Intercropping with Different Companion Plants Affects the Growth and Soil Properties of Chrysanthemum morifolium
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Treatments Description
2.3. Plant Growth Conditions
2.4. Botanical Trait Quantification
2.5. Soil Physical and Chemical Properties and Enzyme Activities
2.6. Phenolic Acid Substances Content in Soil
2.7. Statistical Analysis
3. Results
3.1. Intercropping Affected the Soil Chemical and Physical Properties of Hangju
3.2. Intercropping Affected the Enzyme Activity of Rhizosphere Soil
3.3. Intercropping Affected the Content of Phenolic Acid in Rhizosphere Soil
3.4. Intercropping Affects the Growth of Hangju
3.5. Correlation Analysis Between Growth Index of Hangju and Rhizosphere Soil Characteristics
4. Discussion
4.1. Intercropping Affected the Rhizosphere Soil Properties of Hangju
4.2. Intercropping Affected the Enzyme Activity of Rhizosphere Soil
4.3. Intercropping Affected the Content of Phenolic Acid in Rhizosphere Soil
4.4. Limitations and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEC | Cation exchange capacity |
| CK | Monoculture of Hangju |
| BD | Bulk density |
| EC | Electrical conductivity |
| Hangju | Chrysanthemum morifolium Ramat cv. ‘Hangju’ |
| Soya | Hangju × soya (Glycine max (L.) Merr.) |
| Schizonepeta | Hangju × schizonepeta (Schizonepeta tenuifolia Briq.) |
| Pepper | Hangju × pepper (Capsicum annuum L.) |
| Dandelion | Hangju × dandelion (Taraxacum mongolicum Hand. -Mazz.) |
| Edible amaranth | Hangju × edible amaranth (Amaranthus tricolor L.) |
| Maize | Hangju × maize (Zea mays L.) |
| Purple perilla | Hangju × purple perilla (Perilla frutescens (L.) Britt) |
| LSD | Limit of detection |
| pH | Potential hydrogen |
| RN | Nylon mesh isolation treatment |
| RO | Unisolated treatment |
| RP | Plastic film isolation treatment |
| RSD | Relative standard deviation |
| SWC | Soil water content |
References
- Liu, Y.; Lu, C.; Zhou, J.; Zhou, F.; Gui, A.; Chu, H.; Shao, Q. Chrysanthemum morifolium as a traditional herb: A review of historical development, classification, phytochemistry, pharmacology and application. J. Ethnopharmacol. 2024, 330, 118198. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, Y.; Dai, C. Preliminary study on the causes of continuous cropping obstacles in medicinal chrysanthemum in Yancheng. Soil 2012, 44, 1035–1040. [Google Scholar]
- Liu, J.; Yao, Q.; Li, Y.; Zhang, W.; Mi, G.; Chen, X.; Yu, Z.; Wang, G. Continuous cropping of soybean alters the bulk and rhizospheric soil fungal communities in a Mollisol of Northeast PR China. Land Degrad. Dev. 2019, 30, 1725–1738. [Google Scholar] [CrossRef]
- Du, S.; Bai, G.; Yu, J. Soil properties and apricot growth under intercropping and mulching with erect milk vetch in the loess hilly-gully region. Plant Soil 2015, 390, 431–442. [Google Scholar] [CrossRef]
- Xia, J.; Ren, J.; Zhang, S.; Wang, Y.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Yin, Y.; Zhu, W.; Bai, X.; Zhou, Y. Characteristics of Soil Physicochemical Properties and Microbial Community of Mulberry (Morus alba L.) and Alfalfa (Medicago sativa L.) Intercropping System in Northwest Liaoning. Microorganisms 2023, 1, 114. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Qu, J.; Zhang, D.; Cao, L.; Qin, X.; Li, Z. Intercropping with maize and sorghum-induced saikosaponin accumulation in Bupleurum chinense DC. by liquid chromatography-mass spectrometry-based metabolomics. J. Mass Spectrom. JMS 2024, 59, e5035. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzymes and Their Research Methods; Agricultural Publishing House: Beijing, China, 1986. [Google Scholar]
- Zhao, X.; Arshad, M.; Li, N.; Zare, E.N.; Triantafilis, J. Determination of the optimal mathematical model, sample size, digital data and transect spacing to map CEC (Cation exchange capacity) in a sugarcane field. Comput. Electron. Agric. 2020, 173, 105436. [Google Scholar] [CrossRef]
- Jamal, N.; Munir, A.; Harun, G.; Li, T.; Yuan, C.; Bo, Z.X. Maize/soybean intercropping increases nutrient uptake, crop yield and modifies soil physio-chemical characteristics and enzymatic activities in the subtropical humid region based in Southwest China. BMC Plant Biol. 2024, 24, 434. [Google Scholar] [CrossRef]
- Gurmessa, B.; Cocco, S.; Ashworth, A.J.; Udawatta, R.P.; Cardelli, V.; Serrani, D.; Ilari, A.; Pedretti, E.F.; Fornasier, F.; Corti, G. Enzyme activities and microbial nutrient limitations in response to digestate and compost additions in organic matter poor soils in the Marches, Italy. Soil Tillage Res. 2024, 242, 106136. [Google Scholar] [CrossRef]
- Curtright, A.J.; Tiemann, L.K. Meta-analysis dataset of soil extracellular enzyme activities in intercropping systems. Data Brief 2021, 38, 107284. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liao, X.; Pan, C.; Li, X. Positive effects of intercropping on soil phosphatase activity depend on the application scenario: A meta-analysis. Soil Tillage Res. 2024, 235, 105914. [Google Scholar] [CrossRef]
- Wang, C.; Ning, P.; Li, J.; Wei, X.; Ge, T.; Cui, Y.; Deng, X.; Jiang, Y.; Shen, W. Responses of soil microbial community composition and enzyme activities to long-term organic amendments in a continuous tobacco cropping system. Appl. Soil Ecol. 2022, 169, 104210. [Google Scholar] [CrossRef]
- Greenfield, L.M.; Hill, P.W.; Paterson, E.; Baggs, E.M.; Jones, D.L. Do plants use root-derived proteases to promote the uptake of soil organic nitrogen? Plant Soil 2020, 456, 355–367. [Google Scholar] [CrossRef]
- Pedrol, N.; Puig, C.G. Application of Allelopathy in Sustainable Agriculture. Agronomy 2024, 14, 1362. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y.; Sun, W.; Qiao, B.; Cheng, J.; Shi, S.; Zhao, C.; Li, C. Dynamic response of allelopathic potency of Taxus cuspidata Sieb. et Zucc. mediated by allelochemicals in Ficus carica Linn. root exudates. Sci. Total Environ. 2024, 940, 173663. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.Y.; Zhu, G.X.; Hu, B.W.; Tao, R. Continuous cropping of cut chrysanthemum reduces rhizospheric soil bacterial community diversity and co-occurrence network complexity. Appl. Soil Ecol. 2023, 185, 104801. [Google Scholar] [CrossRef]
- Wilhelm, R.C.; DeRito, C.M.; Shapleigh, J.P.; Madsen, E.L.; Buckley, D.H. Phenolic acid-degrading Paraburkholderia prime decomposition in forest soil. ISME Commun. 2021, 1, 4. [Google Scholar] [CrossRef]
- Mushtaq, W.; Fauconnier, M. Phenolic profiling unravelling allelopathic encounters in agroecology. Plant Stress 2024, 13, 100523. [Google Scholar] [CrossRef]
- Xiao, D.; Surigaoge, S.; Du, Y.; Fu, D.; Yang, X.; Fornara, H.; Zhang, W.; Li, L. Interspecific interactions increase soil aggregate stability through altered root traits in long-term legume/maize intercropping. Soil Tillage Res. 2026, 255, 106808. [Google Scholar] [CrossRef]
- Li, R.; Chen, H.; Yang, Z.; Yuan, S.; Zhou, X. Research status of soybean symbiosis nitrogen fixation. Oil Crop Sci. 2020, 5, 6–10. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Costa, J.; Cayún, Y.; Gallardo, V.; Barría, E.; Caruso, G.R.; Kress, M.R.; Cornejo, P.; Santos, C. Chemical composition and antifungal activity of Capsicum pepper aqueous extracts against plant pathogens and food spoilage fungi. Front. Cell. Infect. Microbiol. 2024, 14, 1451287. [Google Scholar] [CrossRef] [PubMed]
- Yashaswini, M.S.; Nysanth, N.S.; Anith, K.N. Endospore-forming bacterial endophytes from Amaranthus spp. improve plant growth and suppress leaf blight (Rhizoctonia solani Kühn) disease of Amaranthus tricolor L. Rhizosphere 2021, 19, 100387. [Google Scholar] [CrossRef]
- Ainalidou, A.; Bouzoukla, F.; Menkissoglu-Spiroudi, U.; Vokou, D.; Karamanoli, K. Impacts of Decaying Aromatic Plants on the Soil Microbial Community and on Tomato Seedling Growth and Metabolism: Suppression or Stimulation? Plants 2021, 10, 1848. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Chen, Y.; Zhang, X.; Ning, C.; Sun, Y.; Jia, M.; Wu, M.; Tong, X.; Jiang, X.; et al. Effect of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Intercropping with Different Plant Spacing on Blight and Growth of Pepper (Capsicum annuum L.). Phyton-Int. J. Exp. Bot. 2023, 92, 2227–2244. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhang, Y.; Fei, J.; Rong, X.; Peng, J.; Yin, L.; Luo, G. Intercropping enhances maize growth and nutrient uptake by driving the link between rhizosphere metabolites and microbiomes. New Phytol. 2024, 243, 1506–1521. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.; Song, M.; Tian, J.; Song, B.; Hu, Y.; Zhang, J.; Yao, Y. Intercropping with Aromatic Plants Increased the Soil Organic Matter Content and Changed the Microbial Community in a Pear Orchard. Front. Microbiol. 2021, 2, 616932. [Google Scholar] [CrossRef]
- Sun, G.T.; Bao, G.R.; Tai, J.C.; Sa, R.; Liu, N.; Yu, M.; Li, A. Effects of Maize-Peanut Intercropping on Crop and Soil Characteristics. Chin. Agric. Sci. Bull. 2025, 41, 7–12. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, H.; Lu, J.; Liang, Y.; Li, Y.; Xu, L.; Ye, J.; Yang, R.; Li, P.; Jiao, J.; et al. Green manuring with balanced fertilization improves soil ecosystem multifunctionality by enhancing soil quality and enzyme activities in a smooth vetch-maize rotation system. Agric. Ecosyst. Environ. 2025, 387, 109632. [Google Scholar] [CrossRef]
- Ma, Z.; Bork, E.; Li, J.; Chen, G.; Chang, S. Photosynthetic carbon allocation to live roots increases the year following high intensity defoliation across two ecosites in a temperate mixed grassland. Agr. Ecosyst. Environ. 2021, 316, 107450. [Google Scholar] [CrossRef]
- Lin, X.R.; Yang, D.; Wei, Y.F.; Ding, D.; Ou, H.; Yang, S. Amaranth Plants with Various Color Phenotypes Recruit Different Soil Microorganisms in the Rhizosphere. Plants 2024, 13, 2200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, F. Vanillic acid changed cucumber (Cucumis sativus L.) seedling rhizosphere total bacterial, Pseudomonas and Bacillus spp. communities. Sci. Rep. 2018, 8, 4929. [Google Scholar] [CrossRef] [PubMed]
- Shafi, Z.; Shahid, M. Root exudates as molecular architects shaping the rhizobacterial community: A review. Rhizosphere 2025, 36, 101212. [Google Scholar] [CrossRef]
- Kong, C.; Zhang, S.; Li, Y.; Xia, Z.; Yang, X.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef]
- Bao, L.; Liu, Y.; Ding, Y.; Shang, J.; Wei, Y.; Tan, Y.; Zi, F. Interactions Between Phenolic Acids and Microorganisms in Rhizospheric Soil From Continuous Cropping of Panax notoginseng. Front. Microbiol. 2022, 13, 791603. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Chen, L.; Zhang, P.; Liu, Z.; Yang, X.; Shao, J.; Ding, Y.; Mi, Y. Effects of pepper–maize intercropping on the physicochemical properties, microbial communities, and metabolites of rhizosphere and bulk soils. Environ. Microbiome 2024, 19, 108. [Google Scholar] [CrossRef]
- Jalloh, A.A.; Mutyambai, D.M.; Yusuf, A.A.; Subramanian, S.; Khamis, F. Maize edible-legumes intercropping systems for enhancing agrobiodiversity and belowground ecosystem services. Sci. Rep. 2024, 14, 14355. [Google Scholar] [CrossRef]
- Cao, N.; Chen, G.; Wang, S.; Li, H.; Lin, J.; Hu, Q.; Wan, H. Optimizing plant density and nitrogen fertilization in jujube/cotton intercropping systems for sustainable yield and reduced greenhouse gas emissions. Field Crops Res. 2025, 326, 109873. [Google Scholar] [CrossRef]
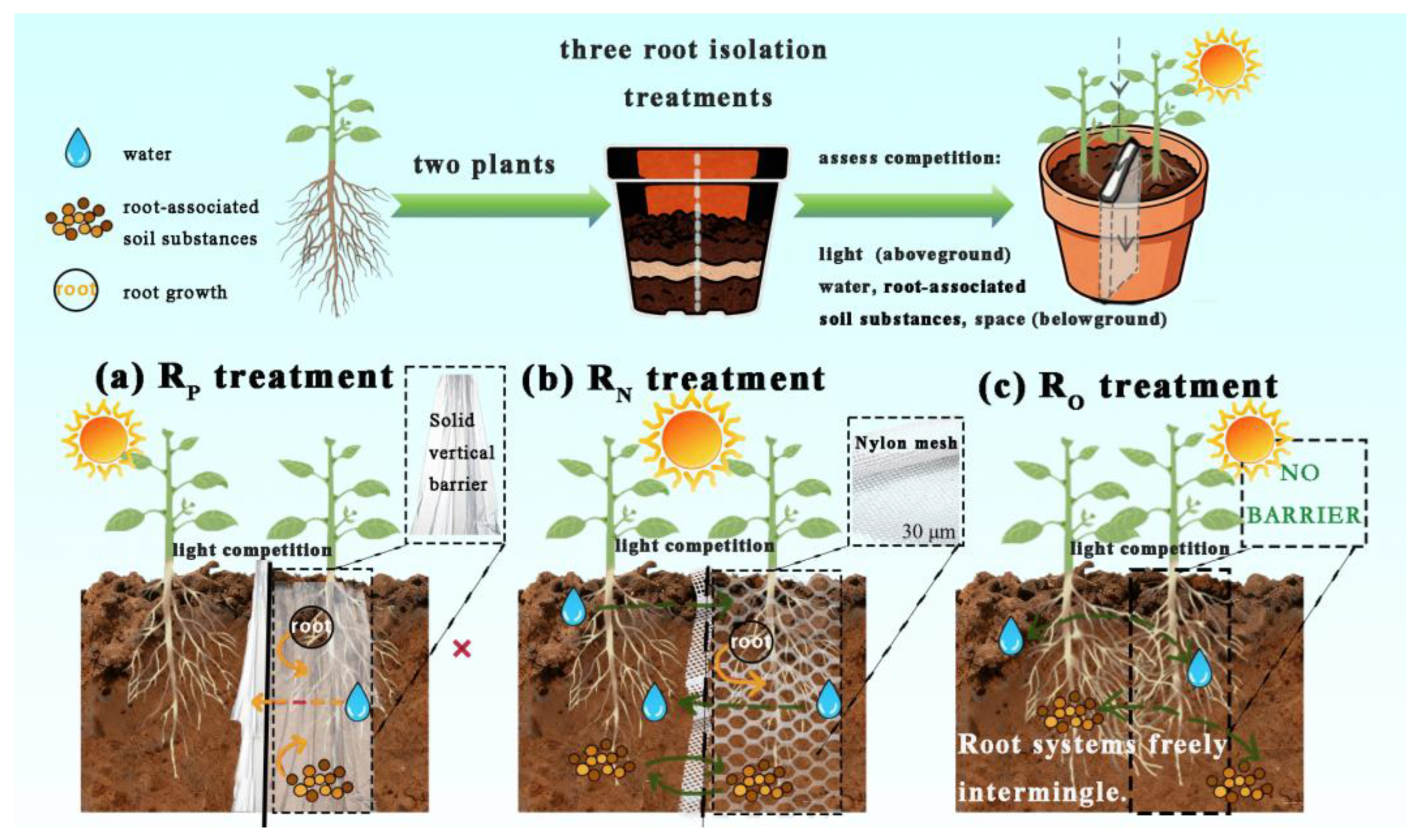
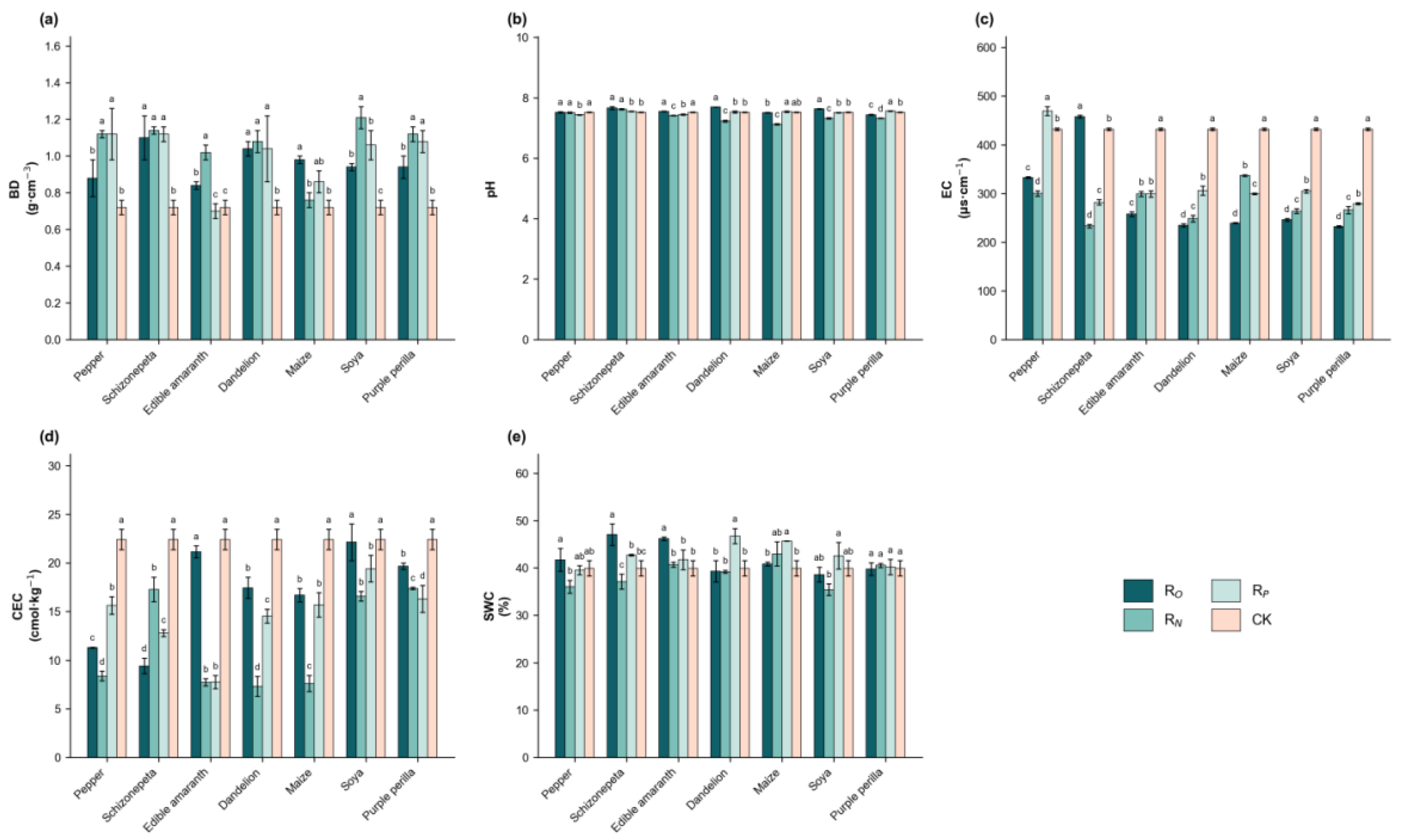
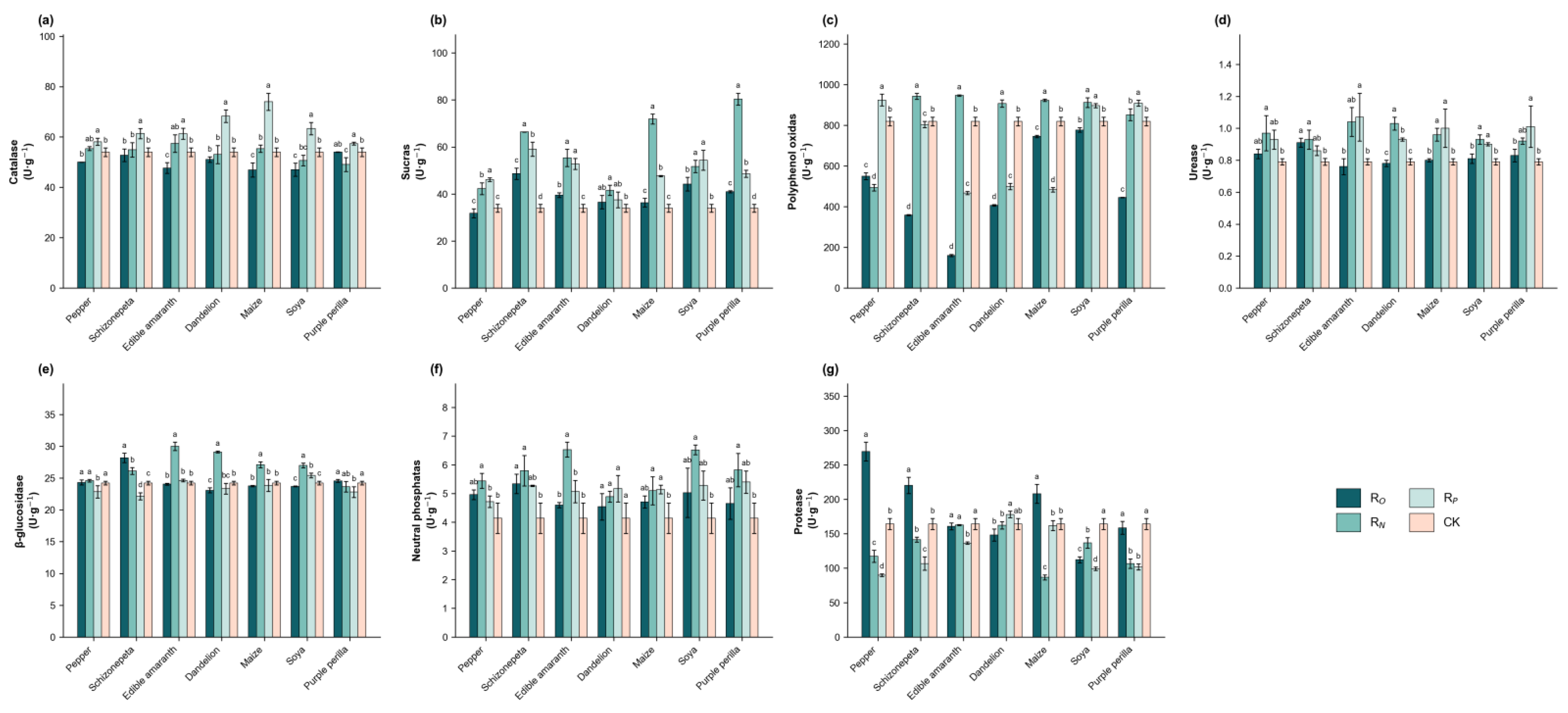
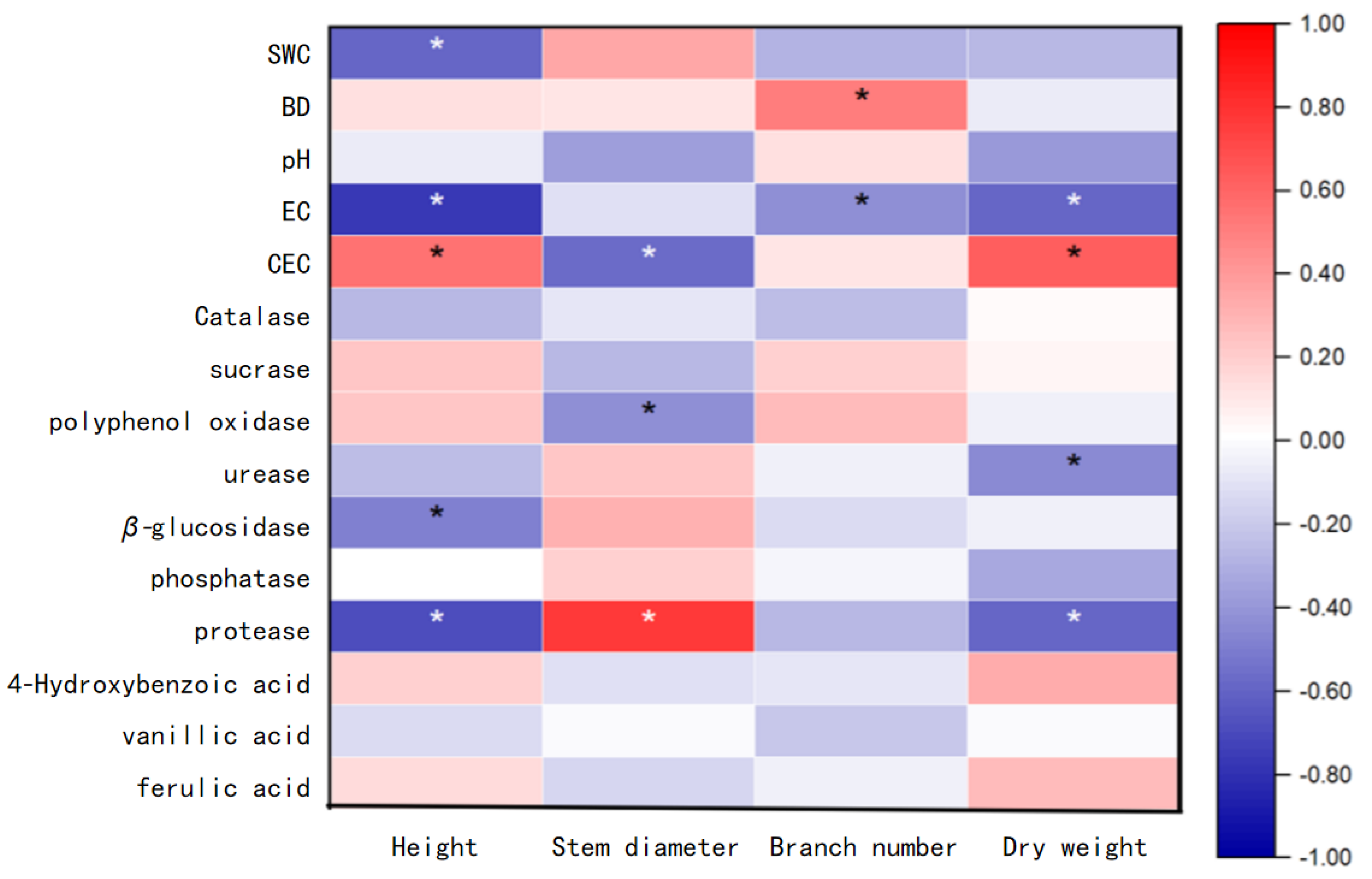
| Group | Soil pH | Electrical Conductivity μs·cm−1 | Cation Exchange Capacity /cmol·kg−1 | Soil Water Content /% | Soil Bulk Density /g·cm−3 |
|---|---|---|---|---|---|
| Pepper | 7.52 ± 0.02 c | 333.00 ± 1.41 c | 11.27 ± 0.05 d | 41.71 ± 2.43 b | 0.88 ± 0.10 b |
| Schizonepeta | 7.66 ± 0.05 ab | 458.00 ± 2.82 a | 9.42 ± 0.78 d | 47.04 ± 2.27 a | 1.10 ± 0.12 a |
| Edible Amaranth | 7.55 ± 0.01 c | 257.67 ± 4.72 d | 21.15 ± 0.60 ab | 46.18 ± 0.36 a | 0.84 ± 0.02 b |
| Dandelion | 7.69 ± 0.01 a | 234.67 ± 3.21 f | 17.44 ± 1.08 c | 39.28 ± 2.27 b | 1.04 ± 0.04 a |
| Maize | 7.51 ± 0.01 c | 239.50 ± 0.70 f | 16.67 ± 0.69 c | 40.85 ± 0.40 b | 0.98 ± 0.02 a |
| Soya | 7.63 ± 0.01 b | 246.00 ± 2.64 e | 22.14 ± 1.88 a | 38.60 ± 1.57 b | 0.94 ± 0.01 b |
| Purple Perilla | 7.44 ± 0.02 d | 232.00 ± 1.73 g | 19.67 ± 0.32 b | 39.78 ± 1.32 b | 0.94 ± 0.06 b |
| Monoculture(CK) | 7.52 ± 0.01 c | 432.00 ± 2.82 b | 22.42 ± 1.05 a | 39.91 ± 1.62 b | 0.72 ± 0.04 c |
| Group | Catalase /U·g−1 | Sucrase /U·g−1 | Polyphenol Oxidase /U·g−1 | Urease /U·g−1 | β-Glucosidase /U·g−1 | Neutral Phosphatase /U·g−1 | Protease /U·g−1 |
|---|---|---|---|---|---|---|---|
| Pepper | 50.00 ± 0.07 ab | 31.83 ± 1.92 d | 550.09 ± 16.96 d | 0.84 ± 0.03 b | 24.32 ± 0.40 bc | 4.96 ± 0.17 a | 269.38 ± 13.67 a |
| Schizonepeta | 52.74 ± 2.54 a | 48.63 ± 2.41 a | 358.74 ± 2.79 g | 0.91 ± 0.02 a | 28.19 ± 0.74 a | 5.34 ± 0.34 a | 220.28 ± 11.73 b |
| Edible Amaranth | 47.66 ± 2.12 b | 39.52 ± 1.02 c | 160.30 ± 5.46 h | 0.76 ± 0.05 c | 24.05 ± 0.14 bc | 4.60 ± 0.09 a | 160.84 ± 4.97 c |
| Dandelion | 51.03 ± 1.05 ab | 36.55 ± 2.89 cd | 406.88 ± 3.67 f | 0.78 ± 0.02 c | 23.08 ± 0.39 c | 4.54 ± 0.46 a | 148.23 ± 8.69 c |
| Maize | 46.89 ± 2.85 b | 36.29 ± 1.84 cd | 745.36 ± 5.93 c | 0.80 ± 0.01 bc | 23.76 ± 0.11 c | 4.71 ± 0.21 a | 207.92 ± 13.77 b |
| Soya | 46.97 ± 2.60 b | 44.20 ± 2.89 b | 777.24 ± 10.67 b | 0.81 ± 0.03 bc | 23.70 ± 0.05 c | 5.03 ± 0.86 a | 112.19 ± 4.54 d |
| Purple Perilla | 54.00 ± 0.07 a | 40.98 ± 0.42 bc | 445.39 ± 1.41 e | 0.83 ± 0.04 bc | 24.57 ± 0.23 b | 4.65 ± 0.55 a | 158.57 ± 9.29 c |
| Monoculture | 53.96 ± 1.70 a | 33.93 ± 1.65 d | 818.96 ± 21.38 a | 0.79 ± 0.02 bc | 24.23 ± 0.30 bc | 4.14 ± 0.53 b | 164.19 ± 7.87 c |
| Group | 4-Hydroxybenzoic Acid /μg·g−1 | Vanillic Acid /μg·g−1 | Ferulic Acid /μg·g−1 |
|---|---|---|---|
| Pepper | 17.58 ± 0.51 e | 3.80 ± 0.29 a | 35.54 ± 2.36 e |
| Schizonepeta | 30.63 ± 0.65 b | 3.87 ± 0.07 a | 67.32 ± 3.95 c |
| Edible Amaranth | 24.97 ± 0.64 c | 3.10 ± 0.02 bc | 63.32 ± 2.03 cd |
| Dandelion | 26.33 ± 1.14 c | 3.26 ± 0.00 b | 63.07 ± 2.66 cd |
| Maize | 22.34 ± 0.98 d | 1.90 ± 0.02 e | 63.16 ± 3.30 cd |
| Soya | 22.10 ± 0.92 d | 2.96 ± 0.06 c | 61.50 ± 1.35 d |
| Purple Perilla | 35.42 ± 2.47 a | 3.01 ± 0.03 c | 96.60 ± 0.96 a |
| Monoculture | 31.10 ± 0.08 b | 2.14 ± 0.09 d | 76.13 ± 3.23 b |
| Group | Height /cm | Stem Diameter /mm | Branch Number | Dry Weight on the Ground /g |
|---|---|---|---|---|
| Pepper | 44.92 ± 0.70 e | 4.11 ± 0.11 d | 8.33 ± 1.15 b | 8.23 ± 0.01 f |
| Schizonepeta | 42.86 ± 0.52 f | 5.61 ± 0.03 bc | 10.00 ± 1.73 b | 10.50 ± 0.61 e |
| Edible Amaranth | 49.04 ± 0.68 d | 5.78 ± 0.06 b | 9.00 ± 1.00 b | 17.64 ± 0.47 bc |
| Dandelion | 53.08 ± 1.45 c | 5.41 ± 0.14 c | 10.67 ± 1.15 ab | 14.53 ± 0.83 d |
| Maize | 53.15 ± 0.49 c | 5.76 ± 0.12 b | 12.67 ± 1.53 a | 19.02 ± 0.28 b |
| Soya | 63.52 ± 1.15 a | 6.10 ± 0.12 a | 11.67 ± 1.15 ab | 16.26 ± 1.33 c |
| Purple Perilla | 60.03 ± 1.08 b | 5.70 ± 0.26 b | 10.67 ± 1.15 ab | 22.34 ± 1.42 a |
| Monoculture | 44.93 ± 0.10 e | 6.24 ± 0.02 a | 8.67 ± 0.58 b | 15.28 ± 1.63 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lei, M.; Zhu, Z.; Wang, C. Intercropping with Different Companion Plants Affects the Growth and Soil Properties of Chrysanthemum morifolium. Agronomy 2026, 16, 119. https://doi.org/10.3390/agronomy16010119
Lei M, Zhu Z, Wang C. Intercropping with Different Companion Plants Affects the Growth and Soil Properties of Chrysanthemum morifolium. Agronomy. 2026; 16(1):119. https://doi.org/10.3390/agronomy16010119
Chicago/Turabian StyleLei, Meng, Zaibiao Zhu, and Changlin Wang. 2026. "Intercropping with Different Companion Plants Affects the Growth and Soil Properties of Chrysanthemum morifolium" Agronomy 16, no. 1: 119. https://doi.org/10.3390/agronomy16010119
APA StyleLei, M., Zhu, Z., & Wang, C. (2026). Intercropping with Different Companion Plants Affects the Growth and Soil Properties of Chrysanthemum morifolium. Agronomy, 16(1), 119. https://doi.org/10.3390/agronomy16010119




