Tenebrio molitor Frass: A Cutting-Edge Biofertilizer for Sustainable Agriculture and Advanced Adsorbent Precursor for Environmental Remediation
Abstract
:1. Introduction
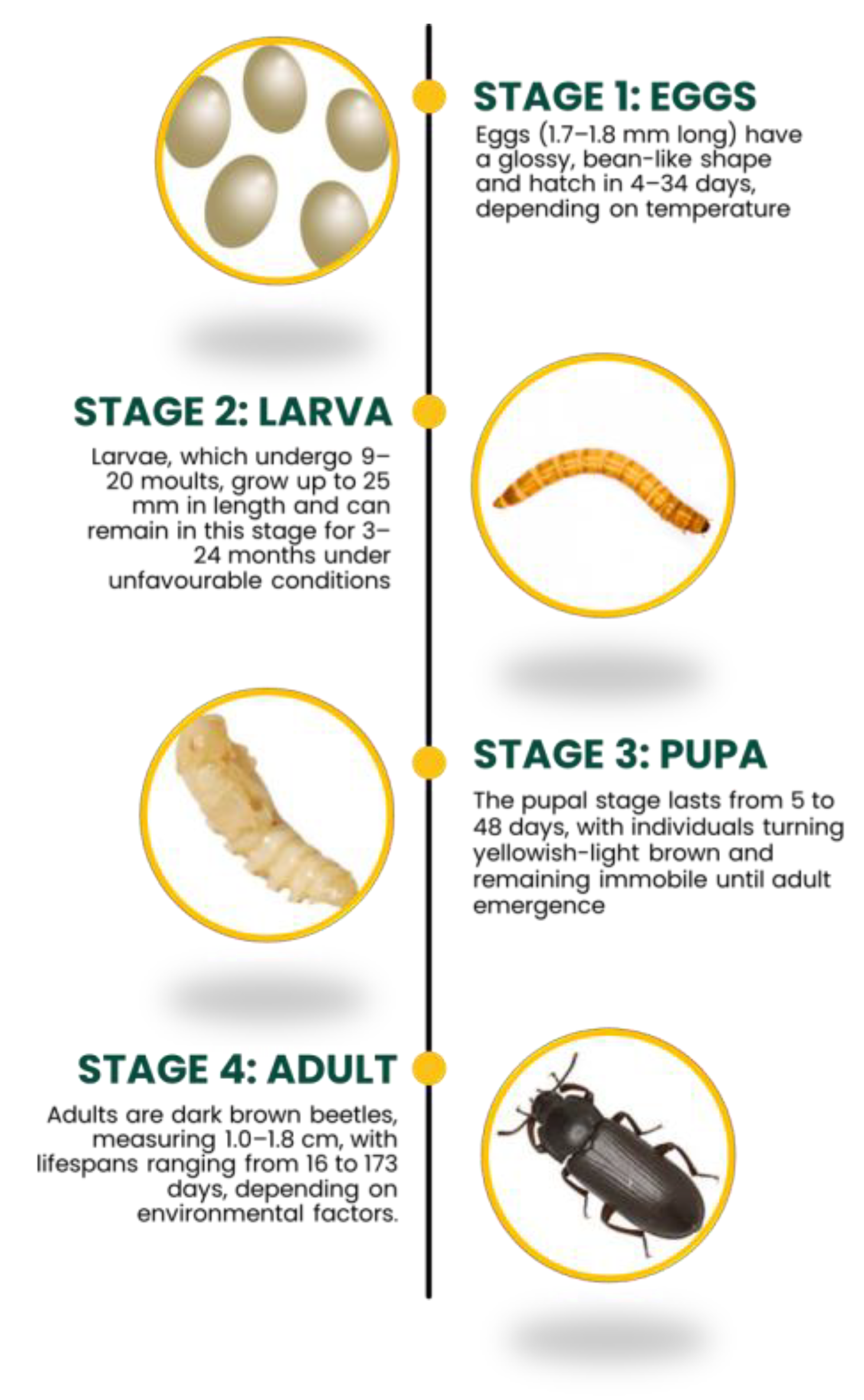
2. Taxonomy and Life Cycle of Tenebrio molitor
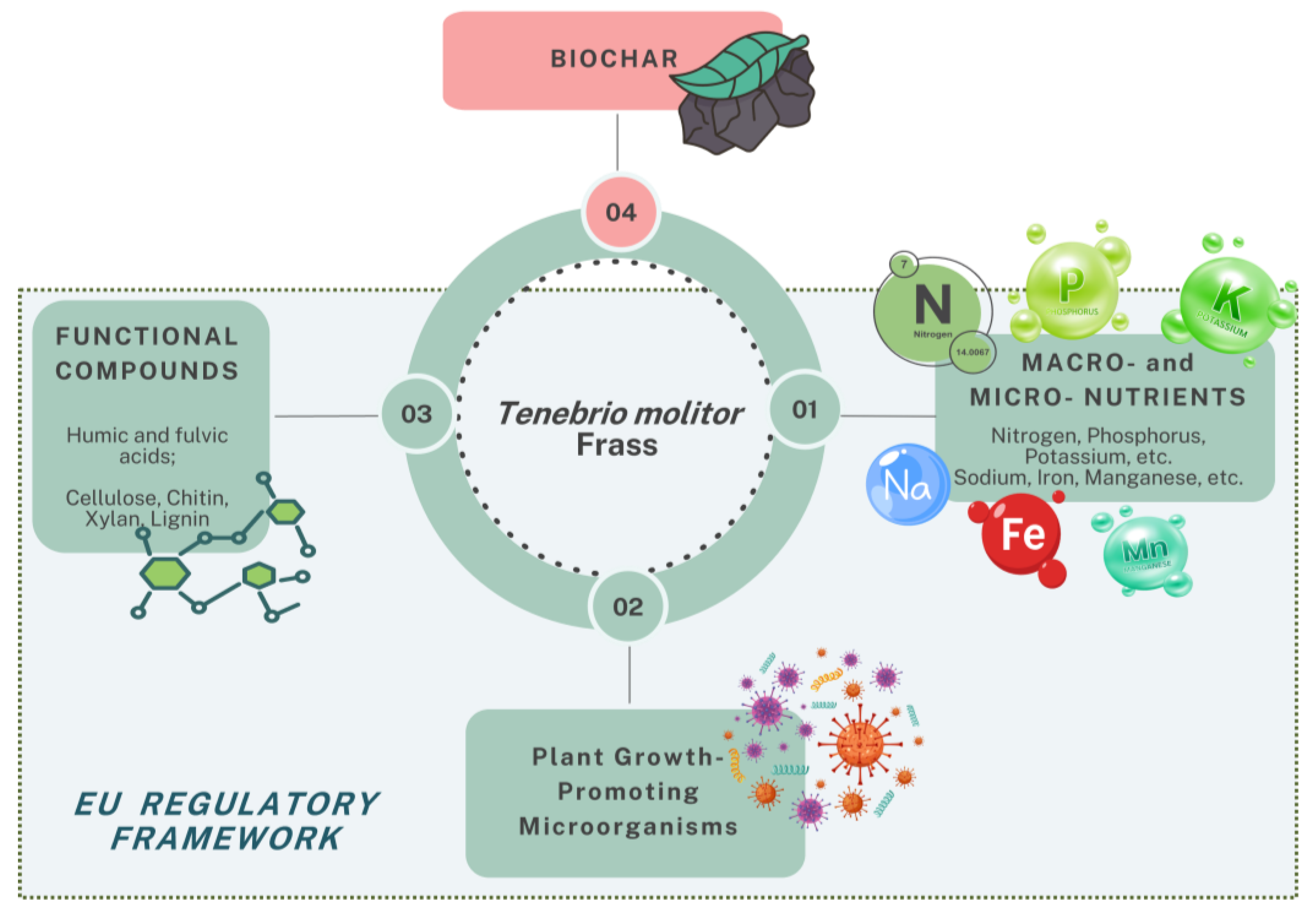
3. Composition and Characteristics of Tenebrio molitor Frass
3.1. Macronutrient and Micronutrient Profile
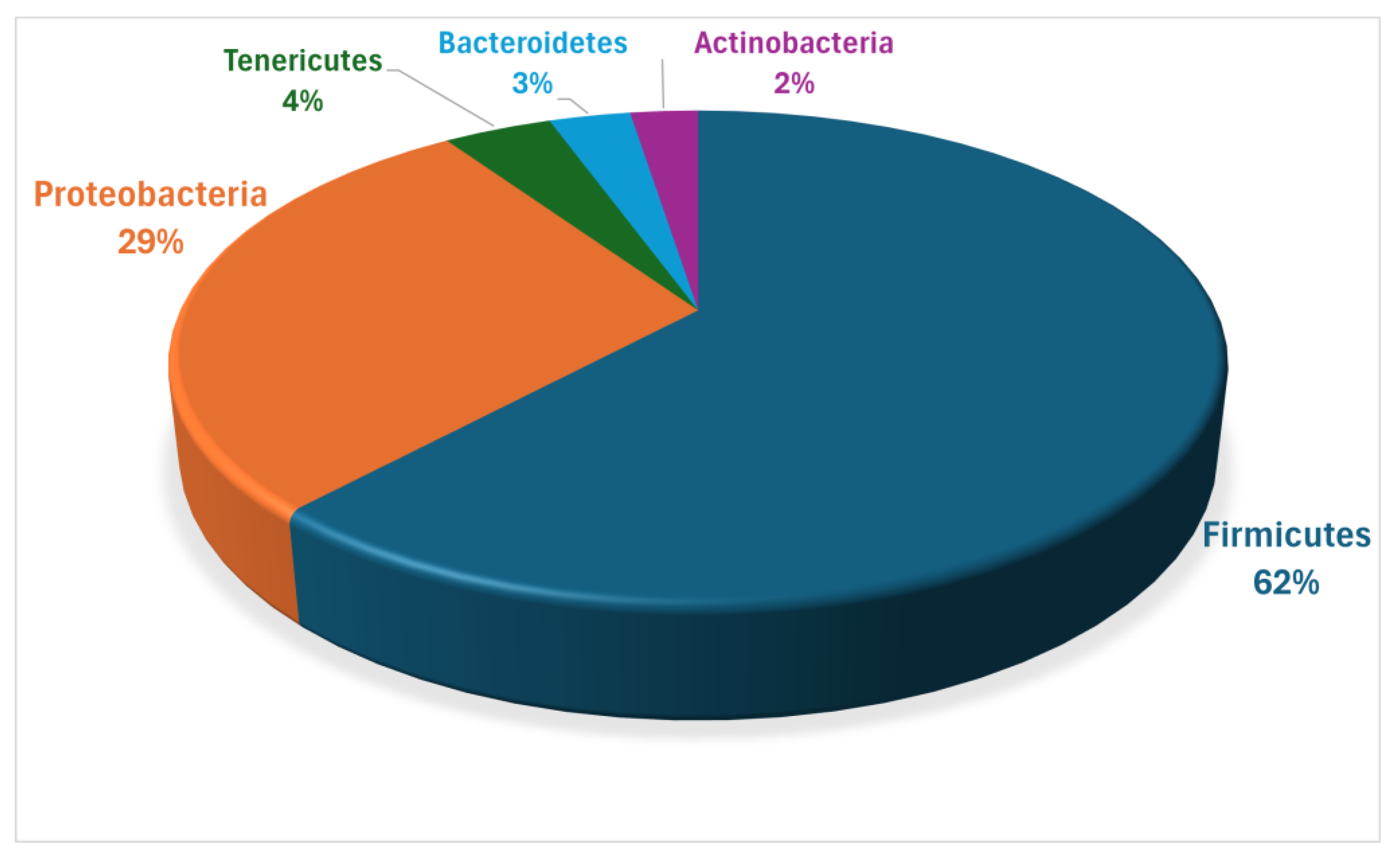
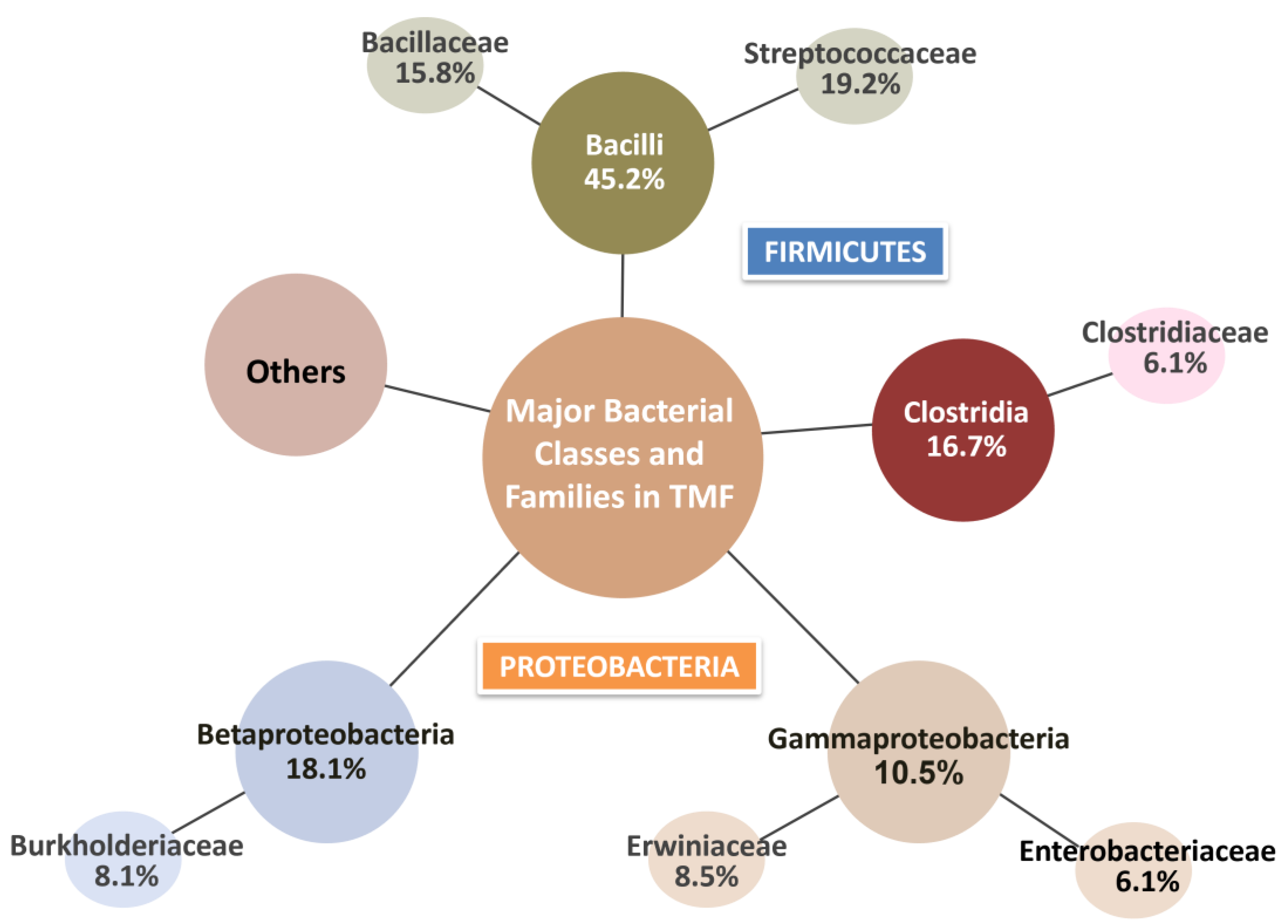
3.2. Microbial Communities
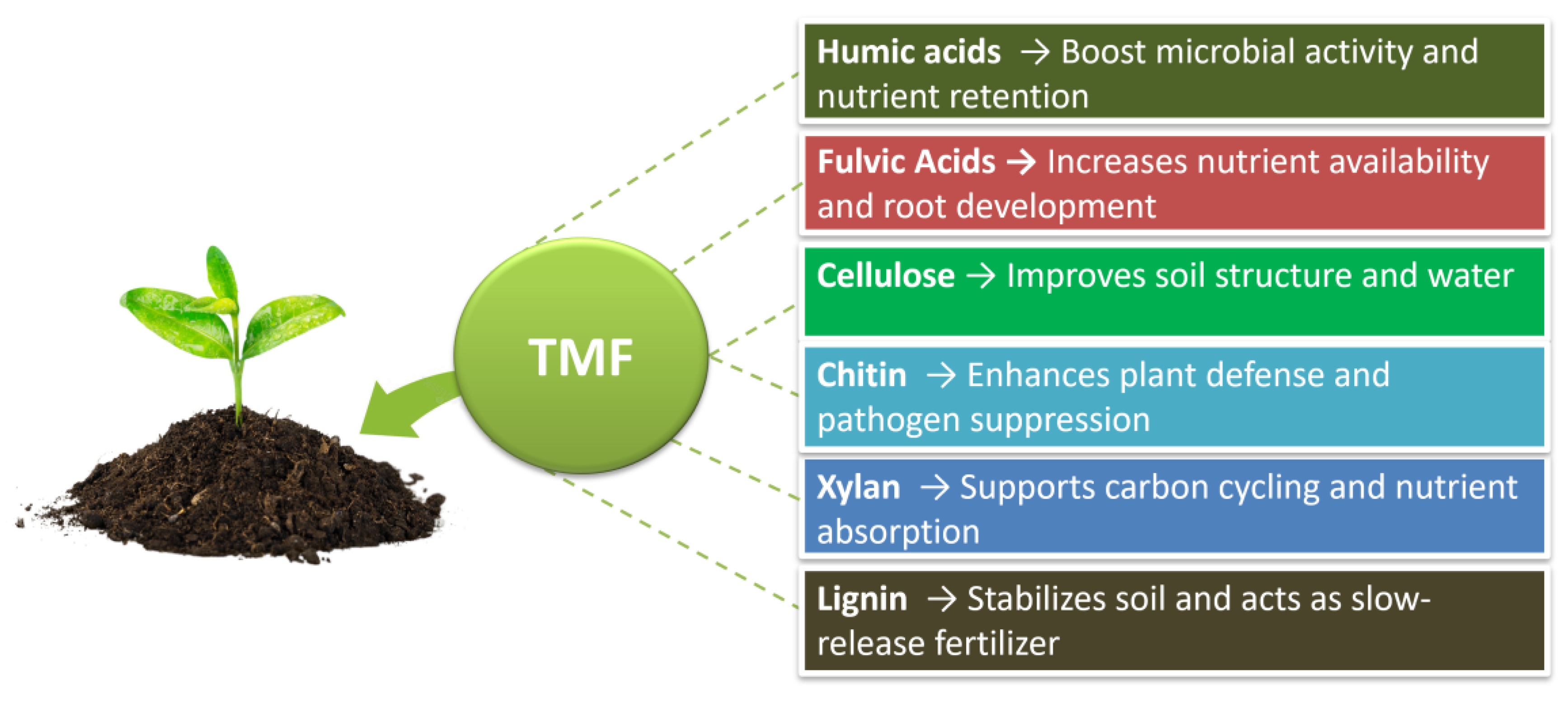
3.3. Functional Compounds
4. Agronomic Benefits
5. Environmental and Economic Impacts and Future Perspectives
- Long-term field studies assessing the effects of TMF on various crops.
- Optimizing application rates and studying its interactions with soil microbiota.
- Evaluating the ecological footprint of TMF production to ensure sustainability [17].
6. TMF-Based Biochar
7. Regulatory Framework for Insect Frass Fertilizers in Europe
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSF | Black Soldier Fly |
| chFE | Chitin-enriched insect frass fertilizer |
| CMC | Constituent Materials Category |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| GHG | Greenhouse gas |
| IPIFF | International Platform of Insects for Food and Feed |
| MAMP | Microbe-associated molecular pattern |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
| PGP | Plant growth-promoting |
| PGPMs | Plant growth-promoting microorganisms |
| TM | Tenebrio molitor |
| TMF | Tenebrio molitor frass |
| TOC | Total organic carbon |
| USD | United States Dollar |
| Al | Aluminum |
| B | Boron |
| Cu | Copper |
| Fe | Iron |
| K | Potassium |
| Mn | Manganese |
| Mo | Molybdenum |
| N | Nitrogen |
| Na | Sodium |
| P | Phosphorus |
| Zn | Zinc |
| NPK | Nitrogen, phosphorus, potassium |
References
- Worldometers. Available online: https://www.worldometers.info/ (accessed on 14 March 2025).
- United Nations. World Population Prospects 2024: Summary of Results; UN DESA/POP/2024/TR/NO. 9; United Nations: New York, NY, USA, 2024.
- FAO. The State of Food Security and Nutrition in the World; FAO Report; FAO: Rome, Italy, 2024. [Google Scholar]
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for World Population and Global Food Availability for Global Health. In The Role of Functional Food Security in Global Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–24. [Google Scholar]
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Calabi-Floody, M.; Medina, J.; Rumpel, C.; Condron, L.M.; Hernandez, M.; Dumont, M.; de la Luz Mora, M. Smart Fertilizers as a Strategy for Sustainable Agriculture. Adv. Agron. 2018, 147, 119–157. [Google Scholar]
- Sharma, A.K.; Sharma, D.; Chopra, A.K. An Overview of Pesticides in the Development of Agriculture Crops. J. Appl. Nat. Sci. 2020, 12, 101–109. [Google Scholar] [CrossRef]
- Srivastav, A.L. Chemical Fertilizers and Pesticides: Role in Groundwater Contamination. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–159. [Google Scholar]
- Rana, A.; Tyagi, M.; Sharma, N. Impact of Chemical Pesticides vs. Biopesticides on Human Health and Environment. Int. J. All Res. Writ. 2019, 2, 45–51. [Google Scholar]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient Quality and Maturity Status of Frass Fertilizer from Nine Edible Insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant–Soil Feedbacks: The Past, the Present and Future Challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Statista. Fertilizer Industry Worldwide—Statistics & Facts. Available online: https://www.statista.com/topics/8956/fertilizer-industry-worldwide/#topicOverview (accessed on 20 February 2025).
- Nakachew, K.; Yigermal, H.; Assefa, F.; Gelaye, Y.; Ali, S. Review on Enhancing the Efficiency of Fertilizer Utilization: Strategies for Optimal Nutrient Management. Open Agric. 2024, 9, 20220356. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Z.; Zhai, Y.; Zhang, L.; Zheng, M.; Yao, H.; Lv, L.; Shen, H.; Zhang, J.; Yao, Y.; et al. Partial Substitution of Chemical Fertilizer by Organic Fertilizer Benefits Grain Yield, Water Use Efficiency, and Economic Return of Summer Maize. Soil Tillage Res. 2022, 217, 105287. [Google Scholar] [CrossRef]
- Muñoz-Seijas, N.; Fernandes, H.; Outeiriño, D.; Morán-Aguilar, M.G.; Domínguez, J.M.; Salgado, J.M. Potential Use of Frass from Edible Insect Tenebrio molitor for Proteases Production by Solid-State Fermentation. Food Bioprod. Process. 2024, 144, 146–155. [Google Scholar] [CrossRef]
- Arévalo Arévalo, H.A.; Menjura Rojas, E.M.; Barragán Fonseca, K.B.; Vásquez Mejía, S.M. Implementation of the HACCP System for Production of Tenebrio molitor Larvae Meal. Food Control 2022, 138, 109030. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Ding, M.-Q.; Li, M.-X.; Ding, J.; Bai, S.-W.; Wu, Q.-L.; Zhao, L.; Cao, G.-L.; Ren, N.-Q.; et al. Sustainable Strategy for Lignocellulosic Crop Wastes Reduction by Tenebrio molitor Linnaeus (Mealworm) and Potential Use of Mealworm Frass as a Fertilizer. J. Clean. Prod. 2021, 325, 129301. [Google Scholar] [CrossRef]
- Deruytter, D.; Coudron, C.L. The Effects of Density on the Growth, Survival and Feed Conversion of Tenebrio molitor Larvae. J. Insects Food Feed 2022, 8, 141–146. [Google Scholar] [CrossRef]
- Blakstad, J.I.; Strimbeck, R.; Poveda, J.; Bones, A.M.; Kissen, R. Frass from Yellow Mealworm (Tenebrio molitor) as Plant Fertilizer and Defense Priming Agent. Biocatal. Agric. Biotechnol. 2023, 53, 102862. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Salcedo, I.; Huérfano, X.; Riga, P.; Estavillo, J.M.; Ávila Blanco, D.; Duñabeitia, M.K. Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants. Agronomy 2023, 13, 1258. [Google Scholar] [CrossRef]
- van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; Dicke, M. Soil Amendment with Insect Frass and Exuviae Affects Rhizosphere Bacterial Community, Shoot Growth and Carbon/Nitrogen Ratio of a Brassicaceous Plant. Plant Soil 2024, 495, 631–648. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; de Boer, W.; Dicke, M. Insect Frass and Exuviae to Promote Plant Growth and Health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef]
- Poveda, J. Insect Frass in the Development of Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Ashworth, A.J.; Arsi, K.; Rojas, M.G.; Morales-Ramos, J.A.; Donoghue, A.; Robinson, K. Insect Frass Composition and Potential Use as an Organic Fertilizer in Circular Economies. J. Econ. Entomol. 2024, 117, 1261–1268. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Amorim, H.C.S.; Drescher, G.L.; Moore, P.A.; Rojas, M.G.; Morales-Ramos, J.; Donoghue, A.M. Insect Frass Fertilizer as Soil Amendment for Improved Forage and Soil Health in Circular Systems. Sci. Rep. 2025, 15, 3024. [Google Scholar] [CrossRef]
- Hénault-Ethier, L.; Reid, B.; Hotte, N.; Paris, N.; Quinche, M.; Lachance, C.; Fortin, A.; Normandin, É.; Laderriere, V.; Vandenberg, G. Growth Trials on Vegetables, Herbs, and Flowers Using Mealworm Frass, Chicken Manure, and Municipal Compost. ACS Agric. Sci. Technol. 2023, 3, 249–259. [Google Scholar] [CrossRef]
- Dean, L. Market Approval Obtained for an Insect-Based Fertilizer. World Fertilizer Magazine. 22 July 2020. Available online: https://www.worldfertilizer.com/special-reports/22072020/market-approval-obtained-for-an-insect-based-fertilizer/ (accessed on 14 March 2025).
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Załuski, D.; Kosewska, A.; Skwierawska, M.; Sienkiewicz, S. The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment. Int. J. Environ. Res. Public Health 2022, 20, 21. [Google Scholar] [CrossRef]
- Chavez, M.; Uchanski, M. Insect Left-over Substrate as Plant Fertiliser. J. Insects Food Feed 2021, 7, 683–694. [Google Scholar] [CrossRef]
- Yang, S.-S.; Chen, Y.; Kang, J.-H.; Xie, T.-R.; He, L.; Xing, D.-F.; Ren, N.-Q.; Ho, S.-H.; Wu, W.-M. Generation of High-Efficient Biochar for Dye Adsorption Using Frass of Yellow Mealworms (Larvae of Tenebrio molitor Linnaeus) Fed with Wheat Straw for Insect Biomass Production. J. Clean. Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Yang, S.-S.; Chen, Y.; Zhang, Y.; Zhou, H.-M.; Ji, X.-Y.; He, L.; Xing, D.-F.; Ren, N.-Q.; Ho, S.-H.; Wu, W.-M. A Novel Clean Production Approach to Utilize Crop Waste Residues as Co-Diet for Mealworm (Tenebrio molitor) Biomass Production with Biochar as Byproduct for Heavy Metal Removal. Environ. Pollut. 2019, 252, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Xu, M.; Yan, X.; Huang, J.; Wang, H. Removal of Neonicotinoid Pesticides by Adsorption on Modified Tenebrio molitor Frass Biochar: Kinetics and Mechanism. Sep. Purif. Technol. 2022, 297, 121506. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Alkoaik, F.N. The Yellow Mealworm as a Novel Source of Protein. Am. J. Agric. Biol. Sci. 2009, 4, 319–331. [Google Scholar] [CrossRef]
- Robinson, W.H. Handbook of Urban Insects and Arachnids; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780511542718. [Google Scholar]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of Various Commodities for the Development of the Yellow Mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor Larva-Based Ingredients for the Food Industry: A Review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Errico, S.; Dimatteo, S.; Moliterni, S.; Baldacchino, F. Effects of Long-Lasting Cold Storage On Tenebrio molitor Larvae (Coleoptera: Tenebrionidae). J. Insects Food Feed 2021, 7, 1111–1116. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New Methods for High-accuracy Insect Chitin Measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Kay, S.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Morphometric Analysis of Instar Variation in Tenebrio molitor (Coleoptera: Tenebrionidae). Ann. Entomol. Soc. Am. 2015, 108, 146–159. [Google Scholar] [CrossRef]
- Selaledi, L.; Mbajiorgu, C.A.; Mabelebele, M. The Use of Yellow Mealworm (T. molitor) as Alternative Source of Protein in Poultry Diets: A Review. Trop. Anim. Health Prod. 2020, 52, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zunzunegui, I.; Martín-García, J.; Santamaría, Ó.; Poveda, J. Analysis of Yellow Mealworm (Tenebrio molitor) Frass as a Resource for a Sustainable Agriculture in the Current Context of Insect Farming Industry Growth. J. Clean. Prod. 2024, 460, 142608. [Google Scholar] [CrossRef]
- Radwan, M.A.; Maggiolino, A.; Hassanien, H.A.M.; Palo, P.D.; El-Kassas, N.E.M.; Abbas, H.S.; Salem, A.Z.M. Dietary Utilization of Mealworm Frass in Rabbit Feeding Regimes and Its Effect on Growth, Carcass Characteristics, and Meat Quality. Front. Vet. Sci. 2023, 10, 1069447. [Google Scholar] [CrossRef]
- Hassanein, H.A.M.; Abou El-Fadel, M.H.; El-Kassas, N.E.M.; Phillip, Y.L.; Tirado-Estrada, G.; Alderey, A.-A.A.; EL-Deghadi, A.S.; Hussein, A.M.; Zayed, M.A.; Radwan, M.A.; et al. Dietary Inclusion of Mealworm Frass: Effect on Blood Metabolites and Growth Performance of Rabbits. J. Agric. Food Res. 2025, 19, 101637. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm Frass as a Potential Biofertilizer and Abiotic Stress Tolerance-Inductor in Plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.-P.; Dulaurent, A.-M. Potential Use of Mealworm Frass as a Fertilizer: Impact on Crop Growth and Soil Properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect Frass as a Novel Organic Soil Fertilizer for the Cultivation of Spinach (Spinacia oleracea): Effects on Soil Properties, Plant Physiological Parameters, and Nutrient Status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Foscari, A.; Dalla Costa, L.; Tulli, F.; Uboni, C.; Fellet, G. Frass from Tenebrio molitor as Alternative to NPK-Mineral Fertilization: Results from a Germination Test and Pot Experiment on Sunflower. Ital. J. Agron. 2024, 19, 100010. [Google Scholar] [CrossRef]
- Behie, S.; Bidochka, M. Insects as a Nitrogen Source for Plants. Insects 2013, 4, 413–424. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Dulaurent, A.-M. Assessment of the Short-Term Fertilizer Potential of Mealworm Frass Using a Pot Experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Watson, C.; Preißing, T.; Wichern, F. Plant Nitrogen Uptake From Insect Frass Is Affected by the Nitrification Rate as Revealed by Urease and Nitrification Inhibitors. Front. Sustain. Food Syst. 2021, 5, 721840. [Google Scholar] [CrossRef]
- Watson, C.; Schlösser, C.; Vögerl, J.; Wichern, F. Excellent Excrement? Frass Impacts on a Soil’s Microbial Community, Processes and Metal Bioavailability. Appl. Soil Ecol. 2021, 168, 104110. [Google Scholar] [CrossRef]
- Mattioli, S.; Paci, G.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Mancini, S. Former Foodstuff in Mealworm Farming: Effects on Fatty Acids Profile, Lipid Metabolism and Antioxidant Molecules. LWT 2021, 147, 111644. [Google Scholar] [CrossRef]
- Porto de Souza Vandenberghe, L.; Marcela Blandon Garcia, L.; Rodrigues, C.; Cândido Camara, M.; Vinícius de Melo Pereira, G.; de Oliveira, J.; Ricardo Soccol, C. Potential Applications of Plant Probiotic Microorganisms in Agriculture and Forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef]
- Pineda, A.; Zheng, S.-J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping Plants to Deal with Insects: The Role of Beneficial Soil-Borne Microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef]
- van Loon, L.C. Plant Responses to Plant Growth-Promoting Rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Kim, M.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil Bacteria Augment Arabidopsis Photosynthesis by Decreasing Glucose Sensing and Abscisic Acid Levels in Planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef]
- Del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC Deaminase in Plant Growth-Promoting Bacteria (PGPB): An Efficient Mechanism to Counter Salt Stress in Crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.G.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and Characterization of Bacteria from the Gut of Bombyx Mori That Degrade Cellulose, Xylan, Pectin and Starch and Their Impact on Digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef]
- Nurfikari, A.; Leite, M.F.A.; Kuramae, E.E.; de Boer, W. Microbial Community Dynamics during Decomposition of Insect Exuviae and Frass in Soil. Soil Biol. Biochem. 2024, 194, 109426. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The Core Gut Microbiome of Black Soldier Fly (Hermetia illucens) Larvae Raised on Low-Bioburden Diets. Fron.t Microbiol. 2020, 11, 993. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; et al. The Bacterial Biota of Laboratory-Reared Edible Mealworms (Tenebrio molitor L.): From Feed to Frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Praeg, N.; Klammsteiner, T. Primary Study on Frass Fertilizers from Mass-Reared Insects: Species Variation, Heat Treatment Effects, and Implications for Soil Application at Laboratory Scale. J. Environ. Manag. 2024, 356, 120622. [Google Scholar] [CrossRef]
- Hung, R.; Lee Rutgers, S. Applications of Aspergillus in Plant Growth Promotion. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–227. [Google Scholar]
- De Tender, C.; Mesuere, B.; Van der Jeugt, F.; Haegeman, A.; Ruttink, T.; Vandecasteele, B.; Dawyndt, P.; Debode, J.; Kuramae, E.E. Peat Substrate Amended with Chitin Modulates the N-Cycle, Siderophore and Chitinase Responses in the Lettuce Rhizobiome. Sci. Rep. 2019, 9, 9890. [Google Scholar] [CrossRef]
- Mamtimin, T.; Han, H.; Khan, A.; Feng, P.; Zhang, Q.; Ma, X.; Fang, Y.; Liu, P.; Kulshrestha, S.; Shigaki, T.; et al. Gut Microbiome of Mealworms (Tenebrio molitor Larvae) Show Similar Responses to Polystyrene and Corn Straw Diets. Microbiome 2023, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the Role of Humic Acids on Crop Performance and Soil Health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Martins, E.M.; Pillajo, J.Q.; Jones, M.L. Humic and Fulvic Acids Promote Growth and Flowering in Petunias at Low and Optimal Fertility. HortScience 2024, 59, 235–244. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and Characterization of Chitin and Chitosan from Fishery Waste by Chemical Method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Wang, B.-T.; Hu, S.; Yu, X.-Y.; Jin, L.; Zhu, Y.-J.; Jin, F.-J. Studies of Cellulose and Starch Utilization and the Regulatory Mechanisms of Related Enzymes in Fungi. Polymers 2020, 12, 530. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Izydorczyk, G.; Taf, R.; Moustakas, K.; Chojnacka, K. Cellulose-Based Fertilizers for Sustainable Agriculture: Effective Methods for Increasing Crop Yield and Soil Health. Ind. Crops Prod. 2023, 205, 117500. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Moliterni, S.; Errico, S.; Spagnoletta, A.; Dimatteo, S. Advanced Technologies for Chitin Recovery from Crustacean Waste. Clean Technol. Recycl. 2023, 3, 4–43. [Google Scholar] [CrossRef]
- Kisaakye, J.; Beesigamukama, D.; Haukeland, S.; Subramanian, S.; Thiongo, P.K.; Kelemu, S.; Tanga, C.M. Chitin-Enriched Insect Frass Fertilizer as a Biorational Alternative for Root-Knot Nematode (Meloidogyne incognita) Management. Front.Plant Sci. 2024, 15, 1361739. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Oldham (Konak), T.; Rogers, R.D. Applications of Chitin in Agriculture. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2019; pp. 125–146. [Google Scholar]
- Ramírez, M.A.; Rodríguez, A.T.; Alfonso, L.; Peniche, C. Chitin and Its Derivatives as Biopolymers with Potential Agricultural Applications. Biotecnol. Apl. 2010, 27, 270–276. [Google Scholar]
- Parada, R.Y.; Egusa, M.; Aklog, Y.F.; Miura, C.; Ifuku, S.; Kaminaka, H. Optimization of Nanofibrillation Degree of Chitin for Induction of Plant Disease Resistance: Elicitor Activity and Systemic Resistance Induced by Chitin Nanofiber in Cabbage and Strawberry. Int. J. Biol. Macromol. 2018, 118, 2185–2192. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, H.; Rosqvist, E.; Lastusaari, M.; Peltonen, J.; Vähäsalo, L.; Xu, C.; Wang, X.; Pranovich, A. Crystalline Nanoxylan from Hot Water Extracted Wood Xylan at Multi-Length Scale: Molecular Assembly from Nanocluster Hydrocolloids to Submicron Spheroids. Carbohydr. Polym. 2024, 335, 122089. [Google Scholar] [CrossRef]
- Curry, T.M.; Peña, M.J.; Urbanowicz, B.R. An Update on Xylan Structure, Biosynthesis, and Potential Commercial Applications. Cell Surf. 2023, 9, 100101. [Google Scholar] [CrossRef]
- Ye, Z.-H.; Zhong, R. Outstanding Questions on Xylan Biosynthesis. Plant Sci. 2022, 325, 111476. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Wu, A.-M. Balanced Xylan Acetylation Is the Key Regulator of Plant Growth and Development, and Cell Wall Structure and for Industrial Utilization. Int. J. Mol. Sci. 2020, 21, 7875. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Blasi, A.; Lopresto, C.G.; Calabrò, V. Bioconversion of Crop Residues Using Alternative Fermentation-Based Approaches. Front. Biosci. 2023, 15, 17. [Google Scholar] [CrossRef]
- Abbas, A.; Wang, Z.; Zhang, Y.; Peng, P.; She, D. Lignin-Based Controlled Release Fertilizers: A Review. Int. J. Biol. Macromol. 2022, 222, 1801–1817. [Google Scholar] [CrossRef]
- Ahmad, U.M.; Ji, N.; Li, H.; Wu, Q.; Song, C.; Liu, Q.; Ma, D.; Lu, X. Can Lignin Be Transformed into Agrochemicals? Recent Advances in the Agricultural Applications of Lignin. Ind. Crops Prod. 2021, 170, 113646. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V.; Nebbioso, A.; Drosos, M.; Nuzzo, A.; Mazzei, P.; Piccolo, A. Humic-like Bioactivity on Emergence and Early Growth of Maize (Zea mays L.) of Water-Soluble Lignins Isolated from Biomass for Energy. Plant Soil 2016, 402, 221–233. [Google Scholar] [CrossRef]
- Popa, V.I.; Dumitru, M.; Volf, I.; Anghel, N. Lignin and Polyphenols as Allelochemicals. Ind. Crops Prod. 2008, 27, 144–149. [Google Scholar] [CrossRef]
- Hemmilä, V.; Sandberg, J.; Sandberg, D. Lignin: An Adhesive Raw Material of the Future or Waste of Research Energy? In Northern European Network for Wood Science and Engineering (WSE): Proceedings of the 9th Meeting; Brischke, C., Meyer, L., Eds.; WSE: Hannover, Germany, 2013; pp. 98–103. [Google Scholar]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in Controlled Release Pesticide Formulations: Prospects to Safer Integrated Pest Management and Sustainable Agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Kosewska, A.; Załuski, D.; Kozera, W.J.; Żarczyński, P.J. Farmed Insect Frass as a Future Organic Fertilizer. Appl. Sci. 2024, 14, 2380. [Google Scholar] [CrossRef]
- Gan, S.K.-E.; Phua, S.-X.; Yeo, J.Y.; Heng, Z.S.-L.; Xing, Z. Method for Zero-Waste Circular Economy Using Worms for Plastic Agriculture: Augmenting Polystyrene Consumption and Plant Growth. Methods Protoc. 2021, 4, 43. [Google Scholar] [CrossRef]
- Nyanzira, A.; Machona, O.; Matongorere, M.; Chidzwondo, F.; Mangoyi, R. Analysis of Frass Excreted by Tenebrio molitor for Use as Fertilizer. Entomol. Appl. Sci. Lett. 2023, 10, 29–37. [Google Scholar] [CrossRef]
- Karkanis, A.; Asprogeraka, A.C.; Paouris, E.; Ntanasi, T.; Karavidas, I.; Rumbos, C.I.; Athanassiou, C.G.; Ntatsi, G. Yellow Mealworm Frass: A Promising Organic Fertilizer for Common Sowthistle (Sonchus oleraceus L.) and Bristly Oxtongue (Helminthotheca echioides (L.) Holub) Cultivation. Heliyon 2024, 10, e35508. [Google Scholar] [CrossRef]
- Chia, S.Y.; van Loon, J.J.A.; Dicke, M. Effects of Frass from Larvae of Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) on Growth and Insect Resistance in Field Mustard (Brassica rapa): Differences between Insect Species and Frass Treatments. Entomol. Exp. Appl. 2024, 172, 394–408. [Google Scholar] [CrossRef]
- Zim, J.; Aitikkou, A.; EL Omari, M.H.; EL Malahi, S.; Azim, K.; Hirich, A.; Nilahyane, A.; Oumouloud, A. A New Organic Amendment Based on Insect Frass for Zucchini (Cucurbita pepo L.) Cultivation. Environ. Sci. Proc. 2022, 16, 28. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Liu, H. Feasibility of Feeding Yellow Mealworm (Tenebrio molitor L.) in Bioregenerative Life Support Systems as a Source of Animal Protein for Humans. Acta Astronaut. 2013, 92, 103–109. [Google Scholar] [CrossRef]
- Moruzzo, R.; Riccioli, F.; Espinosa Diaz, S.; Secci, C.; Poli, G.; Mancini, S. Mealworm (Tenebrio molitor): Potential and Challenges to Promote Circular Economy. Animals 2021, 11, 2568. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B. Environmental Impact of Insect Rearing. In Insects as Animal Feed: Novel Ingredients for Use in Pet, Aquaculture and Livestock Diets; CABI: Surrey, UK, 2021; pp. 53–59. [Google Scholar]
- Sangiorgio, P.; Verardi, A.; Dimatteo, S.; Spagnoletta, A.; Moliterni, S.; Errico, S. Valorisation of Agri-Food Waste and Mealworms Rearing Residues for Improving the Sustainability of Tenebrio molitor Industrial Production. J. Insects Food Feed 2022, 8, 509–524. [Google Scholar] [CrossRef]
- Pandao, M.R.; Rathod, S.R.; Sirsat, D.D.; Lingayat, N.R. The Role of Soil in Carbon Sequestration: Mechanisms and Implications. Asian J. Environ. Ecol. 2024, 23, 66–75. [Google Scholar] [CrossRef]
- van Groenigen, K.J.; van Kessel, C.; Hungate, B.A. Increased Greenhouse-Gas Intensity of Rice Production under Future Atmospheric Conditions. Nat. Clim. Chang. 2013, 3, 288–291. [Google Scholar] [CrossRef]
- Rumpel, C.; Henry, B.; Chenu, C.; Amiraslani, F. Benefits and Trade-Offs of Soil Organic Carbon Sequestration; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; pp. 183–208. [Google Scholar]
- Gitari, M. PanAfrican Agriculture (Magazine), July–September 2023. pp. 31–32. Available online: https://panagrimedia.com/13th-edition/ (accessed on 23 February 2025).
- Foughar, M.; Arrobas, M.; Rodrigues, M.Â. Mealworm Larvae Frass Exhibits a Plant Biostimulant Effect on Lettuce, Boosting Productivity beyond Just Nutrient Release or Improved Soil Properties. Horticulturae 2024, 10, 711. [Google Scholar] [CrossRef]
- van Huis, A. Edible Insects: Challenges and Prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T. Biochar: A Sustainable Solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A.; Chakraborty, S.; Vithanage, M.; Biswas, J.K. Multifaceted Applications of Biochar in Environmental Management: A Bibliometric Profile. Biochar 2023, 5, 11. [Google Scholar] [CrossRef]
- Thalassinos, G.; Levizou, E.; Antoniadis, V. Can Soil Improvers (Biochar, Compost, Insect Frass, Lime, and Zeolite) Achieve Phytostabilization of Potentially Toxic Elements in Heavily Contaminated Soil with the Use of Purslane (Portulaca oleracea)? Agronomy 2023, 13, 2827. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Xiang, H.; Liu, R.; Su, L.; Zhang, L.; Ji, R. Functional Utilization of Biochar Derived from Tenebrio molitor Feces for CO2 Capture and Supercapacitor Applications. RSC Adv. 2022, 12, 22760–22769. [Google Scholar] [CrossRef]
- He, L.; Yang, S.-S.; Bai, S.-W.; Pang, J.-W.; Liu, G.-S.; Cao, G.-L.; Zhao, L.; Feng, X.-C.; Ren, N.-Q. Fabrication and Environmental Assessment of Photo-Assisted Fenton-like Fe/FBC Catalyst Utilizing Mealworm Frass Waste. J. Clean. Prod. 2020, 256, 120259. [Google Scholar] [CrossRef]
- Cara, I.G.; Țopa, D.; Puiu, I.; Jităreanu, G. Biochar a Promising Strategy for Pesticide-Contaminated Soils. Agriculture 2022, 12, 1579. [Google Scholar] [CrossRef]
- Larouche, J.; Campbell, B.; Hénault-Éthier, L.; Banks, I.J.; Tomberlin, J.K.; Preyer, C.; Deschamps, M.-H.; Vandenberg, G.W. The Edible Insect Sector in Canada and the United States. Anim. Front. 2023, 13, 16–25. [Google Scholar] [CrossRef]
- Mazzarelli, S. The Food Safety Law in China: Regulatory Framework and Implications for International Trade. Master’s Thesis, Università Ca’ Foscari, Venezia, Italy, 2015. [Google Scholar]
- International Platform of Insects for Food and Feed (IPIFF). Fact Sheet on Insect Frass; IPIFF: Brussels, Belgium, 2021. [Google Scholar]
- European Commission Regulation (EU) 2021/1925 of 5 November 2021 Amending Regulation (EU) No 142/2011 as Regards the Use of Certain Animal By-Products and Derived Products as Organic Fertilizers and Soil Improvers. Available online: https://eur-lex.europa.eu/eli/reg/2021/1925/oj (accessed on 14 March 2025).
- International Platform of Insects for Food and Feed (IPIFF). Contribution Paper on Application of Insect Frass as Fertilising Product in Agriculture; IPIFF: Brussels, Belgium, 2019. [Google Scholar]
| Elements | Concentration (Units) | Ref. | |
|---|---|---|---|
| Macronutrients | Nitrogen (N) | 3–5% | [44,45,46] |
| Phosphorus (P) | 1.5–2.6% | [24,44,46] | |
| Potassium (K) | 1.1–2.0% | [10,24,44,46] | |
| Protein | 24.3 ± 1.37 g/100 g (dry weight, w/w) | [15] | |
| Lipids | 2.22 ± 0.10 g/100 g (dry weight, w/w) | [15] | |
| Total organic carbon (TOC) | 38.9–49.6% | [10,24,44,46] | |
| Magnesium (Mg) | 0.5–0.7% | [10,24,44,47] | |
| Calcium (Ca) | 0.1–0.3% | [10,24,44,47] | |
| Sulphur (S) | 0.30–0.39% | [19,24] | |
| Micronutrients | Sodium (Na) | 161.8–500.0 mg/kg | [10,19,24] |
| Iron (Fe) | 70.7–140.7 mg/kg 380.0–490.1 mg/kg | [10,19,24,44] | |
| Manganese (Mn) | 146–230 mg/kg | [10,19,47] | |
| Zinc (Zn) | 86–150 mg/kg | [10,19,47] | |
| Copper (Cu) | 11–18 mg/kg | [10,19,24,47] | |
| Boron (B) | 5.35–11.00 mg/kg | [10,19] | |
| Molybdenum (Mo) | 0.56–0.96 mg/kg | [44] | |
| Aluminum (Al) | 0.030 ± 0.03% | [10] |
| Frass Batch 1 | Frass Batch 2 | |
|---|---|---|
| Enterobacteriaceae | 6.8 ± 0.2 a | 7.0 ± 0.1 a |
| Lactic acid bacteria | 8.2 ± 0.1 a | 7.9 ± 0.2 a |
| Total mesophilic aerobes | 8.7 ± 0.1 a | 8.1 ± 0.1 a |
| Spore-forming bacteria | 3.7 ± 0.2 b | 5.4 ± 0.3 a |
| Salmonella spp. | Absence in 25 g | Absence in 25 g |
| Listeria monocytogenes | Absence in 25 g | Absence in 25 g |
| Plant/Substrate Tested | Frass Concentration and Application Method | Key Variables | Results | Ref. |
|---|---|---|---|---|
| Dragon Fruit Cacti | 100%. Cacti grafted on the TMF. | In vitro. Cacti exposed to the substrates for 15 days. | Frass from TM larvae fed solely on polystyrene decreased plant growth by 0.53 cm but enhanced rooting in the cacti compared to the control group (cacti grown on tea leaves). | [90] |
| Arabidopsis, sunflower, and tomato | 2%. Soil incorporation. | In pots. Greenhouse under artificial lights. | Frass added to Arabidopsis does not induce root defence responses. TMF addition to sunflower under nutrient deficiencies compensated for the lack of all nutrients except N. Unsterilized TMF increased the shoot weight of tomato plants. | [19] |
| Lettuce | 1%. Soil incorporation. | In pots. Greenhouse. Soil type: peat. Initial pH of peat + TMF: 5.2. | Frass treatment promoted plant growth and increased lettuce chemical composition (chlorophylls, carotenoids, leaf soluble proteins, and leaf nitrate). | [20] |
| Barley | 10 t/ha. Soil incorporation. | In pots. Controlled greenhouse conditions. Soil type: soil from cultivated land. | +32% biomass compared to the control, increased NPK uptake, enhanced microbial diversity. | [45] |
| Bean and chard plants | 2%. Soil incorporation. | In pots. Greenhouse. Soil type: vermiculite for bean study and fluvial soil mixed with perlite (3/1) for chard plants. | Chlorophyll content (+20%), stem length (+15%), stem width (+10%), and aerial biomass (+25%). There was a 40% reduction in salinity impact on growth. Synthesis of auxins and siderophores. | [44] |
| Spinach | <1%. Soil incorporation. | In pots. At ambient environmental conditions. Soil type: a mixture of loamy soil and perlite. pH of the soil + 1%TMF = 7.44. | 48% increase in both the above-ground tissues and roots compared to the inorganic NPK treatment. | [46] |
| Brussels sprouts | 5 g/kg Soil incorporation. | In pots. Greenhouse conditions. Soil type: agricultural soil. | Frass treatment stimulated plant growth: after 14 days, the leaf surface area of the plants increased to three times that observed in untreated soil. | [21] |
| Italian ryegrass | 1.5%. Soil incorporation. | In pots. Controlled greenhouse conditions. Soil type: 1:1 mixture of quartz sand and agricultural soil. pH of the soil + 3%TMF = 6.6 | TMF enhanced microbial growth and N mineralization. | [50] |
| Wheat | 2%. Top-dressing after every 2 weeks. | In pots. Laboratory conditions. Initial and final pH of soil + 2% TMF = 5.9 and 6.0. | Frass treatment stimulated plant growth especially from the first to the fourth week. | [91] |
| Common sowthistle and bristly oxtongue | 2%. Soil incorporation. | In pots. Laboratory conditions. Soil type: slightly alkaline soil (pH = 7.4). | The number of leaves increased by 25.4 and 29.1% in common sowthistle and bristly oxtongue, respectively. Frass treatment led to the highest P content in above-ground plant tissue and increased root growth. | [92] |
| Field mustard | 2 g/kg. Soil incorporation. | In pots. Greenhouse conditions. Soil type: agricultural soil. | After 14 days of plant growth, TMF after composting increased leaf area compared to the control group. Raw TMF did not reduce the larval survival of two pests of the field mustard (D. radicum and P. xylostella). | [93] |
| Zucchini | 5% Soil incorporation. | In pots. Greenhouse conditions. Soil type: sand. | TMF led to the highest plant height, leaf area, and fresh and dry weight of the vegetative part and enhanced the yield of zucchini plants by 62% compared to control. | [94] |
| Cress, lettuce, tomato, and sunflower | 6.25%. Soil incorporation. | In pots. In growth chamber. Soil type: agricultural soil (pH 6.8). | Frass treatment on sunflowers increased sunflower growth similar to the NPK treatment. | [47] |
| Several vegetables and one herb (arugula, kale, beetroot, carrot, radish, cucumber, sweet corn, cherry tomato, and basil) | 0.5%. Soil incorporation. | In pots. At ambient environmental conditions. Soil type: potting soil with mycorrhizae with 5% municipal compost (pH 6.7). | TMF increased the edible biomass (on average, 16.5 higher than the control treatment). | [26] |
| Flowers (nasturtium, zinnia, and dwarf sunflower) | 0.5%. Soil incorporation. | In pots. At ambient environmental conditions. Soil type: potting soil with mycorrhizae + 5% municipal compost (pH 6.7). | Frass treatment led to a 32-fold increase in flowering of nasturtium and doubled flowering of dwarf sunflower and zinnia. | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verardi, A.; Sangiorgio, P.; Della Mura, B.; Moliterni, S.; Spagnoletta, A.; Dimatteo, S.; Bassi, D.; Cortimiglia, C.; Rebuzzi, R.; Palazzo, S.; et al. Tenebrio molitor Frass: A Cutting-Edge Biofertilizer for Sustainable Agriculture and Advanced Adsorbent Precursor for Environmental Remediation. Agronomy 2025, 15, 758. https://doi.org/10.3390/agronomy15030758
Verardi A, Sangiorgio P, Della Mura B, Moliterni S, Spagnoletta A, Dimatteo S, Bassi D, Cortimiglia C, Rebuzzi R, Palazzo S, et al. Tenebrio molitor Frass: A Cutting-Edge Biofertilizer for Sustainable Agriculture and Advanced Adsorbent Precursor for Environmental Remediation. Agronomy. 2025; 15(3):758. https://doi.org/10.3390/agronomy15030758
Chicago/Turabian StyleVerardi, Alessandra, Paola Sangiorgio, Brigida Della Mura, Stefania Moliterni, Anna Spagnoletta, Salvatore Dimatteo, Daniela Bassi, Claudia Cortimiglia, Raffaella Rebuzzi, Salvatore Palazzo, and et al. 2025. "Tenebrio molitor Frass: A Cutting-Edge Biofertilizer for Sustainable Agriculture and Advanced Adsorbent Precursor for Environmental Remediation" Agronomy 15, no. 3: 758. https://doi.org/10.3390/agronomy15030758
APA StyleVerardi, A., Sangiorgio, P., Della Mura, B., Moliterni, S., Spagnoletta, A., Dimatteo, S., Bassi, D., Cortimiglia, C., Rebuzzi, R., Palazzo, S., & Errico, S. (2025). Tenebrio molitor Frass: A Cutting-Edge Biofertilizer for Sustainable Agriculture and Advanced Adsorbent Precursor for Environmental Remediation. Agronomy, 15(3), 758. https://doi.org/10.3390/agronomy15030758










