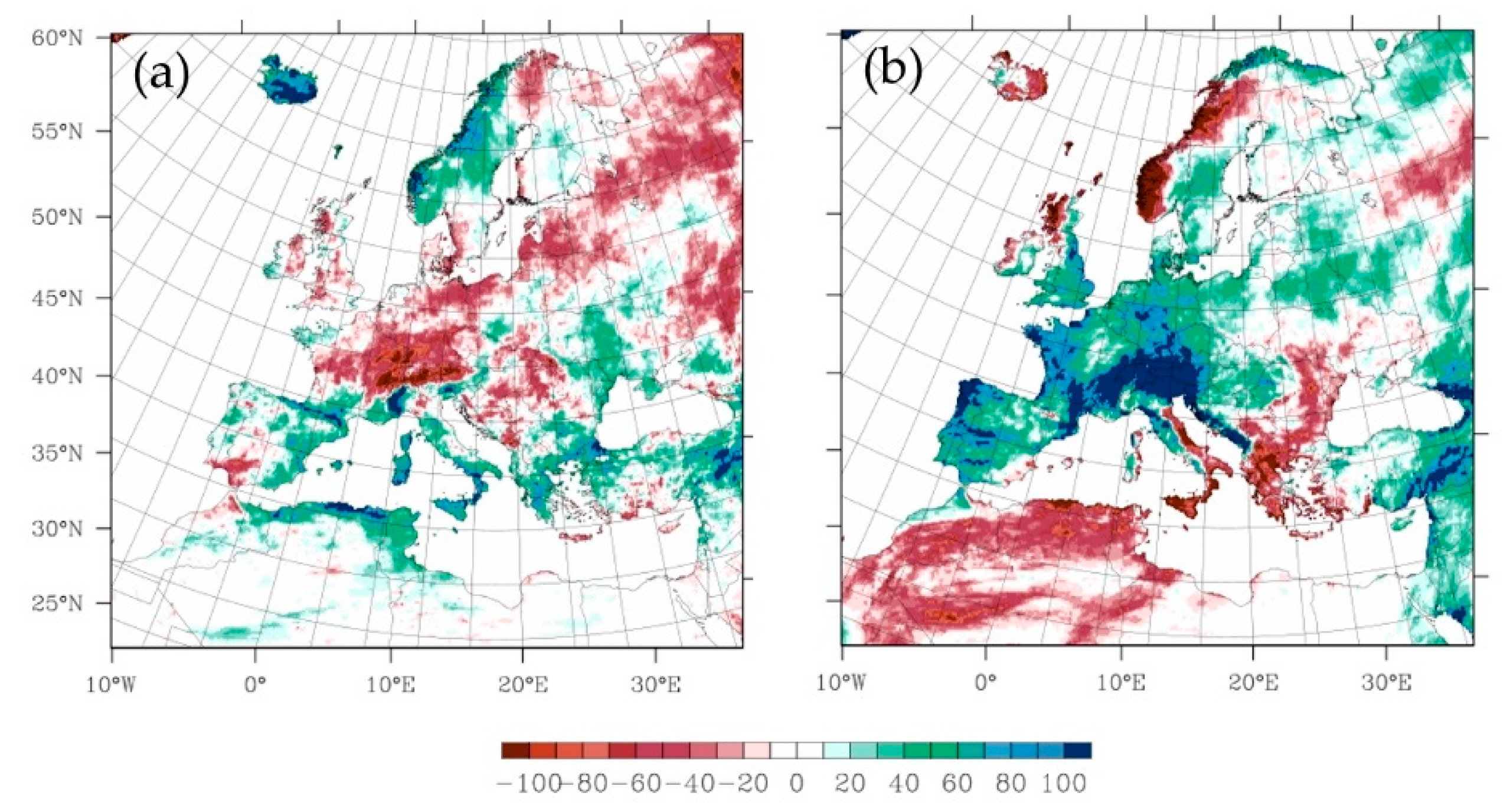
2.3.1. The Projected Climate Change Effects on Agriculture
2.3.2. The Potential Contribution of Bioenergy Cropping Systems to Climate Change Adaptation
- (1)
- Albedo effects could be induced by perennial BCS harvested in winter or spring, such as miscanthus and switchgrass, because the soil of these BCS is covered with senescence and thus brightly colored biomass during winter.
- (2)
2.4. Fostering Rural Development and Sustainable Rural Livelihoods
3. Chances and Challenges for a More Holistic Evaluation of Bioenergy Cropping Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scarlat, N. Highlights of the Conference. In Proceedings of the 27th European Biomass Conference & Exhibition, Lisbon, Portugal, 27–30 May 2019; Available online: http://programme.eubce.com/search.php?close=all (accessed on 31 July 2019).
- Canadell, J.G.; Schulze, E.D. Global potential of biospheric carbon management for climate mitigation. Nat. Commun. 2014, 5, 5282. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Karp, A.; Shield, I. Bioenergy from plants and the sustainable yield challenge. New Phytol. 2008, 179, 15–32. [Google Scholar] [CrossRef]
- Hastilestari, B.R.; Mudersbach, M.; Tomala, F.; Vogt, H.; Biskupek-Korell, B.; Van Damme, P.; Guretzki, S.; Papenbrock, J. Euphorbia tirucalli L.-Comprehensive Characterization of a Drought Tolerant Plant with a Potential as Biofuel Source. PLoS ONE 2013, 8, e63501. [Google Scholar] [CrossRef]
- Hou, Y.-K.; Liu, S.-Y.; Huang, L.; Zhou, H.-J. Selection and evaluation of Bio-diesel tree species in China. For. Res. 2009, 22, 7–13. [Google Scholar]
- Kagunyu, A.F.; Wanjohi, J.G. The emergency of Euphorbia tirucalli as drought feeds for camels in northern Kenya. Pastoralism 2015, 5, 17. [Google Scholar] [CrossRef]
- Khaleghian, A.; Nakaya, Y.; Nazari, H. Biodiesel production from Euphorbia tirucalli L. J. Med. Plant Res. 2011, 5, 4968–4973. [Google Scholar]
- Calabrò, P.S.; Catalán, E.; Folino, A.; Sánchez, A.; Komilis, D. Effect of three pretreatment techniques on the chemical composition and on the methane yields of Opuntia ficus-indica (prickly pear) biomass. Waste Manag. Res. 2018, 36, 17–29. [Google Scholar] [CrossRef]
- Santos, T.D.N.; Dutra, E.D.; Gomes do Prado, A.; Leite, F.C.B.; de Souza, R.D.F.R.; dos Santos, D.C.; Moraes de Abreu, C.A.; Simões, D.A.; de Morais, M.A., Jr.; Menezes, R.S.C. Potential for biofuels from the biomass of prickly pear cladodes: Challenges for bioethanol and biogas production in dry areas. Biomass Bioenergy 2016, 85, 215–222. [Google Scholar] [CrossRef]
- Yang, L.; Lu, M.; Carl, S.; Mayer, J.A.; Cushman, J.C.; Tian, E.; Lin, H. Biomass characterization of Agave and Opuntia as potential biofuel feedstocks. Biomass Bioenergy 2015, 76, 43–53. [Google Scholar] [CrossRef]
- Davis, S.C.; Dohleman, F.G.; Long, S.P. The global potential for Agave as a biofuel feedstock. GCB Bioenergy 2011, 3, 68–78. [Google Scholar] [CrossRef]
- Mason, P.M.; Glover, K.; Smith, J.A.C.; Willis, K.J.; Woods, J.; Thompson, I.P. The potential of CAM crops as a globally significant bioenergy resource: Moving from ‘fuel or food’ to ‘fuel and more food’. Energy Environ. Sci. 2015, 8, 2320–2329. [Google Scholar] [CrossRef]
- Bartholdsen, H.-K.; Eidens, A.; Löffler, K.; Seehaus, F.; Wejda, F.; Burandt, T.; Oei, P.-Y.; Kemfert, C.; von Hirschhausen, C. Pathways for Germany’s Low-Carbon Energy Transformation Towards 2050. Energies 2019, 12, 2988. [Google Scholar] [CrossRef]
- European Commission. A policy framework for climate and energy in the period from 2020 to 2030. Tech. Rep. COM 2014, 15, 2014. [Google Scholar]
- Robinius, M.; Otto, A.; Heuser, P.; Welder, L.; Syranidis, K.; Ryberg, D.S.; Grube, T.; Markewitz, P.; Peters, R.; Stolten, D. Linking the power and transport sectors—Part 1: The principle of sector coupling. Energies 2017, 10, 956. [Google Scholar] [CrossRef]
- Caspeta, L.; Buijs, N.A.A.; Nielsen, J. The role of biofuels in the future energy supply. Energy Environ. Sci. 2013, 6, 1077–1082. [Google Scholar] [CrossRef]
- Kalghatgi, G.; Levinsky, H.; Colket, M. Future transportation fuels. Prog. Energy Combust. Sci. 2018, 69, 103–105. [Google Scholar] [CrossRef]
- Inderwildi, O.R.; King, D.A. Quo vadis biofuels? Energy Environ. Sci. 2009, 2, 343–346. [Google Scholar] [CrossRef]
- Wang, M.; Dewil, R.; Maniatis, K.; Wheeldon, J.; Tan, T.; Baeyens, J.; Fang, Y. Biomass-derived aviation fuels: Challenges and perspective. Prog. Energy Combust. Sci. 2019, 74, 31–49. [Google Scholar] [CrossRef]
- IEA World Energy Outlook 2018—The Gold Standard of Energy Analysis. Available online: https://www.iea.org/weo2018/themes/ (accessed on 8 August 2019).
- David, K.; Ragauskas, A.J. Switchgrass as an energy crop for biofuel production: A review of its ligno-cellulosic chemical properties. Energy Environ. Sci. 2010, 3, 1182–1190. [Google Scholar] [CrossRef]
- Lask, J.; Wagner, M.; Trindade, L.M.; Lewandowski, I. Life cycle assessment of ethanol production from miscanthus: A comparison of production pathways at two European sites. GCB Bioenergy 2019, 11, 269–288. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; Patanè, C.; Mantineo, M.; D’Agosta, G.M. Agronomic, energetic and environmental aspects of biomass energy crops suitable for Italian environments. Ital. J. Agron. 2008, 3, 81–95. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Weiland, P. Production and energetic use of biogas from energy crops and wastes in Germany. Appl. Biochem. Biotechnol. 2003, 109, 263–274. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Pandiyan, K.; Singh, A.; Singh, S.; Saxena, A.K.; Nain, L. Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew. Energy 2019, 132, 723–741. [Google Scholar] [CrossRef]
- Oleskowicz-Popiel, P.; Kádár, Z.; Heiske, S.; Klein-Marcuschamer, D.; Simmons, B.A.; Blanch, H.W.; Schmidt, J.E. Co-production of ethanol, biogas, protein fodder and natural fertilizer in organic farming–evaluation of a concept for a farm-scale biorefinery. Bioresour. Technol. 2012, 104, 440–446. [Google Scholar] [CrossRef]
- Taube, F.; Herrmann, A. Kriterien für einen nachhaltigen Maisanbau zur Biogaserzeugung. Christian-Albrechts-Universität zu Kiel. 2007. Available online: http://www.grassland-organicfarming.uni-kiel.de/gfo/pdf/DMK_Taube07.pdf (accessed on 1 October 2019).
- Serdjuk, M.; Bodmer, U.; Hülsbergen, K.-J. Integration of biogas production into organic arable farming systems: Crop yield response and economic effects. Org. Agric. 2018, 8, 301–314. [Google Scholar] [CrossRef]
- Keegan, D.; Kretschmer, B.; Elbersen, B.; Panoutsou, C. Cascading use: A systematic approach to biomass beyond the energy sector. Biofuels Bioprod. Biorefining 2013, 7, 193–206. [Google Scholar] [CrossRef]
- Philippidis, G.; Bartelings, H.; Helming, J.; M’barek, R.; Smeets, E.; Van Meijl, H. The Good, the Bad and the Uncertain: Bioenergy Use in the European Union. Energies 2018, 11, 2703. [Google Scholar] [CrossRef]
- Zörb, C.; Lewandowski, I.; Kindervater, R.; Göttert, U.; Patzelt, D. Biobased Resources and Value Chains. In Bioeconomy; Lewandowski, I., Ed.; Springer: Cham, Switzerland, 2018; pp. 75–97. [Google Scholar]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind. Crops Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C. Beneficial biofuels—the food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Araújo, K.; Mahajan, D.; Kerr, R.; Silva, M.D. Global biofuels at the crossroads: An overview of technical, policy, and investment complexities in the sustainability of biofuel development. Agriculture 2017, 7, 32. [Google Scholar] [CrossRef]
- Sheppard, A.W.; Gillespie, I.; Hirsch, M.; Begley, C. Biosecurity and sustainability within the growing global bioeconomy. Curr. Opin. Environ. Sustain. 2011, 3, 4–10. [Google Scholar] [CrossRef]
- Elshout, P.M.F.; van Zelm, R.; van der Velde, M.; Steinmann, Z.; Huijbregts, M.A.J. Global relative species loss due to first-generation biofuel production for the transport sector. GCB Bioenergy 2019, 11, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Negri, M.C.; Kozak, J.; Cacho, J.F.; Quinn, J.; Secchi, S.; Ssegane, H. Valuation of ecosystem services in alternative bioenergy landscape scenarios. GCB Bioenergy 2019, 11, 748–762. [Google Scholar] [CrossRef]
- Allen, B.R.; Keegan, D.; Elbersen, B. Biomass and bioenergy in the wider land-use context of the European Union. Biofuels Bioprod. Biorefining 2013, 7, 207–216. [Google Scholar] [CrossRef]
- Valentine, J.; Clifton-Brown, J.; Hastings, A.; Robson, P.; Allison, G.; Smith, P. Food vs. fuel: The use of land for lignocellulosic “next generation” energy crops that minimize competition with primary food production. GCB Bioenergy 2012, 4, 1–19. [Google Scholar] [CrossRef]
- Doelman, J.C.; Stehfest, E.; Tabeau, A.; van Meijl, H. Making the Paris agreement climate targets consistent with food security objectives. Glob. Food Secur. 2019, 23, 93–103. [Google Scholar] [CrossRef]
- Michel, H. Editorial: The Nonsense of Biofuels. Angew. Chem. Int. Ed. 2012, 51, 2516–2518. [Google Scholar] [CrossRef] [PubMed]
- Von Cossel, M.; Elbersen, B.; Von Cossel, V.; Staritsky, I.; Van Eupen, M.; Mantel, S.; Iqbal, I.; Happe, S.; Scordia, D.; Cosentino, S.L.; et al. How to feed the European bioeconomy in the future? Climate change-forced shifts in growth suitability of industrial crops until 2100. manuscript in preparation.
- Winkler, B.; Mangold, A.; Von Cossel, M.; Iqbal, Y.; Kiesel, A.; Lewandowski, I. Implementing miscanthus into sustainable farming systems: A review on agronomic practices, capital and labor demand. Renew. Sustain. Energy Rev. under review.
- Elbersen, B.; Van Eupen, M.; Verzandvoort, S.; Boogaard, H.; Mucher, S.; Cicarreli, T.; Elbersen, W.; Mantel, S.; Bai, Z.; MCallum, I.; et al. Methodological Approaches to Identify and Map Marginal Land Suitable for Industrial Crops in Europe; WUR: Wageningen, The Netherlands, 2018; p. 142. [Google Scholar]
- Fajardy, M.; Chiquier, S.; Mac Dowell, N. Investigating the BECCS resource nexus: Delivering sustainable negative emissions. Energy Environ. Sci. 2018, 11, 3408–3430. [Google Scholar] [CrossRef]
- Elbersen, B.; Fritsche, U.; Petersen, J.-E.; Lesschen, J.P.; Böttcher, H.; Overmars, K. Assessing the effect of stricter sustainability criteria on EU biomass crop potential. Biofuels Bioprod. Biorefining 2013, 7, 173–192. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Wagner, M.; Mangold, A.; Lask, J.; Petig, E.; Kiesel, A.; Lewandowski, I. Economic and environmental performance of miscanthus cultivated on marginal land for biogas production. GCB Bioenergy 2019, 11, 34–49. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Elbersen, H.W.; Cosentino, S.L.; Zatta, A.; Alexopoulou, E.; Monti, A. Agronomic aspects of future energy crops in Europe. Biofuels Bioprod. Biorefining 2010, 4, 674–691. [Google Scholar] [CrossRef]
- Smeets, E.M.W.; Faaij, A.P.C.; Lewandowski, I.M.; Turkenburg, W.C. A bottom-up assessment and review of global bio-energy potentials to 2050. Prog. Energy Combust. Sci. 2007, 33, 56–106. [Google Scholar] [CrossRef]
- Elbersen, B.; Van Verzandvoort, M.; Boogaard, S.; Mucher, S.; Cicarelli, T.; Elbersen, W.; Mantel, S.; Bai, Z.; MCallum, I.; Iqbal, Y.; et al. Definition and Classification of Marginal Lands Suitable for Industrial Crops in Europe (EU Deliverable); WUR: Wageningen, The Netherlands, 2018; p. 44. [Google Scholar]
- Ceotto, E.; Di Candilo, M. Sustainable bioenergy production, land and nitrogen use. In Biodiversity, Biofuels, Agroforestry and Conservation Agriculture; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany, 2010; pp. 101–122. [Google Scholar]
- Cai, X.; Zhang, X.; Wang, D. Land availability for biofuel production. Environ. Sci. Technol. 2011, 45, 334–339. [Google Scholar] [CrossRef]
- Von Cossel, M.; Bauerle, A.; Boob, M.; Thumm, U.; Elsaesser, M.; Lewandowski, I. The Performance of Mesotrophic Arrhenatheretum Grassland under Different Cutting Frequency Regimes for Biomass Production in Southwest Germany. Agriculture 2019, 9, 199. [Google Scholar] [CrossRef]
- Boob, M.; Truckses, B.; Seither, M.; Elsäßer, M.; Thumm, U.; Lewandowski, I. Management effects on botanical composition of species-rich meadows within the Natura 2000 network. Biodivers. Conserv. 2019, 28, 729–750. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef]
- Gützloe, A.; Thumm, U.; Lewandowski, I. Influence of climate parameters and management of permanent grassland on biogas yield and GHG emission substitution potential. Biomass Bioenergy 2014, 64, 175–189. [Google Scholar] [CrossRef]
- Fajardy, M.; Mac Dowell, N. The energy return on investment of BECCS: Is BECCS a threat to energy security? Energy Environ. Sci. 2018, 11, 1581–1594. [Google Scholar] [CrossRef]
- Nakajima, T.; Yamada, T.; Anzoua, K.G.; Kokubo, R.; Noborio, K. Carbon sequestration and yield performances of Miscanthus × giganteus and Miscanthus sinensis. Carbon Manag. 2018, 9, 415–423. [Google Scholar] [CrossRef]
- Searchinger, T.D.; Beringer, T.; Holtsmark, B.; Kammen, D.M.; Lambin, E.F.; Lucht, W.; Raven, P.; van Ypersele, J.-P. Europe’s renewable energy directive poised to harm global forests. Nat. Commun. 2018, 9, 3741. [Google Scholar] [CrossRef] [PubMed]
- Verdade, L.M.; Piña, C.I.; Rosalino, L.M. Biofuels and biodiversity: Challenges and opportunities. Environ. Dev. 2015, 15, 64–78. [Google Scholar] [CrossRef]
- Winkler, B.; Lemke, S.; Ritter, J.; Lewandowski, I. Integrated assessment of renewable energy potential: Approach and application in rural South Africa. Environ. Innov. Soc. Transit. 2017, 24, 17–31. [Google Scholar] [CrossRef]
- Michalscheck, M. On Smallholder Farm and Farmer Diversity. Dissertation, Wageningen University & Research, Wageningen, The Netherlands, 2019. [Google Scholar]
- He, G.; Zhao, Y.; Wang, L.; Jiang, S.; Zhu, Y. China’s food security challenge: Effects of food habit changes on requirements for arable land and water. J. Clean. Prod. 2019, 229, 739–750. [Google Scholar] [CrossRef]
- Fischer, J.; Brosi, B.; Daily, G.C.; Ehrlich, P.R.; Goldman, R.; Goldstein, J.; Lindenmayer, D.B.; Manning, A.D.; Mooney, H.A.; Pejchar, L.; et al. Should agricultural policies encourage land sparing or wildlife-friendly farming? Front. Ecol. Environ. 2008, 6, 380–385. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Danish, K.; Wang, Z. Does biomass energy consumption help to control environmental pollution? Evidence from BRICS countries. Sci. Total Environ. 2019, 670, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M.C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Policy: Sustainable development goals for people and planet. Nature 2013, 495, 305. [Google Scholar] [CrossRef]
- Robert, K.W.; Parris, T.M.; Leiserowitz, A.A. What is sustainable development? Goals, indicators, values, and practice. Environ. Sci. Policy Sustain. Dev. 2005, 47, 8–21. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecology: The Science of Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I.; Montalba, R. Technological Approaches to Sustainable Agriculture at a Crossroads: An Agroecological Perspective. Sustainability 2017, 9, 349. [Google Scholar] [CrossRef]
- Uphoff, N.T.; Altieri, M.A. Alternatives to Conventional Modern Agriculture for Meeting World Needs in the Next Century: Report of a Conference on Sustainable Agriculture, Evaluation of New Paradigms and Old Practices; Cornell International Institute for Food, Agriculture and Development: Bellagio, Italy, 1999. [Google Scholar]
- Elbersen, B.; Van Eupen, M.; Alexopoulou, E.; Bai, Z.; Boogaard, H.; Carrasco, J.E.; Ceccarelli, T.; Ciria Ramos, C.; Ciria, P.; Cosentino, S.L.; et al. Mapping Marginal Land Potentially Available for Industrial Crops in Europe; Visual presentation at the 26th European Biomass Conference & Exhibition, Copenhagen, Denmark, 2018. Available online: https://www.researchgate.net/publication/325272893_Mapping_Marginal_land_potentially_available_for_industrial_crops_in_Europe (accessed on 1 October 2019).
- Ramirez-Almeyda, J.; Elbersen, B.; Monti, A.; Staritsky, I.; Panoutsou, C.; Alexopoulou, E.; Schrijver, R.; Elbersen, W. Assessing the Potentials for Nonfood Crops. In Modeling and Optimization of Biomass Supply Chains; Panoutsou, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–251. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Krasuska, E.; Cadórniga, C.; Tenorio, J.L.; Testa, G.; Scordia, D. Potential land availability for energy crops production in Europe. Biofuels Bioprod. Biorefining 2010, 4, 658–673. [Google Scholar] [CrossRef]
- Ciria, C.S.; Sanz, M.; Carrasco, J.; Ciria, P. Identification of Arable Marginal Lands under Rainfed Conditions for Bioenergy Purposes in Spain. Sustainability 2019, 11, 1833. [Google Scholar] [CrossRef]
- Xue, S.; Lewandowski, I.; Wang, X.; Yi, Z. Assessment of the production potentials of Miscanthus on marginal land in China. Renew. Sustain. Energy Rev. 2016, 54, 932–943. [Google Scholar] [CrossRef]
- McIntyre, B.D. International Assessment of Agricultural Knowledge, Science and Technology for Development (IAASTD): Synthesis Report with Executive Summary: A Synthesis of the Global and Sub-Global IAASTD Reports; IAASTD, Island Press: Washington, DC, USA, 2009. [Google Scholar]
- TEEB. Guidance Manual for TEEB Country Studies-Version 1.0; Institute for European Environmental Policy: London, UK, 2013. [Google Scholar]
- Nabel, M.; Schrey, S.D.; Temperton, V.M.; Harrison, L.; Jablonowski, N.D. Legume Intercropping With the Bioenergy Crop Sida hermaphrodita on Marginal Soil. Front. Plant Sci. 2018, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Nabel, M.; Barbosa, D.B.P.; Horsch, D.; Jablonowski, N.D. Energy Crop (Sida Hermaphrodita) Fertilization Using Digestate under Marginal Soil Conditions: A Dose-response Experiment. Energy Procedia 2014, 59, 127–133. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I. Perennial wild plant mixtures for biomass production: Impact of species composition dynamics on yield performance over a five-year cultivation period in southwest Germany. Eur. J. Agron. 2016, 79, 74–89. [Google Scholar] [CrossRef]
- Emmerling, C. Impact of land-use change towards perennial energy crops on earthworm population. Appl. Soil Ecol. 2014, 84, 12–15. [Google Scholar] [CrossRef]
- Von Cossel, M.; Winkler, B.; Mangold, A.; Lewandowski, I.; Elbersen, B.; Wagner, M.; Magenau, E.; Lask, I.; Staritsky, I.; Van Eupen, M.; et al. Bridging the gap between biofuels and biodiversity for a bioeconomy transition – Social-ecological implications of miscanthus cultivation for isobutanol production. Energy Environ. Sci. under review.
- Kiesel, A.; Wagner, M.; Lewandowski, I. Environmental performance of miscanthus, switchgrass and maize: Can C4 perennials increase the sustainability of biogas production? Sustainability 2017, 9, 5. [Google Scholar] [CrossRef]
- Kalt, G.; Mayer, A.; Theurl, M.C.; Lauk, C.; Erb, K.-H.; Haberl, H. Natural climate solutions versus bioenergy: Can carbon benefits of natural succession compete with bioenergy from short rotation coppice? GCB Bioenergy 2019, in press. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/gcbb.12626 (accessed on 1 October 2019). [CrossRef]
- Secretariat of the Convention on Biological Diversity. Global Biodiversity Outlook 3; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2010. [Google Scholar]
- Grooten, M.; Almond, R.E.A. Living Planet Report 2018. Aiming Higher; WWF: Gland, Switzerland, 2018. [Google Scholar]
- Dauber, J.; Bolte, A. Bioenergy: Challenge or support for the conservation of biodiversity? GCB Bioenergy 2014, 6, 180–182. [Google Scholar] [CrossRef]
- Immerzeel, D.J.; Verweij, P.A.; van der Hilst, F.; Faaij, A.P. Biodiversity impacts of bioenergy crop production: A state-of-the-art review. GCB Bioenergy 2014, 6, 183–209. [Google Scholar] [CrossRef]
- Pedroli, B.; Elbersen, B.; Frederiksen, P.; Grandin, U.; Heikkilä, R.; Krogh, P.H.; Izakovičová, Z.; Johansen, A.; Meiresonne, L.; Spijker, J. Is energy cropping in Europe compatible with biodiversity?—Opportunities and threats to biodiversity from land-based production of biomass for bioenergy purposes. Biomass Bioenergy 2013, 55, 73–86. [Google Scholar] [CrossRef]
- Chmelíková, L.; Wolfrum, S. Mitigating the biodiversity footprint of energy crops–A case study on arthropod diversity. Biomass Bioenergy 2019, 125, 180–187. [Google Scholar] [CrossRef]
- Semere, T.; Slater, F.M. Invertebrate populations in miscanthus (Miscanthus × giganteus) and reed canary-grass (Phalaris arundinacea) fields. Biomass Bioenergy 2007, 31, 30–39. [Google Scholar] [CrossRef]
- Semere, T.; Slater, F.M. Ground flora, small mammal and bird species diversity in miscanthus (Miscanthus × giganteus) and reed canary-grass (Phalaris arundinacea) fields. Biomass Bioenergy 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Haughton, A.J.; Bond, A.J.; Lovett, A.A.; Dockerty, T.; Sünnenberg, G.; Clark, S.J.; Bohan, D.A.; Sage, R.B.; Mallott, M.D.; Mallott, V.E.; et al. A novel, integrated approach to assessing social, economic and environmental implications of changing rural land-use: A case study of perennial biomass crops. J. Appl. Ecol. 2009, 46, 315–322. [Google Scholar] [CrossRef]
- Haughton, A.J.; Bohan, D.A.; Clark, S.J.; Mallott, M.D.; Mallott, V.; Sage, R.; Karp, A. Dedicated biomass crops can enhance biodiversity in the arable landscape. GCB Bioenergy 2016, 8, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Felton, A.; Knight, E.; Wood, J.; Zammit, C.; Lindenmayer, D. A meta-analysis of fauna and flora species richness and abundance in plantations and pasture lands. Biol. Conserv. 2010, 143, 545–554. [Google Scholar] [CrossRef]
- Van der Hilst, F.; Lesschen, J.P.; Van Dam, J.M.C.; Riksen, M.; Verweij, P.A.; Sanders, J.P.M.; Faaij, A.P.C. Spatial variation of environmental impacts of regional biomass chains. Renew. Sustain. Energy Rev. 2012, 16, 2053–2069. [Google Scholar] [CrossRef]
- Werling, B.P.; Dickson, T.L.; Isaacs, R.; Gaines, H.; Gratton, C.; Gross, K.L.; Liere, H.; Malmstrom, C.M.; Meehan, T.D.; Ruan, L. Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Natl. Acad. Sci. USA 2014, 111, 1652–1657. [Google Scholar] [CrossRef]
- Pulighe, G.; Bonati, G.; Colangeli, M.; Morese, M.M.; Traverso, L.; Lupia, F.; Khawaja, C.; Janssen, R.; Fava, F. Ongoing and emerging issues for sustainable bioenergy production on marginal lands in the Mediterranean regions. Renew. Sustain. Energy Rev. 2019, 103, 58–70. [Google Scholar] [CrossRef]
- Williams, M.A.; Feest, A. The Effect of Miscanthus Cultivation on the Biodiversity of Ground Beetles (Coleoptera: Carabidae), Spiders and Harvestmen (Arachnida: Araneae and Opiliones). Agric. Sci. 2019, 10, 903–917. [Google Scholar] [CrossRef][Green Version]
- Von Cossel, M. Agricultural Diversification of Biogas Crop Cultivation. Dissertation, University of Hohenheim, Stuttgart, Germany, 2019. [Google Scholar]
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.; Allan, E.; Beßler, H.; Barnard, R.L.; Buchmann, N.; Buscot, F. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Appl. Ecol. 2017, 23, 1–73. [Google Scholar] [CrossRef]
- Whitaker, J.; Field, J.L.; Bernacchi, C.J.; Cerri, C.E.; Ceulemans, R.; Davies, C.A.; DeLucia, E.H.; Donnison, I.S.; McCalmont, J.P.; Paustian, K. Consensus, uncertainties and challenges for perennial bioenergy crops and land use. GCB Bioenergy 2018, 10, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Dauber, J.; Brown, C.; Fernando, A.L.; Finnan, J.; Krasuska, E.; Ponitka, J.; Styles, D.; Thrän, D.; Van Groenigen, K.J.; Weih, M. Bioenergy from “surplus” land: Environmental and socio-economic implications. BioRisk Biodivers. Ecosyst. Risk Assess. 2012, 7, 5–50. [Google Scholar] [CrossRef]
- Wiens, J.; Fargione, J.; Hill, J. Biofuels and biodiversity. Ecol. Appl. 2011, 21, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.; Taylor, G.; Hanley, E.M. Bioenergy, food production and biodiversity—An unlikely alliance? GCB Bioenergy 2015, 7, 570–576. [Google Scholar] [CrossRef]
- Groom, M.J.; Gray, E.M.; Townsend, P.A. Biofuels and biodiversity: Principles for creating better policies for biofuel production. Conserv. Biol. 2008, 22, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Von Cossel, M.; Mangold, A.; Iqbal, Y.; Hartung, J.; Lewandowski, I.; Kiesel, A. How to Generate Yield in the First Year—A Three-Year Experiment on Miscanthus (Miscanthus × giganteus (Greef et Deuter)) Establishment under Maize (Zea mays L.). Agronomy 2019, 9, 237. [Google Scholar] [CrossRef]
- Himanen, S.; Mäkinen, H.; Rimhanen, K.; Savikko, R. Engaging farmers in climate change adaptation planning: Assessing intercropping as a means to support farm adaptive capacity. Agriculture 2016, 6, 34. [Google Scholar] [CrossRef]
- Gruenewald, H.; Brandt, B.K.V.; Schneider, B.U.; Bens, O.; Kendzia, G.; Hüttl, R.F. Agroforestry systems for the production of woody biomass for energy transformation purposes. Ecol. Eng. 2007, 29, 319–328. [Google Scholar] [CrossRef]
- Volk, T.A.; Abrahamson, L.P.; Nowak, C.A.; Smart, L.B.; Tharakan, P.J.; White, E.H. The development of short-rotation willow in the northeastern United States for bioenergy and bioproducts, agroforestry and phytoremediation. Biomass Bioenergy 2006, 30, 715–727. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Henrik, H.-N.; Alves, B.J.R.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Timsina, J. Can organic sources of nutrients increase crop yields to meet global food demand? Agronomy 2018, 8, 214. [Google Scholar] [CrossRef]
- Weißhuhn, P.; Reckling, M.; Stachow, U.; Wiggering, H. Supporting Agricultural Ecosystem Services through the Integration of Perennial Polycultures into Crop Rotations. Sustainability 2017, 9, 2267. [Google Scholar] [CrossRef]
- Von Cossel, M.; Steberl, K.; Hartung, J.; Agra Pereira, L.; Kiesel, A.; Lewandowski, I. Methane yield and species diversity dynamics of perennial wild plant mixtures established alone, under cover crop maize (Zea mays L.) and after spring barley (Hordeum vulgare L.). GCB Bioenergy. 2019. in press. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/gcbb.12640 (accessed on 1 October 2019). [CrossRef]
- Bybee-Finley, K.A.; Ryan, M.R. Advancing Intercropping Research and Practices in Industrialized Agricultural Landscapes. Agriculture 2018, 8, 80. [Google Scholar] [CrossRef]
- Pagano, M.C.; Correa, E.J.A.; Duarte, N.F.; Yelikbayev, B.; O’Donovan, A.; Gupta, V.K. Advances in Eco-Efficient Agriculture: The Plant-Soil Mycobiome. Agriculture 2017, 7, 14. [Google Scholar] [CrossRef]
- Wienforth, B.; Knieb, A.; Böttcher, U.; Herrmann, A.; Sieling, K.; Taube, F.; Kage, H. Evaluating Bioenergy Cropping Systems towards Productivity and Resource Use Efficiencies: An Analysis Based on Field Experiments and Simulation Modelling. Agronomy 2018, 8, 117. [Google Scholar] [CrossRef]
- Mockshell, J.; Kamanda, J. Beyond the Agroecological and Sustainable Agricultural Intensification Debate: Is Blended Sustainability the Way Forward? Int. J. Agric. Sustain. 2018, 16, 127–149. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Monti, A. Energy crops in rotation. A review. Biomass Bioenergy 2011, 35, 12–25. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Von Cossel, M.; Möhring, J.; Kiesel, A.; Lewandowski, I. Methane yield performance of amaranth (Amaranthus hypochondriacus L.) and its suitability for legume intercropping in comparison to maize (Zea mays L.). Ind. Crops Prod. 2017, 103, 107–121. [Google Scholar] [CrossRef]
- Nurk, L.; Graß, R.; Pekrun, C.; Wachendorf, M. Effect of sowing method and weed control on the performance of maize (Zea mays L.) intercropped with climbing beans (Phaseolus vulgaris L.). Agriculture 2017, 7, 51. [Google Scholar] [CrossRef]
- Von Cossel, M.; Iqbal, Y.; Lewandowski, I. Improving the Ecological Performance of Miscanthus (Miscanthus × giganteus Greef et Deuter) through Intercropping with Woad (Isatis tinctoria L.) and Yellow Melilot (Melilotus officinalis L.). Agriculture 2019, 9, 194. [Google Scholar] [CrossRef]
- Nabel, M.; Temperton, V.M.; Poorter, H.; Lücke, A.; Jablonowski, N.D. Energizing marginal soils—The establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilization, NPK, and legume intercropping. Biomass Bioenergy 2016, 87, 9–16. [Google Scholar] [CrossRef]
- Berti, M.; Samarappuli, D.; Johnson, B.L.; Gesch, R.W. Integrating winter camelina into maize and soybean cropping systems. Ind. Crops Prod. 2017, 107, 595–601. [Google Scholar] [CrossRef]
- Zanetti, F.; Monti, A.; Berti, M.T. Challenges and opportunities for new industrial oilseed crops in EU-27: A review. Ind. Crops Prod. 2013, 50, 580–595. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Valencia-Gredilla, F. Camelina as a rotation crop for weed control in organic farming in a semiarid mediterranean climate. Agriculture 2018, 8, 156. [Google Scholar] [CrossRef]
- Stolzenburg, K.; Bruns, H.; Monkos, A.; Ott, J.; Schickler, J. Produktion von Kosubstraten für die Biogasanlage—Ergebnisse der Versuche mit Durchwachsener Silphie (Silphium perfoliatum L.) in Baden-Württemberg. Landwirtschaftliches Technologiezentrum Augustenberg: Karlsruhe, Germany, 2016. [Google Scholar]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Kuhn, W.; Zeller, J.; Bretschneider-Herrmann, N.; Drenckhahn, K. Energy from Wild Plants—Practical Tips for the Cultivation of Wild Plants to Create Biomass for Biogas Generation Plants, Netzwerk Lebensraum Feldflur c/o Deutsche Wildtier Stiftung, Hamburg, Germany. 2014. Available online: http://cic-wildlife.org/wp-content/uploads/2014/09/English_Praxisratgeber2014.pdf (accessed on 1 October 2019).
- Frick, M.; Pfender, G. AG Wildpflanzen-Biogas Kißlegg. In Biogas aus Wildpflanzen – Chancen und Herausforderungen mehrjähriger Wildpflanzenmischungen zur Biogasnutzung aus Sicht der Forschung und Praxis; University of Hohenheim: Stuttgart, Germany, 2019. [Google Scholar]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.J.; Van Loo, E.N.; Puttick, D.; Monti, A. Agronomic performance and seed quality attributes of Camelina (Camelina sativa L. crantz) in multi-environment trials across Europe and Canada. Ind. Crops Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Tworkowski, J.; Załuski, D.; Kwiatkowski, J.; Szczukowski, S. Camelina and crambe production—Energy efficiency indices depending on nitrogen fertilizer application. Ind. Crops Prod. 2019, 137, 386–395. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Li, D.; Papatheohari, Y.; Siqi, H.; Scordia, D.; Testa, G. How kenaf (Hibiscus cannabinus L.) can achieve high yields in Europe and China. Ind. Crops Prod. 2015, 68, 131–140. [Google Scholar] [CrossRef]
- Väisänen, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of hemp fibers (Cannabis Sativa L.) for composite applications. Ind. Crops Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
- Manninen, P.; Mäkelä, P.; Hartikainen, H.; Santanen, A.; Seppänen, M.; Stoddard, F.; Yli-Halla, M. Growth of hemp and Lupin in chromium, Arsenic and copper contaminated soil. Ital. J. Agron. 2008, 3, 57–58. [Google Scholar]
- Scheliga, M.; Brand, U.; Türk, O.; Gruber, S.; Medina, L.; Petersen, J. Yield and quality of bast fibre from Abutilon theophrasti (Medic.) in southwest Germany depending on the site and fibre extraction method. Ind. Crops Prod. 2018, 121, 320–327. [Google Scholar] [CrossRef]
- da Silva, M.J.; Carneiro, P.C.S.; de Souza Carneiro, J.E.; Damasceno, C.M.B.; Parrella, N.N.L.D.; Pastina, M.M.; Simeone, M.L.F.; Schaffert, R.E.; da Costa Parrella, R.A. Evaluation of the potential of lines and hybrids of biomass sorghum. Ind. Crops Prod. 2018, 125, 379–385. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small-and large-scale experimental plantations in Poland from 2000 to 2017. Renew. Sustain. Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- Iqbal, Y.; Steberl, K.; Hartung, K.; Lewandowski, I. Optimal sampling area determination for willow by evaluating variability in yield and quality. Ind. Crops Prod. 2019, 134, 265–270. [Google Scholar] [CrossRef]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Eberl, V.; Fahlbusch, W.; Fritz, M.; Sauer, B. Screening und Selektion von Amarantsorten und -linien als spurenelementreiches Biogassubstrat; Berichte aus dem TFZ.; Technologie und Förderzentrum im Kompetenzzentrum für Nachwachsende Rohstoffe: Straubing, Germany, 2014; p. 120. [Google Scholar]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Viaud, V.; Durand, P.; Merot, P.; Sauboua, E.; Saadi, Z. Modeling the impact of the spatial structure of a hedge network on the hydrology of a small catchment in a temperate climate. Agric. Water Manag. 2005, 74, 135–163. [Google Scholar] [CrossRef]
- Dietzel, S.; Sauter, F.; Moosner, M.; Fischer, C.; Kollmann, J. Blühstreifen und Blühflächen in der landwirt-schaftlichen Praxis–eine naturschutzfach-liche Evaluation. Anliegen Nat. 2019, 41, 73–86. [Google Scholar]
- Pugesgaard, S.; Schelde, K.; Larsen, S.U.; Lærke, P.E.; Jørgensen, U. Comparing annual and perennial crops for bioenergy production–influence on nitrate leaching and energy balance. GCB Bioenergy 2015, 7, 1136–1149. [Google Scholar] [CrossRef]
- Ruf, T.; Makselon, J.; Udelhoven, T.; Emmerling, C. Soil quality indicator response to land-use change from annual to perennial bioenergy cropping systems in Germany. GCB Bioenergy 2018, 10, 444–459. [Google Scholar] [CrossRef]
- Muylle, H.; Van Hulle, S.; De Vliegher, A.; Baert, J.; Van Bockstaele, E.; Roldán-Ruiz, I. Yield and energy balance of annual and perennial lignocellulosic crops for bio-refinery use: A 4-year field experiment in Belgium. Eur. J. Agron. 2015, 63, 62–70. [Google Scholar] [CrossRef]
- Bradley, B.A.; Oppenheimer, M.; Wilcove, D.S. Climate change and plant invasions: Restoration opportunities ahead? Glob. Change Biol. 2009, 15, 1511–1521. [Google Scholar] [CrossRef]
- Biala, K.; Terres, J.-M.; Pointereau, P.; Paracchini, M.L. Low Input Farming Systems: An opportunity to develop sustainable agriculture. Proc. JRC Summer Univ. Ranco 2007, 2–5. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/low-input-farming-systems-opportunity-develop-sustainable-agriculture-proceedings-jrc-summer (accessed on 1 October 2019).
- Pulighe, G.; Bonati, G.; Fabiani, S.; Barsali, T.; Lupia, F.; Vanino, S.; Nino, P.; Arca, P.; Roggero, P.P. Assessment of the Agronomic Feasibility of Bioenergy Crop Cultivation on Marginal and Polluted Land: A GIS-Based Suitability Study from the Sulcis Area, Italy. Energies 2016, 9, 895. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Meeting US biofuel goals with less land: The potential of Miscanthus. Glob. Change Biol. 2008, 14, 2000–2014. [Google Scholar] [CrossRef]
- Kørup, K.; Lærke, P.E.; Baadsgaard, H.; Andersen, M.N.; Kristensen, K.; Münnich, C.; Didion, T.; Jensen, E.S.; Mårtensson, L.-M.; Jørgensen, U. Biomass production and water use efficiency in perennial grasses during and after drought stress. GCB Bioenergy 2018, 10, 12–27. [Google Scholar] [CrossRef]
- Himken, M.; Lammel, J.; Neukirchen, D.; Czypionka-Krause, U.; Olfs, H.-W. Cultivation of Miscanthus under West European conditions: Seasonal changes in dry matter production, nutrient uptake and remobilization. Plant Soil 1997, 189, 117–126. [Google Scholar] [CrossRef]
- Arthurson, V.; Jäderlund, L. Utilization of natural farm resources for promoting high energy efficiency in low-input organic farming. Energies 2011, 4, 804–817. [Google Scholar] [CrossRef]
- Behnke, G.D.; Pittelkow, C.M.; Nafziger, E.D.; Villamil, M.B. Exploring the Relationships between Greenhouse Gas Emissions, Yields, and Soil Properties in Cropping Systems. Agriculture 2018, 8, 62. [Google Scholar] [CrossRef]
- Felten, D.; Fröba, N.; Fries, J.; Emmerling, C. Energy balances and greenhouse gas-mitigation potentials of bioenergy cropping systems (Miscanthus, rapeseed, and maize) based on farming conditions in Western Germany. Renew. Energy 2013, 55, 160–174. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, W.; Wang, L.; Hui, D.; Grove, J.H.; Yang, X.; Tao, B.; Goff, B. Greenhouse gas emissions and crop yield in no-tillage systems: A meta-analysis. Agric. Ecosyst. Environ. 2018, 268, 144–153. [Google Scholar] [CrossRef]
- Hudiburg, T.W.; Davis, S.C.; Parton, W.; Delucia, E.H. Bioenergy crop greenhouse gas mitigation potential under a range of management practices. GCB Bioenergy 2015, 7, 366–374. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Kollmann, T.; Nabel, M.; Damm, T.; Klose, H.; Müller, M.; Bläsing, M.; Seebold, S.; Krafft, S.; Kuperjans, I.; et al. Valorization of Sida (Sida hermaphrodita) biomass for multiple energy purposes. GCB Bioenergy 2017, 9, 202–214. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Nolot, J.-M.; Raffaillac, D.; Justes, E. Cover crops mitigate nitrate leaching in cropping systems including grain legumes: Field evidence and model simulations. Agric. Ecosyst. Environ. 2015, 212, 1–12. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Raizada, M.N. Challenges in using precision agriculture to optimize symbiotic nitrogen fixation in legumes: Progress, limitations, and future improvements needed in diagnostic testing. Agronomy 2018, 8, 78. [Google Scholar] [CrossRef]
- De Groot, R.S.; Wilson, M.A.; Boumans, R.M. A typology for the classification, description and valuation of ecosystem functions, goods and services. Ecol. Econ. 2002, 41, 393–408. [Google Scholar] [CrossRef]
- De Groot, R.; Brander, L.; Van Der Ploeg, S.; Costanza, R.; Bernard, F.; Braat, L.; Christie, M.; Crossman, N.; Ghermandi, A.; Hein, L. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 2012, 1, 50–61. [Google Scholar] [CrossRef]
- Braat, L.C.; De Groot, R. The ecosystem services agenda: Bridging the worlds of natural science and economics, conservation and development, and public and private policy. Ecosyst. Serv. 2012, 1, 4–15. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef]
- KTBL Web-Anwendungen. Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V., Darmstadt, Germany. 2019. Available online: https://www.ktbl.de/webanwendungen/ (accessed on 22 July 2019).
- Ehmann, A.; Bach, I.-M.; Laopeamthong, S.; Bilbao, J.; Lewandowski, I. Can phosphate salts recovered from manure replace conventional phosphate fertilizer? Agriculture 2017, 7, 1. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Bergfeldt, B.; Morgano, M.T.; Leibold, H.; Richter, F.; Stapf, D. Recovery of phosphorus and other nutrients during pyrolysis of chicken manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef]
- Bilandžija, N.; Krička, T.; Matin, A.; Leto, J.; Grubor, M. Effect of Harvest Season on the Fuel Properties of Sida hermaphrodita (L.) Rusby Biomass as Solid Biofuel. Energies 2018, 11, 3398. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
- Ceotto, E.; Marchetti, R.; Castelli, F. Residual soil nitrate as affected by giant reed cultivation and cattle slurry fertilisation. Ital. J. Agron. 2018, 13, 317–323. [Google Scholar]
- Ehmann, A.; Thumm, U.; Lewandowski, I. Fertilizing Potential of Separated Biogas Digestates in Annual and Perennial Biomass Production Systems. Front. Sustain. Food Syst. 2018, 2, 12. [Google Scholar] [CrossRef]
- Costa, J.; Barbosa, B.; Fernando, A.L. Wastewaters Reuse for Energy Crops Cultivation. In Technological Innovation for Cyber-Physical Systems; DoCEIS, IFIP Advances in Information and Communication Technology; Camarinha-Matos, L.M., Falcão, A.J., Vafaei, N., Najdi, S., Eds.; Springer: Cham, Switzerland, 2016; Volume 470, pp. 507–514. [Google Scholar]
- De Laporte, A.V.; Ripplinger, D.G. Economic viability of perennial grass biomass feedstock in northern climates. Ind. Crops Prod. 2019, 128, 213–220. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; Scalici, G.; Scordia, D.; Testa, G. Soil erosion mitigation by perennial species under Mediterranean environment. BioEnergy Res. 2015, 8, 1538–1547. [Google Scholar] [CrossRef]
- Schulte, L.A.; Asbjornsen, H.; Liebman, M.; Crow, T.R. Agroecosystem restoration through strategic integration of perennials. J. Soil Water Conserv. 2006, 61, 164A–169A. [Google Scholar]
- Jankauskas, B.; Jankauskiene, G. Erosion-preventive crop rotations for landscape ecological stability in upland regions of Lithuania. Agric. Ecosyst. Environ. 2003, 95, 129–142. [Google Scholar] [CrossRef]
- Lewandowski, I.; Schmidt, U. Nitrogen, energy and land use efficiencies of miscanthus, reed canary grass and triticale as determined by the boundary line approach. Agric. Ecosyst. Environ. 2006, 112, 335–346. [Google Scholar] [CrossRef]
- Felten, D.; Emmerling, C. Effects of bioenergy crop cultivation on earthworm communities—A comparative study of perennial (Miscanthus) and annual crops with consideration of graded land-use intensity. Appl. Soil Ecol. 2011, 49, 167–177. [Google Scholar] [CrossRef]
- Zan, C.S.; Fyles, J.W.; Girouard, P.; Samson, R.A. Carbon sequestration in perennial bioenergy, annual corn and uncultivated systems in southern Quebec. Agric. Ecosyst. Environ. 2001, 86, 135–144. [Google Scholar] [CrossRef]
- Bonin, C.L.; Fidel, R.B.; Banik, C.; Laird, D.A.; Mitchell, R.; Heaton, E.A. Perennial biomass crop establishment, community characteristics, and productivity in the upper US Midwest: Effects of cropping systems seed mixtures and biochar applications. Eur. J. Agron. 2018, 101, 121–128. [Google Scholar] [CrossRef]
- Fernando, A.L.; Rettenmaier, N.; Soldatos, P.; Panoutsou, C. Sustainability of Perennial Crops Production for Bioenergy and Bioproducts. In Perennial Grasses for Bioenergy and Bioproducts; Alexopoulou, E., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 245–283. [Google Scholar]
- Alexopoulou, E.; Zanetti, F.; Papazoglou, E.G.; Christou, M.; Papatheohari, Y.; Tsiotas, K.; Papamichael, I. Long-term studies on switchgrass grown on a marginal area in Greece under different varieties and nitrogen fertilization rates. Ind. Crops Prod. 2017, 107, 446–452. [Google Scholar] [CrossRef]
- Rutz, D.; Ugalde, J.M.; Mergner, R.; Janssen, R.; Epp, C.; Leplus, A.; Bernard, J.; Eleftheriadis, I.; Žandeckis, A.; Fištre, Ž.; et al. Short Rotation Woody Crops: Experiences from the eu Project Srcplus, Proceedings of the 25th European Biomass Conference and Exhibition; 25th European Biomass Conference and Exhibition: Stockholm, Sweden, 2017; pp. 143–149. [Google Scholar]
- McElroy, G.H.; Dawson, W.M. Biomass from short-rotation coppice willow on marginal land. Biomass 1986, 10, 225–240. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Bi, M.; Wang, X.; Lu, Y.; Fan, Z. Effects of best management practices on nitrogen load reduction in tea fields with different slope gradients using the SWAT model. Appl. Geogr. 2018, 90, 200–213. [Google Scholar] [CrossRef]
- Vatsa, D.K.; Vyas, N. Modern farm technologies for enhancing work productivity with reduced drudgery of rural women in hill agriculture. AMA Agric. Mech. Asia Afr. Lat. Am. 2018, 49, 32–38. [Google Scholar]
- Pari, L.; Alfano, V.; Garcia-Galindo, D.; Suardi, A.; Santangelo, E. Pruning biomass potential in Italy related to crop characteristics, agricultural practices and agro-climatic conditions. Energies 2018, 11, 1365. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patanè, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Flexas, J.; DIAZ-ESPEJO, A.; GalmES, J.; Kaldenhoff, R.; Medrano, H.; RIBAS-CARBO, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Sánchez, E.; Scordia, D.; Lino, G.; Arias, C.; Cosentino, S.L.; Nogués, S. Salinity and water stress effects on biomass production in different Arundo donax L. clones. BioEnergy Res. 2015, 8, 1461–1479. [Google Scholar] [CrossRef]
- Van Orshoven, J.; Terres, J.-M.; Tóth, T. Updated common bio-physical criteria to define natural constraints for agriculture in Europe—Definition and scientific justification for the common biophysical criteria. JRC Sci. Policy Rep. 2014. [Google Scholar]
- Terres, J.-M.; Hagyo, A.; Wania, A. Scientific contribution on combining biophysical criteria underpinning the delineation of agricultural areas affected by specific constraints: Methodology and factsheets for plausible criteria combinations. JRC Sci. Policy Rep. 2014. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/scientific-contribution-combining-biophysical-criteria-underpinning-delineation-agricultural (accessed on 1 October 2019).
- Volaire, F.; Barkaoui, K.; Norton, M. Designing resilient and sustainable grasslands for a drier future: Adaptive strategies, functional traits and biotic interactions. Eur. J. Agron. 2014, 52, 81–89. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Sanzone, E.; Testa, G.; Copani, V. Response of giant reed (Arundo donax L.) to nitrogen fertilization and soil water availability in semi-arid Mediterranean environment. Eur. J. Agron. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Lelièvre, F.; Seddaiu, G.; Ledda, L.; Porqueddu, C.; Volaire, F. Water use efficiency and drought survival in Mediterranean perennial forage grasses. Field Crops Res. 2011, 121, 333–342. [Google Scholar] [CrossRef]
- Fernando, A.L. Environmental Aspects of Kenaf Production and Use. In Kenaf: A Multi-Purpose Crop for Several Industrial Applications: New insights from the Biokenaf Project; Monti, A., Alexopoulou, E., Eds.; Springer: London, UK, 2013; pp. 83–104. [Google Scholar]
- Reynolds, W.D.; Bowman, B.T.; Drury, C.F.; Tan, C.S.; Lu, X. Indicators of good soil physical quality: Density and storage parameters. Geoderma 2002, 110, 131–146. [Google Scholar] [CrossRef]
- Von Cossel, M.; Iqbal, Y.; Scordia, D.; Cosentino, S.L.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Mantel, S.; Prysiazhniuk, O.; Maliarenko, O.; et al. Low-Input Agricultural Practices for Industrial Crops on Marginal Land. EU-Deliverable; University of Hohenheim: Stuttgart, Germany, 2018. [Google Scholar]
- Ruf, T.; Audu, V.; Holzhauser, K.; Emmerling, C. Bioenergy from periodically waterlogged cropland in Europe: A first assessment of the potential of five perennial energy crops to provide biomass and their interactions with soil. Agronomy 2019, 9, 374. [Google Scholar] [CrossRef]
- Van Orshoven, J.; Terres, J.-M.; Tóth, T. Updated common bio-physical criteria to define natural constraints for agriculture in Europe. JRC Sci. Tech. Rep. 2012. Available online: https://www.semanticscholar.org/paper/Updated-common-bio-physical-criteria-to-define-for-Jos-Jean/1176dee267e586b47dd45be6c568dd6312990735 (accessed on 1 October 2019).
- Obia, A.; Mulder, J.; Hale, S.E.; Nurida, N.L.; Cornelissen, G. The potential of biochar in improving drainage, aeration and maize yields in heavy clay soils. PLoS ONE 2018, 13, e0196794. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.J. Impact of ploughless soil tillage on yield and soil quality: A Scandinavian review. Soil Tillage Res. 1999, 53, 3–14. [Google Scholar] [CrossRef]
- Kharytonov, M.; Pidlisnyuk, V.; Stefanovska, T.; Babenko, M.; Martynova, N.; Rula, I. The estimation of Miscanthus×giganteus’ adaptive potential for cultivation on the mining and post-mining lands in Ukraine. Environ. Sci. Pollut. Res. 2019, 26, 2974–2986. [Google Scholar] [CrossRef]
- Lamb, D.T.; Heading, S.; Bolan, N.; Naidu, R. Use of biosolids for phytocapping of landfill soil. Water. Air. Soil Pollut. 2012, 223, 2695–2705. [Google Scholar] [CrossRef]
- Barbosa, B.; Fernando, A.L. Chapter 9 - Aided Phytostabilization of Mine Waste. In Bio-Geotechnologies for Mine Site Rehabilitation; Prasad, M.N.V., de Favas, P.J.C., Maiti, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–157. [Google Scholar]
- Barbosa, B.; Costa, J.; Fernando, A.L. Production of Energy Crops in Heavy Metals Contaminated Land: Opportunities and Risks. In Land Allocation for Biomass Crops: Challenges and Opportunities with Changing Land Use; Li, R., Monti, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 83–102. [Google Scholar]
- The Council of the European Communities Council directive on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Comm. 1986, 181, 0006–0012.
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. BioEnergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Radojčić Redovniković, I.; De Marco, A.; Proietti, C.; Hanousek, K.; Sedak, M.; Bilandžić, N.; Jakovljević, T. Poplar response to cadmium and lead soil contamination. Ecotoxicol. Environ. Saf. 2017, 144, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Fernando, A.L.; Boléo, S.; Barbosa, B.; Costa, J.; Lino, J.; Tavares, C.; Sidella, S.; Duarte, M.P.; Mendes, B. How sustainable is the production of energy crops in heavy metal contaminated soils. In Proceedings of the 22th European Biomass Conference and Exhibition, Setting the Course for a Biobased Economy; ETA-Renewable Energies: Hamburg, Germany, 2014; pp. 1593–1596. [Google Scholar]
- Papazoglou, E.G.; Fernando, A.L. Preliminary studies on the growth, tolerance and phytoremediation ability of sugarbeet (Beta vulgaris L.) grown on heavy metal contaminated soil. Ind. Crops Prod. 2017, 107, 463–471. [Google Scholar] [CrossRef]
- Sidella, S.; Barbosa, B.; Costa, J.; Cosentino, S.L.; Fernando, A.L. Screening of Giant reed clones for Phytoremediation of lead contaminated soils. In Perennial Biomass Crops for a Resource-Constrained World; Barth, S., Murphy-Bokern, D., Kalinina, O., Taylor, G., Jones, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 191–197. [Google Scholar]
- Fernando, A.L.; Godovikova, V.; Oliveira, J.F.S. Miscanthus x giganteus: Contribution to a sustainable agriculture of a future/present-oriented biomaterial. Trans. Tech. Publ. 2004, 455, 437–441. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lewandowski, I. Inter-annual variation in biomass combustion quality traits over five years in fifteen Miscanthus genotypes in south Germany. Fuel Process. Technol. 2014, 121, 47–55. [Google Scholar] [CrossRef]
- Iqbal, Y.; Kiesel, A.; Wagner, M.; Nunn, C.; Kalinina, O.; Hastings, A.F.S.J.; Clifton-Brown, J.C.; Lewandowski, I. Harvest time optimization for combustion quality of different miscanthus genotypes across Europe. Front. Plant Sci. 2017, 8, 727. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Kiesel, A.; Iqbal, Y.; Muylle, H.; Dolstra, O.; Visser, R.G.F.; Lewandowski, I.; Trindade, L.M. Evaluation of Miscanthus sinensis biomass quality as feedstock for conversion into different bioenergy products. GCB Bioenergy 2017, 9, 176–190. [Google Scholar] [CrossRef]
- Fernando, A.L.; Barbosa, B.; Costa, J.; Papazoglou, E.G. Giant reed (arundo donax l.): A multipurpose crop bridging phytoremediation with sustainable bioeconomy. In Bioremediation and Bioeconomy; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–95. [Google Scholar]
- Gomes, L.; Fernando, A.L.; Santos, F. A toolbox to tackle the technological and environmental constraints associated with the use of biomass for energy from marginal land. In Proceedings of the ECOS 2018, the 31st International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Guimarães, Portugal, 17–22 June 2018. [Google Scholar]
- Lomba, A.; Buchadas, A.; Honrado, J.P.; Moreira, F. Are We Missing the Big Picture? Unlocking the Social-Ecological Resilience of High Nature Value Farmlands to Future Climate Change. In Climate Change-Resilient Agriculture and Agroforestry; Castro, P., Azul, A.M., Leal Filho, W., Azeiteiro, U.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 53–72. [Google Scholar]
- Tuck, G.; Glendining, M.J.; Smith, P.; House, J.I.; Wattenbach, M. The potential distribution of bioenergy crops in Europe under present and future climate. Biomass Bioenergy 2006, 30, 183–197. [Google Scholar] [CrossRef]
- Garbolino, E.; Daniel, W.; Hinojos Mendoza, G. Expected Global Warming Impacts on the Spatial Distribution and Productivity for 2050 of Five Species of Trees Used in the Wood Energy Supply Chain in France. Energies 2018, 11, 3372. [Google Scholar] [CrossRef]
- Samaniego, L.; Thober, S.; Kumar, R.; Wanders, N.; Rakovec, O.; Pan, M.; Zink, M.; Sheffield, J.; Wood, E.F.; Marx, A. Anthropogenic warming exacerbates European soil moisture droughts. Nat. Clim. Change 2018, 8, 421. [Google Scholar] [CrossRef]
- Teuling, A.J. A hot future for European droughts. Nat. Clim. Change 2018, 8, 364. [Google Scholar] [CrossRef]
- Giorgi, F.; Gutowski, W.J. Regional Dynamical Downscaling and the CORDEX Initiative. Annu. Rev. Environ. Resour. 2015, 40, 467–490. [Google Scholar] [CrossRef]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Change 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Soares, P.M.M.; Cardoso, R.M.; Lima, D.C.A.; Miranda, P.M.A. Future precipitation in Portugal: High-resolution projections using WRF model and EURO-CORDEX multi-model ensembles. Clim. Dyn. 2017, 49, 2503–2530. [Google Scholar] [CrossRef]
- Pfeifer, S.; Bülow, K.; Gobiet, A.; Hänsler, A.; Mudelsee, M.; Otto, J.; Rechid, D.; Teichmann, C.; Jacob, D. Robustness of Ensemble Climate Projections Analyzed with Climate Signal Maps: Seasonal and Extreme Precipitation for Germany. Atmosphere 2015, 6, 677–698. [Google Scholar] [CrossRef]
- Huebener, H.; Hoffmann, P.; Keuler, K.; Pfeifer, S.; Ramthun, H.; Spekat, A.; Steger, C.; Warrach-Sagi, K. Deriving user-informed climate information from climate model ensemble results. Adv. Sci. Res. 2017, 14, 261–269. [Google Scholar] [CrossRef]
- Galatsidas, S.; Gounaris, N.; Vlachaki, D.; Dimitriadis, E.; Kiourtsis, F.; Keramitzis, D.; Gerwin, W.; Repmann, F.; Rettenmaier, N.; Reinhardt, G. Revealing Bioenergy Potentials: Mapping Marginal Lands in Europe—The SEEMLA Approach. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–18 May 2018; pp. 31–37. [Google Scholar]
- Gerwin, W.; Repmann, F.; Galatsidas, S.; Vlachaki, D.; Gounaris, N.; Baumgarten, W.; Volkmann, C.; Keramitzis, D.; Kiourtsis, F.; Freese, D. Assessment and quantification of marginal lands for biomass production in Europe using soil-quality indicators. SOIL 2018, 4, 267–290. [Google Scholar] [CrossRef]
- IPCC, Global Warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty [Masson-Delmotte, V., P. Zhai, H.-O. örtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, and T. Waterfield (eds.)]. 2018; in press.
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Poorter, H.; Navas, M.-L. Plant growth and competition at elevated Co2: On winners, losers and functional groups. New Phytol. 2003, 157, 175–198. [Google Scholar] [CrossRef]
- Sage, R.F.; Sage, T.L. C4 Plants. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 361–381. [Google Scholar]
- Seneviratne, S.I.; Corti, T.; Davin, E.L.; Hirschi, M.; Jaeger, E.B.; Lehner, I.; Orlowsky, B.; Teuling, A.J. Investigating soil moisture–climate interactions in a changing climate: A review. Earth-Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Weselek, A.; Ehmann, A.; Zikeli, S.; Lewandowski, I.; Schindele, S.; Högy, P. Agrophotovoltaic systems: Applications, challenges, and opportunities. A review. Agron. Sustain. Dev. 2019, 39, 35. [Google Scholar] [CrossRef]
- Amaducci, S.; Yin, X.; Colauzzi, M. Agrivoltaic systems to optimise land use for electric energy production. Appl. Energy 2018, 220, 545–561. [Google Scholar] [CrossRef]
- MAGIC Marginal Lands for Growing Industrial Crops: Turning a Burden into an Opportunity. 2019. Available online: http://magic-h2020.eu/ (accessed on 14 June 2019).
- Cai, H.; Wang, J.; Feng, Y.; Wang, M.; Qin, Z.; Dunn, J.B. Consideration of land use change-induced surface albedo effects in life-cycle analysis of biofuels. Energy Environ. Sci. 2016, 9, 2855–2867. [Google Scholar] [CrossRef]
- Möndel, A. Ertragsmessungen in Winterroggen-der Ertragseinfluss einer Windschutzanlage in der oberrheinischen Tiefebene. Verbundprojekt: agroforst - neue Optionen für eine nachhaltige Landnutzung, LAP Forchheim, Germany. 2007. Available online: http://docplayer.org/38193544-Ertragsmessungen-in-winterroggen-der-ertragseinfluss-einer-windschutzanlage-in-der-oberrheinischen-tiefebene.html (accessed on 1 October 2019).
- Goetsch, E.; Colinas, F.T. Natural succession of species in agroforestry and in soil recovery. 1992. Available online: media0.agrofloresta.net/static/artigos/agroforestry_1992_gotsch.pdf (accessed on 1 October 2019).
- FAO. Bioenergy and Food Security—The BEFS Analytical Framework; Environment and Natural Resources Management Series; Sales and Marketing Group—Communication Division Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Kaygusuz, K. Energy for sustainable development: A case of developing countries. Renew. Sustain. Energy Rev. 2012, 16, 1116–1126. [Google Scholar] [CrossRef]
- Winkler, B.; Lewandowski, I.; Voss, A.; Lemke, S. Transition towards Renewable Energy Production? Potential in Smallholder Agricultural Systems in West Bengal, India. Sustainability 2018, 10, 801. [Google Scholar] [CrossRef]
- FAO. Evidence-Based Assessment of the Sustainability and Replicability of Integrated Food-Energy Systems—A Guidance Document; Environment and Natural Resources Working Paper; Sales and Marketing Group—Communication Division Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Chen, R. Livestock-biogas-fruit systems in South China. Ecol. Eng. 1997, 8, 19–29. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Y.-X.; Wang, J.-Z.; Chen, G.; Battye, H. Where is the future of China’s biogas? Review, forecast, and policy implications. Pet. Sci. 2016, 13, 604–624. [Google Scholar] [CrossRef]
- MNRE National Biogas and Manure Management Programme. 2019. Available online: https://mnre.gov.in/biogas (accessed on 8 August 2019).
- Amigun, B.; Musango, J.K.; Brent, A.C. Community perspectives on the introduction of biodiesel production in the Eastern Cape Province of South Africa. Energy 2011, 36, 2502–2508. [Google Scholar] [CrossRef]
- Barry, M.-L.; Steyn, H.; Brent, A. Selection of renewable energy technologies for Africa: Eight case studies in Rwanda, Tanzania and Malawi. Renew. Energy 2011, 36, 2845–2852. [Google Scholar] [CrossRef]
- Brent, A.C.; Kruger, W.J.L. Systems analyses and the sustainable transfer of renewable energy technologies: A focus on remote areas of Africa. Renew. Energy 2009, 34, 1774–1781. [Google Scholar] [CrossRef]
- Duku, M.H.; Gu, S.; Hagan, E.B. A comprehensive review of biomass resources and biofuels potential in Ghana. Renew. Sustain. Energy Rev. 2011, 15, 404–415. [Google Scholar] [CrossRef]
- Practical Action Consulting. Small-Scale Bioenergy Initiatives: Brief Description and Preliminary Lessons on Livelihood Impacts from Case Studies in Asia, Latin America and Africa; Practical Action Consulting, Food and Agriculture Organization of the United Nations, Climate Change and Bioenergy Unit: Rome, Italy, 2009. [Google Scholar]
- Stoknes, K.; Scholwin, F.; Krzesiński, W.; Wojciechowska, E.; Jasińska, A. Efficiency of a novel “Food to waste to food” system including anaerobic digestion of food waste and cultivation of vegetables on digestate in a bubble-insulated greenhouse. Waste Manag. 2016, 56, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Kloepffer, W. Life cycle sustainability assessment of products. Int. J. Life Cycle Assess. 2008, 13, 89. [Google Scholar] [CrossRef]
- Fernando, A.L.; Costa, J.; Barbosa, B.; Monti, A.; Rettenmaier, N. Environmental impact assessment of perennial crops cultivation on marginal soils in the Mediterranean Region. Biomass Bioenergy 2018, 111, 174–186. [Google Scholar] [CrossRef]
- Wagner, M.; Kiesel, A.; Hastings, A.; Iqbal, Y.; Lewandowski, I. Novel miscanthus germplasm-based value chains: A Life Cycle Assessment. Front. Plant Sci. 2017, 8, 990. [Google Scholar] [CrossRef]
- Meyer, S.T.; Ebeling, A.; Eisenhauer, N.; Hertzog, L.; Hillebrand, H.; Milcu, A.; Pompe, S.; Abbas, M.; Bessler, H.; Buchmann, N. Effects of biodiversity strengthen over time as ecosystem functioning declines at low and increases at high biodiversity. Ecosphere 2016, 7, e01619. [Google Scholar] [CrossRef]
- Schmidt, T.; Fernando, A.L.; Monti, A.; Rettenmaier, N. Life Cycle Assessment of Bioenergy and Bio-Based Products from Perennial Grasses Cultivated on Marginal Land in the Mediterranean Region. BioEnergy Res. 2015, 8, 1548–1561. [Google Scholar] [CrossRef]
- De Laurentiis, V.; Secchi, M.; Bos, U.; Horn, R.; Laurent, A.; Sala, S. Soil quality index: Exploring options for a comprehensive assessment of land use impacts in LCA. J. Clean. Prod. 2019, 215, 63–74. [Google Scholar] [CrossRef]
- Winter, L.; Lehmann, A.; Finogenova, N.; Finkbeiner, M. Including biodiversity in life cycle assessment—State of the art, gaps and research needs. Environ. Impact Assess. Rev. 2017, 67, 88–100. [Google Scholar] [CrossRef]
- Brankatschk, G.; Finkbeiner, M. Crop rotations and crop residues are relevant parameters for agricultural carbon footprints. Agron. Sustain. Dev. 2017, 37, 58. [Google Scholar] [CrossRef]
- EEX European Emission Allowances (EUA). 2019. Available online: https://www.eex.com/en/market-data/environmental-markets/spot-market/european-emission-allowances (accessed on 21 July 2019).
- Matthey, A.; Bünger, B. Methodenkonvention 3.0 zur Ermittlung von Umweltkosten Kostensätze Stand 02/2019; Für Mensch und Umwelt; Umweltbundesamt: Dessau-Rosslau, Germany, 2019. [Google Scholar]
- Landis, D.A.; Gratton, C.; Jackson, R.D.; Gross, K.L.; Duncan, D.S.; Liang, C.; Meehan, T.D.; Robertson, B.A.; Schmidt, T.M.; Stahlheber, K.A. Biomass and biofuel crop effects on biodiversity and ecosystem services in the North Central US. Biomass Bioenergy 2018, 114, 18–29. [Google Scholar] [CrossRef]
- Breeze, T.D.; Gallai, N.; Garibaldi, L.A.; Li, X.S. Economic Measures of Pollination Services: Shortcomings and Future Directions. Trends Ecol. Evol. 2016, 31, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.M.; Ward, K.L.; Pope, N.; Isaacs, R.; Wilson, J.; May, E.A.; Ellis, J.; Daniels, J.; Pence, A.; Ullmann, K.; et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 2015, 25, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Pizzol, M.; Weidema, B.; Brandão, M.; Osset, P. Monetary valuation in Life Cycle Assessment: A review. J. Clean. Prod. 2015, 86, 170–179. [Google Scholar] [CrossRef]
- Hastings, A.; Mos, M.; Yesufu, J.A.; McCalmont, J.; Schwarz, K.; Shafei, R.; Ashman, C.; Nunn, C.; Schuele, H.; Cosentino, S.; et al. Economic and Environmental Assessment of Seed and Rhizome Propagated Miscanthus in the UK. Front. Plant Sci. 2017, 8, 1058. [Google Scholar] [CrossRef] [PubMed]
- Huth, E.; Paltrinieri, S.; Thiele, J. Bioenergy and its effects on landscape aesthetics–A survey contrasting conventional and wild crop biomass production. Biomass Bioenergy 2019, 122, 313–321. [Google Scholar] [CrossRef]
- Finkbeiner, M.; Schau, E.M.; Lehmann, A.; Traverso, M. Towards Life Cycle Sustainability Assessment. Sustainability 2010, 2, 3309–3322. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; et al. Prospects of Bioenergy Cropping Systems for A More Social-Ecologically Sound Bioeconomy. Agronomy 2019, 9, 605. https://doi.org/10.3390/agronomy9100605
Von Cossel M, Wagner M, Lask J, Magenau E, Bauerle A, Von Cossel V, Warrach-Sagi K, Elbersen B, Staritsky I, Van Eupen M, et al. Prospects of Bioenergy Cropping Systems for A More Social-Ecologically Sound Bioeconomy. Agronomy. 2019; 9(10):605. https://doi.org/10.3390/agronomy9100605
Chicago/Turabian StyleVon Cossel, Moritz, Moritz Wagner, Jan Lask, Elena Magenau, Andrea Bauerle, Viktoria Von Cossel, Kirsten Warrach-Sagi, Berien Elbersen, Igor Staritsky, Michiel Van Eupen, and et al. 2019. "Prospects of Bioenergy Cropping Systems for A More Social-Ecologically Sound Bioeconomy" Agronomy 9, no. 10: 605. https://doi.org/10.3390/agronomy9100605
APA StyleVon Cossel, M., Wagner, M., Lask, J., Magenau, E., Bauerle, A., Von Cossel, V., Warrach-Sagi, K., Elbersen, B., Staritsky, I., Van Eupen, M., Iqbal, Y., Jablonowski, N. D., Happe, S., Fernando, A. L., Scordia, D., Cosentino, S. L., Wulfmeyer, V., Lewandowski, I., & Winkler, B. (2019). Prospects of Bioenergy Cropping Systems for A More Social-Ecologically Sound Bioeconomy. Agronomy, 9(10), 605. https://doi.org/10.3390/agronomy9100605










