Benefits of Transgenic Insect Resistance in Brassica Hybrids under Selection
Abstract
:1. Introduction
- (1)
- the transgene will be beneficial in the presence of herbivores;
- (2)
- the Bt Cry1Ac transgene is costly in the absence of herbivores, and
- (3)
- selection by herbivores for insecticidal transgenes will increase the rate of transgene flow.
2. Results and Discussion
2.1. MANOVA Effects by Year and Univariate ANOVA Effects by Year and Genotype
| 2006 | ABM | ESN | |||
| Between-subject factors | df | F | p | F | p |
| Herbivory (H) | 1 | 0.94 | 0.370 | 2.76 | 0.148 |
| Competition (C) | 2 | 2.49 | 0.125 | 0.47 | 0.638 |
| C X H | 2 | 1.24 | 0.324 | 0.88 | 0.444 |
| Within-subject factors | |||||
| Genotype (G) | 4 | 475.19 | <0.0001 | 130.40 | <0.0001 |
| G X H | 4 | 0.68 | 0.625 | 3.62 | 0.067 |
| G X C | 8 | 0.72 | 0.673 | 2.76 | 0.047 |
| G X C X H | 8 | 0.35 | 0.931 | 1.37 | 0.291 |
| 2007 | ABM | ESN | |||
| Between-subject factors | df | F | p | F | p |
| Herbivory (H) | 1 | 5.89 | 0.051 | 10.56 | 0.018 |
| Competition (C) | 2 | 3.57 | 0.061 | 2.65 | 0.115 |
| C X H | 2 | 0.10 | 0.908 | 0.65 | 0.540 |
| Within-subject factors | |||||
| Genotype (G) | 4 | 407.42 | <0.0001 | 191.96 | <0.0001 |
| G X H | 4 | 3.60 | 0.051 | 9.62 | 0.004 |
| G X C | 8 | 1.03 | 0.452 | 2.15 | 0.092 |
| G X C X H | 8 | 0.35 | 0.936 | 0.39 | 0.907 |
| GM Westar | GM F1 | B. rapa | Non-GM F1 | Westar | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2006 | 2007 | 2006 | 2007 | 2006 | 2007 | 2006 | 2007 | ||
| ABM | Herbivory (H) | 0.537 | 0.720 | 0.391 | 0.863 | 0.727 | 0.012 | 0.927 | 0.016 | 0.613 | 0.587 |
| Competition (C) | 0.718 | 0.867 | 0.278 | 0.030 | 0.711 | 0.235 | 0.094 | 0.194 | 0.532 | 0.397 | |
| H X C | 0.423 | 0.917 | 0.284 | 0.926 | 0.686 | 0.736 | 0.806 | 0.798 | 0.647 | 0.702 | |
| ESN | Herbivory (H) | 0.191 | 0.248 | 0.108 | 0.099 | 0.307 | 0.000 | 0.970 | 0.054 | 0.813 | 0.001 |
| Competition (C) | 0.861 | 0.850 | 0.344 | 0.005 | 0.229 | 0.615 | 0.041 | 0.228 | 0.835 | 0.143 | |
| H X C | 0.630 | 0.859 | 0.439 | 0.322 | 0.774 | 0.942 | 0.838 | 0.988 | 0.618 | 0.426 | |
2.1.1. Heterosis
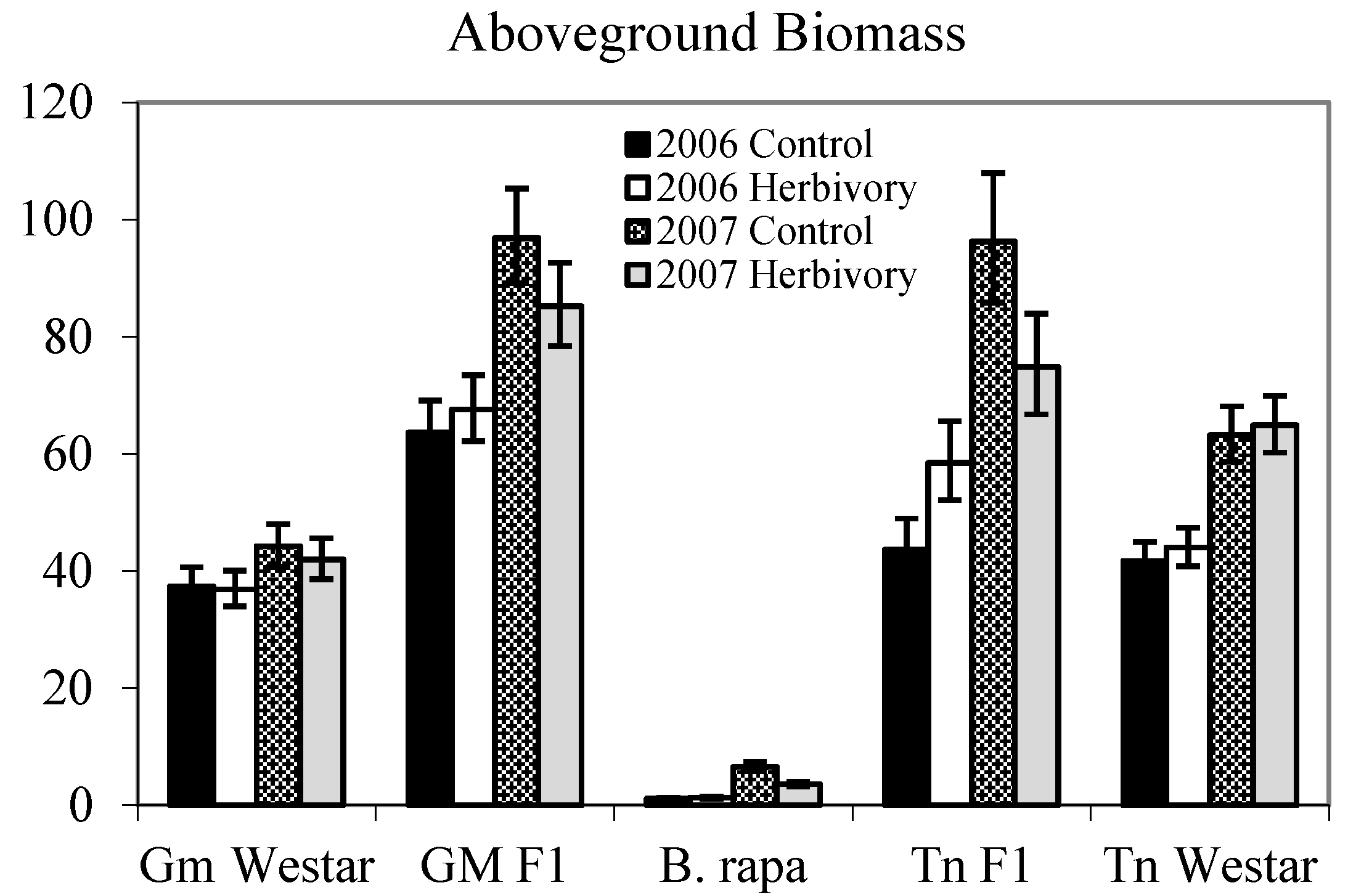
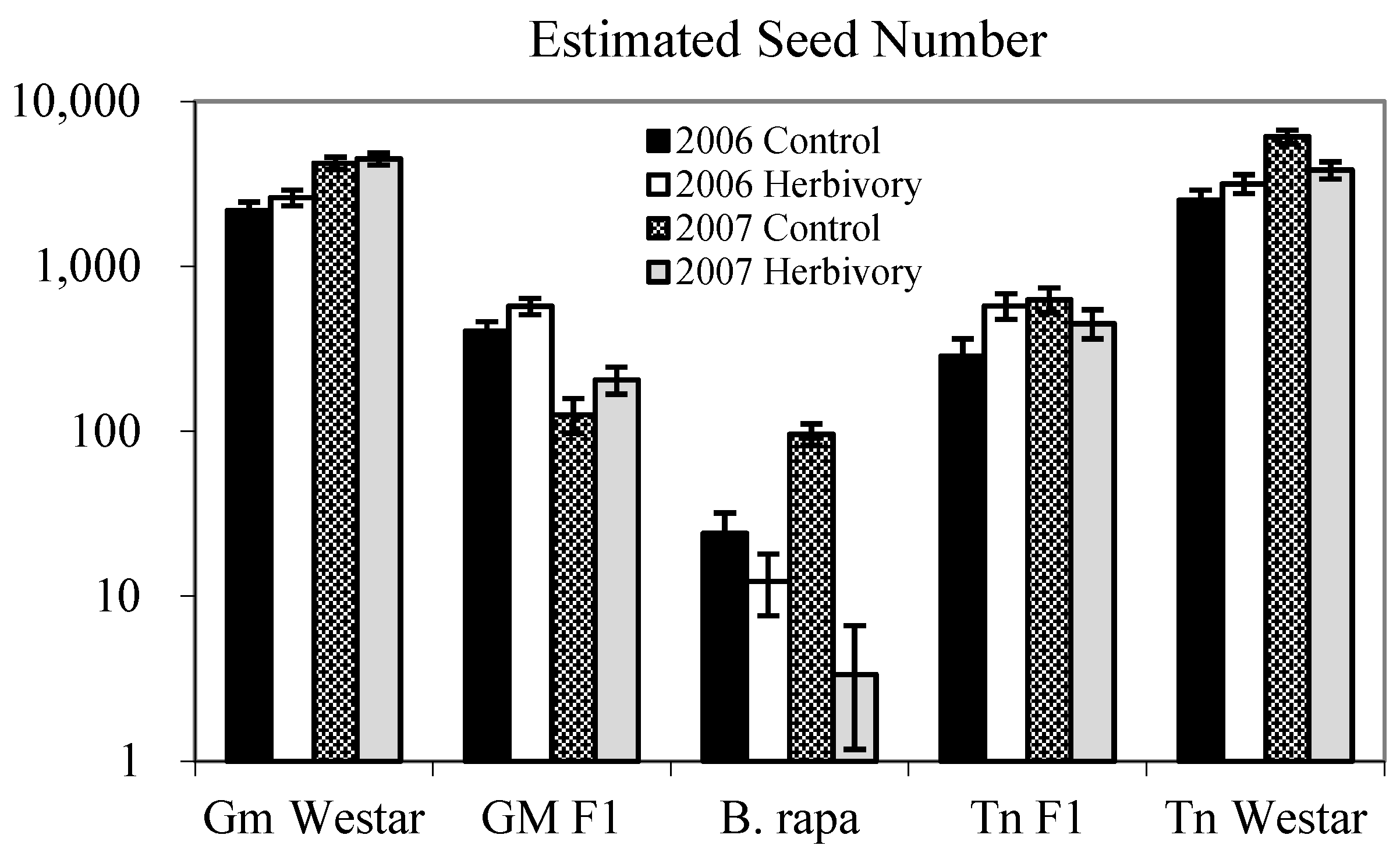
| Contrast | ABM | ABM | ESN | ESN | ||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2006 | 2007 | |||||
| Control | Herbivory | Control | Herbivory | Control | Herbivory | Control | Herbivory | |
| GM Westar vs. GM F1 | 0.0039 | 0.0049 | 0.0001 | 0.0005 | 0.0003 | 0.0001 | 0.0001 | 0.0001 |
| GM Westar vs. non-GM F1 | 0.4526 | 0.0049 | 0.0007 | 0.0136 | 0.0011 | 0.0001 | 0.0001 | 0.0001 |
| GM Westar vs. Westar | 0.372 | 0.1771 | 0.0034 | 0.01 | 0.7 | 0.1849 | 0.0011 | 0.3396 |
| GM Westar vs. B. rapa | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0001 | 0.0001 |
| GM F1 vs. non-GM F1 | 0.0755 | 0.4247 | 0.9272 | 0.1862 | 0.3987 | 0.9542 | 0.0054 | 0.0212 |
| GM F1 vs. Westar | 0.0069 | 0.0232 | 0.0003 | 0.0004 | 0.0003 | 0.0001 | 0.0001 | 0.0001 |
| GM F1 vs. B. rapa | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0012 | 0.0001 | 0.0219 | 0.0001 |
| Non-GM F1 vs. Westar | 0.8273 | 0.1249 | 0.0081 | 0.0715 | 0.0013 | 0.0003 | 0.0001 | 0.0001 |
| Non-GM F1 vs. B. rapa | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0015 | 0.0001 |
| Westar vs. B. rapa | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0001 | 0.0001 |
2.1.2. Herbivory
2.1.3. Costs of Bt Cry1Ac Gene
2.2. Competition
2.3. Transgene Flow under Selection
3. Materials and Methods
3.1. Experimental Design
3.2. Plant Materials
3.3. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Rieseberg, L.H.; Raymond, O.; Rosenthal, D.M.; Lai, Z.; Livingstone, K.; Nakazato, T.; Durphy, J.L.; Schwarzbach, E.E.; Donovan, L.A.; Lexer, C.; et al. Major ecological transitions in annual sunflowers facilitated by hybridization. Science 2003, 301, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Grant, V. Plant Speciation, 2nd ed.; Columbia University Press: New York, NY, USA, 1981; p. 435. [Google Scholar]
- Harlan, J.R. The possible role of weed races in the evolution of cultivated plants. Euphytica 1965, 14, 173–176. [Google Scholar] [CrossRef]
- Harlan, F.R.; de Wet, M.J. Sympatric evolution in sorghum. Genetics 1974, 78, 473–474. [Google Scholar] [PubMed]
- Mallory-Smith, C.; Zapiola, M. Gene flow from glyphosate-resistant crops. Pest. Manag. Sci. 2008, 64, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Watrud, L.S.; Lee, E.H.; Fairbrother, A.; Burdick, C.; Reichman, J.R.; Bollman, M.; Storm, M.; King, G.; van de Water, K.P. Evidence for landscape-level, pollen-mediated gene flow from genetically modified creeping bentgrass with CP4 EPSPS as a marker. Proc. Natl. Acad. Sci. USA 2004, 101, 14533–14538. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.A.; Andow, D.A.; Gepts, P.; Hallerman, E.M.; Power, A.; Tiedje, J.M.; Wolfenbrger, L.L. Genetically engineered organisms and the environment: Current status and recommendations. Ecol. Appl. 2005, 15, 377–404. [Google Scholar] [CrossRef]
- Snow, A.A.; Andersen, B.; Jørgensen, R.B. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy Brassica rapa. Mol. Ecol. 1999, 8, 605–615. [Google Scholar] [CrossRef]
- Bergelson, J.; Purrington, C.B.; Palm, C.J.; Lopez-Gutierrez, J.C. Costs of resistance: A test using transgenic Arabidopsis thaliana. Proc. R. Soc. Lond. B Biol. 1996, 263, 1659–1663. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Rudgers, J.A.; Lau, J.A.; Irwin, R.E. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 2002, 17, 278–285. [Google Scholar] [CrossRef]
- Lenski, R.E. Coevolution of bacteria and phage—Are there endless cycles of bacterial defenses and phage counterdefenses. J. Theor. Biol. 1984, 108, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Darmency, H. Gene flow hampered by low seed size of hybrids between oilseed rape and five wild relatives. Seed Sci. Res. 2008, 18, 115–123. [Google Scholar] [CrossRef]
- Warwick, S.I.; Legere, A.; Simard, M.-J.; James, T. Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Mol. Ecol. 2008, 17, 1387–1395. [Google Scholar]
- Snow, A.A.; Pilson, D.; Rieseberg, L.H.; Paulsen, M.J.; Pleskac, N.; Reagon, M.R.; Wolf, D.E.; Selbo, S.M. A Bt Transgene Reduces Herbivory and Enhances Fecundity in Wild Sunflowers. Ecol. Appl. 2003, 13, 279–286. [Google Scholar] [CrossRef]
- Halfhill, M.D.; Richards, H.A.; Mabon, S.A.; Stewart, C.N., Jr. Expression of GFP and Bt transgenes in Brassica napus and hybridization with Brassica rapa. Theor. Appl. Genet. 2001, 103, 659–667. [Google Scholar] [CrossRef]
- Mercer, K.L.; Andow, D.A.; Wyse, D.L.; Shaw, R.G. Stress and domestication traits increase the relative fitness of crop-wild hybrids in sunflower. Ecol. Lett. 2007, 10, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol. 2009, 184, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Halfhill, M.D.; Sutherland, J.P.; Moon, H.S.; Poppy, G.M.; Warwick, S.I.; Weissinger, A.K.; Ruffy, T.W.; Raymer, P.L.; Stewart, C.N., Jr. Growth, productivity, and competitiveness of introgressed weedy Brassica rapa hybrids selected for the presence of Bt. cry1Ac and GFP transgenes. Mol. Ecol. 2005, 14, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Waschmann, R.S.; Watrud, L.S.; Reece, L.R.; Shiroyama, T. Sunlit mesocosms designed for pollen confinement and risk assessment of transgenic crops. Aerobiologia 2010, 26, 311–325. [Google Scholar] [CrossRef]
- Bigger, D.S.; Fox, L.R. High-density populations of diamondback moth have broader host-plant diets. Oecologia 1997, 112, 179–186. [Google Scholar] [CrossRef]
- Halfhill, M.; Millwood, R.; Weissinger, A.; Warwick, S.; Stewart, C.N., Jr. Additive transgene expression and genetic introgression in multiple green-fluorescent protein transgenic crop × weed hybrid generations. Theor. Appl. Genet. 2003, 107, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, R.B.; Andersen, B. Spontaneous hybridization between oilseed rape (Brassica napus) and weedy Brassica campestris: A risk of growing genetically modified oilseed rape. Am. J. Bot. 1994, 81, 1169–1175. [Google Scholar] [CrossRef]
- Simard, M.-J.; Légère, A.; Warwick, S. Transgenic Brassica napus fields and Brassica rapa weeds in Quebec: Sympatry and weed-crop in situ hybridization. Can. J. Bot. 2006, 84, 1842–1851. [Google Scholar] [CrossRef]
- Rackow, G.; Woods, D.L. Outcrossing in rape and mustard under Saskatchewan prairie conditions. Can. J. Plant Sci. 1987, 678, 147–151. [Google Scholar] [CrossRef]
- Warwick, S.I.; Simard, M.-J.; Légère, A.; Beckie, H.J.; Braun, L.; Zhu, B.; Mason, P.; Séguin-Swartz, G.; Stewart, C.N., Jr. Hybridization between transgenic Brassica napus L. and its wild relatives: B. rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) O.E. Schulz. Theor. Appl. Genet. 2003, 107, 528–539. [Google Scholar] [CrossRef] [PubMed]
- FitzJohn, R.G.; Armstrong, T.T.; Newstrom-Lloyd, E.; Wilton, A.D.; Cochrane, M. Hybridisation within Brassica and allied genera: Evaluation of potential for transgene escape. Euphytica 2007, 58, 209–230. [Google Scholar] [CrossRef]
- Gulden, R.H.; Shirtliffe, S.J.; Thomas, A.G. Harvest losses of canola (Brassica napus) cause large seedbank inputs. Weed Sci. 2003, 51, 83–86. [Google Scholar] [CrossRef]
- López-Granados, F.; Lutman, P. Effect of environmental conditions on the dormancy and germination of volunteer oilseed rape seed (Brassica napus). Weed Sci. 1998, 46, 419–423. [Google Scholar]
- National Agricultural Statistics Service. Available online: http://www.nass.usda.gov/ (accessed on 8 October 2014).
- Harper, B.K.; Mabon, S.A.; Leffel, S.M.; Halfhill, D.; Richards, H.A.; Moyer, K.A.; Stewart, C.N., Jr. Green fluorescent protein in transgenic plants indicates the presence and expression of a second gene. Nat. Biotechnol. 1999, 17, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Lawrence, J.R.; Warwick, S.I.; Mason, P.; Braun, L.; Halfhill, M.D.; Stewart, C.N., Jr. Stable Bacillus thuringiensis (Bt) toxin content in interspecific F1 and backcross populations of wild Brassica rapa after Bt. gene transfer. Mol. Ecol. 2004, 13, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Bautista, N.S.; Sagers, C.L.; Lee, E.H.; Watrud, L.S. Flowering times in genetically modified Brassica hybrids in the absence of selection. Can. J. Plant Sci. 2010, 90, 185–187. [Google Scholar] [CrossRef]
- Londo, J.P.; Bautista, N.S.; Sagers, C.L.; Lee, E.H.; Watrud, L.S. Glyphosate drift promotes changes in fitness and transgene gene flow in canola (Brassica napus) and hybrids. Ann. Bot. 2010, 106, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, G.; Cochran, W. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]
- Kwit, C.; Moon, H.S.; Warwick, S.I.; Stewart, C.N., Jr. Transgene introgression crop relatives: Molecular evidence and mitigation strategies. Trends Biotechnol. 2011, 29, 284–293. [Google Scholar] [CrossRef] [PubMed]
- De Wet, J.M.; Harlan, J.R. Weeds and Domesticates: Evolution in the man-made habitat. Econ. Bot. 1975, 29, 99–108. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagers, C.L.; Londo, J.P.; Bautista, N.; Lee, E.H.; Watrud, L.S.; King, G. Benefits of Transgenic Insect Resistance in Brassica Hybrids under Selection. Agronomy 2015, 5, 21-34. https://doi.org/10.3390/agronomy5010021
Sagers CL, Londo JP, Bautista N, Lee EH, Watrud LS, King G. Benefits of Transgenic Insect Resistance in Brassica Hybrids under Selection. Agronomy. 2015; 5(1):21-34. https://doi.org/10.3390/agronomy5010021
Chicago/Turabian StyleSagers, Cynthia L., Jason P. Londo, Nonnie Bautista, Edward Henry Lee, Lidia S. Watrud, and George King. 2015. "Benefits of Transgenic Insect Resistance in Brassica Hybrids under Selection" Agronomy 5, no. 1: 21-34. https://doi.org/10.3390/agronomy5010021
APA StyleSagers, C. L., Londo, J. P., Bautista, N., Lee, E. H., Watrud, L. S., & King, G. (2015). Benefits of Transgenic Insect Resistance in Brassica Hybrids under Selection. Agronomy, 5(1), 21-34. https://doi.org/10.3390/agronomy5010021





