Abstract
Aminocyclopyrachlor is an herbicide that belongs to the new class of chemicals known as the pyrimidine carboxylic acids, which are used to control broadleaf weeds and brush. However, the environmental behavior and fate of aminocyclopyrachlor are not fully understood. The aim of the present study was thus to evaluate the mineralization, extractable residue and bound residue formation of aminocyclopyrachlor in three tropical soils with different physico-chemical properties. 14C-labeled [pyrimidine-2-14C] aminocyclopyrachlor was used to assess the fate of this herbicide in soil placed in biometer culture flasks. Total mineralization (accumulated 14CO2) of aminocyclopyrachlor was found to be <10% in all soils, decreasing in the following order: Oxisol—Typic Hapludox (clay) > Oxisol—Typic Hapludox (loamy sand) > Plinthosol—Petric (sandy clay). Overall, constant rate of mineralization (k) values for all soils were very low (0.00050% to 0.00079% 14CO2 day−1), with mineralization half-life times (MT50) consequently very high (877 to 1376 days), suggesting potential long persistence in soil. The amount of extractable residues decreased from ~31% to 50% in all soils after 126 days of incubation, indicating an increase in bound residue formation from ~5.0- to 7.5-fold compared to evaluation immediately after herbicide application, suggesting that degradation herbicide is involved in the formation of bound residues. Extractable residues are important factors that control mineralization and bound residue formation from aminocyclopyrachlor in the soil. The present study is the first to assess the fate, distribution, and formation of bound residues of aminocyclopyrachlor in soils. Aminocyclopyrachlor residues were predominantly associated with the OM and clay contents of soil. This effect of soil physico-chemical properties should be considered in environmental risk assessment of aminocyclopyrachlor and its application in the field for weed control.
1. Introduction
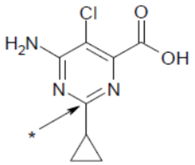
Aminocyclopyrachlor (6-amino-5-chloro-2-cyclopropylpyrimidine-4-carboxylic acid) is an nherbicide that belongs to the new class of chemicals known as the pyrimidine carboxylic acids. It is used in the US since 2010 to control broadleaf weeds and brushes in non-crops areas such as lawns (residential, industrial, and institutional), golf courses, parks, cemeteries, athletic fields, and sod farms [1] and is currently under development in Brazil for the cultivation of sugarcane and pasture [2,3]. Although limited information is known about aminocyclopyrachlor fate in soil, studies reported that this herbicide is highly water soluble, non-volatile, and can be easily leached in soils due to its low sorption capacity and high desorption capacity [4,5]. Its dissipation in the environment is thus expected to occur predominantly via aqueous photolysis and leaching becoming a potential groundwater contaminant [6]. The structural formula and physico-chemical properties of aminocyclopyrachlor are described in Table 1.

Table 1.
Structural formula and physico-chemical properties of aminocyclopyrachlor.
For residual herbicides such as aminocyclopyrachlor, although its persistence in soil is indicative of good weed control, even at low application rates it may damage sensitive plants such as soybean, cotton, sunflower, and alfalfa [9]. Conklin and Lym [10] reported that aminocyclopyrachlor’s 50% dissipation time (DT50) ranged from 3 to >112 days in four US soils. This herbicide thus has the potential for long persistence, although the dissipation rate is dependent on several factors including soil type, moisture content, and temperature. Indeed, aminocyclopyrachlor was found to be biologically active for an even longer period of 150 days in two Brazilian soils [11]. Aminocyclopyrachlor mineralization may take place via two different pathways: degradation of the herbicide in the soil by microorganisms and by photolysis when deposited on the soil surface. [1]
However, the dynamic patterns of mineralization and extractable/bound residues of aminocyclopyrachlor are not fully understood, particularly in tropical soils such as those in Brazil. Herbicide residues in soils can be divided into two types: extractable, which are extractable by organic solvents, and bound (non-extractable) forms, which are residues of the parent herbicide and its metabolites left in the soil after extraction with organic solvents [12,13,14]. It is very important to note that with prolonged interaction between soil and herbicide, the fraction of extractable residues is expected to decrease, while conversely the amount of bound residues should increase [15,16]; the same is expected to be true for aminocyclopyrachlor. When herbicides are available in soil solution, the microorganisms found naturally in these soils can mineralize them and form CO2 [17]. Several studies have been performed using radiometric techniques involving 14C-labeling to examine the environmental behavior and fate of new herbicides, including their mineralization, extractable residues, and soil-bound residue formation [14,18,19].
The aim of the present study was thus to evaluate these patterns for aminocyclopyrachlor in three tropical soils with different physico-chemical properties.
2. Materials and Methods
2.1. Soil Sampling
Soil samples were collected from the surface layer (0–10 cm depth) of areas cultivated with pasture and sugarcane at three different locations untreated with aminocyclopyrachlor, after pre-cleaning the ve cover. The samples were classified as Oxisols (Typic Hapludox, loamy sand, and Typic Hapludox, clay texture, respectively) and a Petric Plinthosol (sandy clay). The soil samples were air-dried for seven days before being sieved through a 2.0-mm mesh and stored at room temperature.
The physico-chemical properties of the samples and their classification according to the Soil Taxonomy and Brazilian Soil Science Society [20] are shown in Table 2. Soil texture was determined using the hydrometer method, soil pH using a 1:2 mixture of soil:CaCl2, and soil OC content by dry combustion at 900 °C and measurement of CO2 evolution. Soil moisture content (w) and field capacity (FC) were recorded at 0.046, 0.178, and 0.242 kg kg−1 and 21.97%, 23.38%, and 31.05% for the Typic Hapludox (loamy sand), Petric Plinthosol, and Typic Hapludox (clay) samples, respectively. In order to maintain these values, the w of the incubated flasks was checked monthly, with distilled water added when the difference between the zero time masses was less than 5%.

Table 2.
Physicochemical properties of three tropical soils (0–10 cm of depth) studied in this experiment.
2.2. Herbicide
The employed 14C-labeled [pyrimidine-2-14C] aminocyclopyrachlor is characterized in Table 1, according to the position of the radiolabel in the structural formula; this herbicide was graciously provided by DuPont (Wilmington, DE, USA). Solutions were prepared in 0.01 mol L−1 CaCl2. The aminocyclopyrachlor exhibited 99.5% radiochemical purity and 1.57 MBq mg−1 specific activity. Herbicide solutions (200 µL and 16.98 kBq radioactivity) were then applied to each experimental unit in doses corresponding to 2.4- and 4.0-times the maximum recommended dose of herbicide applied to sugarcane and pasture, respectively (assuming soil bulk density = 1200 kg m−3, incorporation depth = 0.10 m). This study was carried out at doses higher than the maximum recommended for crops due to the low specific radioactivity of the aminocyclopyrachlor required for quantification.
2.3. Analysis of 14C-Extractable Residue
The experiments were conducted according to the methods established by the Organization for Economic Co-Operation and Development [21] in a completely randomized design with 3 repetitions. Each experimental unit consisted of one 300 mL biometer culture flask (Fisher C-4443-250) equipped with a side tube, which contained 0.2 M sodium hydroxide (NaOH) (10 mL) used as a trap for carbon dioxide (CO2).
Dry soil (50 g) was adjusted to 75% FC, placed in the biometer flask and pre-incubated in the dark at 20 ± 2 °C for seven days to revive soil microbial activity. Aminocyclopyrachlor solutions (200 µL) were then applied to each flask. The amended samples were thoroughly mixed, and the homogeneity of 14C distribution was confirmed by combusting five aliquots (1 g) randomly collected of each soil on a biological oxidizer (OX500, RJ Harvey Instrument Corporation, Tappan, NY, USA) and the release of 14CO2 was measured by liquid scintillation spectrometry (LSS) using a Tri-Carb 2910 TR counter (LSA PerkinElmer, Waltham, MA, USA).
Extractable and bound residue contents were determined by measuring the remaining 14C-aminocyclopyrachlor in the soil at 0, 7, 14, 28, 42, 56, 70, 84, 98, 112, and 126 days after application. Soils were transferred from the flasks to Teflon tubes (250 mL) containing 100 mL acetonitrile:acidified water 0.2% formic acid solution (80:20 v/v), before being homogenized in an ultrasound bath (Branson 2510 ultrasonic, Marshall Scientific, Hampton, NH, USA) at 60 °C for 30 min, according to Nanita et al. [7,22]. Samples were centrifuged (Hitachi CF16RXII centrifuge, Hitachi Koki Co., Ltd., Indaiatuba, SP, Brazil) at 4000 rpm for 15 min. The procedure was performed once more, and then repeated two further times, adding 100 mL of acetonitrile: ammonium acetate 0.15 mmol L−1 solutions (70:30, v/v) on each extraction. The supernatants were then combined in a Schott flask (500 mL) and weighed in a semi-analytical balance. The radioactivity from all extraction steps was summed to obtain the total 14C-extractable residues. This procedure removed all the extractable residues, determined in preliminary studies (unpublished data).
A 1-mL aliquot of the combined supernatants was transferred to a liquid scintillation vial containing 10 mL scintillation cocktail. Radioactivity was measured via LSS. The remaining extract solution was evaporated under vacuum at 40 °C in a rotavapor (V-850 vacuum controller, R-215 rotavapor, B-491 heating bath, Buchi Brazil Ltda, Valinhos, SP, Brazil) and 1 mL aliquots of the concentrated extract analyzed again for radioactivity by LSS.
2.4. Analysis of 14C-Bound Residue
Extracted soil samples were dried at 50 °C and then ground in a mechanical mill (Marconi MA330, Piracicaba, SP, Brazil). Subsamples of each soil type were weighed out in triplicate (0.2 g) and biologically oxidized to determine the amount of non-extractable aminocyclopyrachlor (14C-bound residues); the oxidizer’s efficiency was calculated prior to sample combustion in order to correct any recovery error. The mass balance of radioactivity was then calculated.
2.5. Measurement of Mineralization to 14CO2
Aminocyclopyrachlor mineralization was quantified based on the amount of 14CO2 trapped in the 0.2 M NaOH solution in each flask side-tube. NaOH solutions were analyzed via LSS, with two 1 mL aliquots taken at 0, 7, 14, 28, 42, 56, 70, 84, 98, 112, and 126 days after herbicide application. At each evaluation time, fresh NaOH solution was placed in the side tube of each flask. Entry of atmospheric CO2 into the flasks was prevented using “lime soda” filters.
2.6. Aminocyclopyrachlor Mineralization Model
Data regarding the amount of 14CO2 produced were fit to the first-order kinetic model C = C0 ekt, where C is aminocyclopyrachlor concentration at time t (%); C0 is aminocyclopyrachlor concentration at time zero (%); k is a mineralization rate constant (day−1); and t is the incubation time (day). Mineralization half-life time (MT50), defined as the time required for 50% of the applied herbicide to be mineralized to CO2 was calculated via the following equation: MT50 = ln 2/k. [23,24].
2.7. Statistical Analysis
All measurements were performed in three replicates, with arithmetic means and standard errors of means (means ± SEM) calculated from the repeated measurements. The data were subjected to analysis of variance (ANOVA) using the software Assistat-Statistical Assistance (version 7.3). When significant, averages of aminocyclopyrachlor mineralization were compared using Tukey’s honest significant difference (HSD) test (p < 0.05). Figures of aminocyclopyrachlor distribution in the soil were plotted with Sigma Plot (Version 10.0 for Windows, Systat Software Inc., Richmond, CA, USA).
3. Results and Discussion
3.1. Mass Balance
Mass balances were calculated for 14C-aminocyclopyrachlor (mineralization, extractable residues, and residues bound to soil) averaged for the eleven sampling dates (0 to 126 days) (Figure 1). Recovery of 14C ranged from 93.4% to 99.1%, 90.0% to 100.2%, and 95.4% to 99.3% in the Typic Hapludox (loamy sand), Petric Plinthosol (sandy clay), and Typic Hapludox (clay) samples, respectively. These values are indicative of good mass recoveries for the procedures used in this study, according to OECD guidelines [21], which recommend an acceptable mass balance of radioactivity to be between 90% and 110%.
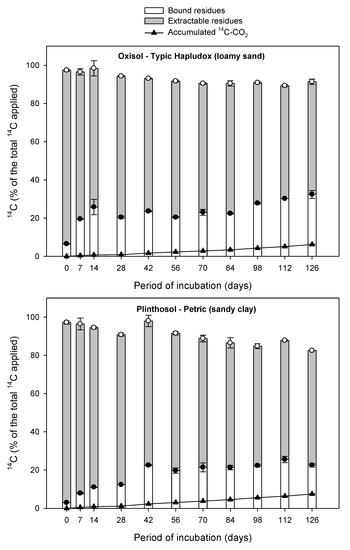
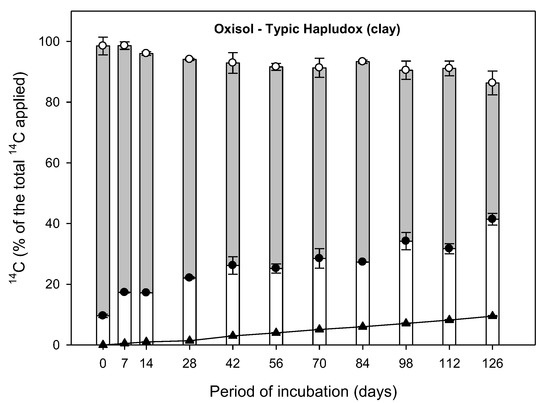
Figure 1.
Distribution of 14C-aminocyclopyrachlor in soil among extractable residues with solvent, mineralized 14C-CO2, and bound residues as a function at the 126 days of incubation time in three Brazilian soil samples. Standard deviation of means (n = 3) are shown for the extractable residue (white circle) and for the bound residue (black circle).
3.2. Aminocyclopyrachlor Mineralization to 14CO2
Low mineralization of herbicide in the soil increases the bound residue formation and bioavailability of extractable residue. The 14CO2 produced directly from 14C-aminocyclopyrachlor mineralization was evaluated weekly for 126 days, and results are shown in Figure 1.
During the first 28 days incubation, the amount of herbicide mineralized was very low, with values of 0.9%, 1.2%, and 1.4% of the initially applied aminocyclopyrachlor recorded in the loamy sand, sandy clay, and clay soil samples, respectively (Figure 1). These results indicated the occurrence of a similar lag phase in all three soils, suggesting that time was needed for the acclimatization of the microbial degraders of aminocyclopyrachlor [18]. As the soils used in the present study were never treated with aminocyclopyrachlor prior to the experiment, we assumed that all three soils did not contain any natural microorganisms able to mineralize this particular herbicide. However, Boivin et al. [15] reported that soils never treated with the herbicide 2,4-d had been known to host significant but low populations of microbial decomposers, and the same principle likely applies here. Assessing the size of the microbial biomass and composition of the microbial community originally present in the tested soils would thus represent a valuable avenue for future investigation.
In all three soils, the added aminocyclopyrachlor underwent slow mineralization, with total accumulated 14CO2 following the order of clay (9.48%) > loamy sand (7.46%) > sandy clay (6.15%) soil (p < 0.05), as shown in Table 3. These data agree with those reported by Durkin [6], who found a maximum percentage of CO2 evolution of 23% at 360 days aminocyclopyrachlor incubation. In the present study, the clay soil had the highest clay (74.1%) and FC content (31.05%), with intermediate OC content (1.7%) and pH (4.7), as shown in Table 2, which could have contributed to the observed higher levels of aminocyclopyrachlor mineralization. According to Oliveira and Brighenti [25], soils with higher OC content present increased biological activity, and therefore higher mineralization rates should be expected in such soils. On the other hand, soils with high OC content can also adsorb herbicides more intensively, promoting the formation of bound residues and thus inhibiting herbicide mineralization. However, the mineralization rate of an herbicide in a soil is a function of multiple factors, such as the density of the microbial population, the physico-chemical properties of the soil and herbicide, soil depth, molecule bioavailability, and climatic conditions [26,27]. Moreover, further research is required regarding the fate of aminocyclopyrachlor in other soil types with different physico-chemical properties and environmental conditions.

Table 3.
14CO2 accumulated at 126 days of incubation (%) and parameters of the first order kinetics (k and MT50) based on 14CO2 released until 126 days of incubation of three tropical soils applied with 14C-aminocyclopyrachlor.
Indeed, mineralization appeared not to be the main aminocyclopyrachlor dissipation process during incubation in the three tropical soils analyzed in the present study, with the first-order kinetics of the herbicide characterized by a declining mineralization rate over time. As shown in Table 3, the mineralization rate (k) was very low throughout the incubation period in all soils, with values ranging from 0.00050% to 0.00079% 14CO2 day−1. MT50 values of aminocyclopyrachlor were higher in sandy clay (1376 days), intermediate in loamy sand (1128 days), and lower in clay soil (877 days) (p < 0.05). The data regarding aminocyclopyrachlor mineralization recorded in this study indicate that the herbicide has a relatively long persistence in all soils and thus could provide prolonged weed control in drought years and this herbicide has high leaching potential, particularly in wet years, since Francisco et al. reported that aminocyclopyrachlor was detected at all depths (0–30 cm) in the agricultural soils and can cause damage to sensitive plants, such as tomatoes [28], soybean, and beet [29].
All aminocyclopyrachlor not mineralized by microorganisms remained in the three soils as residues, in either extractable or non-extractable form, as described in the following sections.
3.3. Dissipation of 14C-Extractable Residue
High dissipation would imply that the herbicide is subject to leaching and groundwater contamination problems, consequently decrease efficacy of the product. The extractable residues of an herbicide are formed by the parent product and its metabolites. Total 14C-extractable residue of aminocyclopyrachlor decreased slowly with incubation time in the three tested soil types in the present study, with values ranging from 90.9% to 59.0%, 94.2% to 60.0%, and 88.9% to 44.9% in loamy sand, sandy clay and clay soil, respectively (Figure 1). At the end of the study after 126 days, the reduction in extractable residue was significantly higher in the clay soil (~50%), followed by the sandy clay (~36%) and the loamy sand (~31%) (p < 0.05). This pattern is likely due to the high clay content (74.1%) of the clay soil compared to that of the other two soils (Table 1).
Francisco et al. [5] also found that aminocyclopyrachlor not only exhibited low sorption capacity (Kf = 0.37–1.34 µmol (1−1/n) L1/n kg−1) and high desorption capacity (Kf = 3.62–5.36 µmol (1−1/n) L1/n kg−1) in the same three tropical soils studied in the present study, but also showed a significant positive correlation with soil clay content. Since this herbicide remains weakly sorbed, it can be easily extracted from the soil using solvents, as proven here.
During the first 28 days of incubation, the fraction of total extractable residues from all extraction steps in the three tested soils was more than 72% of the initially applied 14C-aminocyclopyrachlor (Figure 1). However, after 28 days the amount of extractable herbicide residues gradually decreased. Such a decrease in recovery rate through solvent extraction indicates the tendency of aminocyclopyrachlor to form bound residues in the soil, as detailed below.
3.4. 14C-Bound Residue Formation
High bound residue formation of an herbicide contributes to long persistence of this herbicide in soils. The formation of bound (non-extractable) residues of aminocyclopyrachlor increased slowly with incubation time in all three soils (Figure 1). Total 14C-bound aminocyclopyrachlor residue levels were slightly higher in the clay soil over the incubation period, followed by the reverse order recorded for extractable residues: clay > sandy clay = loamy sand soil (p < 0.05). At the end of the study after 126 days, high levels of bound residues (41.4%) were detected in the clay soil compared to the loamy sand and sandy clay soils (32.4% and 22.5%, respectively), as already justified based on their respective physico-chemical properties.
Bound residues were detected immediately after the application of aminocyclopyrachlor at 6.5%, 3.0%, and 9.6% in the loamy sand, sandy clay and clay soils, respectively, and by the end of the study at 126 days had increased ~5.0-, 7.5-, and 4.3-fold in the same soils (Figure 1). We assume that the formation of bound residues of aminocyclopyrachlor is not related to its low sorption and high desorption rates in the soils studied, because most of this herbicide is bioavailable in the soil solution, according to Francisco et al. [5]. Indeed, Gevao et al. [12] reported that bound residue formation is not equivalent to the strong sorption of compounds because those sorptive processes that are reversible by traditional chemical extraction techniques do not lead to bound residue formation.
A similarly large bound residue fraction was also reported by Wang et al. [19] who found that pyribambenz propyl herbicide residue was consistently non-extractable, accounting for up to 57% in some soil samples, indicating the importance of considering this form of residue in the comprehensive risk assessment of new herbicides.
Barriuso et al. [14] reported that the main issue with this kind of approach is the lack of information regarding the exact nature of the bound residues, which is required to convert 14C-bound residues into 14C-parent herbicide or 14C-metabolites. It should also be noted that bound residues are constituted by the sum of the original compound and its fractions immobilized in the soil colloids, which cannot return to the soil solution and thus become unavailable to plants and soil microorganisms [12]. Consequently, the absorption, mineralization and degradation rates of aminocyclopyrachlor are reduced, leading to the greater persistence of the herbicide in the environment and more available herbicide in soil solution.
4. Conclusions
The present study is the first to assess the fate, distribution, and formation of bound residues of aminocyclopyrachlor in soils. Aminocyclopyrachlor mineralization was very low (<10%) in all three tropical soils evaluated, with MT50 ranging from 877 to 1376 days, suggesting potential long persistence of this herbicide in soils. However, we observed the aminocyclopyrachlor residues to be predominantly associated with the OM and clay contents of soil, with the amount of extractable residues decreasing and bound residue formation increasing, respectively, over time of incubation. This effect of soil physico-chemical properties should be considered in environmental risk assessment of aminocyclopyrachlor and its application in the field for weed control. For a more complete understanding about the bound residue remobilization of aminocyclopyrachlor under environmental conditions, techniques investigations are necessary.
Acknowledgments
The authors would like to thank the Higher Education Personnel Improvement Coordination (CAPES) for the financial support. The authors are deeply grateful to DuPont for supplying the radioactive materials.
Author Contributions
J.G.F. designed the study, carried out statistical analysis, and wrote the paper. V.L.T. was the first author’s advisor, was responsible for training, and assisted in outlining the methodology. R.F.P. carried out the statistical analysis and laboratory analyses. A.C.D.G. provided input to the paper. K.F.M. assisted in outlining the methodology and provided input on the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- USEPA (United States Environmental Protection Agency)/OPPTS (Product Properties Test Guidelines). Ecological Risk Assessment for the Section 3 New Chemical Registration of Aminocyclopyrachlor on Non-Crop Areas and Turf; EPA-HQ-OPP-2009-0789-0004; Off Prevent Pest Tox Sub; Environmental Protection Agency: Washington, DC, USA, 2010. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/000352-00793-20100831.pdf (accessed on 26 September 2017).
- Guerra, N.; Oliveira, R.S., Jr.; Constantin, J.; Oliveira Neto, A.M.; Braz, G.B.P. Aminocyclopyrachlor e indaziflam: Seletividade, controle e comportamento no ambiente. Rev. Bras. Herbic. 2013, 12, 285–295. [Google Scholar] [CrossRef]
- Reis, F.C.; Tornisielo, V.L.; Cason, J.B.; Dias, A.C.R.; Freitas, M.M.; Sotomayor, J.F.M.; Barroso, A.A.M.; Victoria, F.R. Uptake, translocation, and control of trumpet flower (Tecoma stans) with aminocyclopyrachlor. J. Environ. Sci. Health Part B 2015, 50, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.S., Jr.; Alonso, D.G.; Koskinen, C.W. Sorption desorption of aminocyclopyrachlor in selected Brazilian soils. J. Agric. Food Chem. 2011, 59, 4045–4050. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.G.; Mendes, K.F.; Pimpinato, R.F.; Tornisielo, V.L.; Dias, A.C.R. Aminocyclopyrachlor sorption-desorption and leaching from three Brazilian soils. J. Environ. Sci. Health Part B 2017, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Durkin, P.R. Aminocyclopyrachlor: Human Health and Ecological Risk Assessment; Final Report; Syracuse Environmental Research Associates: Manlius, NY, USA, 2012; p. 226, Available online: http://www.fs.fed.us/foresthealth/pesticide/pdfs/Aminocyclopyrachlor.pdf (accessed on 28 March 2017).
- Nanita, S.C.; Stewart, D.J.; Rodgers, C.A.; Tesfai, E.; Schwarz, T.; Robaugh, E.C.; Henze, R.M.; Pentz, A.M.; Moate, T.; Vogl, E.; et al. Analytical method and interlaboratory study for the quantitation of aminocyclopyrachlor residues in vegetation by liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2013, 96, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- PPDB—Pesticide Properties Data Base. Developed by the Agriculture & Environment Research Unit (AERU). University of Hertfordshire. Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 17 March 2017).
- Strachan, S.D.; Nanita, S.C.; Ruggiero, M.; Casini, M.S.; Heldreth, K.M.; Hageman, L.H.; Flanigan, H.A.; Ferry, N.M.; Pentz, A.M. Correlation of chemical analysis of residual levels of aminocyclopyrachlor in soil to biological responses of alfalfa, cotton, soybean, and sunflower. Weed Technol. 2011, 25, 239–244. [Google Scholar] [CrossRef]
- Conklin, K.L.; Lym, R.G. Effect of temperature and moisture on aminocyclopyrachlor soil half-life. Weed Technol. 2013, 27, 552–556. [Google Scholar] [CrossRef]
- Guerra, N.; Oliveira, R.S., Jr.; Constantin, J.; Oliveira, N.A.M.; Gemelli, A.; Pereira, D.M., Jr.; Guerra, A. Persistence of biological activity and leaching potential of herbicides aminocyclopyrachlor and indaziflam in soils with different textures. Planta Daninha 2016, 34, 345–356. [Google Scholar] [CrossRef]
- Gevao, B.; Semple, K.T.; Jones, K.C. Bound pesticide residue in soil: A review. Environ. Pollut. 2000, 108, 3–14. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wu, J.; Huang, P.; Xu, J.; Tang, C. Impact of soil moisture on metsulfuron-methyl residues in Chinese paddy soils. Geoderma 2007, 142, 325–333. [Google Scholar] [CrossRef]
- Barriuso, E.; Benoit, P.; Dubus, I. Formation of pesticide nonextractable (bound) residues in soil: Magnitude, controlling factors and reversibility. Environ. Sci. Technol. 2008, 42, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Boivin, A.; Amellal, S.; Schiavon, M.; van Genuchten, M.T. 2,4-Dichlorophenoxyacetic acid (2,4-d) sorption and degradation dynamics in three agricultural soils. Environ. Pollut. 2005, 138, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mordaunt, C.J.; Gevao, B.; Jones, K.C.; Semple, K.T. Formation of non-extractable pesticide residues: Observations on compound differences, measurement and regulatory issues. Environ. Pollut. 2005, 133, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gonod, L.V.; Chenu, C.; Soulas, G. Spatial variability of 2,4-dichlorophenoxyacetic acid (2,4-d) mineralisation potential at a millimeter scale in soil. Soil Biol. Biochem. 2003, 35, 373–382. [Google Scholar] [CrossRef]
- Wang, H.; Ye, Q.; Yue, L.; Han, A.; Yu, Z.; Wang, W.; Yang, Z.; Lu, L. Fate characterization of a novel herbicide ZJ0273 in aerobic soils using multi-position 14C labeling. Sci. Total Environ. 2009, 407, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yue, L.; Zhang, S.; Ye, Q.; Qi, W.; Wang, H.; Chen, Z. Fate of pyribambenz propyl (ZJ0273) in anaerobic soils revealed by position-specific 14C labeling. J. Hazard. Mater. 2013, 258–259, 151–158. [Google Scholar] [CrossRef] [PubMed]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos, 3rd ed.; EMBRAPA Solos: Brasília, Brazil, 2013; 353p. [Google Scholar]
- OECD—Organisation for Economic Co-operation and Development. OECD guidelines for the testing of chemicals. In Test Number 307: Aerobic and Anaerobic Transformation in Soil; OECD: Paris, France, 2002; p. 17. [Google Scholar]
- Nanita, S.C.; Pentz, A.M.; Grant, J.; Vogl, E.; Devine, T.J.; Henze, R.M. Mass spectrometric assessment and analytical methods for quantitation of the new herbicide aminocyclopyrachlor and its methyl analogue in soil and water. Anal. Chem. 2009, 81, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Picton, P.; Farenhorst, A. Factors influencing 2,4-d sorption and mineralization in soil. Environ. Sci. Health Part B 2004, 39, 367–379. [Google Scholar] [CrossRef]
- Mendes, K.F.; Martins, B.A.B.; Reis, M.R.; Pimpinato, R.F.; Tornisielo, V.L. Quantification of the fate of mesotrione applied alone or in a herbicide mixture in two Brazilian arable soils. Environ. Sci. Pollut. Res. 2017, 24, 8425–8435. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Brighenti, A.M. Comportamento de herbicidas no ambiente. In Biologia e Manejo de Plantas Daninhas; Oliveira, R.S., Jr., Constantin, J., Inoue, M.H., Eds.; Omnipax: Curitiba, Brazil, 2011; pp. 263–304. [Google Scholar]
- Schroll, R.; Becher, H.H.; Dörfler, U.; Gayler, S.; Grundmann, S.; Hartmann, H.P.; Ruoss, J. Quantifying the effect of soil moisture on the aerobic microbial mineralization of selected pesticides in different soils. Environ. Sci. Technol. 2006, 40, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Bending, G.G.; Rodriguez-Cruz, M.S. Microbial aspects of the interaction between soil depth and biodegradation of the herbicide isoproturon. Chemosphere 2007, 66, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.J.; Ruhl, G.E.; Creswell, T.C.; Wan, P.; Scott, D.E.; Becovitz, J.D.; Weisenberger, D.V. Potential damage to sensitive landscape plants from wood chips of aminocyclopyrachlor damaged trees. Weed Technol. 2013, 27, 803–809. [Google Scholar] [CrossRef]
- Guerra, N.; Oliveira, N.A.M.; Oliveira, R.S., Jr.; Constantin, J.; Takano, H.K. Sensibility of plant species to herbicides aminocyclopyrachlor and indaziflam. Planta Daninha 2014, 32, 609–617. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).