Abstract
The noticeable reduction in plant species abundance near industrial hemp (Cannabis sativa L.) highlights the need to investigate its potential allelopathic effects on selected cultivars’ seed germination and seedling growth. Industrial hemp of the “Helena” variety was used to obtain aqueous extracts by conventional (macerate, hydrolate, and post-distillation residue) and green methods (ultrasonic and microwave extracts) in order to treat thirteen most commonly cultivated plant species, including lettuce, kohlrabi, onion, tomato, carrot, pepper, savoy cabbage, rocket, alfalfa, white mustard, pea, sunflower, and parsley. This is the first time that the allelopathic effects of seven different hemp extracts were tested simultaneously on thirteen different species. The extracts were applied at 10, 25, 50, and 100% concentrations. The seed germination percentage and root/shoot length results for all tested plants, except peas, clearly demonstrated an inhibitory effect of higher concentrations of hemp extracts. This effect was observed regardless of variations in chemical composition (CBD, THC, and total polyphenols), suggesting that different extracts have varying impacts on different species. The weakest inhibitory effect on the germination and seedling length for the majority of the tested plant species was noted for PDR, while the strongest inhibitory effect in terms of seedling length was observed in the case of MAE700.
1. Introduction
Industrial hemp (Cannabis sativa L.) is one of the earliest economic crops, historically valued for its edible seeds, fiber, and medicinal resins [1]. Today, it remains a focal point of debate due to its complex legal, ethical, and social implications. These controversies range from concerns about potential health risks and rare fatalities linked to marijuana use to its recognized therapeutic benefits. The diverse applications of hemp, influenced by selective breeding and environmental factors, have led to a rich variety of growth forms and chemical profiles [2]. Hemp produces over 480 compounds, including cannabinoids, terpenes, flavonoids, and phenolic acids, with cannabinoids being the most studied. However, flavonoids and phenolic acids are also crucial, particularly for plant defense against stressors like drought, salinity, and UV radiation. The phytotoxicity of hemp’s terpenes and cannabinoids indicates their role as allelochemicals, substances that can influence the growth of other plants [3,4].
Allelopathy refers to the impact of one plant species on another through secondary metabolites known as allelochemicals [5]. These compounds, which include phenols, flavonoids, alkaloids, and tannins, can be found in all plant parts and affect various physiological processes, such as germination, growth, and root development [6,7]. Allelochemicals can enter the environment through foliar leaching, root exudation, or decomposition of plant residues, disrupting neighboring plants’ physiological processes and potentially offering new avenues for bioherbicide development [8,9]. Examples include juglone from black walnut trees, which inhibits growth under the tree canopy [10]. Cultivated crops like sorghum, wheat, rye, aromatic medicinal plants, and some weed species demonstrate allelopathic effects, suggesting that allelopathy can be harnessed to develop targeted, environmentally friendly herbicides [11,12]. The most commonly observed morphological effects caused by allelochemicals in plants include inhibited or delayed seed germination, as well as impacts on coleoptile elongation and the development of the radicle, shoot, and root [13,14]. The likely allelochemicals present in Cannabis sativa include phytocannabinoids such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), terpenoids like α-pinene, D-limonene, and linalool, and phenylpropanoids. The mentioned phytocannabinoids and terpenoids contribute to the plant’s secondary metabolite profile and have been associated with various biological activities [15]. So far, the allelopathic effects of hemp aqueous leaf extracts on seed germination and seedling growth in durum wheat and barley have been determined [16], as well as the inhibitory effect of water extracts from fiber hemp on the germination energy and rate in case of spring wheat and winter rye [17]. Additionally, the phytotoxic effects of hemp leaf leachate on wheat, itchgrass, lettuce, pea, bean, and hemp species have been noted [18]. The phytotoxic effects of leaf leachates of hemp female plants reduced the seed germination, fresh weight, and pigment content of the Parthenium hysterophorus [19]. In one study, cannabinoids and terpenes were identified in Cannabis sativa as potential allelochemicals. Namely, hemp extract led to a reduction in seed germination, while surface-applied hemp residue significantly delayed and reduced Amaranthus tuberculatus germination and increased its germination time, suggesting its potential use in integrated weed management [4].
Although compounds within plant tissues may have allelopathic potential, they might not be released into the environment to display seedling allelopathy under natural conditions [20]. Therefore, conventional extraction methods, such as Soxhlet extraction, maceration, and hydrodistillation, have long been used to isolate phenolic allelochemicals from plants. These techniques, though well-established, often have limitations in terms of efficiency and environmental impact. Green extraction methods, like microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE), offer more eco-friendly, efficient, and cost-effective alternatives. MAE improves extraction yield through the synergistic effect of heating and mass transfer and can be performed with or without solvents [21]. UAE enhances extraction efficiency by creating cavitation in the solvent, leading to better solute dissolution and diffusion [22]. Despite their promise, these methods face challenges such as scale-up limitations, which could be addressed by optimizing parameters and using pilot-scale equipment.
This study aims to assess the allelopathic effects of different hemp extracts on 13 widely cultivated plant species, including lettuce, kohlrabi, onion, tomato, carrot, pepper, savoy cabbage, rocket, alfalfa, white mustard, pea, sunflower, and parsley. Although the allelopathic effects of hemp on some cultivated plants have been confirmed, the aim of this study was to expand the range of the test plants and determine the differences in the effects of several hemp extracts on these plants. The hemp extracts, obtained through conventional and green extraction methods, were evaluated under controlled lab conditions to determine their influence on seed germination and seedling growth. This research also seeks to identify hemp extracts with potential as natural herbicides beneficial for both conventional and organic agriculture.
2. Materials and Methods
2.1. Plant Raw Material
On the experimental field of the Department for Vegetable and Alternative Plant Species in Bački Petrovac, Institute of Field and Vegetable Crops, Institute of National Importance for the Republic of Serbia from Novi Sad, (45°21′36″ N; 19°35′26″ E), a commercial crop of monoecious industrial hemp (C. sativa L.) cultivar “Helena” was cultivated according to previous recommendations [23]. The top 30 cm of hemp stems with at least one inflorescence (panicle) were sampled during the crop’s flowering stage from the middle of July until the middle of August in 2021 according to the sampling protocol outlined in Chapter 2, Appendix I of EU Regulation 796/200, procedure A [24]. The sampled plant material was dried under ambient conditions in a well-ventilated place without direct sunlight or wind influence. The stems and seeds of various maturities were removed from the dried plant material. At the same time, the remaining aerial parts (leaves, inflorescence, and bracts) were used as raw plant materials for extractions. The aerial parts were ground using a blender (Alpina SF-2813, VCT Electronics, Shenzhen, China) and sifted for 20 min with vibrating sieves (CISA Cedaceria Industrial, Barcelona, Spain). The average size of the ground plant raw material particles was 0.256 mm.
In order to obtain the extracts convenient for the treatment of selected plant species, distilled water was used as an extraction solvent in both conventional and green extraction technologies.
2.2. Chemicals
As analytical standards, the necessary reagents, including ferulic acid (≥99%, batch 1215711), isovitexin (≥98%), and luteolin (≥98%, batch 118K4113), were purchased from Sigma-Aldrich Chem (Steinheim, Germany). Cannabidiol (CBD) and cannabinol (CBN) analytical standards, both with a purity of 99.95%, were sourced from Lipomed GmbH (Weil am Rhein, Germany). All remaining chemicals and solvents utilized were of analytical reagent grade.
2.3. Conventional Methods of Extracting Plant Raw Materials
2.3.1. Maceration
The plant raw material (20 g) underwent extraction in a laboratory incubator shaker (KS 4000, IKA, Staufen, Germany), utilizing 200 mL of distilled water as the solvent. This extraction method maintained a solid–liquid ratio of 1:10 (w/v) and was carried out at room temperature for 24 h, with a rotation speed of 150 rpm. Following extraction, the obtained macerate (MAC) was vacuum filtered (V-700, Büchi, Switzerland) through filter paper with a pore size of 4 to 12 μm (Schleicher and Schuell, Dassel, Germany) and stored at −18 °C until use. A single maceration process yielded 125 mL of extract.
2.3.2. Hydrodistillation
Using a Clevinger-type apparatus for hydrodistillation (2.5 h) of the plant raw material (60 g) and utilizing 800 mL of distilled water as the solvent, three fractions were obtained: essential oil (EO), hydrolate (HYD), and post-distillation residue (PDR). Some authors analyzed the EO fraction for its low polar and aromatic composition, while the by-products of the hydrodistillation, PDR and HYD, were utilized to treat cultivated plant species in this study [2]. PDR was acquired by separating the plant material from distilled water in a glass flask via vacuum (V-700, Büchi, Switzerland) filtration using filter paper with a pore size of 4 to 12 μm (Schleicher and Schuell, Dassel, Germany). HYD was collected from the tube connecting the graduated receiving tube for EO to the glass flask containing the plant material and distilled water. The prepared PDR and HYD samples were stored in a freezer at −18 °C. A single hydrodistillation process yielded 30 mL of HYD and 500 mL of PDR.
2.4. Green Methods of Extracting Hemp Panicles
2.4.1. Microwave-Assisted Extraction (MAE)
In a modified microwave oven (NN-E201, Panasonic, Osaka, Japan), an Erlenmeyer flask equipped with a Liebig condenser was arranged. The flask containing 20 g of plant raw material and distilled water as a solvent, adhering to a solid–liquid ratio of 1:10 (w/v), was carefully positioned to maintain a consistent distance from the magnetron. The extraction process lasted 20 min, with microwave power set at 210 W and 700 W, yielding two extracts—MAE210W and MAE700W. These extracts were subsequently subjected to vacuum filtration (V-700, Büchi, Switzerland) through filter paper with a pore size ranging from 4 to 12 μm (Schleicher and Schuell, Dassel, Germany) and then individually stored at −18 °C until they were utilized in the treatment of cultivated plant species. A single MAE process yielded 125 mL of the MAE210 extract and 125 mL of the MAE700 extract.
2.4.2. Ultrasound-Assisted Extraction (UAE)
The extraction process was conducted using an ultrasonic water bath (EUP540A, EUinstruments, Paris, France), with a duration of 40 min and a constant frequency (40 kHz) and power (240 W) maintained throughout. The ultrasonic water bath features a heater capable of maintaining temperatures from ambient levels up to 80 °C. Each flask contained 20 g of plant raw material combined with distilled water, following a solid–liquid ratio of 1:10 (w/v). Consistently, the flasks were positioned at an equal distance from the ultrasound source. As previously reported [2], the extraction temperature was a crucial determinant in UAE, resulting in the yield of two distinct extracts at 30 °C (UAE30°C) and 60 °C (UAE60°C). The obtained extracts were subsequently subjected to vacuum filtration (V-700, Büchi, Switzerland) through filter paper with a pore size ranging from 4 to 12 μm (Schleicher and Schuell, Dassel, Germany) and then individually stored at −18 °C until they were utilized in the treatment of cultivated plant species. A single UAE process yielded 125 mL of UAE30 and 125 mL of the UAE60 extract.
2.5. Identification and Quantification of Phenolic Compounds
HPLC analysis of hemp extracts was performed using a liquid chromatograph (Agilent 1200 series, Agilent Technologies, Santa Clara, CA, USA) equipped with a diode array detector (DAD) and an Eclipse XDB-C18, 1.8 μm, 4.6 × 50 mm column according to the method explained in detail [25]. Solvents A (methanol) and B (1% formic acid in water (v/v)) were used as the mobile phase, with a flow rate of 1 mL min−1. The solvent gradient was performed by varying the proportion of solvent A to solvent B as follows: at the beginning, 10% A; 0–10 min, 10–25% A; 10–20 min, 25–60% A; 20–30 min, 60–70% A. The column temperature was 30 °C, while the injection volume was 5 μL. The optimized industrial hemp extracts (MAC, PDR, HYD, UAE30, UAE60, MAE210, and MAE700) were properly diluted with a mixture of mobile phases (A:B = 10%:90%; v/v) filtered through a syringe filter (RC; 0.45 μm) and automatically injected into the HPLC system using an autosampler. Detection was carried out at 325 nm. Based on the obtained peak areas, a calibration curve was created for each particular standard (luteolin R2 = 0.9963; ferulic R2 = 0.996; isovitexin R2 = 1) depending on the standard concentration. Vitexin content was determined regarding isovitexin equivalence, while orientin and apigenin contents were evaluated relative to luteolin equivalents. Taking into account the obtained linear concentration dependence, spectrum, retention time, and peak area, the concentrations of individual polyphenolic compounds in the tested samples were calculated. The analyses were performed in two replicates.
2.6. Identification and Quantification of Cannabinoids
The contents of cannabidiol (CBD) and tetrahydrocannabinol (THC) in all the obtained hemp extracts were determined by gas chromatography coupled with mass spectrometry (GC-MS). Methanol in 2.5 mL was added to 0.5 mL of the extract; the mixture was shaken and then centrifuged at 10,000 rpm for 5 min. The resulting sample was then transferred to a GC vial. Decarboxylation of the acid form of CBD and THC was performed using GC-MS at a temperature of 280 °C. Cannabinoids analysis was performed on an Agilent 6890N GC equipped with an Agilent 5975B MS mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The separation was performed on a fused silica capillary column (HP-5MS, 30 m × 0.25 mm i.d. and 0.25 µm film thickness). Helium was used as a carrier gas at a constant flow rate of 1 mL min−1. The temperature program was as follows: an initial temperature of 200 °C for 2 min, then increased to 240 °C at 10 °C min−1 for 10 min. The injector and detector temperatures were set to 280 and 230 °C, respectively. The volume of the injected sample was 1.5 μL, and the split ratio was 1:20. Individual analytical standards for CBD and CBN were used for calibration. The quantification of THC was performed with CBN analytical standard according to [26]. The analyses were performed in two replicates.
2.7. Seed Germination Protocol
The allelopathic influence of different extracts of industrial hemp on seed germination and seedling growth of cultivated plant species was investigated in strictly controlled laboratory conditions in a climate chamber (Binder, model KBWF 720, Tuttlingen, Deutschland), where seeds of the following plant species were germinated: lettuce (Lactuca sativa L.)—“Majska Kraljica”, kohlrabi (Brassica oleracea var. gongylodes L.)—“Bečka Plava”, onion (Allium cepa L.)—“Holandski Žuti”, tomato (Solanum lycopersicum L.)—“Novosadski Jabučar”, carrot (Daucus carota L.)—“Nantes”, pepper (Capsicum annuum L.)—“Šorokšari”, savoy cabbage (Brassica oleracea var. sabauda L.)—“Vertus”, rocket (Eruca sativa L.) (produced by Agrounikum, r.j. Elita, Novi Sad, Serbia), alfalfa (Medicago sativa L.) (produced by NS Seed, Institute of Field and Vegetable Crops, Novi Sad, Serbia), white mustard (Sinapis alba L.) (produced by Hit Spice, Novi Sad, Serbia), pea (Pisum sativum L.) (produced by Agrounikum, r.j. Elita, Novi Sad, Serbia), sunflower (Helianthus annuus L.)—“NS Kruna”, and parsley (Petroselinum crispum L.)—“Berlinski Poludugi”. The listed plant species were selected as the most commonly cultivated ones in the Republic of Serbia. In each of the 13 Petri dishes, 25 seeds of the above-mentioned species were on sterilized filter paper during germination. All seeds were treated with a range of concentrations of previously prepared aqueous extracts of the industrial hemp. All treatments were performed in four replicates (n = 100 ± SD) for each examined plant species, while water was used as a control. Macerate, post-distillation residue, and ultrasonic extracts were applied in concentration ranges of 10, 25, 50, and 100% of stock solution, while hydrolate and microwave extracts were applied in narrower concentration ranges of 10, 25, and 50% of stock solution due to the limited amount obtained by the applied extraction procedures. All Petri dishes were initially moistened with 5 mL of the tested extract [12]. Due to their larger size, pea and sunflower seeds were initially moistened with 8 mL of each tested extract. Tested seeds were germinated in a climate chamber during a 12 h light period (400 μmol m−2 s−1) at 24 °C and during a 12 h dark period at 20 °C, with constant air humidity of 65%. The total duration of the experiment was as follows: for lettuce, 7 days; for peas, 8 days; for alfalfa, kohlrabi, savoy cabbage, rocket, and sunflower, 10 days; for onions, 12 days; for peppers, tomatoes, and carrots, 14 days; and for parsley, 28 days (Rulebook on seed quality of agricultural plants “Official Gazette of the RS”, number 34/2013, Seed testing methods and seed quality standards, point 7, Table 12). Afterward, the germination was read and expressed as a percentage of germinated seeds, while the length of the shoot and root of the seedlings was measured with a caliper and expressed in mm. Complete inhibition is represented by a value of 0% germination and 0 mm length of the root and shoot of the seedlings. The experimental setup with the onion and alfalfa seeds as examples can be observed in Figure 1.
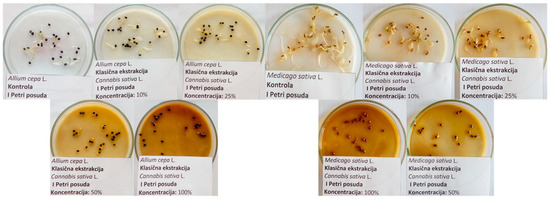
Figure 1.
Experimental setup with the onion and alfalfa seeds as an example (at the end of the experiment).
2.8. Statistical Analysis
The chemical analyses were performed in two replicates, while the germination assays were conducted in quadruple determinations. The mean values for seed germination, as well as the seedlings’ root and shoot lengths, were expressed as the mean value ± standard deviation using MS Office Excel v.2011. The differences between the treatments were analyzed using one-way and factorial analysis of variance (ANOVA), as well as Tukey’s HSD (Honestly Significant Difference) post hoc test (Statistica 14.1.08; TIBCO Software Inc., Palo Alto, CA, USA, University License). The values followed by the same letters indicate the same level of significance (p < 0.05).
3. Results
3.1. Phenolic Content in Conventional and Green Hemp Extracts
The presence of ferulic acid, orientin (luteolin-8-C-glucoside), vitexin (apigenin-8-C-glucoside) and its isomer isovitexin (apigenin-6-C-glucoside), luteolin, and apigenin derivatives in macerate (MAC), post-distillation residue (PDR), hydrolate (HYD), ultrasonic extracts (UAE30, UAE60), and microwave extracts (MAE210, MAE700) has been determined and the results are shown in Table 1. The highest concentrations of the total polyphenols (above 57 mg L−1) were obtained by MAE700, MAE210, and UAE60, in which all the studied phenolic compounds were detected.

Table 1.
Content of phenolic compounds in hemp conventional and green extracts, determined by HPLC.
MAE700 showed the highest concentration of vitexin (20.17 mg L−1), luteolin derivative 1 (19.12 mg L−1), orientin (16.77 mg L−1), apigenin derivative (14.63 mg L−1), and luteolin derivative 2 (8.59 mg L−1), indicating that this green extraction method is the most convenient regarding total polyphenols content and utilized resources. Orientin and vitexin were also identified as the most abundant flavonoids in hemp inflorescences, which together accounted for 70.4–96.0% of total flavonoids, followed by isovitexin (12.6–18.2%) [27]. These three flavonoids are recognized as the characteristic flavonoids of CBD-dominant chemotypes [28]. The microwave extract obtained at a lower input power (MAE210) showed slightly lower total polyphenols content due to decreased orientin and luteolin derivative 1 contents.
Applying ultrasound at two different temperatures delivered extracts with lower total polyphenols content in comparison with MAE210 and MAE700. The ultrasound extraction at 30 °C recovered twice as few polyphenols, confirming the dominance of microwave extraction in this case. By analogy with microwave extracts, the highest concentration of vitexin was detected in UAE30 and UAE60. Vitexin is a flavonoid glycoside derived from apigenin, predominantly found in beet leaves and stalks, but also in other agricultural by-products [29]. According to the literature [30], among the green extraction methods used to recover vitexin, ultrasound extraction is the most frequently employed, especially with water/ethanol as the solvent.
The presence of phenolic compounds was not recorded in the HYD. In contrast, another by-product of hydrodistillation, PDR, recovered total polyphenols content similar to UAE60, with the exception of isovitexin, which was not detected. The MAC was the least potent extract referring to total polyphenols content, in which only ferulic acid and vitexin were noted.
3.2. Cannabinoids Content in Conventional and Green Hemp Extracts
The contents of cannabidiol (CBD) and tetrahydrocannabinol (THC) were examined in the obtained macerate, post-distillation residue, hydrolate, and microwave and ultrasonic extracts and the results are shown in Table 2. The highest CBD content was detected in UAE60 (0.215 ± 0.012 mg mL−1), while it was almost threefold lower in UAE30 (0.075 ± 0.004 mg mL−1), PDR (0.081 ± 0.005 mg mL−1), and microwave extracts (0.072 ± 0.006 mg mL−1 in MAE210; 0.074 ± 0.006 mg mL−1 in MAE700). The CBD content in MAC was somewhere in the middle, between the highest and the lowest detections.

Table 2.
Cannabinoids contents in conventional and green hemp extracts, determined by GC-MS.
In all extracts, the THC levels recorded were twice lower than those for CBD. The THC contents were quite similar in MAC (0.098 ± 0.011 mg mL−1) and UAE60 (0.097 ± 0.003 mg mL−1). In contrast, THC was not detected at all in the HYD.
3.3. Influence of Hemp Extracts on Seed Germination: Conventional vs. Green Extracts (CE vs. GE)
In this study, most of the investigated conventional and green hemp extracts exerted allelopathic inhibitory activity toward seed germination of tested cultivars, except for pea. The most efficient were green extracts, namely UAE30 and UAE60 since they completely inhibited seed germination in 9 out of 13 cultivars at a 100% concentration. At a lower concentration of 50%, UAE30 and UAE60 were less efficient since they only acted, similar to MAC, toward four species, including tomato, rocket, white mustard, and parsley. Within green extracts, all of them decreased the germination percentage of species with an increase in concentration, while on kohlrabi, the effect was concentration-dependent when UAE30 and UAE60 were used as treatment agents. UAE60, as the sample with the highest CBD content, exhibited greater inhibition compared to UAE30 in tomato at a 25% concentration, in carrot at 25 and 50%, in pepper at 10, 25, and 50%, in rocket at 10 and 25%, and alfalfa at 25 and 50%. Sunflower and parsley were the only species in which UAE30 was more potent. HYD applied at a 50% concentration inhibited germination in lettuce, rocket, alfalfa, white mustard, and parsley seeds. In contrast, PDR was ineffective for seed germination since it only caused complete inhibition in rocket. The mean germination values for conventional and green extracts in regard to extract concentration and tested plant species are presented in Figure 2 and Figure 3.

Figure 2.
The mean germination values for conventional extracts in regard to extract concentration and tested plant species.

Figure 3.
The mean germination values for green extracts in regard to extract concentration and tested plant species.
3.3.1. One-Way ANOVA: The Effect of Concentration on Seed Germination of the Tested Plants
The existence of statistical significance was confirmed for the effect of concentration as the independent variable on the seed germination of the tested plants as the dependent variable (p = 0.000000 for p < 0.05). A statistically significant difference was noted between each of the tested concentrations, as well as the control (Figure 4).

Figure 4.
The effect of concentration, hemp extract, and plant species on seed germination of the tested plants (one-way ANOVA). The values followed by the same letters indicate the same level of significance (p < 0.05). HE—hemp extracts; MAC—macerate; PDR—post-distillation residue; HYD—hydrolate, UAE30—ultrasonic extract (at 30 °C); UAE60—ultrasonic extract (at 60 °C); MAE210—microwave extract (at 210 W); MAE700—microwave extract (at 700 W).
3.3.2. One-Way ANOVA: The Effect of Hemp Extract on Seed Germination of the Tested Plants
In the case of the effect of the hemp extract as the independent variable on the seed germination of the tested plants as the dependent variable, the statistical significance was not confirmed (p = 0.070500 for p < 0.05), nor was a statistically significant difference observed between the levels of the independent variable (Figure 4).
3.3.3. One-Way ANOVA: The Effect of Plant Species on Seed Germination of the Tested Plants
The existence of statistical significance was confirmed for the effect of plant species as the independent variable on the seed germination of the tested plants as the dependent variable (p = 0.000000 for p < 0.05). A statistically significant difference was noted between pea (a) and all the other tested plants (b) (Figure 4).
Overall, the one-way ANOVA showed the existence of statistical significance in the effect of the independent variables (concentration, hemp extract, and plant species) on the seed germination of the tested plants as the dependent variable was noted for concentration and plant species, but not for hemp extract (p = 0.070500 for p < 0.05). Also, only in the case of the hemp extract was no statistically significant difference observed between the levels of the independent variable.
3.3.4. Factorial ANOVA: The Combined Effect of Concentration and Plant Species on Seed Germination of the Tested Plants
The factorial ANOVA confirmed the existence of statistical significance for the combined effect of concentration and plant species as independent variables on the seed germination of the tested plants as the dependent variable (p = 0.000000 for p < 0.05). Tukey’s HSD post hoc test determined that the highest mean germination was observed in the control group of lettuce (a) and pea (ab), followed by 10, 25, and 50% hydrolate concentration applied in pea (abc), as well as by the control group of white mustard (abc) and sunflower (abc). The lowest mean germination was mainly observed when the highest hydrolate concentrations were applied in 10 out of 13 tested plant species (q) (Figure 5).
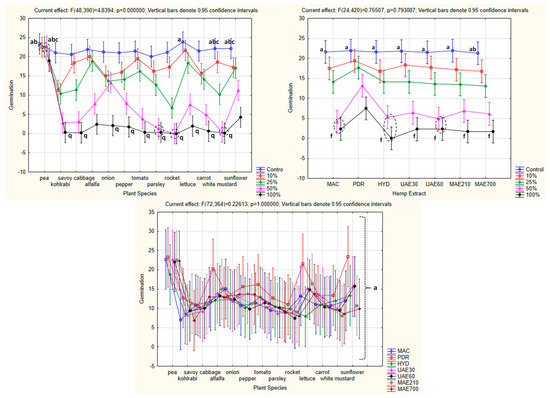
Figure 5.
The combined effect of concentration and plant species, hemp extract and concentration, as well as the hemp extract and plant species, on seed germination of the tested plants (factorial ANOVA). The values followed by the same letters indicate the same level of significance (p < 0.05). Dotted circles indicate closely positioned points sharing the same significance level, distinguishing them from single-point annotations. HE—hemp extracts; MAC—macerate; PDR—post-distillation residue; HYD—hydrolate, UAE30—ultrasonic extract (at 30 °C); UAE60—ultrasonic extract (at 60 °C); MAE210—microwave extract (at 210 W); MAE700—microwave extract (at 700 W).
3.3.5. Factorial ANOVA: The Combined Effect of Hemp Extract and Concentration on Seed Germination of the Tested Plants
The factorial ANOVA did not confirm the existence of statistical significance for the combined effect of hemp extract and concentration as the independent variables on the seed germination of the tested plants as the dependent variable (p = 0.793087 for p < 0.05). Tukey’s HSD post hoc test determined that the highest mean germination was observed in the control group for all the tested hemp hydrolates (a), followed by MAE700 (ab). The lowest mean germination was mainly observed when the highest hydrolate concentrations were applied in six out of seven tested hemp extracts (f) (Figure 5).
3.3.6. Factorial ANOVA: The Combined Effect of Hemp Extract and Plant Species on Seed Germination of the Tested Plants
The factorial ANOVA did not confirm the existence of statistical significance for the combined effect of hemp extract and plant species as independent variables on the seed germination of the tested plants as the dependent variable (p = 1.000000 for p < 0.05). Tukey’s HSD post hoc test did not detect statistically significant differences between the levels of the independent variables (Figure 5).
Overall, the factorial ANOVA showed that the existence of statistical significance for the combined effect of the independent variables was noted only in the case of the combined effect of concentration and plant species on the seed germination of the tested plants (p = 0.000000 for p < 0.05). Tukey’s HSD post hoc test identified that the highest mean germination was mainly observed in the control group, while the lowest mean germination was associated with the highest hydrolate concentrations across the majority of the tested plant species. In the case of the combined effect of hemp extract and plant species, no statistical significance was observed, and no statistically significant differences between the levels of the independent variables were detected.
3.4. Influence of Hemp Extracts on Root/Shoot Length: CE vs. GE
In the case of the root lengths of the seedlings of the 13 tested species, CE led to complete inhibition in 9 out of 13 cultivars, while GE was efficient in 7 cultivars at 50 and 100% concentrations. No hemp extract at a 25% concentration exerted complete inhibition of the root/shoot length among any of the 13 cultivars. CE negatively impacted most cultivars’ root and shoot lengths starting from a 10% concentration, except PDR, which displayed a hormesis effect only in sunflower seedlings. The maximal root length (17.30 mm) in sunflower seedlings was observed at a 50% concentration. Compared with ultrasonic extracts obtained at two temperatures, MAE210 and MAE700 proved more potent in inhibiting sunflower seedlings’ root/shoot lengths.
In the case of pea root and shoot length, despite PDR having a similar total polyphenols and CBD content as UAE60, it exhibited an opposite allelopathic effect. PDR demonstrated a stimulatory effect at a 10% concentration, while UAE60 showed an inhibitory effect at 100%. The pea’s maximum root length (57.16 mm) was recorded on a Petri dish treated with UAE30 at a 25% concentration; thereafter, its length decreased by 50% when increasing the UAE30 concentration up to 100%. PDR at a 50% concentration within CE provided the same pea root length. The maximum shoot length (25.16 mm) was also measured when UAE30 was used at a 25% concentration. The hormesis effect was observed when pea root was treated with HYD, MAC, MAE210, MAE700, and UAE30, while when PDR and UAE60 were used as treatment agents, stimulatory and inhibitory effects occurred, respectively. The allelopathic pattern was the same for pea shoot length, except for MAE700, which exhibited a slight stimulatory effect at 50%. The mean shoot and root lengths for conventional and green extracts in regard to the extract concentration and tested plant species are presented in Figure 6, Figure 7, Figure 8 and Figure 9.

Figure 6.
The mean shoot length for conventional extracts in regard to extract concentration and tested plant species.

Figure 7.
The mean shoot length for green extracts in regard to extract concentration and tested plant species.

Figure 8.
The mean root length for conventional extracts in regard to extract concentration and tested plant species.
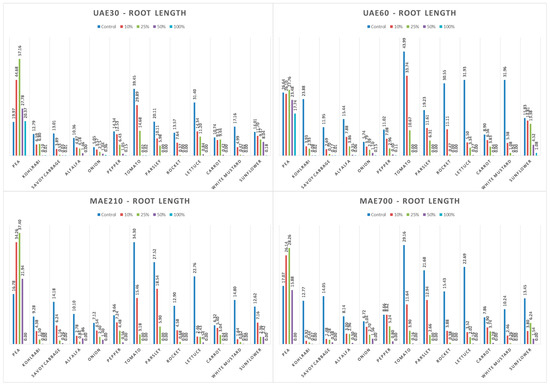
Figure 9.
The mean root length for green extracts in regard to extract concentration and tested plant species.
3.4.1. One-Way ANOVA: The Effect of Concentration on Shoot and Root Lengths of the Tested Plants
The existence of statistical significance was confirmed for the effect of concentration as the independent variable on the shoot and root length as the dependent variables (p = 0.000000 for p < 0.05). In both cases, the control led to the highest mean length, while the highest concentrations led to the lowest mean length (Figure 10).

Figure 10.
The effect of concentration on shoot and root lengths of the tested plants (one-way ANOVA). The values followed by the same letters indicate the same level of significance (p < 0.05).
3.4.2. One-Way ANOVA: The Effect of Hemp Extract on Shoot and Root Lengths of the Tested Plants
In the case of the effect of the hemp extract as the independent variable on the shoot and root lengths as the dependent variables, statistical significance was confirmed only in the case of shoot length (p = 0.034311 for p < 0.05). However, significant differences between the tested hemp extracts were not observed in any of the cases (Figure 11).
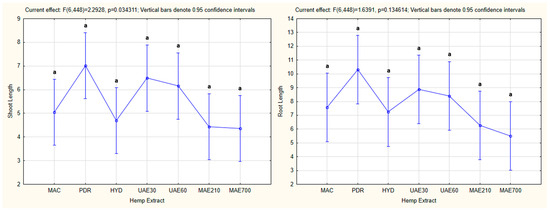
Figure 11.
The effect of hemp extract on shoot and root lengths of the tested plants (one-way ANOVA). The values followed by the same letters indicate the same level of significance (p < 0.05). HE—hemp extracts; MAC—macerate; PDR—post-distillation residue; HYD—hydrolate, UAE30—ultrasonic extract (at 30 °C); UAE60—ultrasonic extract (at 60 °C); MAE210—microwave extract (at 210 W); MAE700—microwave extract (at 700 W).
3.4.3. One-Way ANOVA: The Effect of Plant Species on Shoot and Root Lengths of the Tested Plants
The existence of statistical significance was confirmed for the effect of plant species as the independent variable on the shoot and root lengths as the dependent variables (p = 0.000000 for p < 0.05). In terms of shoot length, a statistically significant difference was noted between the pea and sunflower seedlings (a) compared to the seedlings of all the other tested plants (b). Regarding the root length, a statistically significant difference was noted between the pea seedlings (a), the tomato seedlings (b), the group consisting of parsley, lettuce, and sunflower (bc), and the remaining plant species (c) (Figure 12).

Figure 12.
The effect of plant species on shoot and root lengths of the tested plants (one-way ANOVA). The values followed by the same letters indicate the same level of significance (p < 0.05).
Overall, the one-way ANOVA showed the existence of statistical significance in the effect of the independent variables (concentration, hemp extract, and plant species) on the shoot and root lengths of the tested species as the dependent variables, except for the hemp extract in the case of root lengths (p = 0.134614 for p < 0.05). Also, only in the case of hemp extract was no statistically significant difference observed between the levels of the independent variable.
3.4.4. Factorial ANOVA: The Combined Effect of Concentration and Plant Species on Shoot and Root Lengths of the Tested Plants
The factorial ANOVA confirmed the existence of statistical significance for the combined effect of concentration and plant species as the independent variables on the shoot and root length as the dependent variables (p = 0.000000 for p < 0.05). Tukey’s HSD post hoc test determined that the longest shoots were observed in the control group of sunflower (a), followed by 25 and 50% (ab), as well as 10% hydrolate concentration (abc) in pea. The shortest shoots were mainly observed when the highest hydrolate concentrations were applied in 9 out of 13 tested plant species (n). In the case of root length, the longest roots were noted for the 25% hydrolate concentration in pea and the control group of tomato (a), followed by 10 (ab) and 50% hydrolate concentrations (abc) in pea. The shortest roots were mainly observed when the highest hydrolate concentrations were applied in 11 out of 13 tested plant species (n) (Figure 13).

Figure 13.
The combined effect of concentration and plant species on shoot and root lengths of the tested plants (factorial ANOVA). Dotted circles indicate closely positioned points sharing the same significance level, distinguishing them from single-point annotations. The values followed by the same letters indicate the same level of significance (p < 0.05).
3.4.5. Factorial ANOVA: The Combined Effect of Hemp Extract and Concentration on Shoot and Root Lengths of the Tested Plants
The factorial ANOVA did not confirm the existence of statistical significance for the combined effect of hemp extract and concentration as the independent variables on the shoot and root length as the dependent variables (shoot: p = 0.998480 for p < 0.05; root: p = 0.999204 for p < 0.05). Tukey’s HSD post hoc test determined that the longest shoots were observed in the control group for UAE60 and UAE30 (a) and PDR and MAC (ab). The shortest shoots were observed when a 100% hydrolate concentration was applied in the case of HYD, MAE210, and MAE700 (f). Similar results were obtained in the case of the root length, with the longest roots noted in the control group for UAE60 (a) and MAC, PDR, and UAE30 (ab) and the shortest roots observed when a 100% hydrolate concentration was applied in the case of MAC, HYD, MAE210, and MAE700 (f) (Figure 14).
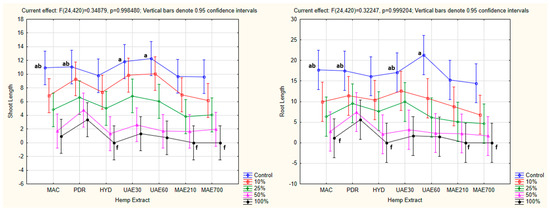
Figure 14.
The combined effect of hemp extract and concentration on shoot and root lengths of the tested plants (factorial ANOVA). The values followed by the same letters indicate the same level of significance (p < 0.05). HE—hemp extracts; MAC—macerate; PDR—post-distillation residue; HYD—hydrolate, UAE30—ultrasonic extract (at 30 °C); UAE60—ultrasonic extract (at 60 °C); MAE210—microwave extract (at 210 W); MAE700—microwave extract (at 700 W).
3.4.6. Factorial ANOVA: The Combined Effect of Hemp Extract and Plant Species on Shoot and Root Lengths of the Tested Plants
The factorial ANOVA did not confirm the existence of statistical significance for the combined effect of hemp extract and plant species as the independent variables on the shoot and root length as the dependent variables (shoot: p = 0.910692 for p < 0.05; root: p = 0.551777 for p < 0.05). Tukey’s HSD post hoc test determined that the longest shoots were observed when PDR was applied in pea (a) and sunflower (ab), followed by UAE30 in pea (abc), as well as MAC, HYD, UAE60, MAE210, and MAE700 in pea; PDR, HYD, and UAE60 in alfalfa; UAE60 in tomato; PDR and UAE30 in lettuce; and MAC, UAE30, and UAE60 in sunflower (abcd). Out of the remaining hemp extract x plant species combinations, 64 were in a homogenous group of the shortest shoots (d). Similar results were noted for the root length. The longest roots were observed in the case of pea, when PDR (a), UAE30 (ab), MAC, HYD, UAE60, MAE210, and MAE700 were applied (bc), followed by MAC, PDR, HYD, UAE30, and UAE60 in tomato (bc) and PDR in sunflower (bc). All of the remaining hemp extract x plant species combinations (78 in total) formed a homogenous group of the shortest roots (c) (Figure 15).
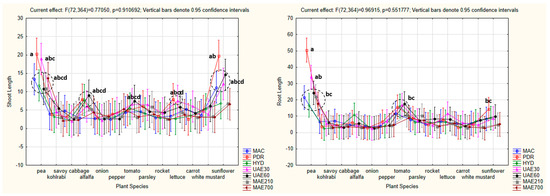
Figure 15.
The combined effect of hemp extract and plant species on shoot and root lengths of the tested plants (factorial ANOVA). The values followed by the same letters indicate the same level of significance (p < 0.05). Dotted circles indicate closely positioned points sharing the same significance level, distinguishing them from single-point annotations. HE—hemp extracts; MAC—macerate; PDR—post-distillation residue; HYD—hydrolate, UAE30—ultrasonic extract (at 30 °C); UAE60—ultrasonic extract (at 60 °C); MAE210—microwave extract (at 210 W); MAE700—microwave extract (at 700 W).
Overall, the factorial ANOVA showed that the existence of statistical significance for the combined effect of the independent variables was noted only in the case of the combined effect of concentration and plant species on both shoot and root lengths of the tested species (p = 0.000000 for p < 0.05). Tukey’s HSD post hoc test identified that the longest shoot and root lengths were mainly observed in the control groups and when lower concentrations were applied, while the shortest shoot and root lengths were associated with the highest hydrolate concentrations across the majority of the tested plant species.
4. Discussion
4.1. Phenolic Content in Conventional and Green Hemp Extracts
Hemp is a source of different phenolic compounds such as phenolic acids, flavonoids (flavonol, rutin, quercetin-3-glucoside, kaempferol-3-O-glucoside, quercetin, kaempferol), flavones (cannflavin A and B, luteolin-7-O-glucoside, apigenin-7-O-glucoside, luteolin, and apigenin), flavanols, and flavanones [31]. Phenolics can enhance seedling growth by elevating the levels of plant hormones like auxins, cytokinins, and gibberellins, which are known to promote growth [32]. To date, the extraction of phenolics in hemp has been reported by various conventional extraction techniques, including maceration, mostly using ethanol as an extraction solvent [33,34,35], as well as advanced extraction methods such as microwave-assisted extraction [36,37] and ultrasound-assisted extraction [36,38]. The presence of ferulic acid, orientin (luteolin-8-C-glucoside), vitexin (apigenin-8-C-glucoside) and its isomer isovitexin (apigenin-6-C-glucoside), luteolin, and apigenin derivatives in macerate (MAC), post-distillation residue (PDR), hydrolate (HYD), ultrasonic extracts (UAE30, UAE60), and microwave extracts (MAE210, MAE700) was confirmed in this study.
According to the total polyphenols content, MAE (MAE700 and MAE210) proved to be the most potent extraction technique. In the case of MAE, the solvent’s dielectric constant plays a crucial role. Even though flavonoids are more soluble in methanol than water [39], water exhibits a higher dielectric constant (ε = 78.4 at 25 °C) compared to solvents like ethanol (ε = 24.55 at 25 °C) and methanol (ε = 32.70 at 25 °C). This characteristic allows water to absorb more energy than it dissipates, leading to a swift rise in temperature within the sample [40]. As a result, the low water solubility of flavonoids was partially compensated by the high dielectric constant of water and consequently by the higher temperatures during MAE. On the other hand, this phenomenon is characteristic only for MAE, giving this technique an advantage over the other tested methods in terms of polyphenols content, despite being the shortest extraction process (Table 2; see Section 2.3 and Section 2.4). The most abundant phenolic compound in all tested extracts, except in MAC, was vitexin. UAE carried out the majority of extraction processes for orientin and vitexin. Orientin and vitexin are flavone 8-C-glycosides that exhibit many health benefits, such as anticancer, antidiabetes, antioxidant, analgesic, and anti-inflammatory effects [41,42]. Increasing the extraction temperature in UAE from 30 °C to 60 °C leads to an increase in total polyphenols extraction since higher temperatures involve faster polyphenol kinetics and improved extraction efficiency [43,44]. The most affected compound by temperature rise was orientin content, which doubled at 60 °C. The ultrasonic circulating extraction was used to recover orientin and vitexin [42]. It was confirmed that the yields of orientin and vitexin increased when the temperature increased from 30 to 60 °C. The influence of extraction time on orientin and vitexin content was also investigated. During the initial 30 min of UAE, a significant increase was observed. However, as the duration of ultrasonic irradiation was extended, the rate of improvement plateaued [42]. Longer ultrasonic exposure durations allow for ample time for cell wall disruption. This extended exposure also enhances solvent penetration into the cells, facilitating the efficient dissolution and release of target compounds from the cells to the surrounding solvent, leading to a higher yield [45].
4.2. Cannabinoids Content in Conventional and Green Hemp Extracts
Phytocannabinoids represent a group of cannabinoids that are synthesized as resin in the glandular trichomes of industrial hemp. The main cannabinoid is Δ9-tetrahydrocannabinol, and it is a structurally analog, although not psychoactive, cannabidiol. It is present in the plant as an inactive acid, while thermal decarboxylation converts it into its non-acidic counterparts [46]. Terpenes found in hemp have been shown to inhibit seed germination [47], while cannabinoids exhibit phytotoxicity in vitro [48]. The highest CBD content was detected in UAE60 (0.215 mg mL−1). The highest extraction yield was at 60 °C by UAE of hemp with 96% ethanol for 50 min [49]. In addition, UAE was more efficient than MAE in terms of the total THC and CBD yield, which is in accordance with our results.
In this study, the highest CBD content was detected in UAE60 (0.215 ± 0.012 mg mL−1), while it was almost threefold lower in UAE30 (0.075 ± 0.004 mg mL−1), PDR (0.081 ± 0.005 mg mL−1), and microwave extracts (0.072 ± 0.006 mg mL−1 in MAE210; 0.074 ± 0.006 mg mL−1 in MAE700), which indicates the influence of temperature in the case of ultrasound extraction.
In another study, microwave extraction was also used to isolate CBD from the same hemp cultivar. It was reported that the highest CBD content (1.8415 mg mL−1) was ten times higher than in the case of the MAC extract in this study, which was obtained when the parameters in microwave extraction were set as 70% ethanol, an extraction time of 20 min, and an S/L ratio of 5 [37].
In all extracts, the THC levels recorded were twice as small as those for CBD. The THC contents were quite similar in MAC (0.098 ± 0.011 mg mL−1) and UAE60 (0.097 ± 0.003 mg mL−1), with a distinction that the latter lasted for 40 min in comparison with 24 h maceration. In contrast, THC was not detected at all in the HYD, which is expected due to its low volatility and water insolubility [50]. However, it was stated [37] that THC contents in the microwave extracts were in the range of 0.0339–0.0637 mg mL−1. The lowest THC content (0.0339 mg mL−1), similar to the one obtained in MAE210, was acquired in the case of 30% ethanol, an extraction time of 20 min, and an S/L ratio of 5.
Some authors have already reported low THC concentrations in hemp essential oils. HYD had neither THC nor any of the phenolic compounds and yet showed allelopathic action. This can only indicate that THC is not the carrier of allelopathic properties and that phenols are not the only source of allelopathic activity in hemp [51].
Previous researchers have investigated the MAE of hemp seeds and identified the optimal conditions as an extraction solvent of methanol, microwave power of 375 W, temperature of 109 °C, and extraction time of 30 min. Compared with conventional extraction methods, MAE achieved the highest extraction yield of total cannabinoids in hemp seeds with the least solvent and lowest time consumption [52]. Maceration with ethanol at room temperature for 45 min was the optimal technique for the recovery of the acidic cannabinoid CBDA. In contrast, MAE at 60 °C for 5 min at an S/L ratio of 1:40 was the most convenient for its neutral counterpart, CBD [53]. The highest CBD content (1.84 mg mL−1) was obtained when MAE parameters were set as 70% ethanol, an extraction time of 20 min, and an S/L ratio of 1:5 [37].
4.3. Influence of Hemp Extracts on Seed Germination: Conventional vs. Green Extracts (CE vs. GE)
Hemp is an efficient crop for suppressing weeds, but the extent of its dominance and its impact on subsequent crops remain unclear [54]. Phenolic compounds, recognized as key allelochemicals in plants, are known to promote seed germination and seedling growth. It has been well-established in the literature that, at low concentrations, these compounds have a stimulatory effect on both germination and initial plant development [17,55,56]. However, higher concentrations of these compounds have been observed to reduce germination rates sharply. To date, research on hemp extracts as a potential source of allelochemicals has primarily focused on those obtained through conventional extraction methods for use against endemic allergen sources [57]. Water extracts of hemp panicles decreased the germination rate of monocot plants such as wheat and rye. The hormesis effect was observed in the germination of rape seeds, where low concentrations of hemp extract stimulated germination, while higher concentrations significantly inhibited it. Additionally, the allelopathic effect of the hemp extract triggered a molecular response in lupine roots, increasing the transcription of genes involved in the isoflavonoid synthesis pathway [54].
The most efficient were green extracts, namely UAE30 and UAE60 since they completely inhibited seed germination in 9 out of 13 cultivars at a 100% concentration. At a lower concentration of 50%, UAE30 and UAE60 were less efficient since they only acted, similar to MAC, toward four species, including tomato, rocket, white mustard, and parsley. However, considering that extraction time in UAE was 40 min compared with the 24 h needed for maceration, UAE would be the method of choice for the allelopathic inhibition of seed germination.
The results of this study demonstrate that concentration and plant species are the primary determinants of seed germination, while hemp extract did not exert a statistically significant effect under the tested conditions. The one-way ANOVA confirmed that concentration and plant species had a significant impact on seed germination, whereas hemp extract did not. Similarly, factorial ANOVA results indicated that only the interaction between concentration and plant species significantly influenced germination, with no significant interactions observed between hemp extract and either variable. Post hoc analysis (Tukey’s HSD test) further revealed that seed germination was the highest in the control group, while the lowest germination rates were recorded when the highest concentrations of the extracts were applied. It also determined that, in the case of the combined effect of hemp extract and plant species, there were no statistically significant differences between the levels of the independent variables. These findings underscore the importance of optimizing concentration levels in relation to plant species in order to enhance germination outcomes.
4.4. Influence of Hemp Extracts on Root/Shoot Length: CE vs. GE
In general, MAE210 and MAE700 have almost identical chemical compositions of phenols and cannabinoids, which correlates with their similar allelopathic activity toward root/shoot length of all tested species.
The weakest inhibitory effect for the majority of the tested plants was observed in the case of PDR, likely due to its lower vitexin content and the absence of isovitexin, both known for their phytotoxic effects [58]. On the other hand, MAE700 exhibited the greatest inhibitory effect for the majority of the tested plants, which can be explained by the fact that it had the highest content of phenolic compounds determined in this study, particularly regarding luteolin derivatives 1 and 2, apigenin derivatives, orientin, and vitexin content. Strong allelopathic effects have previously been confirmed in plants rich in apigenin and luteolin [59].
In all the tested plants, except for pea, root growth inhibition was observed, especially when higher extract concentrations were applied. Only in the case of pea was the root growth stimulated when treated with a 25% concentration of all the extracts, as well as a 50% concentration of PDR, MAC, UAE30, and MAE210. Additionally, the pea seeds exhibited the highest germination rate across all the hemp extract treatments. These results are consistent with the findings of a recently published study [18].
The allelopathic effects of hemp root and shoot extracts on lettuce were investigated. While the shoot extract demonstrated an inhibitory impact on lettuce germination, the root extract did not display any significant effect [12]. Recently, some authors investigated the allelopathic effects of aqueous hemp leaf extracts on seed germination and seedling growth in wheat and barley. They concluded that the phenols in the water hemp extract have a negative impact on wheat and barley seed germination and seedling growth [16].
According to the obtained results, it can be concluded that the applied concentrations of both conventional and green hemp extracts exert mostly allelopathic inhibitory effects on the seed germination of all the above-mentioned cultivars, except for pea. In this study, the pea seed germination percentage was unaffected by various hemp extracts, showing the same values as the control. A possible explanation might be that seed germination efficiency is influenced by size and weight. Thus, larger seeds, such as peas, typically contain more nutrients, enabling faster germination and growth than smaller seeds [60]. Consequently, the strong inhibition activity of hemp-derived phytochemicals on pea seed germination was neutralized. In the case of the pea root and shoot length, despite PDR having similar total polyphenols and CBD contents as UAE60, it exhibited an opposite allelopathic effect. PDR demonstrated a stimulatory effect at 10% concentration, while UAE60 showed an inhibitory effect at 100%. This supports the hypothesis that phenolic compounds and CBD are not the exclusive allelochemicals responsible for the elongation/shortening of root/shoot length of pea seedlings.
The statistical analyses revealed that concentration and plant species significantly influenced shoot and root growth, while hemp extract alone did not exhibit a statistically significant effect. The one-way ANOVA showed that all independent variables (concentration, hemp extract, and plant species) impacted shoot and root lengths, except for hemp extract in the case of the root length, for which statistically significant differences between the levels of the independent variable were also not observed. The factorial ANOVA further confirmed that only the interaction between the concentration and plant species significantly affected shoot and root growth, whereas hemp extract did not demonstrate a statistically significant interaction with either variable. Tukey’s HSD post hoc test clarified that the longest shoots and roots were predominantly observed in the control group and at lower extract concentrations, while the shortest shoots and roots occurred when the highest concentrations of the extracts were applied. These findings emphasize that concentration and plant species are key determinants of plant growth, while hemp extract did not significantly enhance shoot or root development under the tested conditions. Carefully managing the concentration levels according to plant species appears to be crucial for optimizing growth outcomes.
5. Conclusions
Based on the presented results, it can be concluded that industrial hemp possesses certain allelopathic properties toward seed germination and seedling growth of the majority of tested cultivated plants. The results of seed germination percentage and root/shoot length in all tested plants, except for peas, clearly indicate the inhibitory effect of higher concentrations of applied hemp extracts, regardless of differences in chemical composition (CBD, THC, and total polyphenols contents), indicating that diverse extracts have varying effects on different cultivars. The hydrolate contained neither THC nor any phenolic compounds and yet still exhibited some allelopathic activity. This suggests that THC is not responsible for the allelopathic properties and that phenols are not the sole source of allelopathy in hemp. The strongest allelopathic effects were observed for MAE210 and MAE700 extracts, which also had the highest polyphenols content, known to be responsible for allelopathic activity. The allelopathy of hemp extracts is anticipated to be further explored as a weed management tool since it can be a part of a sustainable, ecological, and integrated crop management system. Additionally, it is necessary to confirm pea’s resistance to hemp, as a hemp-based bioherbicide could be valuable for practical application in this crop.
Author Contributions
Conceptualization, M.P.; methodology, A.G. and N.T.; software, T.Z.; validation, M.P. and S.V.; formal analysis, M.K.; investigation, A.K.; resources, S.V.; data curation, M.K.; writing—original draft preparation, M.K. and N.S.; writing—review and editing, N.S. and A.G.; visualization, A.K.; supervision, B.K.; project administration, B.K. and S.V.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work received financial support from the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (Program no. 451-03-66/2024-03/200134, 451-03-66/2024-03/200032, 451-03-136/2025-03/200222 and 451-03-137/2025-03/ 200117).We extend our sincere gratitude to Tijana Stojanović, Teaching Assistant at the University of Novi Sad, Faculty of Agriculture, for her invaluable support in writing, editing, and data analysis following the review process.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
References
- Owusu, N.O.; Arthur, B.; Aboagye, E.M. Industrial hemp as an agricultural crop in Ghana. J. Cannabis Res. 2021, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Drinić, Z.; Vladić, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Tomić, M.; Vidović, S. Application of conventional an high-pressure extraction techniques for the isolation of bioactive compounds from the aerial part of hemp (Cannabis sativa L.) assortment Helena. Ind. Crops Prod. 2021, 171, 113908. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Borah, R.; Sharma, B.; Pandhi, S.; Vijay, T.; Yadav, H.S.; Devi, S.; Patil, U.; et al. Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: An overview. Phytother. Res. 2021, 35, 6010–6029. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, A.; Gage, K.L. Allelopathic Potential of Hemp: Implications for Integrated Weed Management. Sec. Weed Manag. 2022, 4, 832471. [Google Scholar] [CrossRef]
- Mushtaq, W.; Siddiqui, M.B.; Hakeem, K.R. Allelopathy: Potential for Green Agriculture; Springer: Cham, Switzerland, 2020; pp. 1–25. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, S.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant allelochemicals and their various applications. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 441–457. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: Principles and basic aspects for agroecosystem control. In Sustainable Agriculture Reviews 28: Ecology for Agriculture; Springer: Cham, Switzerland, 2018; pp. 47–101. [Google Scholar] [CrossRef]
- Palanivel, H.; Tilaye, G.; Belliathan, S.K.; Benor, S.; Abera, S.; Kamaraj, M. Allelochemicals as natural herbicides for sustainable agriculture to promote a cleaner environment. In Strategies and Tools for Pollutant Mitigation; Aravind, J., Kamaraj, M., Devi, M.P., Rajakumar, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 93–110. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Ferus, P.; Mencik, K.; Konôpková, J. Allelopathic potential of Juglans nigra L. to control the invasive tree-of-heaven (Ailanthus altissima (Mill.) Swingle). Allelopath. J. 2020, 49, 177–188. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Lucić, I. Allelopathic effect of parsley (Petroselinum crispum Mill.) cogermination, water extracts and residues on hoary cress (Lepidium draba (L.) Desv.). Poljoprivreda 2014, 20, 22–26. [Google Scholar]
- Mahmoodzadeh, H.; Ghasemi, M.; Zanganeh, H. Allelopathic effect of medicinal plant Cannabis sativa L. on Lactuca sativa L. seed germination. Acta Agric. Slov. 2015, 105, 233–239. [Google Scholar] [CrossRef]
- Das, C.; Dey, A.; Bandyopadhyay, A. Allelochemicals: An emerging tool for weed management. In Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Springer: Cham, Switzerland, 2021; pp. 249–259. [Google Scholar] [CrossRef]
- Šćepanović, M.; Sarić-Krsmanović, M.; Šoštarčić, V.; Brijačak, E.; Lakić, J.; Špirović Trifunović, B.; Gajić Umiljendić, J.; Radivojević, L.J. Inhibitory Effects of Brassicaceae Cover Crop on Ambrosia artemisiifolia Germination and Early Growth. Plants 2021, 10, 794. [Google Scholar] [CrossRef]
- Prvulović, D.; Gvozdenac, S.; Latković, D.; Peić Tukuljac, M.; Sikora, V.; Kiprovski, B.; Mišan, A.; Chrysargyris, A.; Tzortzakis, N.; Ovuka, J. Phytotoxic and insecticidal activity of industrial hemp (Cannabis sativa L.) extracts against Plodia interpunctella Hübner—A potential sunflower grain protectant. Agronomy 2023, 13, 2456. [Google Scholar] [CrossRef]
- Patanè, C.; Pellegrino, A.; Cosentino, S.L.; Testa, G. Allelopathic effects of Cannabis sativa L. aqueous leaf extracts on seed germination and seedling growth in durum wheat and barley. Agronomy 2023, 13, 454. [Google Scholar] [CrossRef]
- Pudełko, K.; Majchrzak, L.; Narožna, D. Allelopathic effect of fibre hemp (Cannabis sativa L.) on monocot and dicot plant species. Ind. Crops Prod. 2014, 56, 191–199. [Google Scholar] [CrossRef]
- Poonsawat, T.; Srilasak, N.; Koodkaew, I. Allelopathic characterization and allelochemicals identification of hemp (Cannabis sativa L.) leaf residue. Ind. Crops Prod. 2024, 222, 120003. [Google Scholar] [CrossRef]
- Singh, N.B.; Thapar, R. Allelopathic influence of Cannabis sativa on growth and metabolism of Parthenium hysterophorus. Allelopath. J. 2003, 12, 61–70. [Google Scholar]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Distribution and exudation of allelochemicals in wheat Triticum aestivum. J. Chem. Ecol. 2000, 26, 2141–2154. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Wang, C.; Qi, J.; Liu, Q.; Wang, Y.; Wang, H. Allelopathic potential of aqueous extracts from fleagrass (Adenosma buchneroides Bonati) against two crop and three weed species. Agriculture 2022, 12, 1103. [Google Scholar] [CrossRef]
- Bocsa, I.; Karus, M. The Cultivation of Hemp: Botany, Varieties, Cultivation and Harvesting; Hemptech: Sebastopol, CA, USA, 1998; pp. 1–184. ISBN 978-1-886874-03-9. [Google Scholar]
- Callaway, J.C. A more reliable evaluation of hemp THC levels is necessary and possible. J. Ind. Hemp. 2008, 13, 117–144. [Google Scholar] [CrossRef]
- Mišan, A.; Mimica-Dukić, N.; Mandić, A.; Sakač, M.; Milovanović, I.; Sedej, I. Development of a rapid resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Open Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Poortman-Van Der Meer, A.J.; Huizer, H. A contribution to the improvement of accuracy in the quantitation of THC. Forensic Sci. Int. 1999, 101, 1–8. [Google Scholar] [CrossRef]
- Beleggia, R.; Iannucci, A.; Menga, V.; Quitadamo, F.; Suriano, S.; Citti, C.; Pecchioni, N.; Trono, D. Impact of chitosan-based foliar application on the phytochemical content and the antioxidant activity in hemp (Cannabis sativa L.) inflorescences. Plants 2023, 12, 3692. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of chemotypic markers in three chemotype categories of cannabis using secondary metabolites profiled in inflorescences, leaves, stem bark, and roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef] [PubMed]
- Lorizola, I.M.; Furlan CP, B.; Portovedo, M.; Milanski, M.; Botelho, P.B.; Bezerra RM, N.; Sumere, B.R.; Rostagno, M.A.; Capitani, C.D. Beet stalks and leaves (Beta vulgaris L.) protect against high-fat diet-induced oxidative damage in the liver in mice. Nutrients 2018, 10, 872. [Google Scholar] [CrossRef]
- de Freitas Marinho, L.; Sganzerla, W.G.; Ferreira, V.C.; Moreno, J.A.J.; Rostagno, M.A.; Forster-Carneiro, T. Advances in green extraction methods, biological properties, and applications of betanin and vitexin: An updated review and bibliometric analysis. Biocatal. Agric. Biotechnol. 2023, 51, 102744. [Google Scholar] [CrossRef]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical analysis of minor bioactive components and cannabidiolic acid in commercial hemp seed oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar] [CrossRef]
- Drinić, Z.; Vidović, S.; Vladić, J.; Koren, A.; Kiprovski, B.; Sikora, V. Effect of extraction solvent on total polyphenols content and antioxidant activity of Cannabis sativa L. Lek. Sirovine 2018, 38, 17–21. [Google Scholar] [CrossRef]
- Gallo, M.; Formato, A.; Ciaravolo, M.; Formato, G.; Naviglio, D. Study of the kinetics of extraction process for the production of hemp inflorescences extracts by means of conventional maceration (CM) and rapid solid-liquid dynamic extraction (RSLDE). Separations 2020, 7, 20. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, Soxhlet, UAE and RSLDE techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of medical cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef]
- Drinić, Z.; Vladić, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Kiprovski, B.; Vidović, S. Microwave-assisted extraction of cannabinoids and antioxidants from Cannabis sativa aerial parts and process modeling. J. Chem. Technol. Biotechnol. 2020, 95, 831–839. [Google Scholar] [CrossRef]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-assisted extraction of cannabinoids from Cannabis sativa L. optimized by response surface methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef]
- Dong, X.; Li, X.; Ruan, X.; Kong, L.; Wang, N.; Gao, W.; Wang, R.; Sun, Y.; Jin, M. A deep insight into the structure-solubility relationship and molecular interaction mechanism of diverse flavonoids in molecular solvents, ionic liquids, and molecular solvent/ionic liquid mixtures. J. Mol. Liq. 2023, 385, 122359. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Liu, J.; Gu, H.; Yang, L. An efficient approach for the extraction of orientin and vitexin from Trollius chinensis flowers using ultrasonic circulating technique. Ultrason. Sonochem. 2017, 37, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Miron, T.L.; Plaza, M.; Bahrim, G.; Ibáñez, E.; Herrero, M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. J. Chromatogr. A 2011, 1218, 4918–4927. [Google Scholar] [CrossRef]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef]
- Meng, Q.; Buchanan, B.; Zuccolo, J.; Poulin, M.M.; Gabriele, J.; Baranowski, D.C. A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products. PLoS ONE 2018, 13, e0196396. [Google Scholar] [CrossRef]
- De Martino, L.D.; Mancini, E.; de Almeida LF, R.; Feo, V.D. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, A. Understanding the Role of Secondary Metabolites in Hemp-Weed Interactions. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2021. [Google Scholar]
- Sirikantaramas, S.; Taura, F.; Tanaka, Y.; Ishikawa, Y.; Morimoto, S.; Shoyama, Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 2005, 46, 1578–1582. [Google Scholar] [CrossRef]
- De Vita, D.; Madia, V.N.; Tudino, V.; Saccoliti, F.; De Leo, A.; Messore, A.; Roscilli, P.; Botto, A.; Pindinello, I.; Santilli, G.; et al. Comparison of different methods for the extraction of cannabinoids from cannabis. Nat. Prod. Res. 2020, 34, 2952–2958. [Google Scholar] [CrossRef]
- Malingre, T.; Hendriks, H.; Batterman, S.; Bos, R.; Visser, J. The essential oil of Cannabis sativa. Planta Med. 1975, 28, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, V.; Steinemann, S. Essential oil of Cannabis sativa L. strains. J. Int. Hemp Assoc. 1997, 4, 80–82. [Google Scholar] [CrossRef]
- Chang, C.W.; Yen, C.C.; Wu, M.T.; Hsu, M.C.; Wu, Y.T. Microwave-assisted extraction of cannabinoids in hemp nut using response surface methodology: Optimization and comparative study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L.(hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Tanase, C.; Bujor, O.C.; Popa, V.I. Influence on physiological processes in plants. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–55. [Google Scholar] [CrossRef]
- Hegab, M.M.; Khodary SE, A.; Hammouda, O.; Ghareib, H.R. Autotoxicity of chard and its allelopathic potentiality on germination and some metabolic activities associated with growth of wheat seedlings. Afr. J. Biotechnol. 2008, 7, 884–892. [Google Scholar]
- Gharib, F.A.; Hegazi, A.Z. Salicylic acid ameliorates germination, seedling growth, phytohormone and enzymes activity in bean (Phaseolus vulgaris L.) under cold stress. J. Am. Sci. 2010, 6, 675–683. [Google Scholar]
- Putnam, A.R. Allelochemicals from plants as herbicides. Weed Technol. 1988, 2, 510–518. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Lombardo, S.; Pesce, G.R.; Mauromicale, G. Allelopathic potential of leaf aqueous extracts from Cynara cardunculus L. on the seedling growth of two cosmopolitan weed species. Ital. J. Agron. 2019, 14, 1373. [Google Scholar] [CrossRef]
- Sadeghi, H.; Khazaei, F.; Sheidaei, S.; Yari, L. Effect of seed size on seed germination behavior of safflower (Carthamus tinctorius L.). ARPN J. 2011, 6, 5–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).