Mulch-Based No-Tillage Effects on Weed Community and Management in an Organic Vegetable System
Abstract
1. Introduction
2. Materials and Methods
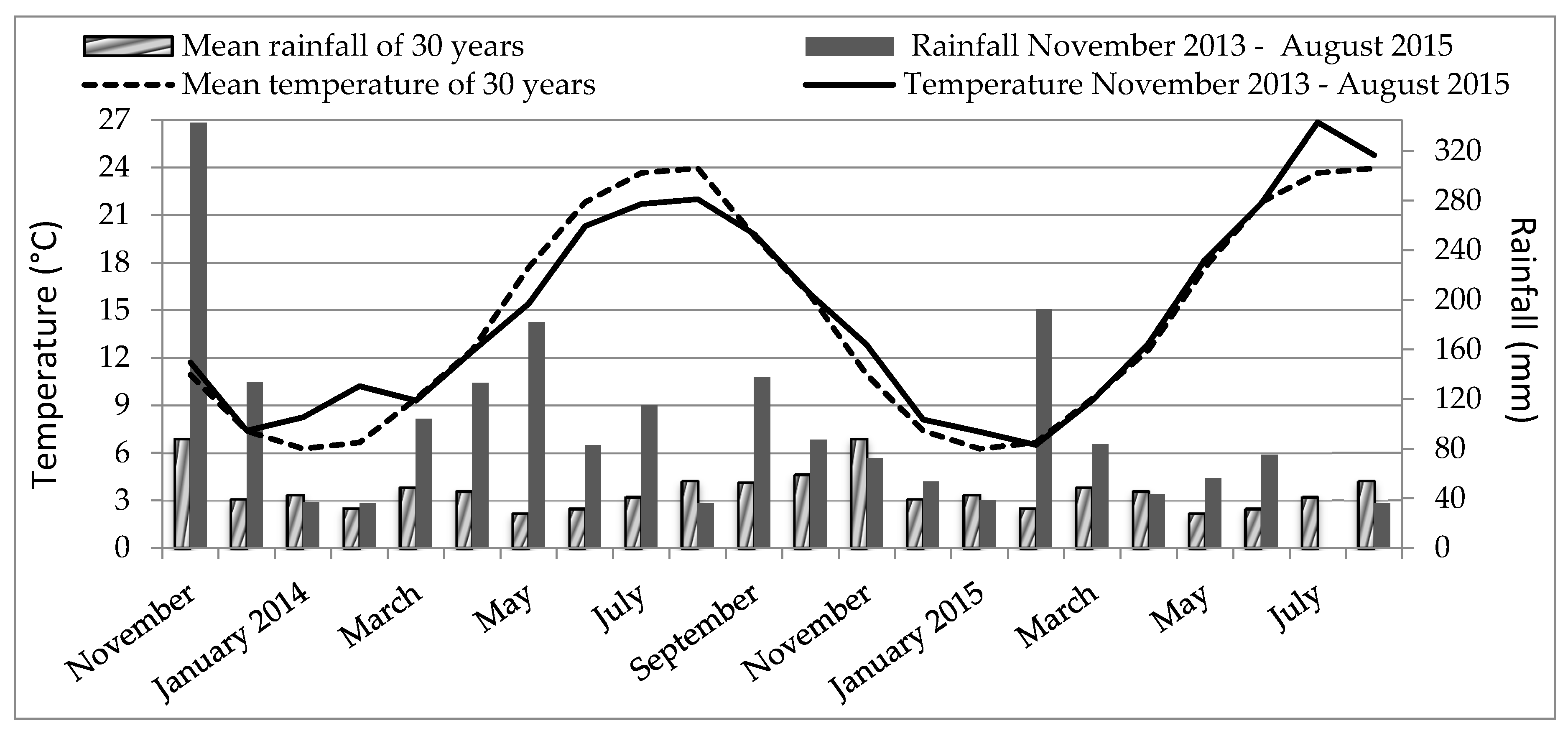
2.1. Site, Climate, and Soil
2.2. Experiment Setup and Treatments
2.3. Measurements
2.3.1. ASC and Cash Crop Yields
2.3.2. Soil Mineral Nitrogen
2.3.3. Weed Biomass and Density
2.3.4. Ecological Characterization of Weed Community
H’ = −∑ (pi * ln pi)
i = 1
2.4. Statistical Analysis
3. Results
3.1. ASC and Cash Crop Yields
3.2. Soil Mineral Nitrogen
3.3. Ecological Characterization of Weed Community
3.3.1. Principal Component Analysis
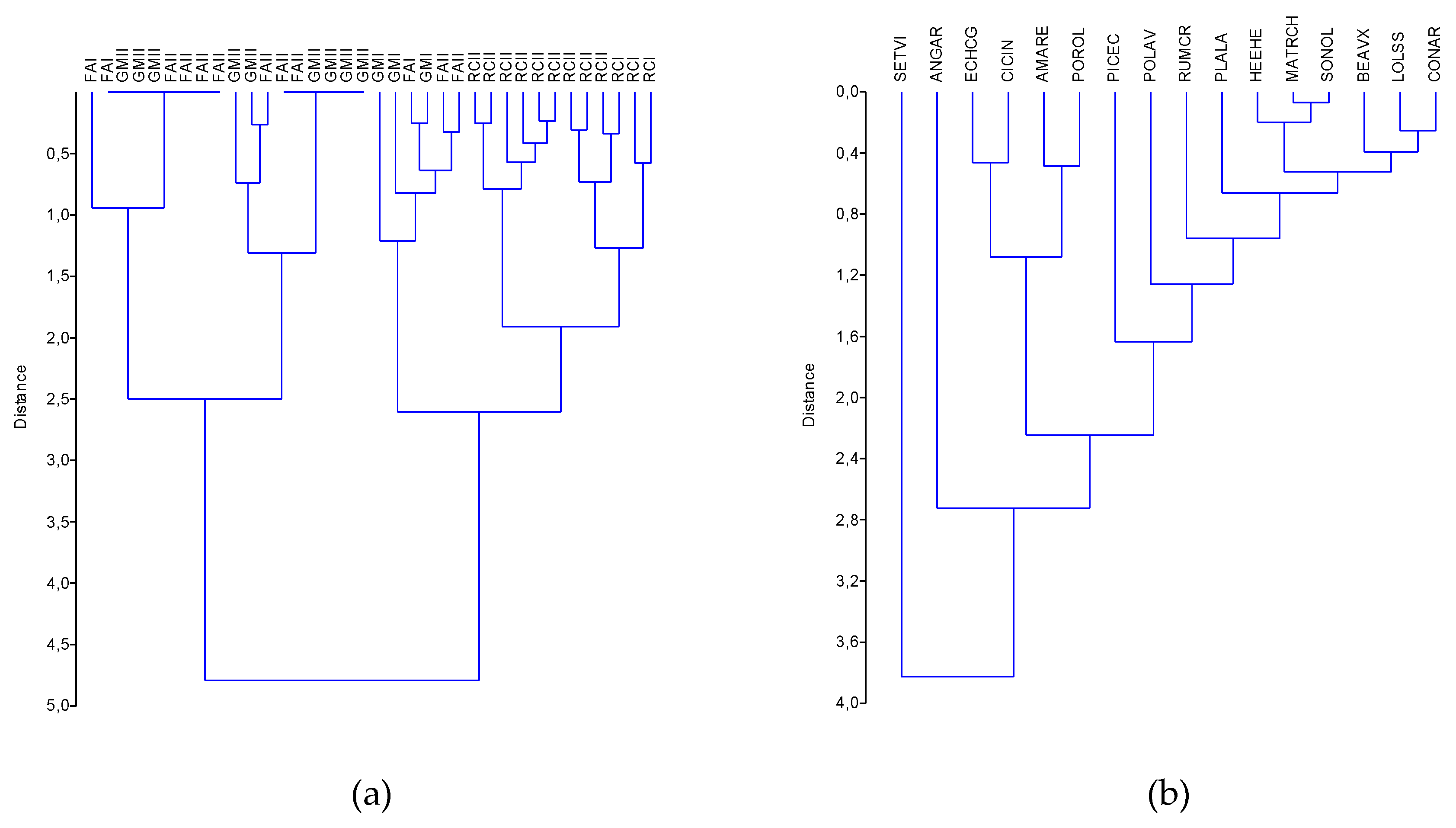
3.3.2. Hierarchical Clustering
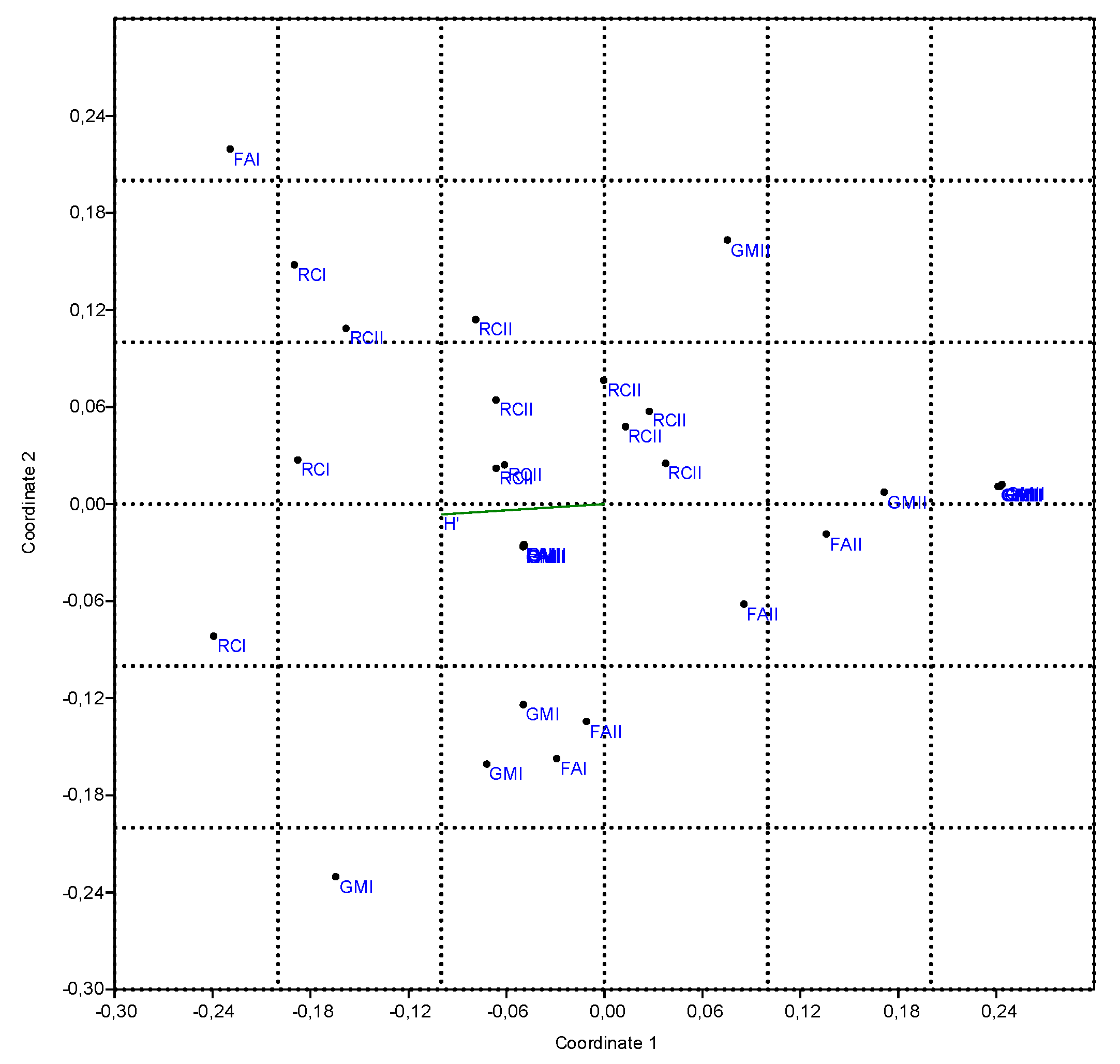
3.3.3. Non-Metric Multi-Dimensional Scaling
3.3.4. Correlation Analysis
4. Discussion
4.1. Zucchini and Barley Yields, Soil Mineral N
4.2. Weed Community and Management
Author Contributions
Funding
Conflicts of Interest
References
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Ciaccia, C.; La Torre, A.; Ferlito, F.; Testani, E.; Battaglia, V.; Salvati, L.; Roccuzzo, G. Agroecological Practices and Agrobiodiversity: A Case Study on Organic Orange in Southern Italy. Agronomy 2019, 9, 85. [Google Scholar] [CrossRef]
- Wan, K.; Tao, Y.; Li, R.; Pan, J.; Tang, L.; Chen, F. Influences of long-term different types of fertilization on weed community biodiversity in rice paddy fields. Weed Biol. Manag. 2012, 12, 12–21. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Canali, S.; Diacono, M.; Campanelli, G.; Montemurro, F. Organic No-Till with Roller Crimpers: Agro-ecosystem Services and Applications in Organic Mediterranean Vegetable Productions. Sustain. Agric. Res. 2015, 4, 70–79. [Google Scholar] [CrossRef]
- Ciaccia, C.; Testani, E.; Campanelli, G.; Sestili, S.; Leteo, F.; Tittarelli, F.; Riva, F.; Trinchera, A. Ecological service providing crops effect on melon-weed competition and allelopathic interactions. Org. Agric. 2015, 5, 199–207. [Google Scholar] [CrossRef]
- Magagnoli, S.; Depalo, L.; Masetti, A.; Campanelli, G.; Canali, S.; Leteo, F.; Burgio, G. Influence of agro-ecological service crop termination and synthetic biodegradable film covering on Aphis gossypii Glover (Rhynchota: Aphididae) infestation and natural enemy dynamics. Renew. Agric. Food Syst. 2018, 33, 386–392. [Google Scholar] [CrossRef]
- Yin, L.; Cai, Z.; Zhong, W. Changes in weed community diversity of maize crops due to long-term fertilization. Crop. Prot. 2006, 25, 910–914. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Magid, J.; Jensen, L.S. Catch crops and green manures as biological tools in nitrogen management in temperate zones. Adv. Agron. 2003, 79, 227–302. [Google Scholar]
- Allison, F.E. The fate of nitrogen applied to soils. Adv. Agron. 1966, 18, 219–258. [Google Scholar] [CrossRef]
- Liebman, A.M.; Grossman, J.; Brown, M.; Wells, M.S.; Reberg-Horton, S.C.; Shi, W. Legume cover crops and tillage impact nitrogen dynamics in organic corn production. Agron. J. 2018, 110, 1046–1057. [Google Scholar] [CrossRef]
- Fried, G.; Chauvel, B.; Reboud, X. A functional analysis of large-scale temporal shifts from 1970 to 2000 in weed assemblages of sunflower crops in France. J. Veg. Sci. 2009, 20, 49–58. [Google Scholar] [CrossRef]
- Demjanovà, E. Effects of crop rotation and tillage systems on weed populations, density and diversity in maize (Zea mays L.). Acta Fytotech. Zootech. 2004, 7, 61–63. [Google Scholar]
- Blackshaw, R.E.; Moyer, J.R.; Doram, R.C.; Boswell, A.L. Yellow sweetclover, green manure, and its residues effectively suppress weeds during fallow. Weed Sci. 2001, 49, 406–413. [Google Scholar] [CrossRef]
- De Albuquerque, M.B.; dos Santos, R.C.; Lima, L.M.; de Albuquerque Melo Filho, P.; Nogueira, R.J.M.C.; Da Câmara, C.A.G.; de Rezende Ramos, A. Allelopathy, an alternative tool to improve cropping systems. A review. Agron. Sustain. Dev. 2011, 31, 379–395. [Google Scholar] [CrossRef]
- Buhler, D.D.; Hartzler, R.G.; Forcella, F. Implications of weed seedbank dynamics to weed management. Weed Sci. 1997, 45, 329–336. [Google Scholar] [CrossRef]
- Canali, S.; Campanelli, G.; Ciaccia, C.; Leteo, F.; Testani, E.; Montemurro, F. Conservation tillage strategy based on the roller crimper technology for weed control in Mediterranean vegetable organic cropping systems. Eur. J. Agron. 2013, 50, 11–18. [Google Scholar] [CrossRef]
- Ciaccia, C.; Canali, S.; Campanelli, G.; Testani, E.; Montemurro, F.; Leteo, F.; Delate, K. Effect of roller-crimper technology on weed management in organic zucchini production in a Mediterranean climate zone. Renew. Agric. Food Syst. 2016, 31, 1–11. [Google Scholar] [CrossRef]
- Campanelli, G.; Testani, E.; Canali, S.; Ciaccia, C.; Leteo, F.; Trinchera, A. Effects of cereals as agro-ecological service crops and no-till on organic melon, weeds and N dynamics. Biol. Agric. Hortic. 2019. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Mirsky, S.B.; Spargo, J.T.; Cavigellia, M.A.; Maula, J.E. Reduced-Tillage Organic Corn Production in a Hairy Vetch Cover Crop. Agron. J. 2012, 104, 621–628. [Google Scholar] [CrossRef]
- Tittarelli, F.; Campanelli, G.; Leteo, F.; Farina, R.; Napoli, R.; Ciaccia, C.; Canali, S.; Testani, E. Mulch Based No-Tillage and Compost Effects on Nitrogen Fertility in Organic Melon. Agron. J. 2018, 110, 1482–1491. [Google Scholar] [CrossRef]
- Mirsky, S.B.; Ryan, M.R.; Teasdale, J.R.; Curran, W.S.; Reberg-Horton, C.S.; Spargo, J.T.; Scott Wells, M.; Keene, C.L.; Moyer, J.W. Overcoming weed management challenges in cover crop–based organic rotational no-till soybean production in the eastern United States. Weed Technol. 2013, 27, 193–203. [Google Scholar] [CrossRef]
- Nalewaja, J.D. Weeds and conservation agriculture. In Conservation Agriculture; García-Torres, L., Benites, J., Martínez-Vilela, A., Holgado-Cabrera, A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 201–210. [Google Scholar] [CrossRef]
- Armengot, L.; Blanco-Moreno, J.M.; Bàrberi, P.; Bocci, G.; Carlesi, S.; Aendekerk, R.; Bernerb, A.; Celettee, F.; Grossef, M.; Huitingg, H.; et al. Tillage as a driver of change in weed communities: A functional perspective. Agric. Ecosyst. Environ. 2016, 222, 276–285. [Google Scholar] [CrossRef]
- Barberi, P.; Cozzani, A.; Macchia, M.; Bonari, E. Size and composition of the weed seedbank under different management systems for continuous maize cropping. Weed Res. 1998, 38, 319–334. [Google Scholar] [CrossRef]
- Cardina, J.; Herms, C.P.; Doohan, D.J. Crop rotation and tillage system effects on weed seedbanks. Weed Sci. 2002, 50, 448–460. [Google Scholar] [CrossRef]
- Zanin, G.; Otto, S.; Riello, L.; Borin, M. Ecological interpretation of weed flora dynamics under different tillage systems. Agric. Ecosyst. Environ. 1997, 66, 177–188. [Google Scholar] [CrossRef]
- Gibson, K.D.; McMillan, J.; Hallett, S.G.; Jordan, T.; Weller, S.C. Effect of a living mulch on weed seed banks in tomato. Weed Technol. 2011, 25, 245–251. [Google Scholar] [CrossRef]
- USDA. Keys to Soil Taxonomy. Soil Survey Staff, 7th ed.; U.S. Dept. of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 1996; p. 644.
- Campanelli, G.; Canali, S. Crop production and environmental effects in conventional and organic vegetable farming systems: The case of a long-term experiment in Mediterranean conditions (Central Italy). J. Sustain. Agric. 2012, 36, 599–619. [Google Scholar] [CrossRef]
- Krom, M.D. Spectrophotometric determination of ammonia: A study of a modified Berthelot reaction using salicylate and dichloroisocyanurate. Analyst 1980, 105, 305–316. [Google Scholar] [CrossRef]
- Henriksen, A.; Selmer-Olsen, A.R. Automatic methods for determining nitrate and nitrite in water and soil extracts. Analyst 1970, 95, 514–518. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Plant Sociology: The Study of Plant Communities, 3rd ed.; Fuller, G.D., Conard, H.S., Eds.; (Authorized English translation of Pflanzensociologie: Grundzuge der Vegetationskunde); Springer: Berlin/Heidelberg, Germany; McGraw-Hill: New York, NY, USA, 1932. [Google Scholar]
- Pignatti, S. Geobotanica. In Trattato di Botanica; Cappelletti, C., Ed.; UTET: Turin, Italy, 1976; pp. 801–997. [Google Scholar]
- Wikum, D.A.; Shanholtzer, G. Application of the Braun–Blanquet cover-abundance scale for vegetation analysis in land development studies. Environ. Manag. 1978, 2, 323–329. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1948. [Google Scholar] [CrossRef]
- Smith, W.G. Raunkiaer’s “life-forms” and statistical methods. J. Ecol. 1913, 1, 16–26. [Google Scholar] [CrossRef]
- Pignatti, S. Valori di bioindicazione delle piante vascolari della flora d’Italia. Braun Blanquetia 2005, 39, 3–97. [Google Scholar]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Murungu, F.S.; Chiduza, C.; Muchaonyerwa, P.; Mnkeni, P.N.S. Decomposition, nitrogen and phosphorus mineralization from winter-grown cover crop residues and suitability for a smallholder farming system in South Africa. Nutr. Cycl. Agroecosys 2011, 89, 115–123. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Mohler, C.L. Light transmittance, soil temperature, and soil moisture under residue of hairy vetch and rye. Agron. J. 1993, 85, 673–680. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Mohler, C.L. The quantitative relationship between weed emergence and the physical properties of mulches. Weed Sci. 2000, 48, 385–392. [Google Scholar] [CrossRef]
- Peigné, J.; Ball, B.C.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Buhler, D.D.; Stoltenberg, D.E.; Becker, R.L.; Gunsolus, J.L. Perennial weed populations after 14 years of variable tillage and cropping practices. Weed Sci. 1994, 42, 205–209. [Google Scholar] [CrossRef]
- Shrestha, A.; Lanini, T.; Wright, S.; Vargas, R.; Mitchell, J. Conservation Tillage and Weed Management; Division of Agricultural and Natural Resources, University of California: Oakland, CA, USA, 2006. [Google Scholar] [CrossRef]
- Moreau, D.; Milard, G.; Munier-Jolain, N. A plant nitrophily index based on plant leaf area response to soil nitrogen availability. Agron. Sustain. Dev. 2013, 33, 809–815. [Google Scholar] [CrossRef]
- Moreau, D.; Busset, H.; Matejicek, A.; Munier-Jolain, N. The ecophysiological determinants of nitrophily in annual weed species. Weed Res. 2014, 54, 335–346. [Google Scholar] [CrossRef]
- Perry, L.G.; Blumenthal, D.M.; Monaco, T.A.; Paschke, M.W.; Redente, E.F. Immobilizing nitrogen to control plant invasion. Oecologia 2010, 163, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Willi, J.C.; Mountford, J.O.; Sparks, T.H. The modification of ancient woodland ground flora at arable edges. Biodivers. Conserv. 2005, 14, 3215–3233. [Google Scholar] [CrossRef]
- Qasem, J.R. Herbicide resistant weeds: The technology and weed management. In Herbicides-Current Research and Case Studies in Use; InTech: London, UK, 2013; pp. 445–471. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crop. Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26. [Google Scholar] [CrossRef]

| Levels | Barley Biomass | Zucchini Yield | Weed Biomass | Weed Density | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mg ha−1 (dry matter) | N m−2 | ||||||||
| Y 1 | 2014 | 16.86 | a | 17.13 | b | 0.93 | 33.91 | ||
| 2015 | 10.52 | b | 22.11 | a | 1.23 | 79.26 | |||
| Sig. | * | ** | n.s. | n.s. | |||||
| Man 2 | No_ASC 3 | 33.25 | a | 0.45 | b | 88.33 | |||
| GM 4 | 17.00 | b | 0.97 | b | 70.22 | ||||
| RC 5 | 8.60 | c | 1.81 | a | 84.67 | ||||
| Sig. | *** | ** | n.s. | ||||||
| Y × Man | No_ASC I | 31.28 | a | 113.33 | ab | ||||
| GM I | 11.53 | c | 96.00 | ab | |||||
| RC I | 8.57 | c | 41.33 | b | |||||
| No_ASC II | 35.22 | a | 63.33 | ab | |||||
| GM II | 22.47 | b | 44.44 | b | |||||
| RC II | 8.64 | c | 128.00 | a | |||||
| Sig. | * | n.s. | * | ||||||
| Factor | Level | SMN (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|
| Barley Termination | Zucchini Flowering | Zucchini Fruit Set | Zucchini Harvest | ||||
| DAT 3 2014/2015 | −13/−11 | 23/13 | 49/44 | 90/88 | |||
| Y 1 | 2014 | 26.76 | 14.24 | b | 26.34 | 16.75 | b |
| 2015 | 24.12 | 61.33 | a | 28.72 | 40.53 | a | |
| Sig. | n.s. | *** | n.s. | *** | |||
| Man 2 | No_ASC 4 | 28.80 | 47.27 | a | 24.47 | 26.82 | |
| GM 5 | 24.15 | 29.22 | b | 32.96 | 28.85 | ||
| RC 6 | 23.37 | 36.86 | b | 25.16 | 30.26 | ||
| Sig. | n.s. | ** | n.s. | n.s. | |||
| Y × Man | Sig. | n.s. | n.s. | n.s. | n.s. | ||
| Species | Common English Name | EPPO 1 Code | Raunkiaer BG 2 | Habit | N 12 | I Year | II Year |
|---|---|---|---|---|---|---|---|
| Amaranthus retroflexus L. | Redroot pigweed | AMARE | T 3 scap 7 | Erect | 9 | * | * |
| Anagallis arvensis L. | Scarlet pimpernel | ANGAR | T rept 8 | Prostrate | 6 | * | * |
| Beta vulgaris L. | Beet | BEAVX | H 4 scap | Erect | 5 | * | |
| Cichorium intybus L. | Common chicory | CICIN | H scap | Erect | 5 | * | |
| Convolvulus arvensis L. | Field bindweed | CONAR | G 5 rhiz 9 | Prostrate/Creeping | 5 | * | |
| Echinocloa crus-galli (L.) P. Beauv. | Cockspur | ECHCG | T scap | Erect | 8 | * | |
| Hedera helix L. | Common ivy | HEEHE | P 6 lian 10 | Creeping | x | * | |
| Helminthotheca echioides (L.) Holub | Bristly oxtongue | PICEC | T scap | Erect | 2 | * | |
| Lolium spp | Tufted grass | LOLSS | T scap | Erect | 6 | * | |
| Matricaria chamomilla L. | Chamomile | MATCH | T scap | Erect | 5 | * | |
| Plantago lanceolata L. | Ribwort plantain | PLALA | H ros 11 | Erect | x | * | |
| Polygonum aviculare L. | Common knotgrass | POLAV | T rept | Prostrate/Creeping | 1 | * | * |
| Portulaca oleracea L | Common purslane | POROL | T scap | Prostrate/Creeping | 7 | * | * |
| Rumex crispus L. | Curly dock | RUMCR | H scap | Erect | 5 | * | * |
| Setaria viridis L. | Green foxtail | SETVI | T scap | Erect | 7 | * | |
| Sonchus oleraceus L. | Common sowthistle | SONOL | T scap | Erect | 6 | * | * |
| Species | EPPO 1 Code | Pearson | Spearman |
|---|---|---|---|
| Amaranthus retroflexus L. | AMARE | 0.30 | 0.33 |
| Anagallis arvensis L. | ANGAR | 0.14 | 0.39 |
| Beta vulgaris L. | BEAVX | 0.20 | 0.21 |
| Cichorium intybus L. | CICIN | 0.10 | 0.19 |
| Convolvulus arvensis L. | CONAR | 0.43 | 0.53 |
| Echinocloa crus-galli (L.) P. Beauv. | ECHCG | 0.21 | 0.21 |
| Hedera helix L. | HEEHE | −0.02 | −0.04 |
| Helminthotheca echioides (L.) Holub | LOLSS | 0.29 | 0.29 |
| Lolium spp | MATCH | 0.30 | 0.33 |
| Matricaria chamomilla L. | PICEC | 0.60 | 0.65 |
| Plantago lanceolata L. | PLALA | 0.49 | 0.61 |
| Polygonum aviculare L. | POLAV | 0.15 | 0.25 |
| Portulaca oleracea L | POROL | 0.32 | 0.31 |
| Rumex crispus L. | RUMCR | 0.02 | 0.02 |
| Setaria viridis L. | SETVI | −0.32 | −0.15 |
| Sonchus oleraceus L. | SONOL | 0.20 | 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testani, E.; Ciaccia, C.; Campanelli, G.; Leteo, F.; Salvati, L.; Canali, S. Mulch-Based No-Tillage Effects on Weed Community and Management in an Organic Vegetable System. Agronomy 2019, 9, 594. https://doi.org/10.3390/agronomy9100594
Testani E, Ciaccia C, Campanelli G, Leteo F, Salvati L, Canali S. Mulch-Based No-Tillage Effects on Weed Community and Management in an Organic Vegetable System. Agronomy. 2019; 9(10):594. https://doi.org/10.3390/agronomy9100594
Chicago/Turabian StyleTestani, Elena, Corrado Ciaccia, Gabriele Campanelli, Fabrizio Leteo, Luca Salvati, and Stefano Canali. 2019. "Mulch-Based No-Tillage Effects on Weed Community and Management in an Organic Vegetable System" Agronomy 9, no. 10: 594. https://doi.org/10.3390/agronomy9100594
APA StyleTestani, E., Ciaccia, C., Campanelli, G., Leteo, F., Salvati, L., & Canali, S. (2019). Mulch-Based No-Tillage Effects on Weed Community and Management in an Organic Vegetable System. Agronomy, 9(10), 594. https://doi.org/10.3390/agronomy9100594






