Abstract
The projected growth of the global population to over 10 billion by 2080 necessitates groundbreaking sustainable agricultural solutions that enhance productivity while mitigating environmental impacts. Tenebrio molitor frass (TMF), derived from larval excrement and exuviae, has emerged as a promising organic fertilizer. Enriched with macro- and micronutrients, TMF enhances soil functions through microbial communities that promote nutrient cycling, decompose organic matter, and suppress soilborne pathogens. Additionally, functional compounds like chitin, cellulose, xylans, and lignin improve the soil structure, foster beneficial microbes, and activate natural plant defence responses. The synergy of microbial activity and bioactive compounds positions TMF as a valuable resource for enhancing plant growth and soil health. Its role as a nutrient source, biostimulant, and soil amendment aligns with circular economy principles by recycling agro-industrial by-products and reducing reliance on synthetic fertilizers. TMF also contributes to sustainable agriculture by improving soil fertility, microbial biodiversity, and plant stress resilience, while mitigating greenhouse gas emissions and nutrient runoff. Additionally, TMF-derived biochar offers the potential for environmental remediation as an effective adsorbent. Despite its advantages, TMF faces challenges in scalability, cost, and regulations, requiring advancements in processing, enrichment, and supportive policies to maximize its potential in sustainable farming.
1. Introduction
As of March 2025, the global population has reached 8.2 billion people [1], marking a significant increase from 5 billion in 1986 and 7.7 billion in 2019 [2]. According to the latest United Nations World Population Prospects (2024), global population growth is projected to peak at 10.3 billion in the mid-2080s before gradually declining to 10.2 billion by 2100, driven largely by declining fertility in recent years in large countries such as China [2].
According to the Food and Agriculture Organization (FAO), achieving “zero hunger” by 2030 requires a 50% increase in food production compared to 2012 levels [3], while meeting the food demands of the growing global population by 2050 will necessitate a 70% increase in agricultural productivity [4]. These ambitious goals call for profound transformations in agricultural systems, prioritizing sustainable practices and addressing climate change challenges [5].
Currently, global agriculture mainly depends on using pesticides and chemical fertilizers [6,7], but their widespread use poses significant environmental and health risks [8]. Chemical pesticides have been linked to immunotoxicity, respiratory disorders, reproductive system alterations, hormonal imbalances, and increased carcinogenicity risks [9]. The production of chemical fertilizers, also known as mineral fertilizers, is highly energy-intensive and accounts for 2% of global greenhouse gas emissions [10]. Additionally, while these fertilizers are pivotal in modern agriculture for their ability to provide essential macronutrients—nitrogen (N), phosphorus (P), and potassium (K)—their excessive or improper use has been linked to several adverse environmental impacts. These include soil acidification, disruption of ionic balance, inhibition of beneficial microbial activity, accumulation of harmful substances, salinization, groundwater contamination caused by nutrient leaching, and soil degradation [11]. Despite these drawbacks, the global fertilizer market is projected to grow significantly, from USD 190 billion in 2021 to USD 240 billion by 2030, driven by increasing demand from a growing population [12]. This situation highlights the urgent need to develop sustainable agricultural models that minimize dependence on non-renewable resources and adopt strategies aligned with the principles of the circular economy. The urgency is underscored by food shortages reported in 2018, affecting nations such as the United States (2.3%), Canada (4.6%), the United Kingdom (8.2%), Germany (2.6%), Japan (2.9%), Ethiopia (23.4%), Ivory Coast (22.4%), Bangladesh (12.7%), Pakistan (17.2%), Haiti (45.6%), and India (14.3%) [13].
To tackle these challenges, organic or natural fertilizers have emerged as a viable alternative to chemical fertilization, with their use increasingly recognized as a key strategy for achieving sustainable agriculture [14]. Derived from natural resources such as manure, compost, or organic waste, biofertilizers recycle nutrients while enhancing soil health. Among these, insect frass—a by-product of insect farming—has attracted considerable attention for its potential agricultural applications, especially considering the growing large-scale insect production in Europe and the global insect market. The latter was valued at around USD 1.2 billion in 2023 [15].
Tenebrio molitor (TM) has been identified as one of the most promising insect species for large-scale industrial farming [16]. Frass is one of the main by-products of TM rearing, accumulating during the growth process of insects. TM larvae, reared under standard conditions (temperature 27 ± 1 °C; humidity 65 ± 5%; photoperiod dark: light = 24 h:0 h, fed on bran), produce, on average, 2 to 3 times their weight of frass, equating to approximately 200–300 g of TMF for every 100 g of TM larval biomass [17,18,19].
Composed of a mixture of insect excrement, shed exoskeletons, and undigested feed, TMF is dry, friable, and odorless, with a nutrient profile rich in readily mineralizable macronutrients (NPK) and micronutrients [10,20]. Its rapid mineralization rate further enhances its suitability for agricultural use. Beyond its nutritional properties, TMF hosts plant growth-promoting microorganisms (PGPMs) that improve soil fertility and plant development [21,22,23]. This distinguishes TMF from conventional mineral fertilizers, which lack the benefits of microbes for soil health. Additionally, TMF contains functional compounds such as humic and fulvic acids, cellulose, chitin, xylan, and lignin, which significantly contribute to agronomic performance [15,20].
The use of frass can produce results for crop production that are comparable to, or even better than, those of other organic fertilizers. For example, TMF compared to poultry litter showed 93% more total carbon, 60% more total nitrogen, and an intermediate carbon/nitrogen ratio, indicating its potential as an effective soil amendment [24]. Additionally, TMF application at a rate of 6800 kg ha−1 increased soil nitrogen, potassium, and magnesium by 12%, 30%, and 35%, respectively, compared to poultry litter application at 3400 kg ha−1, resulting in similar yield and quality of Bermudagrass [Cynodon dactylon (L.) Pers.] [25].
Beyond poultry litter, TMF has also been compared with hen manure and compost. A study by Hénault-Ethier et al. [26] evaluated TMF, hen manure, and compost across nine vegetable crops, one herb, and three flower species. The results showed that TMF performed similarly to hen manure, while plants fertilized with TMF exhibited a 16-fold increase in edible biomass and produced larger and more abundant flowers compared to those grown with compost [26].
TMF is a competitive alternative to mineral NPK fertilizers and does not exhibit the negative environmental effects commonly associated with synthetic fertilizers. Moreover, it shares characteristics with biofertilizers, improving soil health, enhancing microbial activity, and increasing carbon content. Unlike many other organic fertilizers, TMF is virtually odorless, dry, and easy to store and transport, making it a practical and sustainable soil amendment [27].
The use of organic waste in TM’s diet also aligns frass production with circular economy principles, reducing the dependency on external inputs and minimizing waste. While frass from other insects, such as black soldier flies, or BSF (Hermetia illucens), has widely been studied, TMF offers distinct advantages due to its composition and production scalability [28,29].
Moreover, TMF can serve as a precursor for creating biochar-based adsorbents used in environmental remediation [30]. Biochars derived from TMF pyrolysis exhibit unique properties, making them particularly effective at removing organic and inorganic contaminants from soil and water, often outperforming biochars derived from other organic materials [31]. This approach offers a viable and sustainable alternative to simply composting TMF, given the large quantities of frass generated by TM rearings, the low cost of the material, and the cost-effectiveness of the biochar production process [32].
Figure 1 provides an overview of the key components and applications of TMF, highlighting its role in soil fertility, plant growth, environmental remediation, and its alignment with the EU regulatory framework.
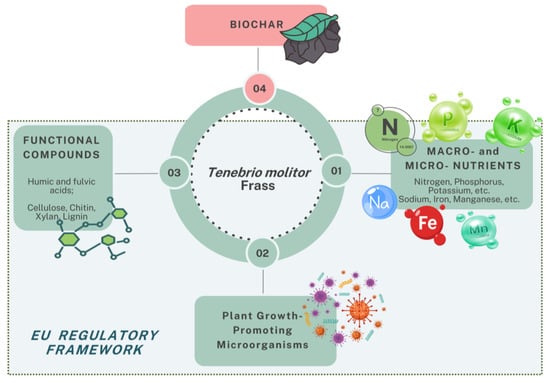
Figure 1.
Overview of TMF applications and functions across different research areas.
Based on this premise, this review underscores the growing importance of TMF as an effective organic fertilizer. It emphasizes TMF’s ability to enhance soil health, increase plant productivity, and promote environmental sustainability due to its rich nutrient composition, functional compounds, and microbial communities. Additionally, it highlights the potential of TMF-derived biochar as an efficient adsorbent for environmental remediation. By critically evaluating the benefits and limitations of TMF, and addressing legislative considerations, this study aims to clarify the potential for integrating TMF into sustainable agricultural practices and environmental recovery.
2. Taxonomy and Life Cycle of Tenebrio molitor
The insect TM, commonly known as the yellow mealworm, belongs to the order Coleoptera and the family Tenebrionidae, also referred to as “darkling beetles”. Like many members of this family, TM is a nocturnal insect that thrives in dark and damp environments, including feed sacks, grain silos, and food storage facilities [33]. It feeds on broken or damaged cereal grains and milling products [34], making it one of the largest beetles commonly found in stored agricultural products such as grains, flour, and bran [35]. This insect is holometabolic, meaning its development involves a complete metamorphosis consisting of four distinct stages: eggs, larvae, pupae, and adults.
The duration of the entire life cycle varies significantly, ranging from 60 days to 1–2 years, depending on factors such as temperature, humidity, diet, and population density [36], although the average period indicated is usually 30–120 days [37].
The eggs (length: 1.7–1.8 mm, width 0.6–0.7 mm), have a glossy appearance and a bean-like shape. Coated with a sticky secretory fluid, they can be laid either singly or in clusters. The time required for the egg’s hatching into larvae is highly temperature-dependent, ranging from 4 days at 26–30 °C to 34 days at 15 °C, with the optimal hatching period being approximately 2 weeks at 25 °C [36]. The fully developed larvae have an elongated cylindrical shape, measuring approximately 25 mm in length and weighing about 0.2 g. They possess a well-sclerotized structure, six legs positioned behind the head, and two short appendages at the tip of the abdomen [33,38]. As the larvae mature, their color turns from pale whitish to yellowish-brown [36]. TM larvae moult many times during the larval phase, from 9 to 20 times (on average, at least 12 times), before becoming pupae, losing the exuviae each time [39]. Following the larval stage, the pupal phase begins. The duration of the larval stage can vary significantly, ranging from 3–6 months to up to 2 years under unfavorable environmental conditions that hinder the transition to pupae, particularly during winter [33]. The larvae assume a characteristic “C” shape during their transition to the pupal stage [40], which can range from 5 to 48 days, depending on the temperature [34]. The pupal stage can vary from 5 to 48 days, depending on the temperature [34]. The pupae are yellowish-light brown, about 1 cm long, and lack a mouth and anus, a condition that lasts until they become adults [41]. In the adult stage, a brown beetle measuring 1.0 to 1.8 cm in length emerges from the pupa, gradually darkening as it matures [36]. Its lifetime in this stage ranges from 37 to 96 days [33] or from 16 to 173 days [40]. The TM life cycle is illustrated in Figure 2.
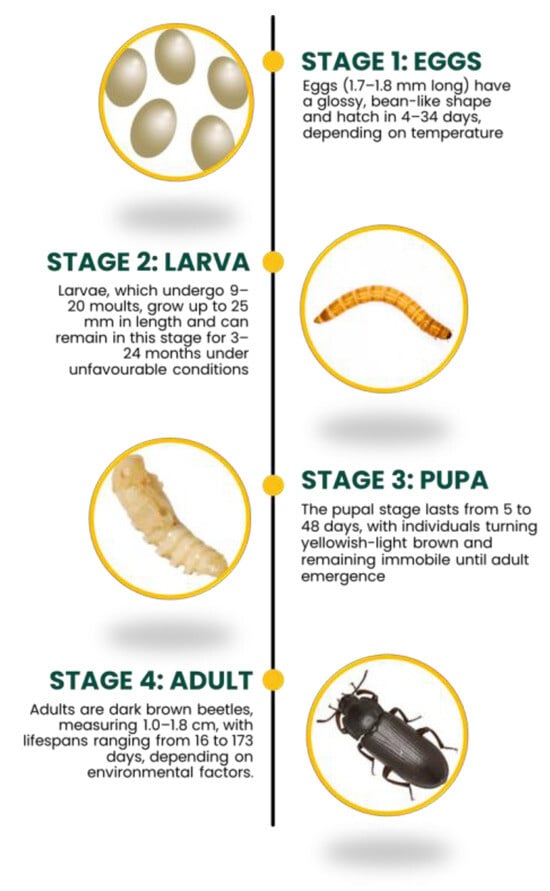
Figure 2.
TM life cycle illustrating the four main development stages.
3. Composition and Characteristics of Tenebrio molitor Frass
3.1. Macronutrient and Micronutrient Profile
TMF is generally characterized by a rich abundance of micro- and macronutrients, making it a valuable resource for food and feed applications [23]. For example, TMF has been explored as a dietary supplement, supporting the growth of mammals such as rabbits. These studies highlight the adaptability of TMF as an alternative protein and nutrient source for these animals, leading to similar growth performance results compared to the control group [42,43].
In terms of plants, insect frass provides nutrients that are easily absorbed by roots (Table 1).

Table 1.
Nutritional composition of Tenebrio molitor frass.
N, K, and P, often present in high concentrations in plant tissues, are selectively excreted by insects to maintain internal equilibrium. As a result, insect frass contains high levels of these elements. Applying TMF to the soil can effectively supply the required amounts of N, P, and K, providing vital support for plant growth [41]. This characteristic is particularly valuable given that N, a critical plant resource, is frequently scarce due to inefficient agricultural practices, soil erosion, and biological processes like denitrification and microbial competition. By serving as an N reservoir and contributing to the soil N cycle, insects play a vital role at the ecosystem level [48]. P and K in TMF are present in bioavailable forms, allowing for immediate uptake by plants upon application in the soil. The N content from TM frass has been observed to undergo gradual mobilization into the soil (e.g., frass NPK: 5-2-1.7) [49]. The mobilization speed of N should be evaluated with the crop’s capacity to assimilate the released minerals. For instance, a study on ryegrass found that the N release from TMF was too rapid for the plant to fully absorb it (frass NPK: 4-1.5-3). To address this, Watson et al. [50] proposed the addition of nitrification inhibitors alongside frass to enhance N uptake and minimize N losses.
In another study using horticultural peat as a substrate, the application of TMF at a rate of 5–10 g/dm³ significantly increased the N content of the growing medium compared to other nutrients such as P, K, Mg, and Na. However, a decrease in the Ca content was observed two weeks after application [28]. A longer-term study (4 weeks) also reported an increase in the soil mineral N content released from TMF (frass NPK: 2.3-2.6-10), highlighting the time-dependent nature of the nutrient release [51]. Additionally, composting has been shown to further enhance N release from frass. A 32-day composting experiment demonstrated a significant increase in the N availability from TMF [17]. Thus, the release of nutrients, particularly N, from TMF is strongly influenced by the duration of its presence in the soil. However, the dynamics of N transition from TMF to soil and plants are complex and warrant further investigation. Future studies should consider different soil types and crop species to better understand and optimize this process.
The nutritional composition of TMF primarily depends on the insect’s diet [41]. In addition, Poveda et al. [44] demonstrated that the effectiveness of TMF as a fertilizer is directly dependent on the insect’s diet, underlining the importance of feed quality in determining the agronomic value of TMF.
Similarly, Mattioli et al. [52] explored the influence of dietary substrates on the physicochemical properties of TMF. The study demonstrated that the fatty acid profile of the frass closely mirrored the dietary intake of the larvae, emphasizing the strong link between the larval diet and the composition of TMF. Substrates rich in saturated fatty acids and monounsaturated fatty acids (MUFAs) led to the persistence of these fatty acids in the frass, as larvae showed limited conversion capabilities. In contrast, diets higher in polyunsaturated fatty acids (PUFAs) resulted in a proportional increase in PUFAs in the frass. Notably, the MUFA content in larvae was positively correlated with MUFA deposition in the frass and inversely correlated with the PUFA levels in feces. This highlights the importance of dietary composition in tailoring the nutritional profile of frass for specific applications [52].
3.2. Microbial Communities
In addition to supplying essential nutrients, insect frass enhances plant resilience to both abiotic stresses (such as salinity, drought, and flooding) and biotic stresses caused by pathogens and pests (including viruses, bacteria, fungi, nematodes, insects, arachnids, and weeds) that can disrupt plant metabolism [19]. This enhanced resilience is due to the beneficial microorganisms present in insect frass, including TMF, which act as bioprotectants, biocontrollers, biofertilizers, and biostimulants [19,23,45,51,53]. PGPMs, for instance, effectively bolster plant defences against pests and diseases, supporting sustainable agricultural practices [21,22,44]. Many PGPMs improve nutrient uptake and availability by solubilizing otherwise inaccessible nutrients (e.g., rock phosphate), fixing atmospheric nitrogen [44,45,49], and promoting zinc absorption [44].
Some of them also synthesize growth-promoting hormones, including indole-3-acetic acid, cytokinins, auxins, and gibberellins [22,44,54,55], as well as jasmonic acid, which induces salinity stress tolerance [44], and salicylic acid, which plays a key role in plant innate immunity [44]. Additionally, these microorganisms: (i) modulate endogenous sugar and abscisic acid signalling to enhance photosynthesis [54,56]; (ii) produce essential enzymes, such as glucanases, chitinases, and 1-aminocyclopropane-1-carboxylic acid deaminase, which support plant growth, and enhance tolerance to various stress conditions [44,57]; and (iii) release siderophores, small molecules with a high capacity to bind iron, aiding in plant nutrition [23].
Insect frass hosts a diverse range of microbial communities that play a significant role in key processes, such as cellulose degradation, breaking down plant-derived cellulose into simple sugars [58], and supplying essential nutrients like vitamins and amino acids.
Although the characterization of microbial communities in TMF is a significant area of research, several studies have mainly focused on the gut microbial composition of TM and the impact of TMF on soil microbial diversity to assess its potential beneficial effects as a biofertilizer. For example, applying frass to the soil increases microbial diversity and activity, stimulating processes like organic matter decomposition and nutrient mineralization. Moreover, frass can suppress soil-borne pathogens by enhancing beneficial microbial populations with antagonistic properties [59].
Soil treatment with TMF was observed to increase the metabolic activity and diversity of the soil microbial population, with the enrichment of chitinolytic microbes belonging to Gammaproteobacteria, Bacilli, Actinobacteria, and Mortierellomycetes [45,59]. Furthermore, Nurfikari et al. [59] observed an enrichment of Gammaproteobacteria in TMF-amended soils, particularly of Pseudomonas, Massilia, and Lysobacter, along with a strong stimulation of Bacillus and Pseudarthrobacter. Regarding the fungal population, significant changes were observed in the abundance of Mortierellomycetes (Mortierella), Sordariomycetes (Humicola), and Tremellomycetes (Saitozyma).
While specific data on the microbial communities in mealworm frass are limited, it is established that its microbial composition varies depending on environmental factors, such as habitat, diet, developmental stage, and host phylogeny. The microbial diversity of frass originates primarily from the insect gut, which typically contains a few dozen species [45,60]. Since frass consists of a combination of excrement and moulted skins (exuviae), its microbiota can also be enriched by microbes colonizing the insects’ surface, interior, and surrounding environment [61].
Recently, Praeg and Klammsteiner [62] highlighted that the physiochemical and microbial composition of frass from BSF, TM, and Jamaican field cricket (Gryllus assimilis) depends on the insect species. Differences in frass microbiomes persisted even in heat-treated frass samples, which effectively reduced the viable microbial population without significantly affecting the fertilizer properties of the frass.
Despite its potential importance, the microbial composition of TMF remains relatively underexplored, with only a limited number of studies addressing its characterization. Osimani et al. [61] investigated the TMF microbiota using microbiological and molecular approaches. Their microbiological analysis primarily focused on Enterobacteriaceae, lactic acid bacteria (LAB), total mesophilic aerobes, and spore-forming bacteria, with viable counts reported in Table 2. They did not detect Salmonella or Listeria monocytogenes. The 16S rRNA amplicon sequencing revealed three predominant bacterial groups, belonging to the genera Enterococcus and Lactococcus, and the family Enterobacteriaceae. Further analyses identified specific Lactococcus species (L. garviae and L. lactis) and genera within the Enterobacteriaceae family, such as Enterobacter, Escherichia, Klebsiella, Trabulsiella, and Erwinia. Additionally, Clostridiales was detected within the bacterial population.

Table 2.
Microbial counts in TMF from two different batches [61].
Poveda et al. [44] conducted more extensive metagenomic analyses of TMF, identifying several key bacterial taxa, predominantly belonging to the phylum Firmicutes, followed by Proteobacteria. Smaller proportions were attributed to the phyla Tenericutes, Bacteroidetes, and Actinobacteria (Figure 3).
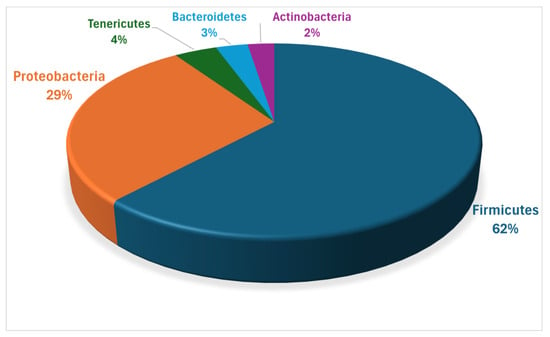
Figure 3.
Relative abundance of bacterial phyla in TMF.
Within the Firmicutes phylum, the dominant classes were Bacilli and Clostridia. At the family level, Streptococcaceae, Clostridiaceae, and Bacillaceae were the most prevalent, with Lactococcus, Clostridium, and Bacillus being the most detected genera. Notably, Bacillus species were identified at a high abundance and are recognized for promoting root development, enhancing nutrient assimilation, and suppressing plant pathogens [44]. Among these, the Gram-positive species Bacillus thuringiensis and Bacillus cereus were detected. These species are well known for their antagonistic activity against a variety of plant pathogens and are already widely used commercially for biological pest control [41]. Within the Proteobacteria phylum, the main classes observed were Gammaproteobacteria and Betaproteobacteria (Figure 4) [44].
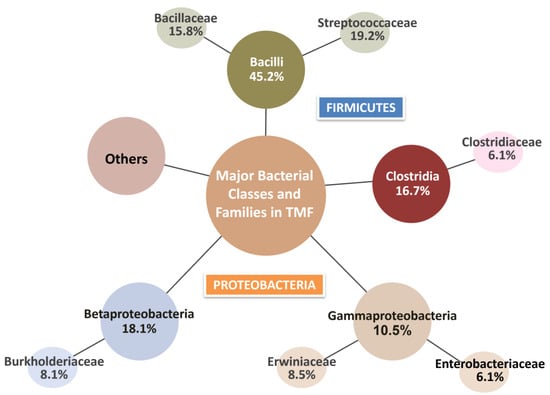
Figure 4.
Representation of major bacterial classes and families identified in TMF. The diagram highlights Firmicutes (Bacilli and Clostridia) and Proteobacteria (Betaproteobacteria and Gammaproteobacteria) as the dominant bacterial groups, with their respective families. The relative abundance of each class and family is indicated as a percentage.
Among the numerous beneficial effects exerted by the microbial community on plant growth and development, Barragán-Fonseca et al. [22] highlight the ability of many bacteria, particularly those belonging to the phyla Bacteroidetes and Actinobacteria, and the class Gammaproteobacteria, to reduce the incidence of diseases caused by root pathogenic fungi such as Verticillium dahliae, Fusarium oxysporum, and Rhizoctonia solani.
Poveda et al. [44] also investigated fungal communities, which were predominantly classified within the phylum Ascomycota (98.4%), while Basidiomycota represented approximately 1.6% of the total analyzed sequences. At the family level, the fungal community was largely dominated by Aspergillaceae (97.1%), with Aspergillus emerging as the most abundant genus [44]. Aspergillus species exert synergistic effects on various aspects of plant growth and development. They stimulate systemic resistance and significantly alleviate the stress experienced by plants, thereby enhancing their overall health and resilience [63].
Recent research has demonstrated that TMF can positively influence soil microbial communities, particularly by increasing the bacterial abundance in amended soil, in comparison with untreated soils, especially in terms of the enrichment of chitin-degrading bacteria like Gammaproteobacteria, Bacilli, and Actinobacteria [59]. The breakdown of chitin into short-chain oligomers is a crucial step in the activation of plant genes linked to growth and development [64].
The microbial composition of TMF is influenced by dietary inputs and environmental conditions. Studies have shown that altering the larval diet significantly affects the microbial community in frass, with high-fibre diets promoting cellulolytic bacteria and nitrogen-rich diets enhancing the abundance of N-fixing bacteria [41,44,65]. Moreover, this diet-driven microbial variability may also impact microbial stability after processing, as certain bacterial taxa, such as Firmicutes, appear more resilient to heat treatment than Proteobacteria-dominated communities like those found in BSF frass [62]. These findings suggest that dietary manipulation during insect rearing could be a key strategy to optimize frass microbiota for specific agricultural applications.
It is important to underline that most of the microbes occurring in the frass could disappear after a heat treatment of at least 70 °C for 1 h, which is currently mandatory for the placing of insect frass on the European markets (see section on regulatory framework). While this process significantly reduces the microbial viability, studies suggest that TMF retains a substantial portion of its microbial community post-treatment, unlike BSF frass, which experiences a more pronounced microbial decline [62]. The predominance of Firmicutes in TMF after heat treatment suggests that its functional properties, such as organic matter decomposition and biocontrol potential, may be better preserved compared to those of BSF frass [23,44,60,61]. This could make TMF a more effective biofertilizer in post-treatment applications, especially in soils requiring microbial-driven nutrient cycling and pathogen suppression.
3.3. Functional Compounds
In addition to its microbial composition and activity, TMF contains several functional compounds that enhance plant growth, soil health, and agricultural sustainability [47,59,60,61]. Fuertes-Mendizábal et al. [20] identified humic acids (7.52%) and fulvic acids (19.6%) in TMF obtained after growing mealworm larvae fed with whole wheat flour supplemented with vegetables in open trays for 9 weeks. These organic compounds improve the soil structure, nutrient retention, microbial activity, and plant growth, enhancing key agronomic parameters [66,67]. TMF also contains structural polysaccharides (expressed as mean values ± standard deviation in g/100 g dry weight, w/w), including cellulose (13.6 ± 0.65), chitin (7.40 ± 0.37), xylan (16.4 ± 0.75), and lignin (10.9 ± 0.04) [15], which provide various agronomic benefits. These concentrations were determined using validated analytical methods: cellulose, hemicellulose, and lignin were quantified following the National Renewable Energy Laboratory (NREL) Technical Report [68], while the chitin content was determined using the modified method by Kumari et al. [69].
In TMF, cellulose—a major constituent of plant biomass and the most abundant polysaccharide on Earth [70,71]—can help enhance crop productivity, nutrient accessibility, and soil health. Additionally, it has been associated with positive effects on key soil parameters, including organic matter content, microbial activity, and water retention capacity [72].
Chitin, a key component of TMF, plays a dual role in sustainable agriculture by improving plant growth and offering natural pest protection [73]. Kisaakye et al. [74] emphasized the potential of chitin-enriched insect frass fertilizer (chFE) as an effective solution for plant protection, particularly in managing nematodes and other pests. chFE has been shown to suppress Meloidogyne incognita (root-knot nematodes), while significantly enhancing spinach growth, increasing the root biomass by 54–74% and shoot biomass by 39–58% compared to commercial nematicides [74]. Moreover, chFE improves soil health, promotes nutrient release, and supports beneficial microbial activity, fostering a healthier growing environment that strengthens plant resilience and productivity under varying environmental conditions. Similarly, Barragán-Fonseca et al. [22] highlighted the benefits of incorporating chFE into agricultural soils to enhance plant resistance. Recognized by plants as a microbe-associated molecular pattern (MAMP), chitin triggers a range of defence responses, including the systemic expression of defence-related genes, programmed cell death, and the release of reactive oxygen species [22]. Its effectiveness in stimulating plant defences against pathogens has been demonstrated in various systems, whether applied as a soil amendment or as a foliar spray [75,76]. Poveda [23] further emphasizes the role of chitin, particularly when found in insect frass, as a crucial signalling molecule that activates plants’ natural defences. When recognized as an MAMP, chitin triggers a cascade of reactions that activate systemic resistance through pathways involving salicylic acid, jasmonic acid, and ethylene, all essential for plant protection [23]. Parada et al. [77] demonstrated that chitin and its derivatives enhance resistance to pathogens such as Alternaria brassicicola and Colletotrichum fructicola. Shamshina et al. [75] investigated chitin as a natural fertilizer that stimulates plant growth. The study highlighted that, when biodegraded in the soil by bacterial enzymes (chitinases), chitin releases compounds like ammonia, which have fertilizing effects and promote the growth of beneficial microorganisms. This decomposition process is driven by chitinase enzymes, which function at specific temperature and pH conditions. Notably, bacteria and fungi such as Flavobacterium and Fusarium oxysporum play a pivotal role in this process [75]. Ramírez et al. [76] demonstrated that chitin promotes the growth and development of beneficial microorganisms, such as mycorrhizae and Rhizobium species, which form synergistic relationships with plants. Furthermore, these authors reported that chitin-induced increases in microbial populations and soil activity enhance nutrient properties and availability. They also showed that, as growth regulators, chitin and its derivatives accelerate seed germination, boost plant growth, and improve agricultural yields [76].
Xylan, another major polysaccharide in TMF, contributes to soil carbon cycling and plant structural integrity [78,79]. A distinctive feature of xylan is the presence of acetyl groups in its molecular structure, which influence plant growth, environmental responsiveness, and pathogen defence [80]. The degree and position of xylan acetylation affect its functionality: higher deacetylation can enhance the plant’s mechanical strength and improve its defence against certain pathogens, while reduced acetylation facilitates xylan degradation. This process enhances the bioavailability of its hydrolysis products, making them more accessible to soil microorganisms and plant roots, thereby improving nutrient uptake and supporting plants [81].
TMF contains lignin, a structurally complex natural polymer that enhances soil stability [82] and serves as a carrier for controlled nutrient release [83]. According to Ahmad et al. [84], lignin could be used to develop slow-release N-fertilizers, phosphate fertilizers, compound fertilizers, and chelated micro-fertilizers. Modified with various elements, lignin can offer long-term stability, resistance to leaching, low pollution, high fertilizer efficiency, low cost, and enhanced biological activity. Thanks to its chemical structure rich in functional groups, lignin ensures the slow degradation and gradual release of nutrients, improving efficiency and reducing pollution. In addition, lignin-based fertilizers are more economical compared to traditional ones, with a price reduction of 20–30% [84]. Furthermore, Savy et al. [85] demonstrated that water-soluble lignin can stimulate the emergence and early growth of maize seedlings, while Popa et al. [86] suggest its potential as a plant growth promoter in agriculture. Among its other important applications, the lignin contained in TMF plays a key role in the formulation of pesticides, where it serves as a dispersant and adhesive [87]. Singh et al. [88] demonstrated that lignin-based pesticides could significantly reduce soil leaching, soil pollution, and groundwater contamination.
Therefore, TMF’s cellulose, chitin, xylan, and lignin contribute to soil health, support plant growth, and enhance overall crop productivity, reinforcing TMF’s potential as a sustainable agricultural input (Figure 5).
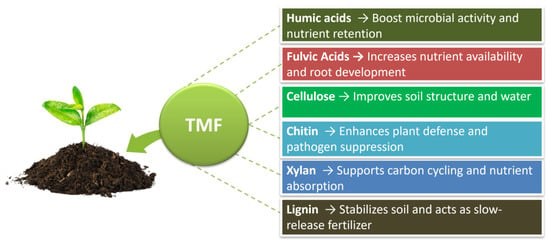
Figure 5.
Functional compounds in TMF and their agronomic benefits.
4. Agronomic Benefits
Agricultural practices often deplete soil nutrients and increase the risk of plant diseases, leading to crop losses and environmental damage. While mineral fertilizers can quickly provide an ideal balance of NPK, they often disrupt the soil ionic balance, inhibit microbial activity, and introduce harmful substances, ultimately reducing soil fertility [11,19,89]. Conversely, organic fertilizers release nutrients more slowly due to microbial activity, which can benefit some crops but may fail to meet the immediate nutritional demands of others [14,19].
Insect frass has emerged as a nutrient-rich organic fertilizer and soil amendment. Among different insects’ frasses, TMF is not only a source of essential macronutrients and micronutrients but also harbours PGPMs [20,89]. Studies have demonstrated that TMF performs comparably or even better than inorganic fertilizers, particularly when used in combination with them.
Table 3 presents the results from studies on the effectiveness of TMF as a fertilizer for various plants, along with the testing conditions and the quantity of frass utilized.

Table 3.
Effectiveness of TMF as a fertilizer.
One of the first studies to explore TMF as an organic fertilizer was conducted by Poveda et al. [44] on chard plants (Beta vulgaris var. cicla). The TMF applied (dosage: 2%, v/v) had an NPK profile of 3-1.5-2 and contained S, Ca, Mg, Mn, Fe, and Mo as essential micronutrients for plant growth and development. By administering these nutrients through TMF, the chard plants achieved a higher chlorophyll content, fresh weight, shoot length, and collar thickness, all of which are economically valuable [44]. Since then, the growth-promoting effects attributed to the nutrient supply from TMF have been documented in various other plants and crops. According to Houben et al. [45], TMF at a dosage of 10 mg/ha, with an NPK balance of 5-2-2, increased the biomass production in barley. Similarly, Nyanzira et al. [91] observed an improvement in grain yield after applying TMF at a dose of 2% (w/v), with high-quality, golden-brown seeds. These authors investigated wheat (Triticum spp.) cultivated in soils treated with TMF alone, TMF combined with mineral fertilizers, and untreated soils. Their results revealed that TMF alone produced growth, root development, and seed quality outcomes like or better than those achieved with conventional NPK fertilizers, with the best results obtained when combined with mineral fertilizers [91].
The high labile carbon content and low recalcitrant carbon in TMF make it readily degradable, enabling the efficient and immediate release of N into the soil. Houben et al. [49] demonstrated that applying TMF at a rate of 10 t ha−1 significantly increased the N soil availability and crop yields compared to other organic fertilizers, which do not often deliver short-term nutrient benefits.
TMF has shown advantages in horticultural substrates. Nogalska et al. [28] found that TMF reduced the acidifying effects of urea in peat-based substrates, likely due to its alkaline elements such as calcium and magnesium. Moreover, peat enriched with TMF demonstrated improved microbiological quality, avoiding issues like salinization or excessive N accumulation [28].
The nutrient profile of TMF closely resembles that of poultry manure, making it a viable and environmentally friendly alternative. While poultry manure poses water pollution risks through nitrate and orthophosphate runoff, TMF presents significantly fewer environmental hazards. Hénault-Ethier et al. [26] confirmed that TMF and poultry manure produced similar growth rates and yields across a variety of crops and flowers, emphasizing its potential as a sustainable substitute.
While insect frass offers numerous benefits, excessive application can lead to negative effects such as phytotoxicity and eutrophication due to high N concentrations. For example, Watson et al. [50] observed that increasing the TMF application rates from 1.5% to 3% inhibited seed germination in Italian ryegrass (Lolium multiflorum), potentially due to salinity or ammonia toxicity. Optimizing application rates and monitoring salinity levels are critical to mitigating these issues [50].
TMF contains valuable nutrients and microbial communities that boost plant resilience to abiotic and biotic stresses. PGPMs in TMF can solubilize phosphate, produce siderophores, and enhance plant defences. For instance, TMF has been shown to improve drought tolerance, salinity resistance, and waterlogging resilience in crops [44].
The combination of TMF with plant growth-promoting (PGP) biostimulants can amplify its beneficial effects. Fuertes-Mendizábal et al. [20] demonstrated that lettuce (Lactuca sativa L.) treated with TMF and PGP biostimulants exhibited higher biomass, enhanced chlorophyll content, and reduced nitrate accumulation compared to treatments using either product alone. This synergy promotes nutrient uptake, supports beneficial microbial populations like Trichoderma spp., and suppresses pathogenic fungi [20].
In the scientific literature, articles highlighting the negative effects of treatment with TMF on plants have been emerging. Li et al. [95] studied the application of aqueous extracts of mealworm frass on wheat, simulating closed space conditions such as those of a space station. The results showed a strong inhibitory effect on germination and plant growth, probably due to certain fatty acids.
Several studies have shown that pre-treating TMF, before adding it to the growing substrate, can limit its phytotoxicity [28,95]. In this regard, Chia et al. [93] studied the performance of TMF on field mustard under different conditions: as is (raw frass), after composting, and after incubation in the soil. Composted and soil-matured frass performed better than raw frass.
5. Environmental and Economic Impacts and Future Perspectives
Insect farming, particularly for TM production, is pivotal in closing the loop on agricultural waste management. TM larvae can efficiently upcycle organic residues, such as vegetable scraps, crop residues, and food processing by-products, into nutrient-rich frass [96]. This process not only reduces the volume of agricultural waste but also mitigates its associated environmental issues, such as eutrophication and GHG emissions caused by improper disposal [97]. By repurposing waste streams that would otherwise pose disposal challenges, TM farming aligns with circular economy principles and promotes resource efficiency [98]. The bioconversion of waste into frass provides a sustainable alternative to traditional organic fertilizers like poultry manure, which often carry risks of water pollution through nitrate and phosphate leaching [26]. Additionally, TMF production requires less energy than synthetic fertilizer manufacturing, which is heavily dependent on energy-intensive processes like the Haber–Bosch method for N fixation [49]. Furthermore, the use of TMF contributes to carbon sequestration when incorporated into soil, enhancing long-term soil organic carbon storage and promoting sustainable agricultural practices [46]. This effect is attributed to TMF’s high organic matter content, its stimulation of microbial activity, and its role in forming organo-mineral complexes that stabilize carbon in the soil [99]. Recent studies have estimated that the application of insect-derived organic amendments can increase the soil organic carbon stocks by 0.2–0.6 Mg C ha−1 yr−1, corresponding to 0.73–2.2 Mg CO2-equivalent sequestered per hectare annually [100,101]. Additionally, soil CO2 flux measurements suggest that incorporating insect frass may reduce short-term carbon emissions compared to other organic fertilizers, thereby contributing to a more stable and long-term soil carbon balance [23]. These findings highlight the potential of TMF as a valuable soil amendment, enhancing both soil fertility and climate change mitigation through improved carbon sequestration.
The cost-effectiveness of TMF as a fertilizer lies in its multifunctionality as a nutrient source, biostimulant, and soil amendment. While the initial production costs for TMF may be higher than those for synthetic fertilizers due to the specialized infrastructure required for insect farming, its long-term economic benefits are notable. TMF improves soil health and fertility, reducing the need for additional input over time [44]. Moreover, the slow-release nature of nutrients in TMF minimizes nutrient runoff, which is not only environmentally harmful but also represents a loss of investment in synthetic fertilizers [89]. The additional benefits of TMF, such as enhanced microbial activity and plant resilience to stress, further contribute to its economic value by improving crop productivity and reducing dependency on chemical pesticides [41].
Regarding specific cost data, no economic results have yet been published on TMF. However, previous research on insect-based fertilizers has demonstrated potential economic benefits. For example, research carried out in Africa by Beesigamukama et al. [10] reported that fertilization with BSF frass costs approximately USD 390 per hectare per season, compared to USD 854 per hectare per season for synthetic fertilizers, leading to significant savings for farmers. Additionally, the study found that BSF frass could increase crop production profits by 10 to 154% and boost farmers’ gross margins by 35% [102].
While TMF has demonstrated a comparable or superior agronomic performance to BSF frass in several studies, particularly in terms of plant growth, biomass yield, and plant health [10,93,103], its economic feasibility has not yet been assessed. Future research should focus on large-scale field trials to evaluate its cost-effectiveness in different agricultural systems.
Maintaining competitive costs between TMF and synthetic fertilizers on a large scale is challenging. To scale up TMF production, investments are needed in the automation and optimization of larval rearing systems. These improvements can help reduce costs and ensure consistent quality for large-scale agricultural applications. Despite these challenges, the agronomic and environmental benefits of TMF make it a promising option for integrating sustainable practices into modern agricultural systems [104].
The future of TMF lies in advancing production scalability, innovating processing methods, and integrating its use within circular and sustainable agricultural systems. Furthermore, innovations in frass processing and enrichment, such as biofortification with additional nutrients or microbial consortia, could expand its utility as a targeted biostimulant and soil amendment. Such enhancements may improve TMF efficacy in diverse cropping systems while addressing specific nutrient deficiencies [20,49].
Integrating TMF into regenerative farming practices further supports the transition towards low-input and eco-friendly agricultural systems, reducing reliance on synthetic agrochemicals and promoting resource efficiency [104]. TMF represents a promising tool for addressing global challenges in food security and environmental conservation by closing the nutrient loop and enhancing the ecological sustainability of agricultural practices.
However, despite its promise, TMF remains underexplored compared to other insect frasses, such as BSF frass. Much of the existing research has been conducted in controlled environments, which limits its applicability in large-scale agricultural practices. To fully unlock the potential of TMF, future research should prioritize the following:
- Long-term field studies assessing the effects of TMF on various crops.
- Optimizing application rates and studying its interactions with soil microbiota.
- Evaluating the ecological footprint of TMF production to ensure sustainability [17].
Continued interdisciplinary research, policy support, and stakeholder collaboration will be critical to fully realizing TMF’s potential as a cornerstone of sustainable agricultural systems.
6. TMF-Based Biochar
In addition to traditional agronomic applications, TMF in the form of biochar has also demonstrated its potential in environmental remediation. The pyrolysis process, involving the thermal degradation of biomass in oxygen-free conditions, stabilizes nutrients and creates carbon-rich biochar [105,106,107]. Biochar derived from TMF has shown promise as a bio-adsorbent for wastewater detoxification and soil remediation [30,31].
TMF biochar can adsorb organic contaminants through various mechanisms that depend on its intrinsic properties—affected by the initial frass and the pyrolysis conditions—as well as the structures of the contaminants and environmental conditions, particularly pH. The presence of numerous oxygen-containing functional groups (such as --C--O, C=O, --OH, and --COOH) on the surface of the biochar facilitates adsorption through several interactions, including electrostatic attraction, hydrophobic interactions, π-π electron donor–acceptor interactions, and hydrogen bonding. Additionally, the structure and texture of biochar, with its specific surface area and porosity, play a key role in physical adsorption, which can be categorized into surface adsorption and pore filling [32].
For instance, biochar from the frass of TM larvae fed on wheat straw exhibited superior adsorption of malachite green, a toxic cationic dye, with a capacity of 1738.6 mg/g. This efficiency resulted more from chemisorption and electrostatic interaction than from a pore filling effect. In contrast to cationic dyes such as crystal violet and malachite green, the anionic Congo red showed the lowest adsorption capacity. This phenomenon is due to the electrostatic repulsion between the anionic dye and the negatively charged surface of TMF-based biochar due to the presence of hydroxide ions [30]. In another research study, a KOH-activated TMF biochar resulted in an optimal and sustainable adsorbent to remove neonicotinoid pesticides, one of the most widely used categories of insecticides globally, from aquatic environments [32]. Biochar prepared by the pyrolysis of TMF and activated with KOH at 750 °C exhibited the highest specific surface area (1858.80 m2/g) and the highest pore volume (1.11 cm3/g). These physical properties along with the abundant oxygen-containing functional groups endowed TMF biochar with an excellent adsorption capacity for neonicotinoid pesticides. However, the authors admit that further research is needed to study their modified TMF-based biochar for pesticide adsorption in real wastewater [32].
Regarding metal contamination, Yang et al. [31] studied the heavy metal adsorption capacities of biochars derived from frasses of TM fed on five lignocellulosic crop residues and compared them to those of biochars obtained from the original crop residues. The study demonstrated that the performance of TMF biochar outperforms crop-derived biochar in adsorbing heavy metals. Metal adsorption onto TMF biochars follows different mechanisms, including electrostatic interactions, complexation, cation exchange, co-precipitation, and reduction. The results showed that frass-based biochars had higher adsorption capacities for Pb(II), Cd(II), Zn(II), and Cr(VI) while improving soil properties like water retention and nutrient stabilization [31].
Various studies show that TMF biochar contributes to carbon sequestration, supporting circular economy-based initiatives and mitigating greenhouse gas emissions. Wang et al. [108] studied the CO2 capture capacity and electrochemical performance of biochar produced from the frass of TM fed on waste fruits. They activated the biochar using KOH at 600, 700, and 800 °C. The results of the CO2 adsorption isotherms indicate that all of the biochars captured 90% of the CO2 within 20 min. Among these, the biochar activated at 700 °C demonstrated the highest CO2 capture capacity and a promising electrochemical performance (335.8 F g−1 at 0.5 A g−1). The CO2 capture and supercapacitor performance of the tested TMF-based biochars are comparable to some modified biochars and have excellent recycling performance [108].
TMF-based biochar can serve as a green and versatile support for promising catalysts, offering an effective method for degrading contaminants in aquatic environments. In this context, He et al. [109] prepared a Fenton-type photo-assisted catalyst from TMF-based biochar by Fe immobilization. The obtained iron (Fe)-loaded TMF-based biochar resulted in an efficient catalyst characterized by a high surface area (90.65 m2 g−1) and good recycling performance. In the Fenton-type photo-assisted heterogeneous system, this catalyst demonstrated the excellent removal efficiency of malachite green dye, achieving a 67% reduction in the TOC within 5 min. In this research, the authors also evaluated the environmental impacts through a life cycle assessment study of two scenarios: the production of the tested catalyst and the disposal of TMF via composting. Producing the frass-based biochar catalyst had a lower environmental footprint compared to the composting of the frass [109].
In conclusion, many authors emphasize that improved adsorption capacities are obtained by activating TMF-based biochars with alkali or loading them with ions or metals such as iron [108,109]. This modification improves their effectiveness in adsorbing contaminants and facilitates the degradation and decontamination processes in soils and aquatic environments. It is necessary, however, to deepen the mechanisms of action with which these modified biochars exert their beneficial functions [32,110].
Moreover, the results obtained so far indicate that TMF biochar can compete with those made from lignocellulosic biomass (rice, wheat, and corn straws) or manure from various animals (cow, chicken, and swine manure), sometimes proving to be better. These encouraging results were primarily attributed to the increased Brunauer–Emmett–Teller surface area of the produced TMF biochars and the higher total pore volume compared to other biochars [30,31,32].
7. Regulatory Framework for Insect Frass Fertilizers in Europe
The regulatory status of insect frass varies globally, with Europe leading in the establishment of specific legislation. In contrast, regulations in North America and Asia remain less defined, often classifying insect frass under general organic fertilizer policies. In the United States, frass is generally regulated as a soil amendment under USDA and FDA guidelines, while in Canada, it falls under CFIA (Canadian Food Inspection Agency, Ottawa, ON, Canada) regulations for organic fertilizers [111]. In China, insect frass is subject to general organic fertilizer policies under the Ministry of Agriculture and Rural Affairs (MARA), without dedicated legislation [112]. Despite the growing interest in insect-based fertilizers, no harmonized international framework currently exists.
Given this landscape, our study focuses primarily on the European regulatory framework, as it offers the most detailed and structured approach to the commercialization and agricultural use of insect frass. The European Union has taken significant steps to regulate insect frass, ensuring its safe and effective application while fostering the expansion of the insect farming sector. Insect frass, including that derived from TM, is classified as a by-product of animal origin under European legislation and is not considered waste. Consequently, it is independent of the Waste Framework Directive (Directive 2008/98/EC). The regulatory framework governing insect frass significantly evolved with the adoption of EU Regulation 2021/1925 on 5 November 2021, which amended EU Regulation 142/2011. This regulation classifies frass as a Category 2 material (medium risk), similar to processed manure, and establishes specific requirements for its commercialization as a fertilizer. These requirements include heat treatment at a minimum of 70 °C for at least 60 min, compliance with microbiological thresholds for Escherichia coli and Enterococcaceae to ensure hygienic safety, and controlled storage measures to prevent secondary contamination or rehydration, such as the use of well-sealed and insulated silos or hermetically sealed packaging (EU Regulation 142/2011, Annex XI, Chapter I, Section 2, letters ‘b,’ ‘d,’ and ‘e’).
Before November 2021, EU Regulation 142/2011, which implemented Regulation 1069/2009 on animal by-products, defined “manure” but did not explicitly include insect frass. This regulatory gap led to inconsistencies across EU Member States, with some classifying insect frass under the generic category of manure [113]. The introduction of Regulation 2021/1925 resolved this ambiguity by providing the first official definition of insect frass: “A mixture of excrement derived from farmed insects, the feeding substrate, parts of farmed insects, dead eggs, and with a content of dead farmed insects not exceeding 5% by volume and 3% by weight” [114].
In 2021, significant progress was also made in the use of insect frass in organic farming. EU Regulation 1165/2021 authorized its application in organic agriculture, aligning with sustainability objectives and expanding its potential use in environmentally conscious farming practices. The International Platform of Insects for Food and Feed (IPIFF) played a pivotal role in advocating for the regulatory recognition of frass, particularly given the rapid growth of insect farming in Europe and the associated increase in by-products like frass [115]. However, IPIFF and some researchers argue that the current heat treatment requirement of 70 °C for 60 min may not be optimal for insect frass, as it could potentially impact its quality and functional properties. They advocate for tailored standards that address the specific characteristics of frass while maintaining stringent hygiene and safety conditions.
Despite its recognition as a fertilizer, insect frass is not yet fully integrated into EU Regulation 2019/1009, which harmonizes rules for various fertilizer categories and promotes the use of biostimulants derived from organic waste. Given its composition—larvae droppings, undigested organic waste, and exuviae—frass could reasonably be included in Constituent Materials Category 10 (CMC 10), which pertains to derivative products. However, the absence of a comprehensive list of derivative products under CMC 10 remains a significant regulatory gap. A forthcoming delegated act by the European Commission is expected to address this issue, and the IPIFF is actively advocating for the inclusion of frass in CMC 10 to streamline national authorization procedures and facilitate intra-EU trade [113].
Currently, no regulation specifies the allowable application rates of insect frass for agricultural use. This is partly due to the need for further research to establish optimal rates, which depend on various factors, including the insect species, their diet, and the crops being fertilized [20]. Additional studies are necessary to evaluate the long-term environmental impacts of frass and its interactions with different soil and crop systems, particularly for TMF. Developing comprehensive and standardized regulatory frameworks will be critical to realizing its full potential as a sustainable agricultural input.
8. Conclusions
TMF offers a unique and versatile solution for sustainable agriculture and environmental recovery. Its nutrient-rich composition includes essential elements such as N, P, and K, alongside a range of functional compounds, including humic and fulvic acids, cellulose, chitin, xylans, and lignin. These compounds enhance soil properties, nutrient availability, and agronomic performance. The presence in TMF of PGPMs further stimulates soil fertility and disease resistance, setting TMF apart from conventional mineral fertilizers, which lack these microbial benefits. Additionally, incorporating organic by-products into TM’s diet reduces reliance on external inputs and minimizes waste, supporting sustainable goals.
Besides serving as a sustainable fertilizer, TMF offers the potential for creating adsorbents, such as biochars, through pyrolysis, which have proven to be particularly effective at removing organic and inorganic contaminants from soil and water. Biochars derived from TMF are more efficient than those made from other organic materials, representing a valid and sustainable alternative to simple composting.
Then, the use of TMF not only addresses the environmental drawbacks of conventional fertilizers but also enhances soil health, plant productivity, and resilience to abiotic and biotic stressors. TMF production, based on resource efficiency and waste reduction, strengthens its role in regenerative farming practices, offering a sustainable alternative to conventional fertilizers.
Despite its promise, the scalability of TMF production and its competitiveness with synthetic fertilizers remain significant challenges. Advances in automation, optimized larval rearing, and innovative frass processing methods, such as biofortification, are crucial for reducing production costs and improving its agronomic value. Additionally, a harmonized regulatory framework and long-term field studies are necessary to ensure its safe and effective application across diverse agricultural systems.
As global agricultural demands increase, TMF stands out as a pivotal resource for promoting resource efficiency, reducing environmental impact, and fostering sustainable food production. Continued interdisciplinary collaboration among researchers, policymakers, and industry stakeholders will be the key to unlocking its full potential and contributing to the global transition toward sustainable and resilient agricultural systems. Future research should focus on genomic studies of TMF microbial communities to better understand their contribution to soil fertility, plant health, and environmental remediation. Investigating the molecular mechanisms of plant growth promotion and pathogen suppression will provide deeper insights into how TMF-derived microbial and biochemical interactions enhance crop productivity. Additionally, developing efficient TMF processing technologies, including advanced drying, pelletization, and microbial biofortification, will be essential for optimizing its stability, application efficiency, and overall agronomic performance. Addressing these aspects will be essential for harnessing TMF’s full potential as a sustainable agricultural input.
Author Contributions
Conceptualization, A.V. and P.S.; methodology, A.V., P.S. and B.D.M.; validation, A.V., P.S. and S.E.; formal analysis, A.V. and P.S.; investigation, A.V., P.S., B.D.M., S.M., S.D., A.S. and S.E.; resources, A.V., P.S., B.D.M., S.M., A.S., S.D., C.C., D.B., R.R., S.P. and S.E.; data curation, A.V. and P.S.; writing—original draft preparation, A.V., P.S., B.D.M., S.M., A.S. and S.E.; writing—review and editing, A.V., P.S., C.C., D.B. and S.E.; visualization, A.V., P.S., D.B., C.C. and B.D.M.; supervision, A.V. and P.S.; project administration, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge the Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for proposals No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union—Next Generation EU; Award Number: Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP I83C22001790001, Project title “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BSF | Black Soldier Fly |
| chFE | Chitin-enriched insect frass fertilizer |
| CMC | Constituent Materials Category |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| GHG | Greenhouse gas |
| IPIFF | International Platform of Insects for Food and Feed |
| MAMP | Microbe-associated molecular pattern |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
| PGP | Plant growth-promoting |
| PGPMs | Plant growth-promoting microorganisms |
| TM | Tenebrio molitor |
| TMF | Tenebrio molitor frass |
| TOC | Total organic carbon |
| USD | United States Dollar |
| Al | Aluminum |
| B | Boron |
| Cu | Copper |
| Fe | Iron |
| K | Potassium |
| Mn | Manganese |
| Mo | Molybdenum |
| N | Nitrogen |
| Na | Sodium |
| P | Phosphorus |
| Zn | Zinc |
| NPK | Nitrogen, phosphorus, potassium |
References
- Worldometers. Available online: https://www.worldometers.info/ (accessed on 14 March 2025).
- United Nations. World Population Prospects 2024: Summary of Results; UN DESA/POP/2024/TR/NO. 9; United Nations: New York, NY, USA, 2024.
- FAO. The State of Food Security and Nutrition in the World; FAO Report; FAO: Rome, Italy, 2024. [Google Scholar]
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for World Population and Global Food Availability for Global Health. In The Role of Functional Food Security in Global Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–24. [Google Scholar]
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Calabi-Floody, M.; Medina, J.; Rumpel, C.; Condron, L.M.; Hernandez, M.; Dumont, M.; de la Luz Mora, M. Smart Fertilizers as a Strategy for Sustainable Agriculture. Adv. Agron. 2018, 147, 119–157. [Google Scholar]
- Sharma, A.K.; Sharma, D.; Chopra, A.K. An Overview of Pesticides in the Development of Agriculture Crops. J. Appl. Nat. Sci. 2020, 12, 101–109. [Google Scholar] [CrossRef]
- Srivastav, A.L. Chemical Fertilizers and Pesticides: Role in Groundwater Contamination. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–159. [Google Scholar]
- Rana, A.; Tyagi, M.; Sharma, N. Impact of Chemical Pesticides vs. Biopesticides on Human Health and Environment. Int. J. All Res. Writ. 2019, 2, 45–51. [Google Scholar]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient Quality and Maturity Status of Frass Fertilizer from Nine Edible Insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant–Soil Feedbacks: The Past, the Present and Future Challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Statista. Fertilizer Industry Worldwide—Statistics & Facts. Available online: https://www.statista.com/topics/8956/fertilizer-industry-worldwide/#topicOverview (accessed on 20 February 2025).
- Nakachew, K.; Yigermal, H.; Assefa, F.; Gelaye, Y.; Ali, S. Review on Enhancing the Efficiency of Fertilizer Utilization: Strategies for Optimal Nutrient Management. Open Agric. 2024, 9, 20220356. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Z.; Zhai, Y.; Zhang, L.; Zheng, M.; Yao, H.; Lv, L.; Shen, H.; Zhang, J.; Yao, Y.; et al. Partial Substitution of Chemical Fertilizer by Organic Fertilizer Benefits Grain Yield, Water Use Efficiency, and Economic Return of Summer Maize. Soil Tillage Res. 2022, 217, 105287. [Google Scholar] [CrossRef]
- Muñoz-Seijas, N.; Fernandes, H.; Outeiriño, D.; Morán-Aguilar, M.G.; Domínguez, J.M.; Salgado, J.M. Potential Use of Frass from Edible Insect Tenebrio molitor for Proteases Production by Solid-State Fermentation. Food Bioprod. Process. 2024, 144, 146–155. [Google Scholar] [CrossRef]
- Arévalo Arévalo, H.A.; Menjura Rojas, E.M.; Barragán Fonseca, K.B.; Vásquez Mejía, S.M. Implementation of the HACCP System for Production of Tenebrio molitor Larvae Meal. Food Control 2022, 138, 109030. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Ding, M.-Q.; Li, M.-X.; Ding, J.; Bai, S.-W.; Wu, Q.-L.; Zhao, L.; Cao, G.-L.; Ren, N.-Q.; et al. Sustainable Strategy for Lignocellulosic Crop Wastes Reduction by Tenebrio molitor Linnaeus (Mealworm) and Potential Use of Mealworm Frass as a Fertilizer. J. Clean. Prod. 2021, 325, 129301. [Google Scholar] [CrossRef]
- Deruytter, D.; Coudron, C.L. The Effects of Density on the Growth, Survival and Feed Conversion of Tenebrio molitor Larvae. J. Insects Food Feed 2022, 8, 141–146. [Google Scholar] [CrossRef]
- Blakstad, J.I.; Strimbeck, R.; Poveda, J.; Bones, A.M.; Kissen, R. Frass from Yellow Mealworm (Tenebrio molitor) as Plant Fertilizer and Defense Priming Agent. Biocatal. Agric. Biotechnol. 2023, 53, 102862. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Salcedo, I.; Huérfano, X.; Riga, P.; Estavillo, J.M.; Ávila Blanco, D.; Duñabeitia, M.K. Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants. Agronomy 2023, 13, 1258. [Google Scholar] [CrossRef]
- van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; Dicke, M. Soil Amendment with Insect Frass and Exuviae Affects Rhizosphere Bacterial Community, Shoot Growth and Carbon/Nitrogen Ratio of a Brassicaceous Plant. Plant Soil 2024, 495, 631–648. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; de Boer, W.; Dicke, M. Insect Frass and Exuviae to Promote Plant Growth and Health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef]
- Poveda, J. Insect Frass in the Development of Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Ashworth, A.J.; Arsi, K.; Rojas, M.G.; Morales-Ramos, J.A.; Donoghue, A.; Robinson, K. Insect Frass Composition and Potential Use as an Organic Fertilizer in Circular Economies. J. Econ. Entomol. 2024, 117, 1261–1268. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Amorim, H.C.S.; Drescher, G.L.; Moore, P.A.; Rojas, M.G.; Morales-Ramos, J.; Donoghue, A.M. Insect Frass Fertilizer as Soil Amendment for Improved Forage and Soil Health in Circular Systems. Sci. Rep. 2025, 15, 3024. [Google Scholar] [CrossRef]
- Hénault-Ethier, L.; Reid, B.; Hotte, N.; Paris, N.; Quinche, M.; Lachance, C.; Fortin, A.; Normandin, É.; Laderriere, V.; Vandenberg, G. Growth Trials on Vegetables, Herbs, and Flowers Using Mealworm Frass, Chicken Manure, and Municipal Compost. ACS Agric. Sci. Technol. 2023, 3, 249–259. [Google Scholar] [CrossRef]
- Dean, L. Market Approval Obtained for an Insect-Based Fertilizer. World Fertilizer Magazine. 22 July 2020. Available online: https://www.worldfertilizer.com/special-reports/22072020/market-approval-obtained-for-an-insect-based-fertilizer/ (accessed on 14 March 2025).
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Załuski, D.; Kosewska, A.; Skwierawska, M.; Sienkiewicz, S. The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment. Int. J. Environ. Res. Public Health 2022, 20, 21. [Google Scholar] [CrossRef]
- Chavez, M.; Uchanski, M. Insect Left-over Substrate as Plant Fertiliser. J. Insects Food Feed 2021, 7, 683–694. [Google Scholar] [CrossRef]
- Yang, S.-S.; Chen, Y.; Kang, J.-H.; Xie, T.-R.; He, L.; Xing, D.-F.; Ren, N.-Q.; Ho, S.-H.; Wu, W.-M. Generation of High-Efficient Biochar for Dye Adsorption Using Frass of Yellow Mealworms (Larvae of Tenebrio molitor Linnaeus) Fed with Wheat Straw for Insect Biomass Production. J. Clean. Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Yang, S.-S.; Chen, Y.; Zhang, Y.; Zhou, H.-M.; Ji, X.-Y.; He, L.; Xing, D.-F.; Ren, N.-Q.; Ho, S.-H.; Wu, W.-M. A Novel Clean Production Approach to Utilize Crop Waste Residues as Co-Diet for Mealworm (Tenebrio molitor) Biomass Production with Biochar as Byproduct for Heavy Metal Removal. Environ. Pollut. 2019, 252, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Xu, M.; Yan, X.; Huang, J.; Wang, H. Removal of Neonicotinoid Pesticides by Adsorption on Modified Tenebrio molitor Frass Biochar: Kinetics and Mechanism. Sep. Purif. Technol. 2022, 297, 121506. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Alkoaik, F.N. The Yellow Mealworm as a Novel Source of Protein. Am. J. Agric. Biol. Sci. 2009, 4, 319–331. [Google Scholar] [CrossRef]
- Robinson, W.H. Handbook of Urban Insects and Arachnids; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780511542718. [Google Scholar]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of Various Commodities for the Development of the Yellow Mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor Larva-Based Ingredients for the Food Industry: A Review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Errico, S.; Dimatteo, S.; Moliterni, S.; Baldacchino, F. Effects of Long-Lasting Cold Storage On Tenebrio molitor Larvae (Coleoptera: Tenebrionidae). J. Insects Food Feed 2021, 7, 1111–1116. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New Methods for High-accuracy Insect Chitin Measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Kay, S.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Morphometric Analysis of Instar Variation in Tenebrio molitor (Coleoptera: Tenebrionidae). Ann. Entomol. Soc. Am. 2015, 108, 146–159. [Google Scholar] [CrossRef]
- Selaledi, L.; Mbajiorgu, C.A.; Mabelebele, M. The Use of Yellow Mealworm (T. molitor) as Alternative Source of Protein in Poultry Diets: A Review. Trop. Anim. Health Prod. 2020, 52, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zunzunegui, I.; Martín-García, J.; Santamaría, Ó.; Poveda, J. Analysis of Yellow Mealworm (Tenebrio molitor) Frass as a Resource for a Sustainable Agriculture in the Current Context of Insect Farming Industry Growth. J. Clean. Prod. 2024, 460, 142608. [Google Scholar] [CrossRef]
- Radwan, M.A.; Maggiolino, A.; Hassanien, H.A.M.; Palo, P.D.; El-Kassas, N.E.M.; Abbas, H.S.; Salem, A.Z.M. Dietary Utilization of Mealworm Frass in Rabbit Feeding Regimes and Its Effect on Growth, Carcass Characteristics, and Meat Quality. Front. Vet. Sci. 2023, 10, 1069447. [Google Scholar] [CrossRef]
- Hassanein, H.A.M.; Abou El-Fadel, M.H.; El-Kassas, N.E.M.; Phillip, Y.L.; Tirado-Estrada, G.; Alderey, A.-A.A.; EL-Deghadi, A.S.; Hussein, A.M.; Zayed, M.A.; Radwan, M.A.; et al. Dietary Inclusion of Mealworm Frass: Effect on Blood Metabolites and Growth Performance of Rabbits. J. Agric. Food Res. 2025, 19, 101637. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm Frass as a Potential Biofertilizer and Abiotic Stress Tolerance-Inductor in Plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.-P.; Dulaurent, A.-M. Potential Use of Mealworm Frass as a Fertilizer: Impact on Crop Growth and Soil Properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect Frass as a Novel Organic Soil Fertilizer for the Cultivation of Spinach (Spinacia oleracea): Effects on Soil Properties, Plant Physiological Parameters, and Nutrient Status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Foscari, A.; Dalla Costa, L.; Tulli, F.; Uboni, C.; Fellet, G. Frass from Tenebrio molitor as Alternative to NPK-Mineral Fertilization: Results from a Germination Test and Pot Experiment on Sunflower. Ital. J. Agron. 2024, 19, 100010. [Google Scholar] [CrossRef]
- Behie, S.; Bidochka, M. Insects as a Nitrogen Source for Plants. Insects 2013, 4, 413–424. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Dulaurent, A.-M. Assessment of the Short-Term Fertilizer Potential of Mealworm Frass Using a Pot Experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Watson, C.; Preißing, T.; Wichern, F. Plant Nitrogen Uptake From Insect Frass Is Affected by the Nitrification Rate as Revealed by Urease and Nitrification Inhibitors. Front. Sustain. Food Syst. 2021, 5, 721840. [Google Scholar] [CrossRef]
- Watson, C.; Schlösser, C.; Vögerl, J.; Wichern, F. Excellent Excrement? Frass Impacts on a Soil’s Microbial Community, Processes and Metal Bioavailability. Appl. Soil Ecol. 2021, 168, 104110. [Google Scholar] [CrossRef]
- Mattioli, S.; Paci, G.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Mancini, S. Former Foodstuff in Mealworm Farming: Effects on Fatty Acids Profile, Lipid Metabolism and Antioxidant Molecules. LWT 2021, 147, 111644. [Google Scholar] [CrossRef]
- Porto de Souza Vandenberghe, L.; Marcela Blandon Garcia, L.; Rodrigues, C.; Cândido Camara, M.; Vinícius de Melo Pereira, G.; de Oliveira, J.; Ricardo Soccol, C. Potential Applications of Plant Probiotic Microorganisms in Agriculture and Forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef]
- Pineda, A.; Zheng, S.-J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping Plants to Deal with Insects: The Role of Beneficial Soil-Borne Microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef]
- van Loon, L.C. Plant Responses to Plant Growth-Promoting Rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Kim, M.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil Bacteria Augment Arabidopsis Photosynthesis by Decreasing Glucose Sensing and Abscisic Acid Levels in Planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef]
- Del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC Deaminase in Plant Growth-Promoting Bacteria (PGPB): An Efficient Mechanism to Counter Salt Stress in Crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.G.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and Characterization of Bacteria from the Gut of Bombyx Mori That Degrade Cellulose, Xylan, Pectin and Starch and Their Impact on Digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef]
- Nurfikari, A.; Leite, M.F.A.; Kuramae, E.E.; de Boer, W. Microbial Community Dynamics during Decomposition of Insect Exuviae and Frass in Soil. Soil Biol. Biochem. 2024, 194, 109426. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The Core Gut Microbiome of Black Soldier Fly (Hermetia illucens) Larvae Raised on Low-Bioburden Diets. Fron.t Microbiol. 2020, 11, 993. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; et al. The Bacterial Biota of Laboratory-Reared Edible Mealworms (Tenebrio molitor L.): From Feed to Frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Praeg, N.; Klammsteiner, T. Primary Study on Frass Fertilizers from Mass-Reared Insects: Species Variation, Heat Treatment Effects, and Implications for Soil Application at Laboratory Scale. J. Environ. Manag. 2024, 356, 120622. [Google Scholar] [CrossRef]
- Hung, R.; Lee Rutgers, S. Applications of Aspergillus in Plant Growth Promotion. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–227. [Google Scholar]
- De Tender, C.; Mesuere, B.; Van der Jeugt, F.; Haegeman, A.; Ruttink, T.; Vandecasteele, B.; Dawyndt, P.; Debode, J.; Kuramae, E.E. Peat Substrate Amended with Chitin Modulates the N-Cycle, Siderophore and Chitinase Responses in the Lettuce Rhizobiome. Sci. Rep. 2019, 9, 9890. [Google Scholar] [CrossRef]
- Mamtimin, T.; Han, H.; Khan, A.; Feng, P.; Zhang, Q.; Ma, X.; Fang, Y.; Liu, P.; Kulshrestha, S.; Shigaki, T.; et al. Gut Microbiome of Mealworms (Tenebrio molitor Larvae) Show Similar Responses to Polystyrene and Corn Straw Diets. Microbiome 2023, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the Role of Humic Acids on Crop Performance and Soil Health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Martins, E.M.; Pillajo, J.Q.; Jones, M.L. Humic and Fulvic Acids Promote Growth and Flowering in Petunias at Low and Optimal Fertility. HortScience 2024, 59, 235–244. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and Characterization of Chitin and Chitosan from Fishery Waste by Chemical Method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Wang, B.-T.; Hu, S.; Yu, X.-Y.; Jin, L.; Zhu, Y.-J.; Jin, F.-J. Studies of Cellulose and Starch Utilization and the Regulatory Mechanisms of Related Enzymes in Fungi. Polymers 2020, 12, 530. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Izydorczyk, G.; Taf, R.; Moustakas, K.; Chojnacka, K. Cellulose-Based Fertilizers for Sustainable Agriculture: Effective Methods for Increasing Crop Yield and Soil Health. Ind. Crops Prod. 2023, 205, 117500. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Moliterni, S.; Errico, S.; Spagnoletta, A.; Dimatteo, S. Advanced Technologies for Chitin Recovery from Crustacean Waste. Clean Technol. Recycl. 2023, 3, 4–43. [Google Scholar] [CrossRef]
- Kisaakye, J.; Beesigamukama, D.; Haukeland, S.; Subramanian, S.; Thiongo, P.K.; Kelemu, S.; Tanga, C.M. Chitin-Enriched Insect Frass Fertilizer as a Biorational Alternative for Root-Knot Nematode (Meloidogyne incognita) Management. Front.Plant Sci. 2024, 15, 1361739. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Oldham (Konak), T.; Rogers, R.D. Applications of Chitin in Agriculture. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2019; pp. 125–146. [Google Scholar]
- Ramírez, M.A.; Rodríguez, A.T.; Alfonso, L.; Peniche, C. Chitin and Its Derivatives as Biopolymers with Potential Agricultural Applications. Biotecnol. Apl. 2010, 27, 270–276. [Google Scholar]
- Parada, R.Y.; Egusa, M.; Aklog, Y.F.; Miura, C.; Ifuku, S.; Kaminaka, H. Optimization of Nanofibrillation Degree of Chitin for Induction of Plant Disease Resistance: Elicitor Activity and Systemic Resistance Induced by Chitin Nanofiber in Cabbage and Strawberry. Int. J. Biol. Macromol. 2018, 118, 2185–2192. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, H.; Rosqvist, E.; Lastusaari, M.; Peltonen, J.; Vähäsalo, L.; Xu, C.; Wang, X.; Pranovich, A. Crystalline Nanoxylan from Hot Water Extracted Wood Xylan at Multi-Length Scale: Molecular Assembly from Nanocluster Hydrocolloids to Submicron Spheroids. Carbohydr. Polym. 2024, 335, 122089. [Google Scholar] [CrossRef]
- Curry, T.M.; Peña, M.J.; Urbanowicz, B.R. An Update on Xylan Structure, Biosynthesis, and Potential Commercial Applications. Cell Surf. 2023, 9, 100101. [Google Scholar] [CrossRef]
- Ye, Z.-H.; Zhong, R. Outstanding Questions on Xylan Biosynthesis. Plant Sci. 2022, 325, 111476. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Wu, A.-M. Balanced Xylan Acetylation Is the Key Regulator of Plant Growth and Development, and Cell Wall Structure and for Industrial Utilization. Int. J. Mol. Sci. 2020, 21, 7875. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Blasi, A.; Lopresto, C.G.; Calabrò, V. Bioconversion of Crop Residues Using Alternative Fermentation-Based Approaches. Front. Biosci. 2023, 15, 17. [Google Scholar] [CrossRef]
- Abbas, A.; Wang, Z.; Zhang, Y.; Peng, P.; She, D. Lignin-Based Controlled Release Fertilizers: A Review. Int. J. Biol. Macromol. 2022, 222, 1801–1817. [Google Scholar] [CrossRef]
- Ahmad, U.M.; Ji, N.; Li, H.; Wu, Q.; Song, C.; Liu, Q.; Ma, D.; Lu, X. Can Lignin Be Transformed into Agrochemicals? Recent Advances in the Agricultural Applications of Lignin. Ind. Crops Prod. 2021, 170, 113646. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V.; Nebbioso, A.; Drosos, M.; Nuzzo, A.; Mazzei, P.; Piccolo, A. Humic-like Bioactivity on Emergence and Early Growth of Maize (Zea mays L.) of Water-Soluble Lignins Isolated from Biomass for Energy. Plant Soil 2016, 402, 221–233. [Google Scholar] [CrossRef]
- Popa, V.I.; Dumitru, M.; Volf, I.; Anghel, N. Lignin and Polyphenols as Allelochemicals. Ind. Crops Prod. 2008, 27, 144–149. [Google Scholar] [CrossRef]
- Hemmilä, V.; Sandberg, J.; Sandberg, D. Lignin: An Adhesive Raw Material of the Future or Waste of Research Energy? In Northern European Network for Wood Science and Engineering (WSE): Proceedings of the 9th Meeting; Brischke, C., Meyer, L., Eds.; WSE: Hannover, Germany, 2013; pp. 98–103. [Google Scholar]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in Controlled Release Pesticide Formulations: Prospects to Safer Integrated Pest Management and Sustainable Agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Kosewska, A.; Załuski, D.; Kozera, W.J.; Żarczyński, P.J. Farmed Insect Frass as a Future Organic Fertilizer. Appl. Sci. 2024, 14, 2380. [Google Scholar] [CrossRef]
- Gan, S.K.-E.; Phua, S.-X.; Yeo, J.Y.; Heng, Z.S.-L.; Xing, Z. Method for Zero-Waste Circular Economy Using Worms for Plastic Agriculture: Augmenting Polystyrene Consumption and Plant Growth. Methods Protoc. 2021, 4, 43. [Google Scholar] [CrossRef]
- Nyanzira, A.; Machona, O.; Matongorere, M.; Chidzwondo, F.; Mangoyi, R. Analysis of Frass Excreted by Tenebrio molitor for Use as Fertilizer. Entomol. Appl. Sci. Lett. 2023, 10, 29–37. [Google Scholar] [CrossRef]
- Karkanis, A.; Asprogeraka, A.C.; Paouris, E.; Ntanasi, T.; Karavidas, I.; Rumbos, C.I.; Athanassiou, C.G.; Ntatsi, G. Yellow Mealworm Frass: A Promising Organic Fertilizer for Common Sowthistle (Sonchus oleraceus L.) and Bristly Oxtongue (Helminthotheca echioides (L.) Holub) Cultivation. Heliyon 2024, 10, e35508. [Google Scholar] [CrossRef]
- Chia, S.Y.; van Loon, J.J.A.; Dicke, M. Effects of Frass from Larvae of Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) on Growth and Insect Resistance in Field Mustard (Brassica rapa): Differences between Insect Species and Frass Treatments. Entomol. Exp. Appl. 2024, 172, 394–408. [Google Scholar] [CrossRef]
- Zim, J.; Aitikkou, A.; EL Omari, M.H.; EL Malahi, S.; Azim, K.; Hirich, A.; Nilahyane, A.; Oumouloud, A. A New Organic Amendment Based on Insect Frass for Zucchini (Cucurbita pepo L.) Cultivation. Environ. Sci. Proc. 2022, 16, 28. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Liu, H. Feasibility of Feeding Yellow Mealworm (Tenebrio molitor L.) in Bioregenerative Life Support Systems as a Source of Animal Protein for Humans. Acta Astronaut. 2013, 92, 103–109. [Google Scholar] [CrossRef]
- Moruzzo, R.; Riccioli, F.; Espinosa Diaz, S.; Secci, C.; Poli, G.; Mancini, S. Mealworm (Tenebrio molitor): Potential and Challenges to Promote Circular Economy. Animals 2021, 11, 2568. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B. Environmental Impact of Insect Rearing. In Insects as Animal Feed: Novel Ingredients for Use in Pet, Aquaculture and Livestock Diets; CABI: Surrey, UK, 2021; pp. 53–59. [Google Scholar]
- Sangiorgio, P.; Verardi, A.; Dimatteo, S.; Spagnoletta, A.; Moliterni, S.; Errico, S. Valorisation of Agri-Food Waste and Mealworms Rearing Residues for Improving the Sustainability of Tenebrio molitor Industrial Production. J. Insects Food Feed 2022, 8, 509–524. [Google Scholar] [CrossRef]
- Pandao, M.R.; Rathod, S.R.; Sirsat, D.D.; Lingayat, N.R. The Role of Soil in Carbon Sequestration: Mechanisms and Implications. Asian J. Environ. Ecol. 2024, 23, 66–75. [Google Scholar] [CrossRef]
- van Groenigen, K.J.; van Kessel, C.; Hungate, B.A. Increased Greenhouse-Gas Intensity of Rice Production under Future Atmospheric Conditions. Nat. Clim. Chang. 2013, 3, 288–291. [Google Scholar] [CrossRef]
- Rumpel, C.; Henry, B.; Chenu, C.; Amiraslani, F. Benefits and Trade-Offs of Soil Organic Carbon Sequestration; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; pp. 183–208. [Google Scholar]
- Gitari, M. PanAfrican Agriculture (Magazine), July–September 2023. pp. 31–32. Available online: https://panagrimedia.com/13th-edition/ (accessed on 23 February 2025).
- Foughar, M.; Arrobas, M.; Rodrigues, M.Â. Mealworm Larvae Frass Exhibits a Plant Biostimulant Effect on Lettuce, Boosting Productivity beyond Just Nutrient Release or Improved Soil Properties. Horticulturae 2024, 10, 711. [Google Scholar] [CrossRef]
- van Huis, A. Edible Insects: Challenges and Prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T. Biochar: A Sustainable Solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A.; Chakraborty, S.; Vithanage, M.; Biswas, J.K. Multifaceted Applications of Biochar in Environmental Management: A Bibliometric Profile. Biochar 2023, 5, 11. [Google Scholar] [CrossRef]
- Thalassinos, G.; Levizou, E.; Antoniadis, V. Can Soil Improvers (Biochar, Compost, Insect Frass, Lime, and Zeolite) Achieve Phytostabilization of Potentially Toxic Elements in Heavily Contaminated Soil with the Use of Purslane (Portulaca oleracea)? Agronomy 2023, 13, 2827. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Xiang, H.; Liu, R.; Su, L.; Zhang, L.; Ji, R. Functional Utilization of Biochar Derived from Tenebrio molitor Feces for CO2 Capture and Supercapacitor Applications. RSC Adv. 2022, 12, 22760–22769. [Google Scholar] [CrossRef]
- He, L.; Yang, S.-S.; Bai, S.-W.; Pang, J.-W.; Liu, G.-S.; Cao, G.-L.; Zhao, L.; Feng, X.-C.; Ren, N.-Q. Fabrication and Environmental Assessment of Photo-Assisted Fenton-like Fe/FBC Catalyst Utilizing Mealworm Frass Waste. J. Clean. Prod. 2020, 256, 120259. [Google Scholar] [CrossRef]
- Cara, I.G.; Țopa, D.; Puiu, I.; Jităreanu, G. Biochar a Promising Strategy for Pesticide-Contaminated Soils. Agriculture 2022, 12, 1579. [Google Scholar] [CrossRef]
- Larouche, J.; Campbell, B.; Hénault-Éthier, L.; Banks, I.J.; Tomberlin, J.K.; Preyer, C.; Deschamps, M.-H.; Vandenberg, G.W. The Edible Insect Sector in Canada and the United States. Anim. Front. 2023, 13, 16–25. [Google Scholar] [CrossRef]
- Mazzarelli, S. The Food Safety Law in China: Regulatory Framework and Implications for International Trade. Master’s Thesis, Università Ca’ Foscari, Venezia, Italy, 2015. [Google Scholar]
- International Platform of Insects for Food and Feed (IPIFF). Fact Sheet on Insect Frass; IPIFF: Brussels, Belgium, 2021. [Google Scholar]
- European Commission Regulation (EU) 2021/1925 of 5 November 2021 Amending Regulation (EU) No 142/2011 as Regards the Use of Certain Animal By-Products and Derived Products as Organic Fertilizers and Soil Improvers. Available online: https://eur-lex.europa.eu/eli/reg/2021/1925/oj (accessed on 14 March 2025).
- International Platform of Insects for Food and Feed (IPIFF). Contribution Paper on Application of Insect Frass as Fertilising Product in Agriculture; IPIFF: Brussels, Belgium, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).