Bacillus simplex—A Little Known PGPB with Anti-Fungal Activity—Alters Pea Legume Root Architecture and Nodule Morphology When Coinoculated with Rhizobium leguminosarum bv. viciae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nitrogen Fixation Tests
| Strain | Relevant Characteristics | Source/Reference |
|---|---|---|
| Bacillus simplex 30N-5 | Isolated from the rhizosphere of a Podocarpus nagi tree growing in the MEMBG. | This work |
| Bacillus subtilis 30VD-1 | Isolated from soil in the palm section of the MEMBG, adjacent to Brahea edulis (Aracaceae; Guadalupe Palm), a tree indigenous to Guadalupe Island in Mexico. | This work |
| Bacillus subtilis BAL218 | JH642 trpC2, pheA1 | Beth A. Lazazzera (University of California, Los Angeles) |
| Sinorhizobium (Ensifer) meliloti 1021 | Wild-type Sinorhizobium (Ensifer) meliloti, Strr | Laboratory strain |
| Rhizobium leguminosarum bv. viciae 128C53 | Wild-type Rlv | Laboratory strain |
| Rhizobium leguminosarum bv. viciae 128C53/pHC60 | Wild-type Rlv with GFP, Tetr | This work |
| Fusarium oxysporum forma specialis conglutinans race 2 PHW 808 | Isolated from cabbage. | [46] |
| Fusarium oxysporum forma specialis matthioli race 2 PHW 726 | Isolated from Mathiola incana. | [46] |
| Fusarium verticillioides NRRL 13993 | Isolated from maize ear. | [47] |
| Nectria hematococca 77-113-4 | Pea pathogen, member of the Fusarium solani complex | Martha C. Hawes (University of Arizona) |
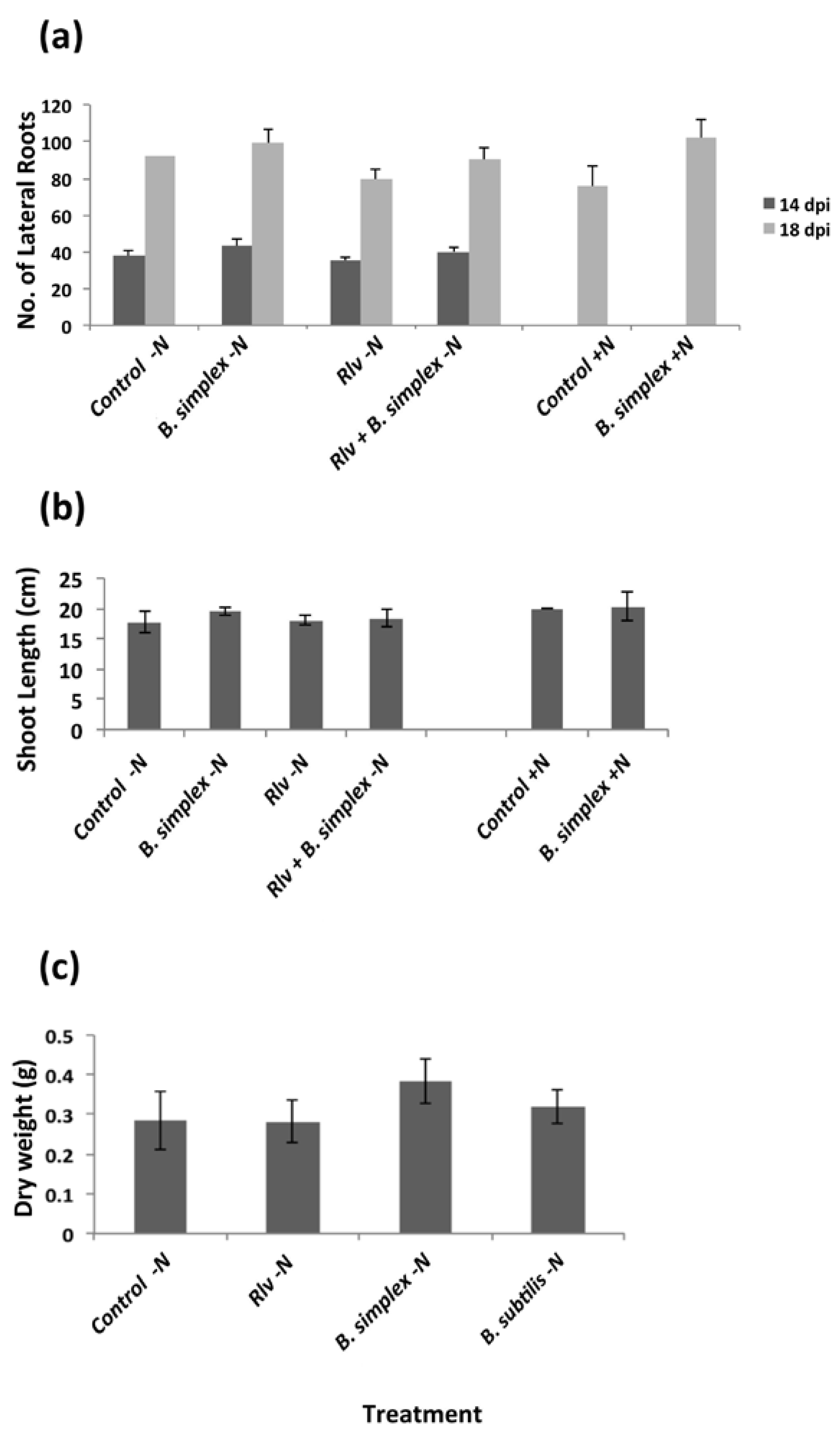
2.2. Inoculation with B. simplex 30N-5 Alters Root Architecture

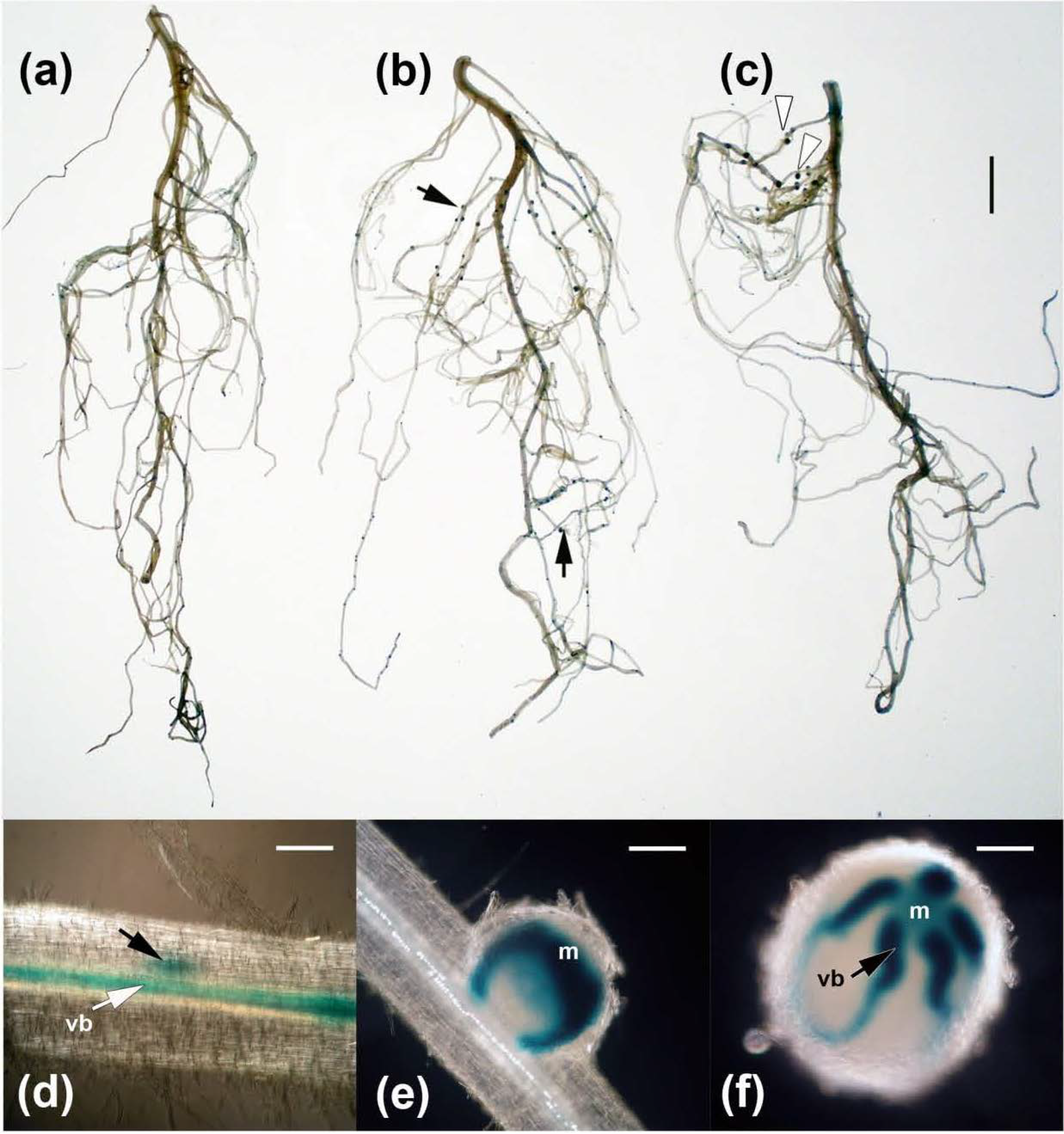
2.3. Effects on Nodulation in Response to Rhizobium leguminosarum bv. viciae
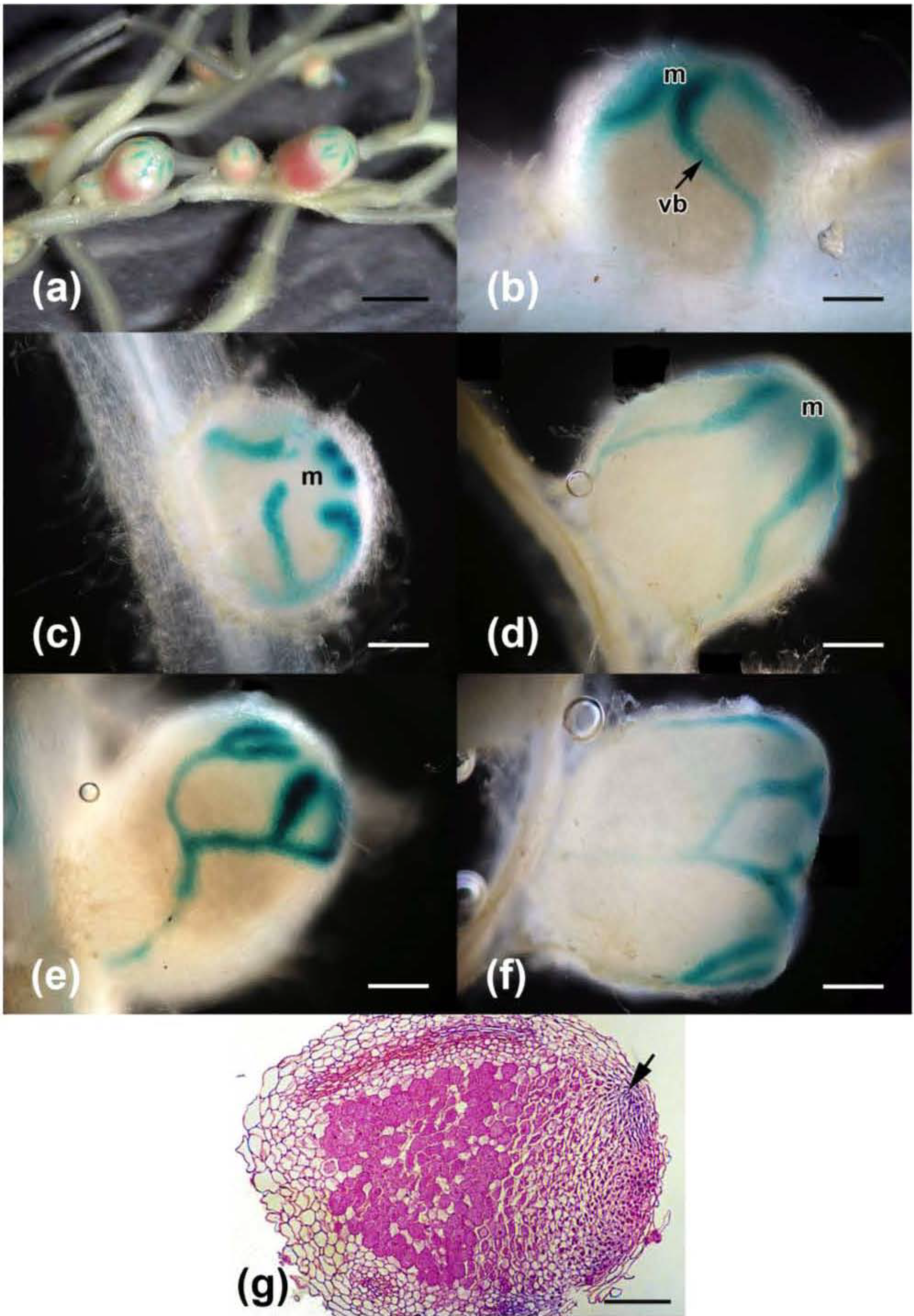
2.4. Nodulation in Response to Coinoculation
| Dilution | Rlv/pHC60-inoculated | Coinoculated 1 | Coinoculated 2 | Coinoculated 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Total # colonies | Non-GFP colonies | Total # colonies | Non-GFP colonies | Total # colonies | Non-GFP colonies | Total # colonies | Non-GFP colonies | |
| 1:100 | 247 | 0 | n.d. * | n.d. | 435 | 1 | n.d. | n.d. |
| 1:1000 | n.d. | n.d. | 88 | 3 | 126 | 2 | 136 | 1 |
2.5. Mechanisms by Which B. simplex 30N-5 and B. subtilis 30VD-1 Could Promote Plant Growth

3. Experimental Section
3.1. Routine Bacterial Growth Conditions
3.2. Amplification, Purification, and Sequencing of the 16S rRNA Gene
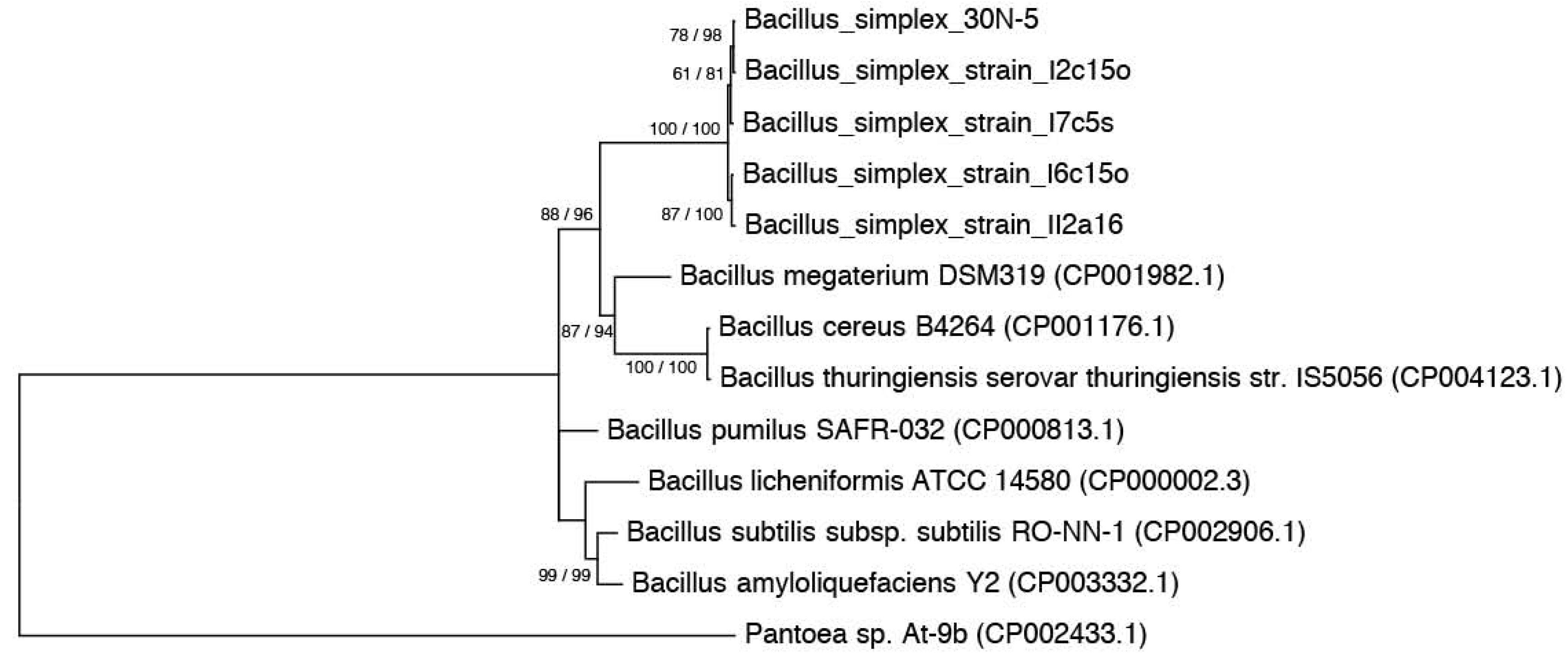
3.3. Phylogenies
3.4. Amplification, Purification, and Sequencing of a Putative nifH Gene
3.5. PCR Amplification of a Putative acdS Gene
3.6. ACC Utilization Assay
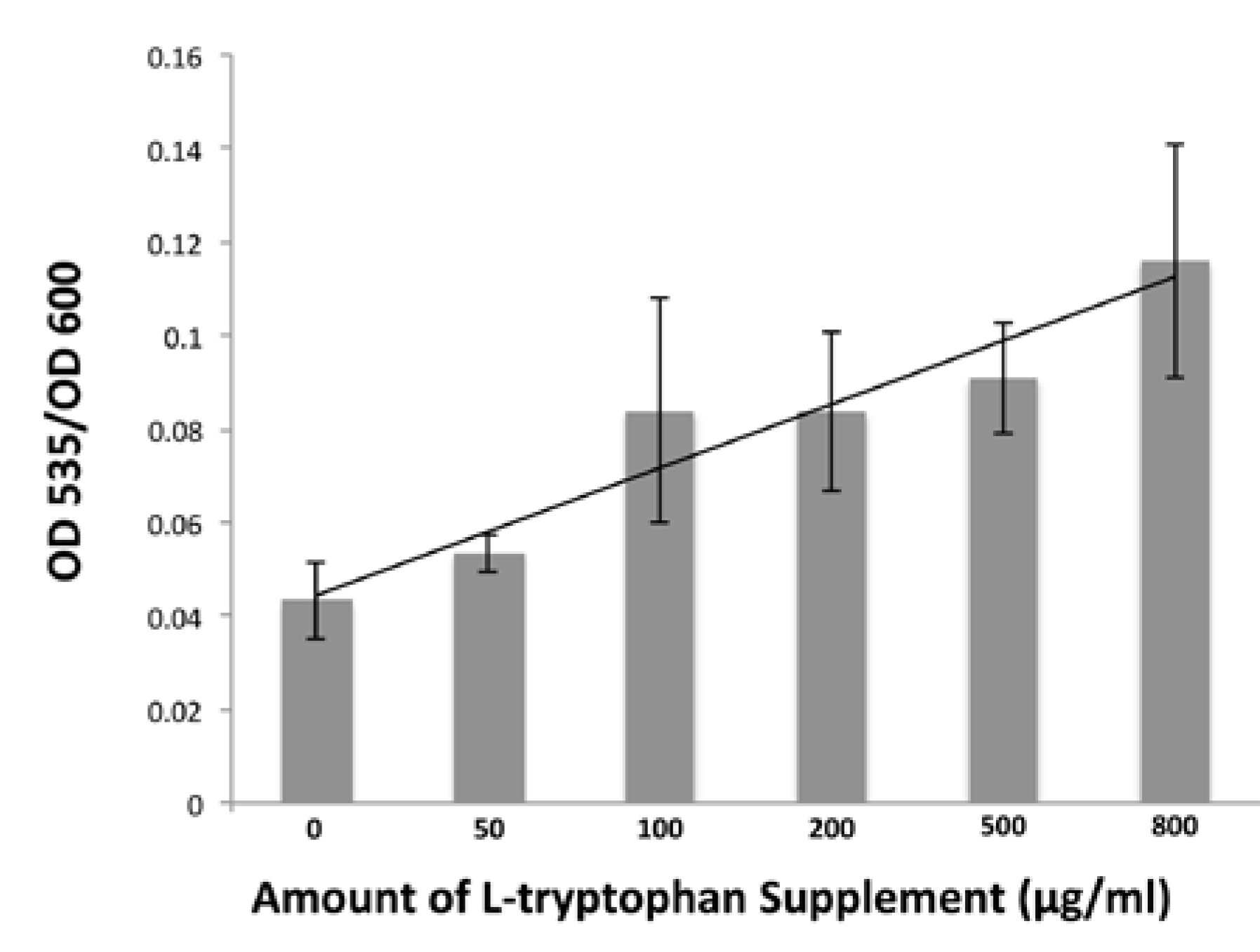
3.7. Detecting IAA in B. simplex 30N-5 Growth Medium Supernatant
3.8. Pisum sativum Growth Experiments and GUS Staining
3.9. Nodules Squashes and Microscopy
3.10. Phosphate Solubilization Assay
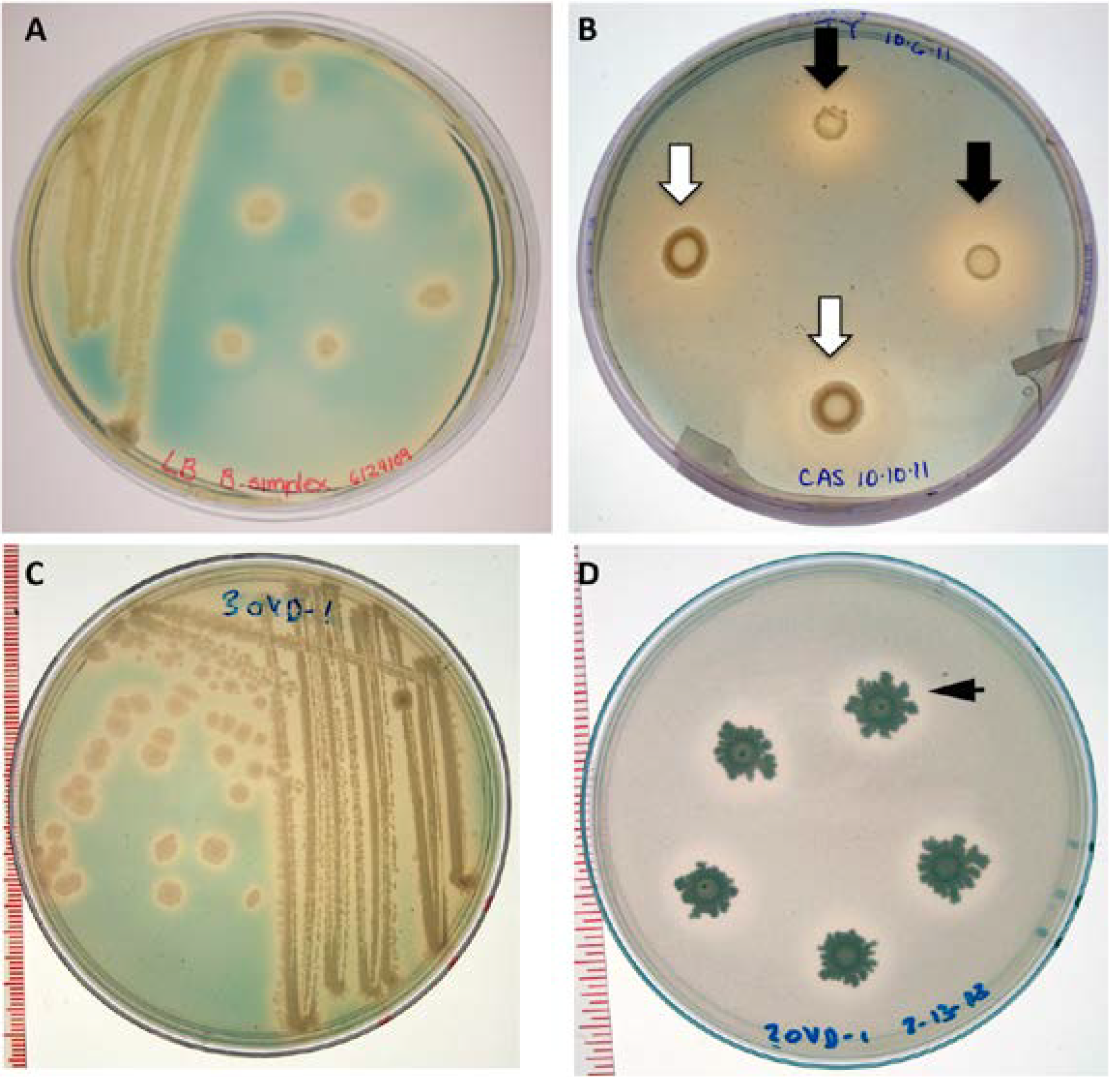
3.11. Siderophore Assay
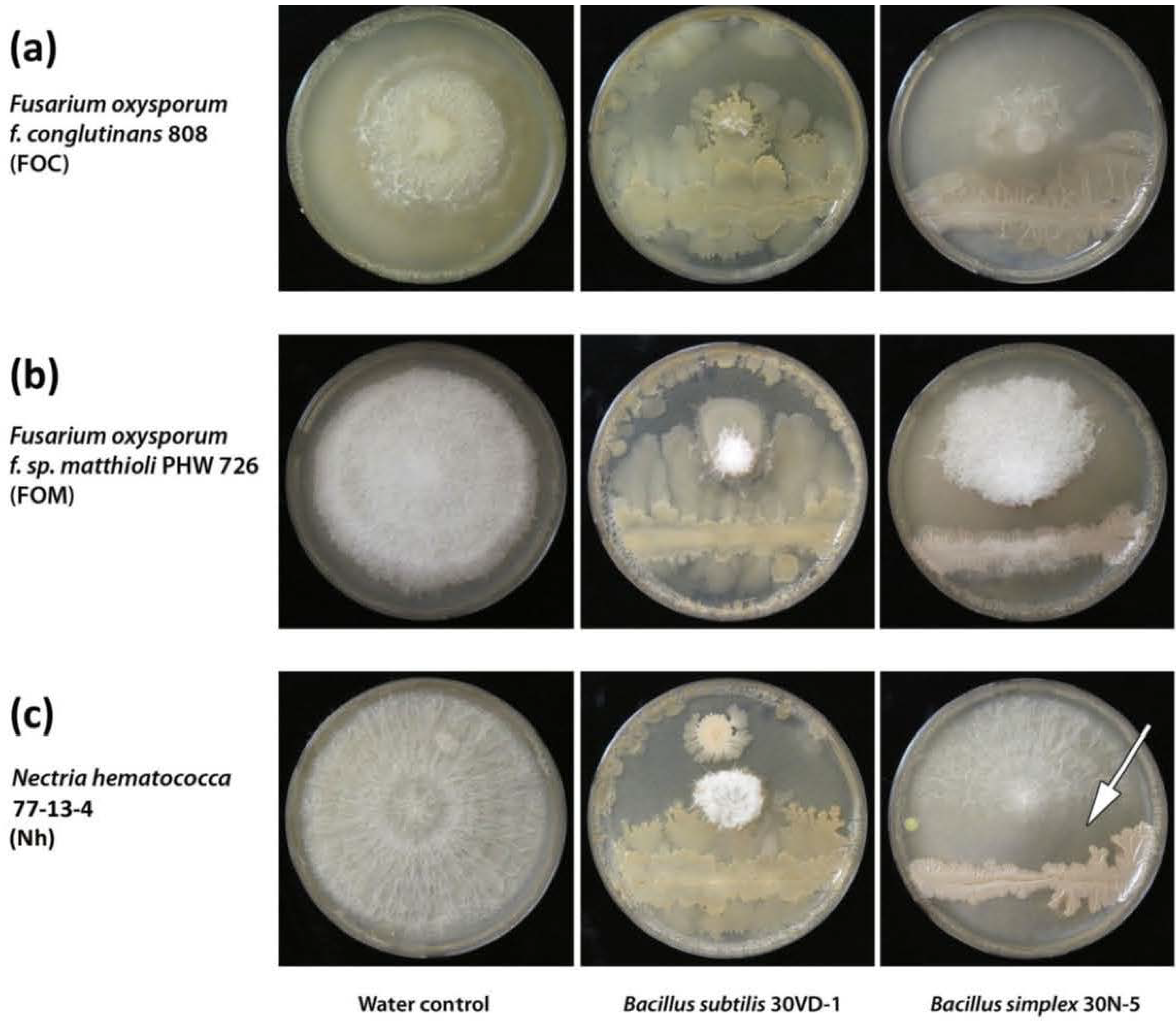
3.12. Analysis of Anti-Fungal Activity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bashan, Y.; Holguin, G. Proposal for the division of plant growth-promoting rhizobacteria into two classifications: Biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 1998, 30, 1225–1228. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Ann. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Fravel, D. Commercialization and implementation of biocontrol. Ann. Re. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.-S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef]
- Zhang, H.; Murzello, C.; Sun, Y.; Kim, M.-S.; Xie, X.; Jeter, R.M.; Zak, J.C.; Dowd, S.E.; Paré, P.W. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol. Plant-Microbe Interact. 2010, 23, 1097–1104. [Google Scholar] [CrossRef]
- Zhang, J.; Howell, C.; Starr, J. Suppression of Fusarium colonization of cotton roots and Fusarium wilt by seed treatments with Gliocladium virens and Bacillus subtilis. Biocontrol Sci. Technol. 1996, 6, 175–188. [Google Scholar] [CrossRef]
- Idris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- Benhamou, N.; Kloepper, J.W.; Quadt-Hallman, A.; Tuzun, S. Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol. 1996, 112, 919–929. [Google Scholar]
- Probanza, A.; Mateos, J.; García, J.L.; Ramos, B.; de Felipe, M.; Mañero, F.G. Effects of inoculation with PGPR Bacillus and Pisolithus tinctorius on Pinus pinea l. Growth, bacterial rhizosphere colonization, and mycorrhizal infection. Microbial. Ecol. 2001, 41, 140–148. [Google Scholar]
- Bai, Y.M.; Zhou, X.M.; Smith, D.L. Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci. 2003, 43, 1774–1781. [Google Scholar] [CrossRef]
- Handelsman, J.; Raffel, S.; Mester, E.H.; Wunderlich, L.; Grau, C.R. Biological control of damping-off of alfalfa seedlings with Bacillus-cereus UW85. Applied and Environmental Microbiology 1990, 56, 713–718. [Google Scholar]
- López-Bucio, J.; Campos-Cuevas, J.C.; Hernández-Calderón, E.; Velásquez-Becerra, C.; Farías-Rodríguez, R.; Macías-Rodríguez, L.I.; Valencia-Cantero, E. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin-and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2007, 20, 207–217. [Google Scholar] [CrossRef]
- Francis, I.; Holsters, M.; Vereecke, D. The Gram-positive side of plant–microbe interactions. Environ. Microbiol. 2010, 12, 1–12. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With SPECIAL reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ma, Y.; Freitas, H. Characterization of metal-resistant plant-growth promoting Bacillus weihenstephanensis isolated from serpentine soil in Portugal. J. Basic Microbiol. 2008, 48, 500–508. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, F.M.; López-Bucio, J.; Altamirano-Hernández, J.; Valencia-Cantero, E.; de la Cruz, H.R.; Macías-Rodríguez, L. Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 2010, 51, 75–83. [Google Scholar] [CrossRef]
- Lamsal, K.; Kim, S.W.; Kim, Y.S.; Lee, Y.S. Application of rhizobacteria for plant growth promotion effect and biocontrol of anthracnose caused by Colletotrichum acutatum on pepper. Mycobiology 2012, 40, 244–251. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar]
- Rashid, S.; Charles, T.C.; Glick, B.R. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl. Soil Ecol. 2012, 61, 217–224. [Google Scholar] [CrossRef]
- Guiñazú, L.B.; Andrés, J.A.; Del Papa, M.F.; Pistorio, M.; Rosas, S.B. Response of alfalfa (Medicago sativa L.) to single and mixed inoculation with phosphate-solubilizing bacteria and Sinorhizobium meliloti. Biol. Fertil. Soils 2010, 46, 185–190. [Google Scholar] [CrossRef]
- Rojas, A.; Holguin, G.; Glick, B.R.; Bashan, Y. Synergism between Phyllobacterium sp. (N2-fixer) and Bacillus licheniformis (P-solubilizer), both from a semiarid mangrove rhizosphere. FEMS Microbiol. Ecol. 2001, 35, 181–187. [Google Scholar] [CrossRef]
- Vivas, A.; Marulanda, A.; Ruiz-Lozano, J.M.; Barea, J.M.; Azcón, R. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza 2003, 13, 249–256. [Google Scholar] [CrossRef]
- Bacon, C.W.; Yates, I.E.; Hinton, D.M.; Meredith, F. Biological control of Fusarium moniliforme in maize. Environ. Health Perspect. 2001, 109, 325–332. [Google Scholar]
- Zhang, Y.Z.; Chen, W.F.; Li, M.; Sui, X.H.; Liu, H.-C.; Zhang, X.X.; Chen, W.X. Bacillus endoradicis sp. nov., an endophytic bacterium isolated from soybean root. Int. J. Syst. Evol. Microbiol. 2012, 62, 359–363. [Google Scholar] [CrossRef]
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar]
- Hassen, A.I.; Labuschagne, N. Root colonization and growth enhancement in wheat and tomato by rhizobacteria isolated from the rhizoplane of grasses. World J. Microbiol. Biotechnol. 2010, 26, 1837–1846. [Google Scholar] [CrossRef]
- Ash, C.; Farrow, J.A.E.; Wallbanks, S.; Collins, M.D. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative-analysis of small-subunit-ribosomal RNA sequences. Lett. Appl. Microbiol. 1991, 13, 202–206. [Google Scholar]
- Koeppel, A.; Perry, E.B.; Sikorski, J.; Krizanc, D.; Warner, A.; Ward, D.M.; Rooney, A.P.; Brambilla, E.; Connor, N.; Ratcliff, R.M. Identifying the fundamental units of bacterial diversity: A paradigm shift to incorporate ecology into bacterial systematics. Proc. Natl. Acad. Sci. USA 2008, 105, 2504–2509. [Google Scholar] [CrossRef]
- Xu, D.; Côte, J.-C. Phylogenetic relationships between Bacillus species and related genera inferred from comparison of 3′ end 16S rDNA and 5′ end 16S–23S ITS nucleotide sequences. Int. J. Syst. Evol. Microbiol. 2003, 53, 695–704. [Google Scholar] [CrossRef]
- Petersen, D.J.; Srinivasan, M.; Chanway, C.P. Bacillus polymyxa stimulates increased Rhizobium etli populations and nodulation when co-resident in the rhizosphere of Phaseolus vulgaris. FEMS Microbiol. Lett. 1996, 142, 271–276. [Google Scholar] [CrossRef]
- Srinivasan, M.; Holl, F.; Petersen, D. Nodulation of Phaseolus vulgaris by Rhizobium etli is enhanced by the presence of Bacillus. Can. J. Microbiol. 1997, 43, 1–8. [Google Scholar] [CrossRef]
- Turner, J.; Backman, P. Factors relating to peanut yield increases after seed treatment with Bacillus subtilis. Plant Dis. 1991, 75, 347–353. [Google Scholar] [CrossRef]
- Rajendran, G.; Sing, F.; Desai, A.J.; Archana, G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresour. Technol. 2008, 99, 4544–4550. [Google Scholar] [CrossRef]
- Halverson, L.J.; Handelsman, J. Enhancement of soybean nodulation by Bacillus cereus UW85 in the field and in a growth chamber. Appl. Environ. Microbiol. 1991, 57, 2767–2770. [Google Scholar]
- Hirsch, A.M. Developmental biology of legume nodulation. New Phytologist 1992, 122, 211–237. [Google Scholar] [CrossRef]
- DeMason, D.A.; Polowick, P.L. Patterns of DR5::GUS expression in organs of pea (Pisum sativum). Int. J. Plant Sci. 2009, 170, 1–11. [Google Scholar] [CrossRef]
- Sikorski, J.; Nevo, E. Adaptation and incipient sympatric speciation of Bacillus simplex under microclimatic contrast at “Evolution Canyons” I and II, Israel. Proc. Natl. Acad. Sci. USA 2005, 102, 15924–15929. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Liu, Y.; Chen, S. Isolation and identification of nitrogen-fixing Bacilli from plant rhizospheres in Beijing region. J. Appl. Microbiol. 2005, 99, 1271–1281. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Chen, S. Colonization of maize and rice plants by strain Bacillus megaterium C4. Curr. Microbiol. 2006, 52, 186–190. [Google Scholar]
- Xie, G.H.; Cui, Z.; Yu, J.; Yan, J.; Hai, W.; Steinberger, Y. Identification of nif genes in N2-fixing bacterial strains isolated from rice fields along the Yangtze river plain. J. Basic Microbiol. 2006, 46, 56–63. [Google Scholar] [CrossRef]
- Achouak, W.; Normand, P.; Heulin, T. Comparative phylogeny of rrs and nifH genes in the Bacillaceae. Int. J. Syst. Microbiol. 1999, 49, 961–967. [Google Scholar]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Kistler, H.C.; Bosland, P.W.; Benny, U.; Leong, S.; Williams, P. Relatedness of strains of Fusarium oxysporum from crucifers measured by examination of mitochondrial and ribosomal DNA. Phytopathology 1987, 77, 1289–1293. [Google Scholar] [CrossRef]
- O’Donnell, K.; Gueidan, C.; Sink, S.; Johnston, P.R.; Crous, P.W.; Glenn, A.; Riley, R.; Zitomer, N.C.; Colyer, P.; Waalwijk, C. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 2009, 46, 936–948. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Z.; Glick, B.R. The presence of a 1-aminocyclopropane-1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol. Lett. 2009, 296, 131–136. [Google Scholar] [CrossRef]
- Paz, I.; Santin, R.; Guimarães, A.; Rosa, O.; Dias, A.; Quecine, M.; Azevedo, J.; Matsumura, A. Eucalyptus growth promotion by endophytic Bacillus spp. Genet. Mol. Res. 2012, 11, 3711–3720. [Google Scholar] [CrossRef]
- Guinel, F.C. Getting around the legume nodule: I. The structure of the peripheral zone in four nodule types. Botany-Botanique 2009, 87, 1117–1138. [Google Scholar] [CrossRef]
- Schwartz, D.; Munger, R.; Lazzaro, B.; Lumb, G. A modified Brown and Hopps stain for identification of Gram-positive and Gram-negative microorganisms in glycol methacrylate-embedded tissues. Arch. Pathol. Lab. Med. 1989, 113, 181–183. [Google Scholar]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Bianco, C.; Defez, R. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl. Environ. Microbiol. 2010, 76, 4626–4632. [Google Scholar] [CrossRef]
- Imperlini, E.; Bianco, C.; Lonardo, E.; Camerini, S.; Cermola, M.; Moschetti, G.; Defez, R. Effects of indole-3-acetic acid on Sinorhizobium meliloti survival and on symbiotic nitrogen fixation and stem dry weight production. Appl. Microbiol. Biotechnol. 2009, 83, 727–738. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Miller, P.M. V-8 juice agar as a general purpose medium for fungi and bacteria. Phytopathology 1955, 45, 461–462. [Google Scholar]
- Atlas, R. Alphabetical Listing of Media. In Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 1993; pp. 455–462. [Google Scholar]
- Tzean, S.; Torrey, J.G. Spore germination and the life cycle of Frankia in vitro. Can. J. Microbiol. 1989, 35, 801–806. [Google Scholar] [CrossRef]
- Davis, K.E.R.; Joseph, S.J.; Janssen, P.H. Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Appl. Environ. Microbiol. 2005, 71, 826–834. [Google Scholar] [CrossRef]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972; p. 433. [Google Scholar]
- Beringer, J.E. R-factor transfer in Rhizobium legumunosarum. J. Gen. Microbiol. 1974, 84, 188–198. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of the Root-Nodule Bacteria; IBP Handbook No. 15; Blackwell: Oxford and Edinburgh, UK, 1970; p. 6. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bact. 1991, 173, 697–703. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evolution. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Angus, A.A.; Lee, A.S.; Lum, M.R.; Shehayeb, M.; Hessabi, R.; Fujishige, N.A.; Yerrapragada, S.; Kano, S.; Song, N.; Yang, P.; et al. Nodulation and effective nitrogen fixation of Macroptilium atropurpureum (siratro) by Burkholderia tuberum, a beta-proteobacterium, are influenced by environmental factors. Plant Soil 2013, 362, 543–562. [Google Scholar]
- Machlis, L.; Torrey, J.G. Plants in Action: A Laboratory Manual of Plant Physiology; W.H. Freeman and Company: San Francisco, CA, USA, 1956; p. 44. [Google Scholar]
- Cheng, H.P.; Walker, G.C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 1998, 180, 5183–5191. [Google Scholar]
- Jefferson, R. The GUS reporter gene system. Nature 1989, 342, 837–838. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.; Fernández, F. O-CAS, a fast and universal method for siderophore detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Schmidt, S.; Blom, J.F.; Pernthaler, J.; Berg, G.; Baldwin, A.; Mahenthiralingam, E.; Eberl, L. Production of the antifungal compound pyrrolnitrin is quorum sensing-regulated in members of the Burkholderia cepacia complex. Environ. Microbiol. 2009, 11, 1422–1437. [Google Scholar] [CrossRef]
- Contesto, C.; Milesi, S.; Mantelin, S.; Zancarini, A.; Desbrosses, G.; Varoquaux, F.; Bellini, C.; Kowalczyk, M.; Touraine, B. The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium. Phyllobacterium brassicacearum Planta 2010, 232, 1455–1470. [Google Scholar]
Appendix
| Gene | Sequence | PCR Conditions | Size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA | 5'-CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG-3' 5'-CCCGGGATCCAAGCTTAAGGAGGTGATCCAGCC-3' | 5 min at 95 °C, 30 cycles of 30 s at 94 °C, 30 s at 55 °C, 1.5 min at 68 °C, and 10 min at 68 °C | 832 | [64] |
| nifH | 5'-CTGVGCCTTGTTYTCGCGGGATSGGCATGGC-3’ 5’-GGCTGCGATCCVAAGGCCGAYTCVACCCG-3’. | same as above | ca. 300 | [42] |
| acdS | 5'-TACAAAGCTTATGAACCTGCAACGATTCC-3' 5'-AATGGATCCTTAGCCGTTGCGGAAAAATG-3 | same as above | 1017 | [49] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schwartz, A.R.; Ortiz, I.; Maymon, M.; Herbold, C.W.; Fujishige, N.A.; Vijanderan, J.A.; Villella, W.; Hanamoto, K.; Diener, A.; Sanders, E.R.; et al. Bacillus simplex—A Little Known PGPB with Anti-Fungal Activity—Alters Pea Legume Root Architecture and Nodule Morphology When Coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 2013, 3, 595-620. https://doi.org/10.3390/agronomy3040595
Schwartz AR, Ortiz I, Maymon M, Herbold CW, Fujishige NA, Vijanderan JA, Villella W, Hanamoto K, Diener A, Sanders ER, et al. Bacillus simplex—A Little Known PGPB with Anti-Fungal Activity—Alters Pea Legume Root Architecture and Nodule Morphology When Coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy. 2013; 3(4):595-620. https://doi.org/10.3390/agronomy3040595
Chicago/Turabian StyleSchwartz, Allison R., Irma Ortiz, Maskit Maymon, Craig W. Herbold, Nancy A. Fujishige, Janahan A. Vijanderan, William Villella, Kayoko Hanamoto, Andrew Diener, Erin R. Sanders, and et al. 2013. "Bacillus simplex—A Little Known PGPB with Anti-Fungal Activity—Alters Pea Legume Root Architecture and Nodule Morphology When Coinoculated with Rhizobium leguminosarum bv. viciae" Agronomy 3, no. 4: 595-620. https://doi.org/10.3390/agronomy3040595
APA StyleSchwartz, A. R., Ortiz, I., Maymon, M., Herbold, C. W., Fujishige, N. A., Vijanderan, J. A., Villella, W., Hanamoto, K., Diener, A., Sanders, E. R., DeMason, D. A., & Hirsch, A. M. (2013). Bacillus simplex—A Little Known PGPB with Anti-Fungal Activity—Alters Pea Legume Root Architecture and Nodule Morphology When Coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy, 3(4), 595-620. https://doi.org/10.3390/agronomy3040595





