Transcriptome and Physio-Biochemical Profiling Reveals Differentially Expressed Genes in Seedlings from Aerial and Subterranean Seeds Subjected to Drought Stress in Amphicarpaea edgeworthii Benth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought Treatment
2.2. Physiological Index Measurements Under Drought Stress
2.3. RNA Extraction and Illumina RNA-Seq
2.4. Raw Data Processing, Read Mapping, and Differentially Expressed Gene Analysis
2.5. Functional Annotation of DEGs
2.6. Quantitative Real-Time PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
3.1. Phenotypic and Physiological Alterations in A. edgeworthii Under Drought Stress
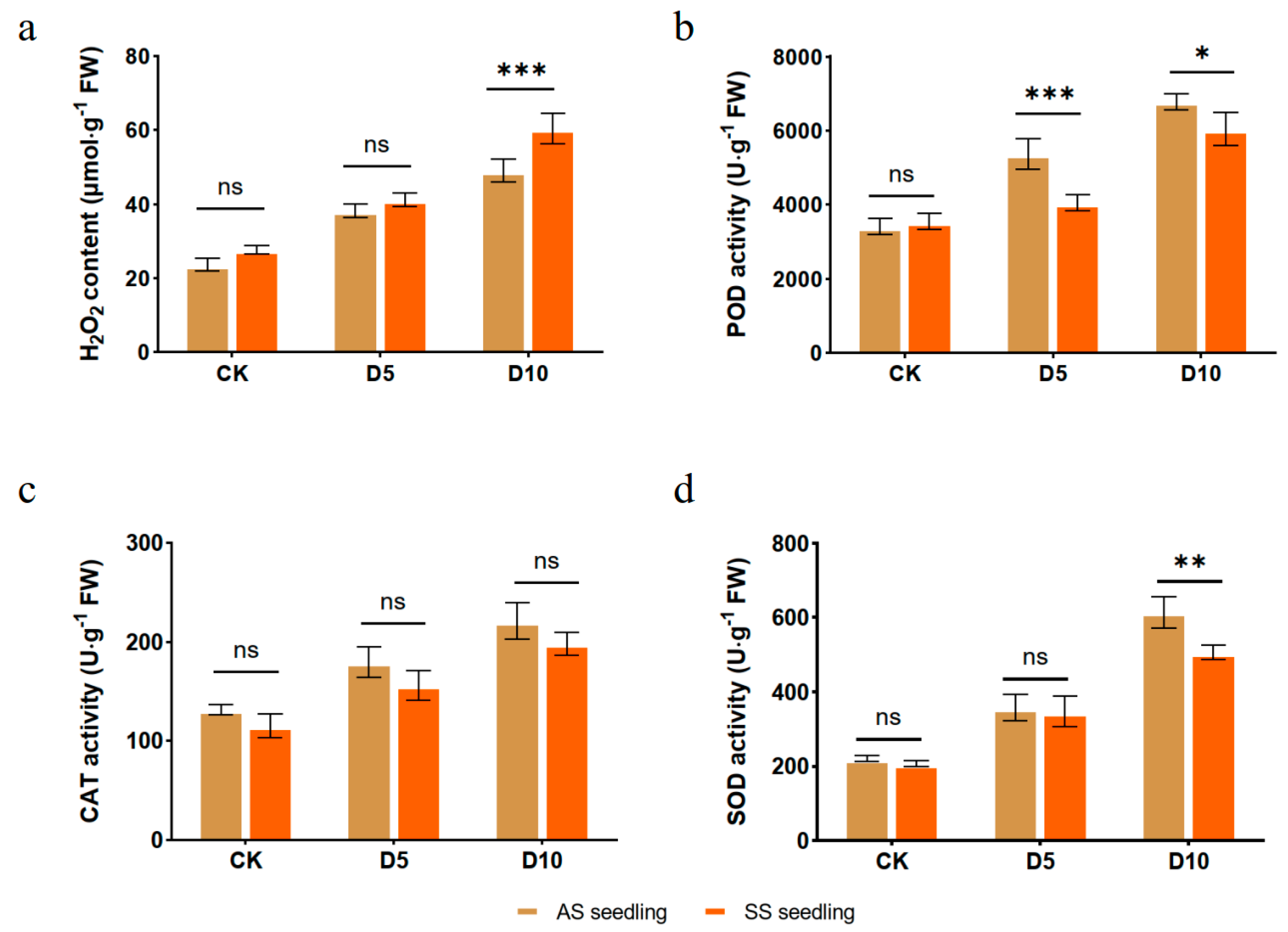
3.2. Effect of Drought Stress on ROSs and Antioxidant Activities in A. edgeworthii
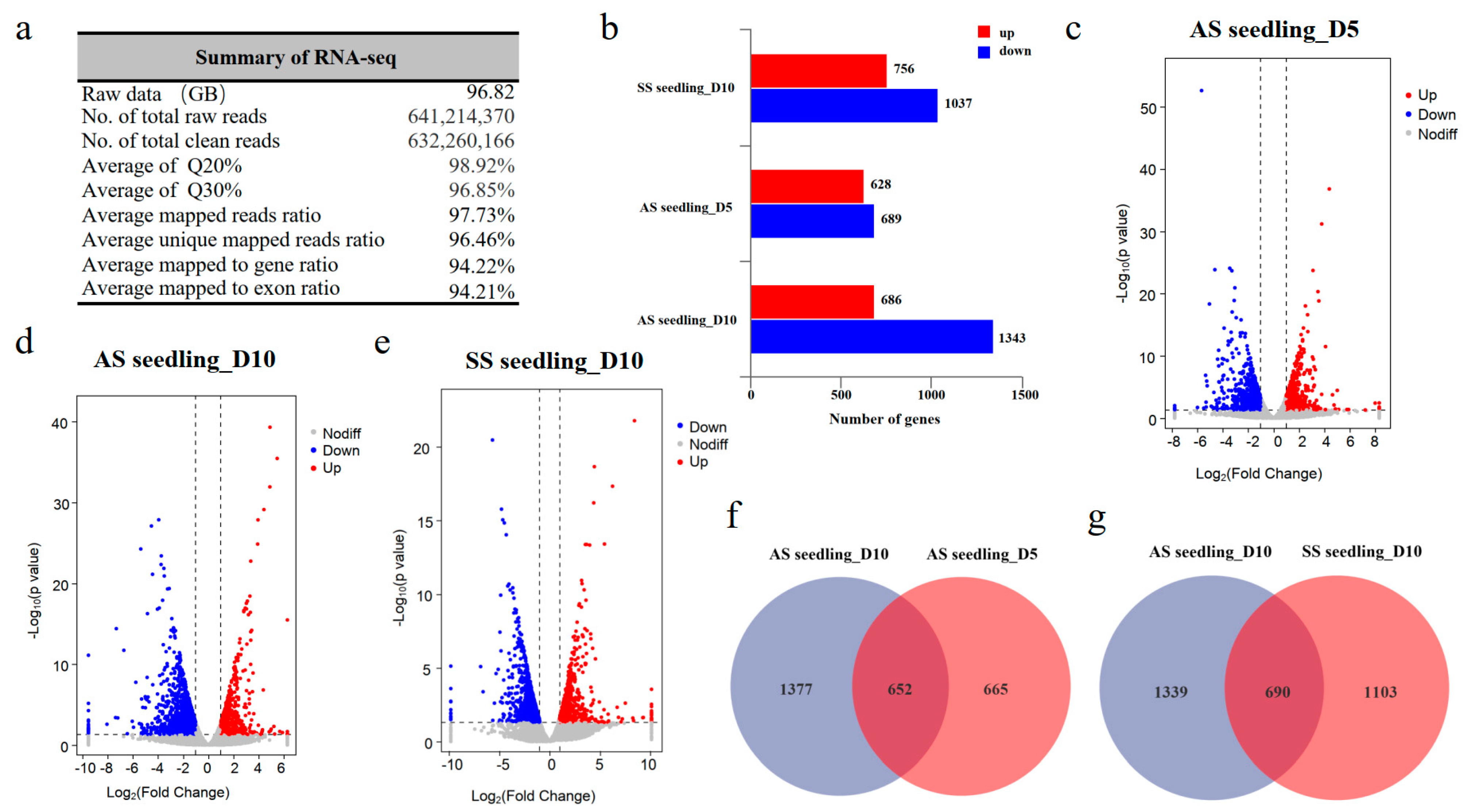
3.3. RNA-Seq Analysis and Screening of DEGs in AS and SS Seedlings Under Drought Stress
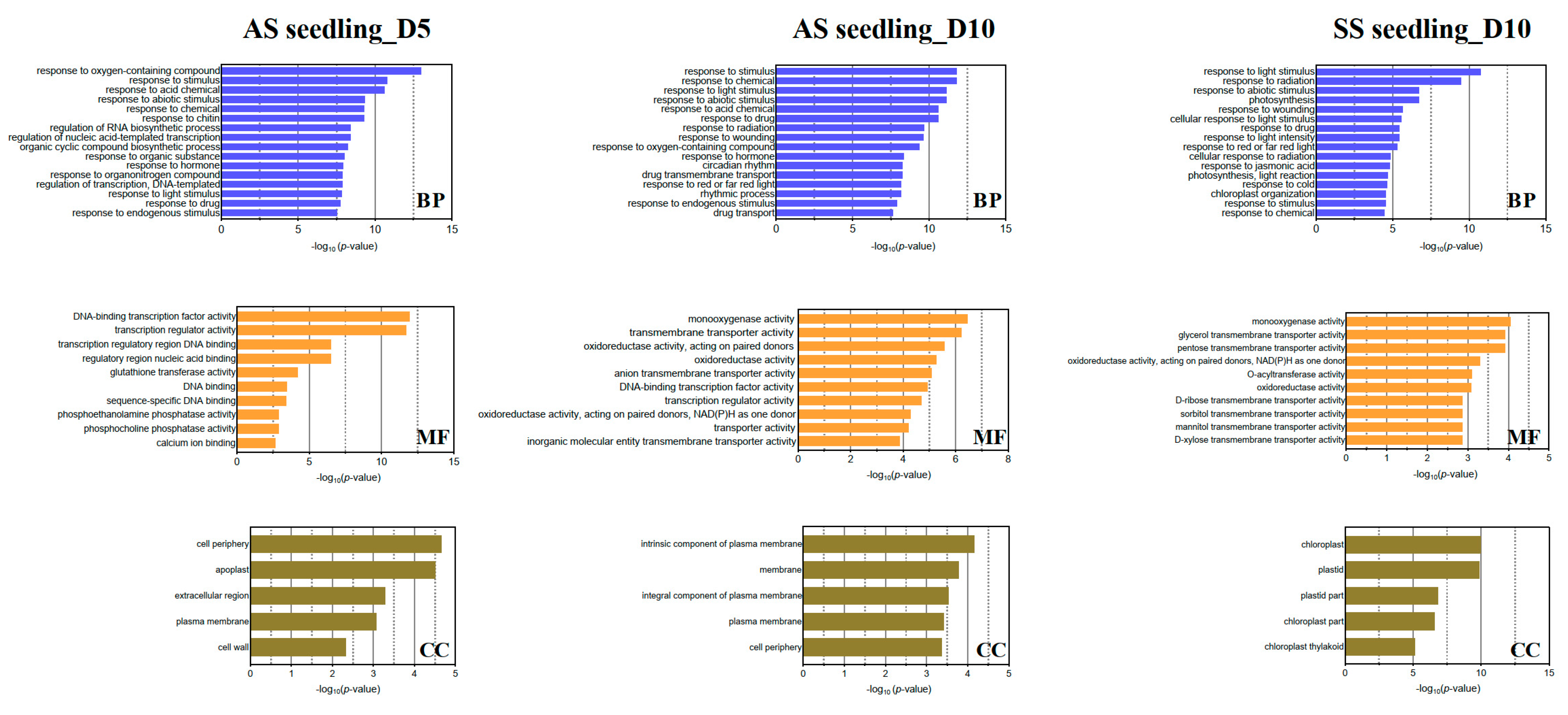
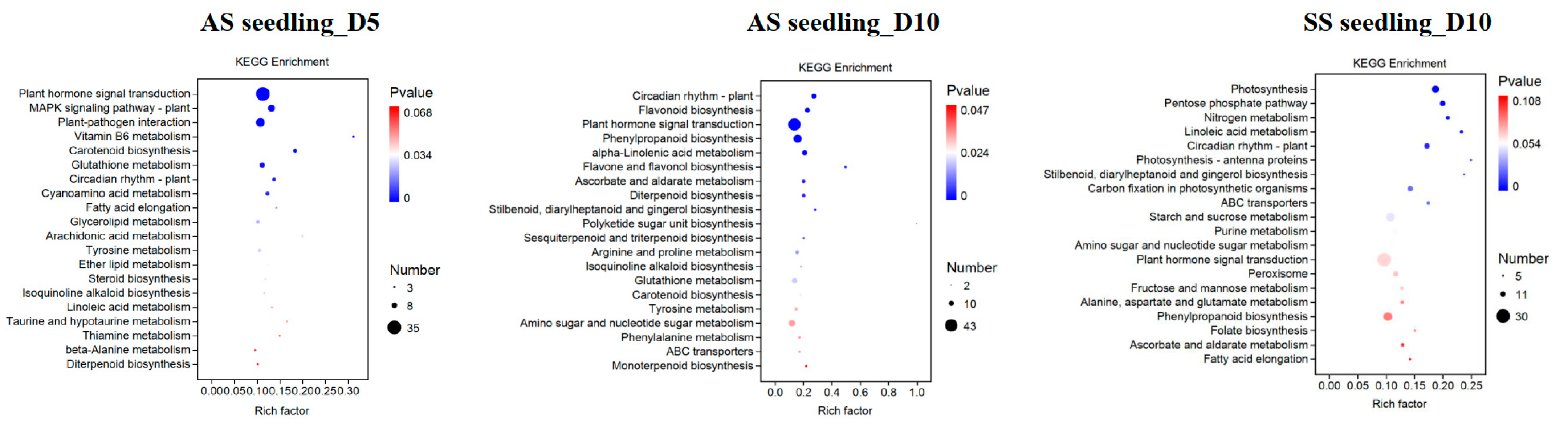
3.4. Functional Enrichment Analyses of Drought-Responsive DEGs
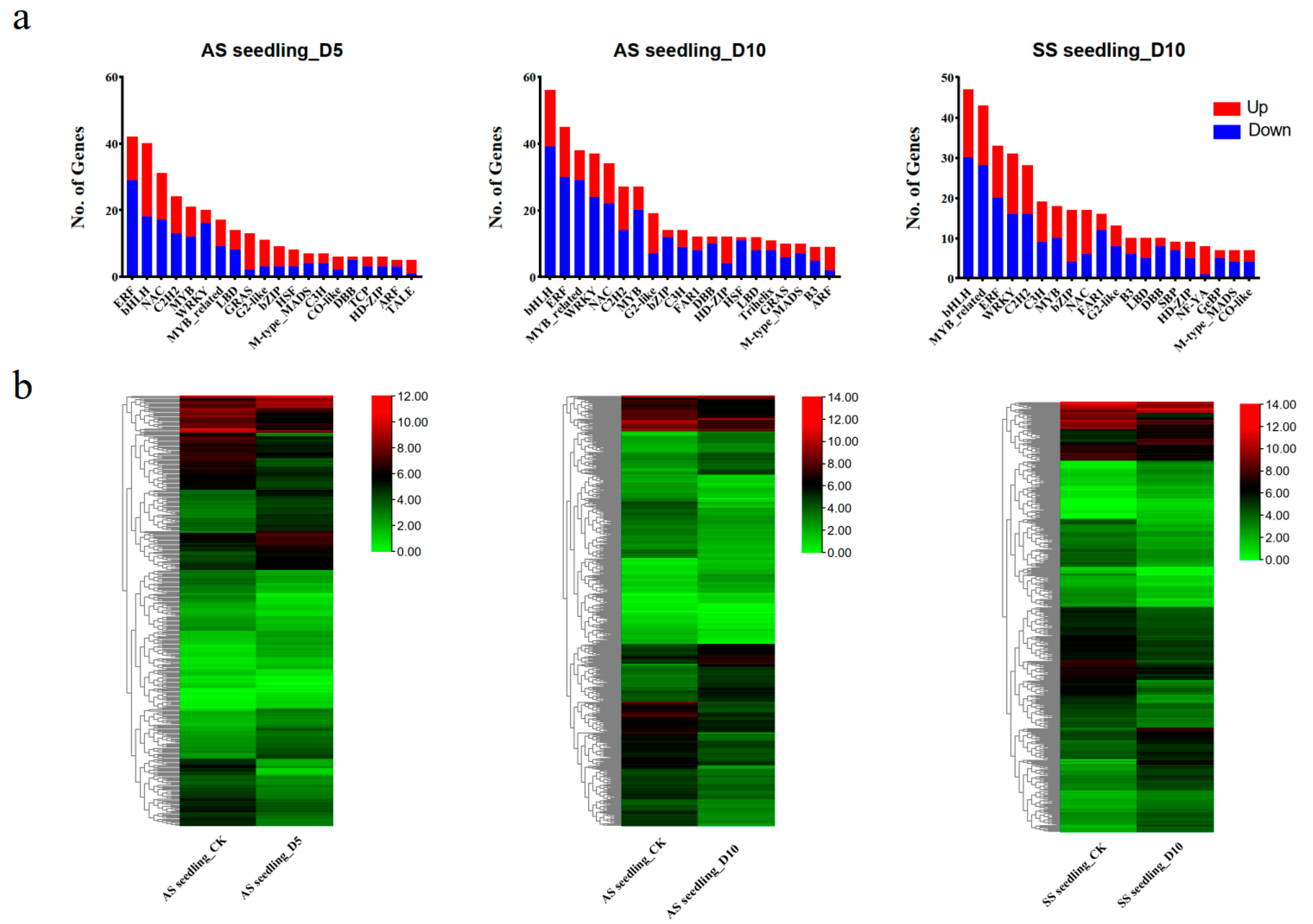
3.5. Distribution of Differentially Expressed Transcription Factors
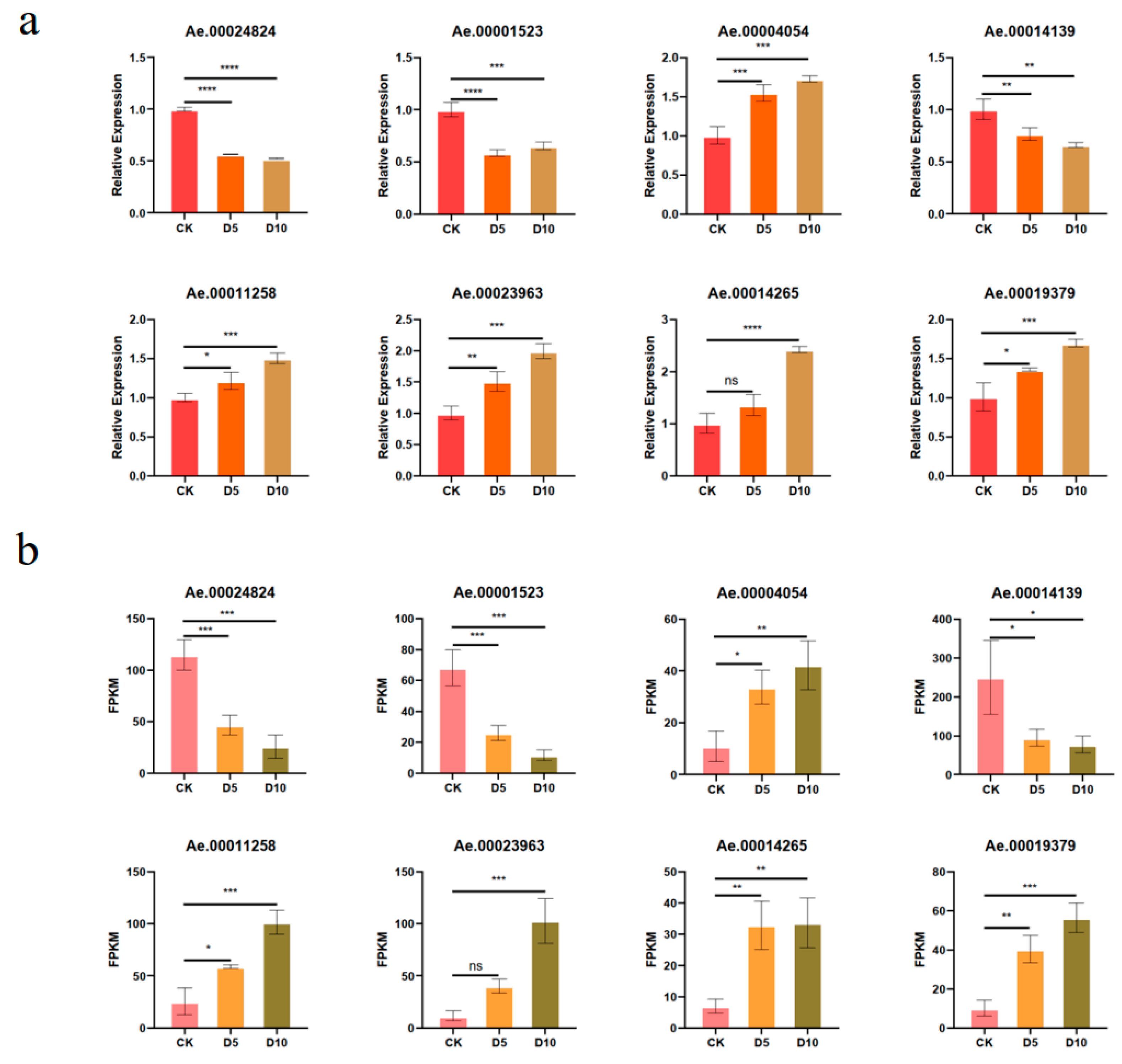
3.6. Validation of DEG Expression Patterns via RT-qPCR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | Aerial seeds |
| SS | Subterranean seeds |
| RNA-seq | RNA sequencing |
| DEG | Differentially expressed gene |
| TF | Transcription factor |
| ROS | Reactive oxygen species |
References
- Zhang, Y.; Yang, J.; Rao, G.-Y. Genetic Diversity of an Amphicarpic Species, Amphicarpaea edgeworthii Benth. (Leguminosae) Based on RAPD Markers. Biochem. Syst. Ecol. 2005, 33, 1246–1257. [Google Scholar] [CrossRef]
- Zhang, K.; Baskin, J.M.; Baskin, C.C.; Yang, X.; Huang, Z. Lack of Divergence in Seed Ecology of Two Amphicarpaea (Fabaceae) Species Disjunct between Eastern Asia and Eastern North America. Am. J. Bot. 2015, 102, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Han, K.; Li, R.; Xu, G.; Han, Y.; Cui, F.; Fan, S.; Seim, I.; Fan, G.; et al. Insights into Amphicarpy from the Compact Genome of the Legume Amphicarpaea edgeworthii. Plant Biotechnol. J. 2021, 19, 952–965. [Google Scholar] [CrossRef]
- Zhang, K.; Baskin, J.M.; Baskin, C.C.; Yang, X.; Huang, Z. Effect of Seed Morph and Light Level on Growth and Reproduction of the Amphicarpic Plant Amphicarpaea edgeworthii (Fabaceae). Sci. Rep. 2017, 7, 39886. [Google Scholar] [CrossRef]
- González, E.M. Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2023, 24, 6562. [Google Scholar] [CrossRef]
- Valliyodan, B.; Nguyen, H.T. Understanding Regulatory Networks and Engineering for Enhanced Drought Tolerance in Plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, A.; Ambavaram, M.M.R.; Klumas, C.; Krishnan, A.; Batlang, U.; Myers, E.; Grene, R.; Pereira, A. Effects of Drought on Gene Expression in Maize Reproductive and Leaf Meristem Tissue Revealed by RNA-Seq. Plant Physiol. 2012, 160, 846–867. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of Drought on Photosynthesis, Total Antioxidant Capacity, Bioactive Component Accumulation, and the Transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Cheng, J. Reduced Chilling Injury in Cucumber by Nitric Oxide and the Antioxidant Response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Bhatnagar-Mathur, P.; Vadez, V.; Jyostna Devi, M.; Lavanya, M.; Vani, G.; Sharma, K.K. Genetic Engineering of Chickpea (Cicer arietinum L.) with the P5CSF129A Gene for Osmoregulation with Implications on Drought Tolerance. Mol. Breed. 2009, 23, 591–606. [Google Scholar] [CrossRef]
- Chen, Y.; Chi, Y.; Meng, Q.; Wang, X.; Yu, D. GmSK1, an SKP1 Homologue in Soybean, Is Involved in the Tolerance to Salt and Drought. Plant Physiol. Biochem. 2018, 127, 25–31. [Google Scholar] [CrossRef]
- Privitera, G.F.; Treccarichi, S.; Nicotra, R.; Branca, F.; Pulvirenti, A.; Lo Piero, A.R.; Sicilia, A. Comparative Transcriptome Analysis of B. oleracea L. Var. Italica and B. macrocarpa Guss. Genotypes under Drought Stress: De Novo vs Reference Genome Assembly. Plant Stress 2024, 14, 100657. [Google Scholar] [CrossRef]
- Behera, S.; Voshall, A.; Moriyama, E.N. Plant Transcriptome Assembly: Review and Benchmarking. In Bioinformatics; Nakaya, H.I., Ed.; Exon Publications: Brisbane, Australia, 2021; pp. 109–130. ISBN 978-0-6450017-1-6. [Google Scholar]
- Ding, N.; Zhao, Y.; Wang, W.; Liu, X.; Shi, W.; Zhang, D.; Chen, J.; Ma, S.; Sun, Q.; Wang, T.; et al. Transcriptome Analysis in Contrasting Maize Inbred Lines and Functional Analysis of Five Maize NAC Genes under Drought Stress Treatment. Front. Plant Sci. 2023, 13, 1097719. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Dun, B.; Li, L.; Ma, X.; Zhang, H.; Su, Y.; Wu, D. Metabolomic and Transcriptomic Responses of Adiantum (Adiantum nelumboides) Leaves under Drought, Half-Waterlogging, and Rewater Conditions. Front. Genet. 2023, 14, 1113470. [Google Scholar] [CrossRef]
- Singh, V.; Gupta, K.; Singh, S.; Jain, M.; Garg, R. Unravelling the Molecular Mechanism Underlying Drought Stress Response in Chickpea via Integrated Multi-Omics Analysis. Front. Plant Sci. 2023, 14, 1156606. [Google Scholar] [CrossRef]
- Azzouz-Olden, F.; Hunt, A.G.; Dinkins, R. Transcriptome Analysis of Drought-Tolerant Sorghum Genotype SC56 in Response to Water Stress Reveals an Oxidative Stress Defense Strategy. Mol. Biol. Rep. 2020, 47, 3291–3303. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Sun, A.; Wang, L.; Ren, C.; Liu, J.; Gao, X. Transcriptome Analysis Reveals Key Drought-Stress-Responsive Genes in Soybean. Front. Genet. 2022, 13, 1060529. [Google Scholar] [CrossRef]
- Aleem, M.; Raza, M.M.; Haider, M.S.; Atif, R.M.; Ali, Z.; Bhat, J.A.; Zhao, T. Comprehensive RNA-seq Analysis Revealed Molecular Pathways and Genes Associated with Drought Tolerance in Wild Soybean (Glycine soja Sieb. and Zucc.). Physiol. Plant. 2021, 172, 707–732. [Google Scholar] [CrossRef]
- Abdeen, A.; Schnell, J.; Miki, B. Transcriptome Analysis Reveals Absence of Unintended Effects in Drought-Tolerant Transgenic Plants Overexpressing the Transcription Factor ABF3. BMC Genom. 2010, 11, 69. [Google Scholar] [CrossRef]
- Jia, X.; Sun, C.; Zuo, Y.; Li, G.; Li, G.; Ren, L.; Chen, G. Integrating Transcriptomics and Metabolomics to Characterise the Response of Astragalus membranaceus Bge. var. Mongolicus (Bge.) to Progressive Drought Stress. BMC Genom. 2016, 17, 188. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Alekseyev, Y.O.; Fazeli, R.; Yang, S.; Basran, R.; Maher, T.; Miller, N.S.; Remick, D. A Next-Generation Sequencing Primer—How Does It Work and What Can It Do? Acad. Pathol. 2018, 5, 2374289518766521. [Google Scholar] [CrossRef]
- Liu, E.; Xu, L.; Luo, Z.; Li, Z.; Zhou, G.; Gao, H.; Fang, F.; Tang, J.; Zhao, Y.; Zhou, Z.; et al. Transcriptomic Analysis Reveals Mechanisms for the Different Drought Tolerance of Sweet Potatoes. Front. Plant Sci. 2023, 14, 1136709. [Google Scholar] [CrossRef]
- Tamang, B.G.; Li, S.; Rajasundaram, D.; Lamichhane, S.; Fukao, T. Overlapping and Stress-specific Transcriptomic and Hormonal Responses to Flooding and Drought in Soybean. Plant J. 2021, 107, 100–117. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Ma, Y.; Xie, H.; Chang, F.; Guan, C.; Yang, B.; Ma, Y. Proline Metabolism in Response to Climate Extremes in Hairgrass. Plants 2024, 13, 1408. [Google Scholar] [CrossRef]
- Čakar, U.; Čolović, M.; Milenković, D.; Pagnacco, M.; Maksimović, J.; Krstić, D.; Đorđević, B. Strawberry and Drupe Fruit Wines Antioxidant Activity and Protective Effect Against Induced Oxidative Stress in Rat Synaptosomes. Antioxidants 2025, 14, 155. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Zulfiqar, U.; Yong, J.W.H.; Ishfaq, M.; Din, K.U.; Ullah, A.; Abbas, A.; Awan, M.I.; Moussa, I.M.; et al. Thiourea Improves Yield and Quality Traits of Brassica napus L. by Upregulating the Antioxidant Defense System under High Temperature Stress. Sci. Rep. 2024, 14, 12195. [Google Scholar] [CrossRef]
- Das, D.; Chowdhury, N.; Sharma, M.; Suma, R.; Saikia, B.; Velmurugan, N.; Chikkaputtaiah, C. Screening for Brown-Spot Disease and Drought Stress Response and Identification of Dual-Stress Responsive Genes in Rice Cultivars of Northeast India. Physiol. Mol. Biol. Plants 2024, 30, 647–663. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Sun, Y.; Zhang, J.; Li, C. Influence of Drought Hardening on the Resistance Physiology of Potato Seedlings under Drought Stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, T.; Li, X.; Song, C.-P.; Zhu, J.-K.; Chen, L.; Zhao, Y. Phosphorylation of SWEET Sucrose Transporters Regulates Plant Root:Shoot Ratio under Drought. Nat. Plants 2022, 8, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, C.; Cao, Y.; Wang, C.; Sun, X.; Zhang, L.; Wang, W.; Song, S. Comparative Physiological and Transcriptomic Analysis of Two Contrasting Soybean Genotypes Reveals Complex Mechanisms Involved in Drought Avoidance. Crop Sci. 2024, 64, 788–802. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Ullah, F.; Ben-Hur, A.; Reddy, A.S.N. Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress. Int. J. Mol. Sci. 2020, 21, 772. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, T.; Kang, S.; Zhang, J.; Zhang, S.; Yang, B.; Ma, X.; Guo, L.; Li, M.; Jin, L. Integrated Physiological Characterisation and Transcriptomics Reveals Drought Tolerance Differences between Two Cultivars of A. Sinensis at Seedling Stage. Mol. Biol. Rep. 2025, 52, 283. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Feng, C. Genome-Wide Analysis of MYB Genes in Primulina eburnea (Hance) and Identification of Members in Response to Drought Stress. Int. J. Mol. Sci. 2023, 25, 465. [Google Scholar] [CrossRef]
- Tao, R.; Liu, Y.; Chen, S.; Shityakov, S. Meta-Analysis of the Effects of Overexpressed bZIP Transcription Factors in Plants under Drought Stress. Plants 2024, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Wang, S. Comprehensive Analysis and Discovery of Drought-Related NAC Transcription Factors in Common Bean. BMC Plant Biol. 2016, 16, 193. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Wang, L.; Wang, S. Genome-Wide Investigation of WRKY Transcription Factors Involved in Terminal Drought Stress Response in Common Bean. Front. Plant Sci. 2017, 8, 380. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wu, M.; Li, L.; Li, C.; Han, Z.; Yuan, J.; Chen, C.; Song, W.; Wang, C. Genome-Wide Identification of AP2/ERF Transcription Factors in Cauliflower and Expression Profiling of the ERF Family under Salt and Drought Stresses. Front. Plant Sci. 2017, 8, 946. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, J.; Su, Y.; Lei, T.; Hou, S.; Tian, J.; Li, M.; Zhang, S.; Ding, X.; Li, Q.; Xiao, J. Transcriptome and Physio-Biochemical Profiling Reveals Differentially Expressed Genes in Seedlings from Aerial and Subterranean Seeds Subjected to Drought Stress in Amphicarpaea edgeworthii Benth. Agronomy 2025, 15, 735. https://doi.org/10.3390/agronomy15030735
Kou J, Su Y, Lei T, Hou S, Tian J, Li M, Zhang S, Ding X, Li Q, Xiao J. Transcriptome and Physio-Biochemical Profiling Reveals Differentially Expressed Genes in Seedlings from Aerial and Subterranean Seeds Subjected to Drought Stress in Amphicarpaea edgeworthii Benth. Agronomy. 2025; 15(3):735. https://doi.org/10.3390/agronomy15030735
Chicago/Turabian StyleKou, Jiancheng, Yue Su, Tianyu Lei, Siqi Hou, Jiali Tian, Minglong Li, Shuzhen Zhang, Xiaodong Ding, Qiang Li, and Jialei Xiao. 2025. "Transcriptome and Physio-Biochemical Profiling Reveals Differentially Expressed Genes in Seedlings from Aerial and Subterranean Seeds Subjected to Drought Stress in Amphicarpaea edgeworthii Benth" Agronomy 15, no. 3: 735. https://doi.org/10.3390/agronomy15030735
APA StyleKou, J., Su, Y., Lei, T., Hou, S., Tian, J., Li, M., Zhang, S., Ding, X., Li, Q., & Xiao, J. (2025). Transcriptome and Physio-Biochemical Profiling Reveals Differentially Expressed Genes in Seedlings from Aerial and Subterranean Seeds Subjected to Drought Stress in Amphicarpaea edgeworthii Benth. Agronomy, 15(3), 735. https://doi.org/10.3390/agronomy15030735






