Abstract
Licorice (Glycyrrhiza spp.) is a medicinal plant belonging to the Fabaceae family. In Korean Pharmacopoeia, three species of G. uralensis, G. glabra, and G. inflata are listed as licorice. Recently, G. korshinskyi has been registered in the Korean Pharmacopoeia, but there is no comprehensive monograph covering its agronomic characteristics. This research evaluated the agronomic characteristics of G. korshinskyi through growth characteristics, character correlations analysis, and principal component analysis (PCA) using 50 lines. We evaluated growth characteristics of the stem, root, stolon (rhizome), the emergence rate, and glycyrrhizin content. Correlation analysis showed that plant height and root diameter strong positively correlated with root weight and glycyrrhizin content. PCA was useful for understanding the agronomic characteristics of G. korshinskyi, with plant height, root diameter, root weight, stolon diameter, glycyrrhizin content, stolon length, stolon number, and stolon weight as key factors. Cluster analysis grouped G. korshinskyi lines into three groups. Group III contained nine lines with a high plant height, leaf length, leaf width, root diameter, root weight, and glycyrrhizin content. In conclusion, this research evaluated the agronomic characteristics of G. korshinskyi resources through growth traits, correlation analysis, and principal component analysis. This research establishes a foundation for future breeding programs and functional studies.
1. Introduction
Licorice (Glycyrrhiza spp.) is a medicinal plant belonging to the legume family (Fabaceae). There are 22 species worldwide, which are distributed in arid and semi-arid regions of Asia, the Americas, and Europe [1,2,3,4,5,6]. Traditionally, licorice has been used as an antidote, analgesic, and to harmonize the efficacy of medicinal herbs [7,8]. Recent studies have shown that glycyrrhizin, a triterpenoid saponin, primarily accumulates in the roots and stolons of Glycyrrhiza spp. and serves as a key marker for its agricultural evaluation, It exhibits antiviral [9], antibacterial [10], anti-inflammatory [11], and anticancer [12,13] properties. Therefore, licorice has been used in the pharmaceutical and food industries [14].
However, licorice is not native to Korea and is mostly dependent on imports. Historically, multiple attempts have been made to cultivate licorice domestically, but these efforts were largely unsuccessful. The Annals of the Joseon Dynasty contain 41 records related to licorice, many of which describe unsuccessful cultivation attempts [15]. To address this issue, the Rural Development Administration developed the licorice cultivars ‘Wongam’ [16] and ‘Dagam’ [17] specifically for Korea’s climate. These cultivars, belonging to Glycyrrhiza korshinskyi, were cultivated in Korea’s hot and humid climate. G. korshinskyi was distinguished by its high yield and glycyrrhizin content compared to other licorice species. However, the use of medicinal plants in Korea is strictly regulated by the Korean Pharmacopoeia, and only species recognized as origin plants can be supplied to farmers. Therefore, we evaluated the safety of G. korshinskyi and its efficacy equivalence to the original species. Through this process, we registered it in the Korean Pharmacopoeia, laying the foundation for the localization of licorice cultivation.
Previous studies on G. korshinskyi have primarily focused on its chemical composition and pharmacological effects, while limited studies have explored its cultivation and breeding. This research evaluated the agronomic characteristics of G. korshinskyi through evaluation of growth characteristics, correlation analysis, and principal component analysis (PCA). This research aim to expand the agricultural potential of G. korshinskyi and establish a foundation for future breeding programs and further functional research.
2. Materials and Methods
2.1. Plant Materials and Propagation
In this research, fifty G. korshinskyi lines were selected from China, Kazakhstan, and Korea. The 50 lines were cultivated in a medicinal crop test field. During cultivation, stolons were cut into 7 cm lengths, planted in April 2019, and harvested in November 2020.
The field was fertilized before planting with N:P2O:K2O: compost at a ratio of 17:11:14: 2000 kg/10a [18]. The width of the furrow was set at 100 cm and the height at 50 cm. The replanting distance was 50 cm between rows and 20 cm between plants, and the experiment was laid out in a three-replicate randomized block design. The weather conditions in the test field were an annual average temperature of 12.3 °C, an annual precipitation of 795.5 mm, an average humidity of 67.8%, and an annual sunshine duration of 2261.9 h in 2019, and an annual average temperature of 12.1 °C, an annual precipitation of 1446.5 mm, an average humidity of 70.2%, and an annual sunshine duration of 2222.0 h in 2020.
2.2. Evalution of Growth Characteristics
Growth characteristics were evaluated using 20 randomly selected plants with three replications. Aboveground growth characteristics were investigated in July 2020, including plant height, stem diameter, number of nodes, leaf length, leaf width, leaf number, leaflet length, leaflet width, and leaflet number per plant. The floral characteristics were investigated using a Vernier caliper (CD-20CP, Microtech, Kanagawa, Japan) at the time when flowering rate reached 40–50%, including floret length, stamen length, and pistil length. The underground growth characteristics were investigated after harvesting in November 2020, including root length, root diameter, root number, stolon length, stolon diameter, stolon number, dry root weight, dry stolon weight, and dry yield.
2.3. Glycyrrhizin Analysis
Glycyrrhizin was analyzed according to the standard method specified in the Korean Pharmacopoeia [19]. Sample (100 mg of powdered material) was added to 10 mL of 80% MeOH, sealed with parafilm, and sonicated for 15 min, 3 times. The extract was filtered through a membrane filter (PTFE, 0.2 μm, syringe filter) and used as a sample. Glycyrrhizin analysis was performed using a Dionex Ultimate 3000 (Thermo Fisher Scientific, Gardena, CA, USA) equipped with a Waters XBridge C18 (3.5 μm, 4.6 mm × 150 mm, Stoughton, MA, USA), and Dionex UltiMate 3000 DAD-3000 (Thermo Fisher Scientific, Gardena, CA, USA). The detection wavelength was 254 nm, the flow rate was 1 mL/min, and the column oven temperature was set at 35 °C. The sample was injected in 5 μL using an autosampler. The analysis of glycyrrhizin content was performed using a mobile phase consisting of water with 0.1% formic acid (Solvent A) and acetonitrile with 0.1% formic acid (Solvent B). The solvent condition for B started at 5% and increased to 100% by 57 min, was maintained at 100% until 67 min, then reduced to 5% and held for 5 min.
Glycyrrhizin standard obtained from Coresciences (Coresciences Co., Ltd., Seoul, Republic of Korea) was used in this research. The standards were prepared by diluting with 80% MeOH and preparing standard solutions in steps of 0.005–100 µL/mL and calibration curves were drawn. The amount of each component (µg/mL) was converted by substituting the HPLC peak area analyzed in each sample into the calibration line regression equation, and the yield was calculated and quantified. The correlation coefficient R2 of the HPLC conditions was 0.9998.
2.4. Statistical Analysis
Correlation, principal component analysis, and cluster analysis were used in the statistical analysis using SAS Enterprise Guide 7.1 (Statistical analysis system, 2009, Cray, SAS Institute Inc., Cray, NC, USA). Correlation analysis was performed using Pearson’s correlation coefficient to indicate significance. Principal Component Analysis (PCA) was performed, followed by cluster analysis. The cluster analysis was performed by the average linkage cluster method, and the significance test between clusters was performed by Duncan’s multiple range test (DMRT) analysis.
3. Results and Discussion
3.1. Growth Characteristics
The emergence rates of lines cultivated by stolons ranged from 20.0% to 93.0% according to the characteristics of the lines (Table 1). The 7 lines was mergence rates above 86% (Figure 1). Vegetative propagation was the advantage of maintaining genetic characteristics, but it was the disadvantage of a low multiplication rate compared to seed propagation. Therefore, emergence rates affected yield characteristics, and lines with emergence rates above 86% showed higher yield characteristics.

Table 1.
Stem and leaf characteristics of G. korshinskyi.
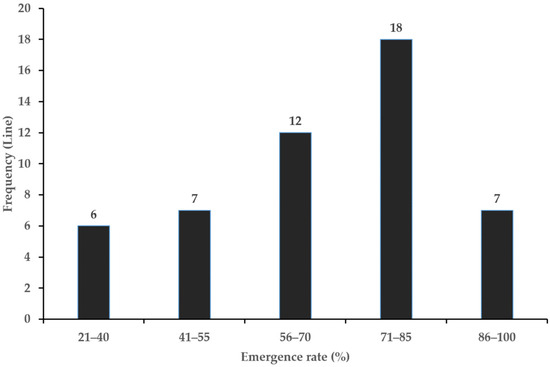
Figure 1.
Frequency distributions of emergence rate in G. korshinskyi lines. Seven lines exhibited an emergence rate of 86% or higher.
The stem was semi-erect, green, and covered with trichomes on the surface (Figure 2). Over time, the trichomes changed into hard spines, and the base of the stem became brown and woody. Plant height ranged from 62.3 to 175.0 cm, with an average stem diameter of 9.1 mm and an average number of 37 nodes (Table 1). The plant form was semi-erect. Thicker stems were closely related to lodging resistance [20,21,22].
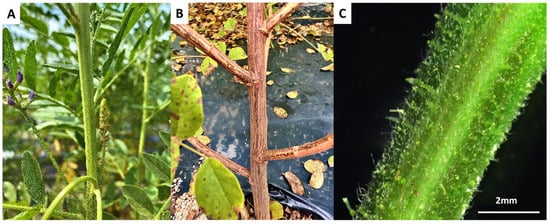
Figure 2.
Stem characteristics of G. korshinskyi. (A) The green stem was shown with its overall structure. (B) The stem base turned brown and became woody in September. (C) The stem surface was covered with trichomes, as seen under an electron microscope. Scale bar = 2 mm.
The leaf was oblong-elliptical, with an odd-pinnate (Figure 3). The leaf length averaged 16.3 cm, and the leaf width averaged 7.6 cm (Table 1). The leaflets were elliptic or ovoid, with acute or emarginate apex, rounded or oblique bases. The margin of leaflet was entire or wavy types (Figure 3). They showed morphological characteristics of both G. uralensis, with its cuspidate and wavy features, and G. glabra, with its emarginate and entire features [23]. The leaflet length averaged 3.6 cm, the width averaged 2.0 cm, and the number of leaflets ranged from 7 to 15 (Table 1). The back side of leaflets had white trichomes. We confirmed that the leaflets of G. korshinskyi exhibited species-specific characteristics, incorporating characteristics from both G. uralensis and G. glabra.
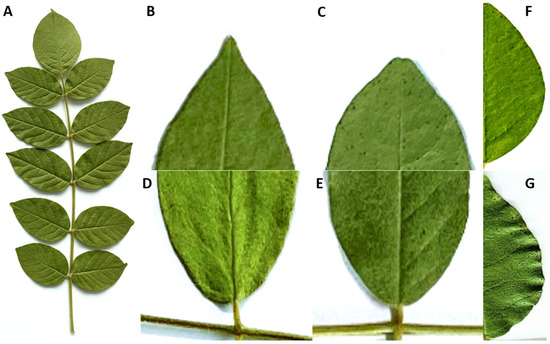
Figure 3.
Leaf characteristics of G. korshinskyi lines. (A) Leaf, (B) acute leaf apex, (C) emarginate leaf apex, (D) rounded leaf base, (E) oblique leaf base, (F) entire leaf margin, (G) wavy leaf margin.
The flower was a raceme (Figure 4). The flowering period of G. korshinskyi was generally reported as June to July [24], but in Korea, G. korshinskyi bloomed from late May to late June. Depending on climatic conditions, some lines bloomed again in September after the rainy season. However, the lines bloomed in September did not set pods. This might have been due to the drop in temperature affecting flower pollination and pod formation, as the average temperature in September was 22.4 °C, 4.1 °C lower than in the previous month. The flower was composed of a banner, side petal, keel, pistil, stamens, and calyx (Figure 4) [25,26]. The floret length ranged from 11.6 to 16.6 mm, and the pistils were 7.2 to 10.8 mm. This was larger than G. glabra and smaller than G. uralensis [27]. The stamens ranged in length from 6.5 to 9.6 mm (Table 2). We confirmed the floral characteristics of G. korshinskyi as an important morphological feature for classifying related species.
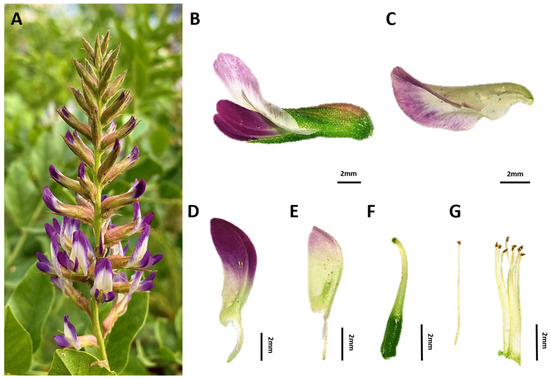
Figure 4.
Floral morphology and structural characteristics of G. korshinskyi (A) Flower, (B) Floret, (C) Banner petal, (D) Wing petal, (E) Keel petal, (F) Pistil, (G) Stamens. Scale bar = 2 mm.

Table 2.
Floral and pod characteristics of G. korshinskyi.
The pods (legumes) were observed 14 days after flowering. The pods of G. korshinskyi had a green surface, which turned brown at maturity. Depending on the characteristics of the line, the pods of G. korshinskyi varied in form, taking the form of ring-shaped, semi-ring-shaped, or erect (Figure 5). These exhibited species-specific characteristics, including characteristics of G. uralensis, which had trichomes and a ring shape, and G. glabra, which lacked trichomes and was erect [28,29]. Pod length averaged 26.2 mm, and width averaged 6.1 mm (Table 2). We proposed that in flowers and pods, floret length, pistil, and pod characteristics can serve as important morphological characteristics to distinguish species within the genus Glycyrrhiza.
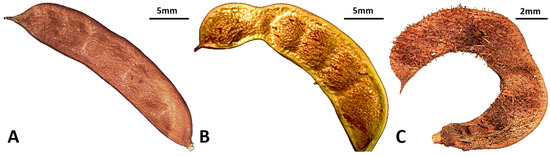
Figure 5.
Pod morphology of G. korshinskyi lines, showing three distinct shapes. (A) Erect, (B) Semi-ring-shaped, (C) Ring-shaped. Scale bars = 5 mm (A,B), 2 mm (C).
Seeds were kidney-shaped (Figure 6), ranging from 2.7 to 3.3 mm in length and 2.5 to 3.0 mm in width, with an average weight of 11.5 g (Table 2). Hard seeds had a low emergence rate due to their hard coat, which made water absorption difficult. Physical wounding was necessary to increase the emergence rate, with pinprick-type seed coat wounding shown to increase the emergence rate to 90% [30].

Figure 6.
Seed morphology and cross-section of G. korshinskyi.
The roots were columnar, with transverse lenticels on the surface. The root colors were classified as brown in 31 lines, red brown in 15 lines, and gray brown in 4 lines (Figure 7). Root length ranged from 45.7 to 99.6 cm, with an average root diameter of 20.3 mm. Root diameter ranged between 15 to 26 mm for 98% of the G. korshinskyi lines (Figure 7). The root diameter of G. uralensis cultivated in Korea averaged 14.6 mm, which was smaller than that of G. korshinskyi [17], potentially contributing to yield differences. The rootlet diameter averaged 6.5 mm, with the number of rootlets ranging from 3 to 11 (Table 3). G. korshinskyi was characterized by a high number of rootlets and a large root diameter, which were important for nutrient uptake and growth during early growth stages [31,32,33]. These root characteristics could be evaluated as important characteristics that contributed to the cultivation adaptability and yield of G. korshinskyi resources in Korea.
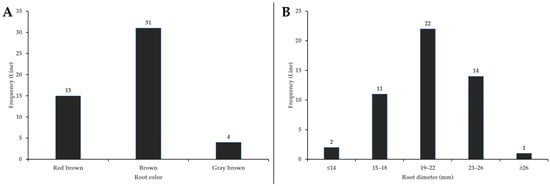
Figure 7.
Frequency distributions of root color and root diameter in G. korshinskyi lines. (A) Most lines had brown roots, followed by red brown and gray brown. (B) Root diameter was in the range of 15 to 26 mm for 98% of the G. korshinskyi lines.

Table 3.
Root and stolon characteristics of G. korshinskyi.
The stolon (rhizome) grows underground, with limited growth in the first year, followed by development in the second year [34]. Stolon length ranges from 41.5 to 175.3 cm, and stolon diameter ranges from 4.2 to 11.3 mm (Table 4). This variation was attributed to genetic differences between lines, even under the same cultivation conditions. A stolon diameter of 7 mm or more was observed in 60% of the stolon, with the number of stolons averaging 3 per plant (Figure 8, Table 4). Stolon diameter was closely related to emergence rate, with stolon diameters of 7 mm or more showing an emergence rate of approximately 70% [35]. Therefore, we propose that G. korshinskyi, with most stolon diameters exceeding 7 mm and a high emergence rate, could be a promising resource that can contribute to increased yield.

Table 4.
Yield characteristics and glycyrrhizin content of G. korshinskyi.
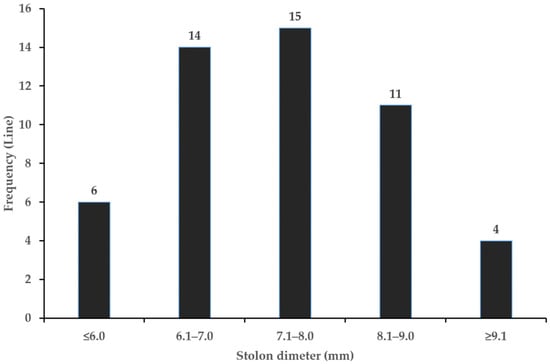
Figure 8.
Frequency distributions of stolon diameter in G. korshinskyi lines. Stolon diameter of 7 mm or more was observed in 30 lines.
The dry root weight ranged from 33.9 to 159.4 g, with an average dry yield of 53.4%, varying by line. In comparison, the average dry root weight of G. uralensis was 54.9 g, while G. korshinskyi was 214% higher at 117.5 g. The dry stolon weight ranged from 18.7 to 128.6 g, with an average dry yield of 48.8%. The average dry yield of roots with stolons removed was 503.7 kg/10a (Table 4). The yield of G. korshinskyi was about 136% higher than that of G. uralensis, which averaged 213.7 kg/10a [17]. Therefore, we propose that G. korshinskyi is more suitable for cultivation in the Korean environment than G. uralensis, as it offered higher dry root weight and yield.
Glycyrrhizin is a standard ingredient in licorice and belongs to the triterpenoid saponin group [36]. Glycyrrhizin content must be at least 2.5% for medicinal use in Korea [18]. Glycyrrhizin content averaged 1.5%, ranging from 0.5% to 2.6% depending on the line (Table 4). Previous studies have also reported variability in glycyrrhizin content. In Japan, the glycyrrhizin content of G. uralensis cultivated over 5 years ranged from 0.5% to 4.7% [37], while in northern China, it ranged from 1.0% to 1.9% after 2 years of cultivation [38]. Thus, glycyrrhizin content is known to be influenced not only by the characteristics of the resource but also by environmental factors such as geographic location, temperature, and soil conditions [39,40]. In 2020, Korea experienced a monsoon season lasting 49 days, which was estimated to have negatively impacted crop growth and glycyrrhizin content. However, accurately assessing glycyrrhizin content was challenging due to the short study period and limited lines, necessitating further research under diverse environmental conditions. Therefore, further studies on the interaction between environmental factors and glycyrrhizin content are needed.
3.2. Correlation Analysis
This research analyzed the correlations among 14 agronomic characteristics of G. korshinskyi.
Plant height showed a strong correlation with root weight (r = 0.65) and a moderate correlation with glycyrrhizin content (r = 0.40). This result showed that plant height is a characteristic that increases root weight and glycyrrhizin content. Leaf length and width showed a moderate correlation with root weight (r = 0.59), indicating that larger leaves enhance photosynthesis and contribute to root growth. Plant height, leaf length and leaf width increased root weight, and can be used as an indicator for predicting root yield based on above-ground traits.
Root diameter showed a strong correlation with root weight (r = 0.75), indicating that as root diameter increases, root weight also increases. However, the moderate correlation between root weight and glycyrrhizin content (r = 0.51) suggests that glycyrrhizin content was influenced by physiological or genetic factors. Stolon length showed a strong correlation with stolon weight (r = 0.66), indicating that longer stolons contribute to vegetative growth. Stolon diameter showed a moderate correlation with root weight (r = 0.49) and a weak correlation with glycyrrhizin content (r = 0.37). Stolon length and stolon weight were characteristics that do not affect glycyrrhizin content (Table 5).

Table 5.
Correlation coefficients among G. korshinskyi characteristics.
Plant height, root diameter, leaf length, leaf width, and stolon diameter can be used as a selection criteria to increase root yield. These characteristics also showed moderate correlations with glycyrrhizin content, suggesting their possible role in glycyrrhizin accumulation. However, glycyrrhizin content varies depending on genetic and physiological factors, requiring further research.
3.3. Principal Component Analysis
Principal component analysis (PCA) was conducted on 13 characteristics. Table 6 presents the eigenvalues and contributions of each principal component. PC1 had an eigenvalue of 4.25, explaining 32.69% of the total variance. PC2 had an eigenvalue of 2.01, accounting for 15.48%, PC3 had an eigenvalue of 1.47, explaining 11.29%, PC4 had an eigenvalue of 1.14, explaining 8.74%, and PC5 had an eigenvalue of 1.02, accounting for 7.85%, respectively. The cumulative contribution rates were 32.69%, 48.17%, 59.46%, 68.20%, and 76.05% for PC1 through PC5, respectively, reaching 100% at PC13 (Table 6). Based on the scree plot of the PCA (Figure 9) and following the method, principal components with eigenvalues of 1 or higher were selected [41]. In this research, the principal components up to PC5 explained 76.05% of the total variance. PC1 showed positive loadings for plant height, root diameter, root weight, stolon diameter, and glycyrrhizin content, indicating its association with growth and quality traits. PC2 showed positive loadings for stolon length, stolon number, stolon weight, and root weight, indicating its association with stolon-related characteristics (Table 7). Plant height, root diameter, root weight, stolon diameter, glycyrrhizin content, stolon length, stolon number, and stolon weight were useful traits for understanding the agronomic characteristics of G. korshinskyi.

Table 6.
Eigenvalue and contribution obtained from G. korshinskyi principal component analysis.
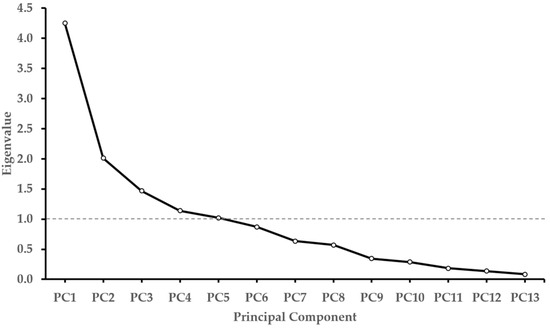
Figure 9.
Scree plot of eigenvalues in G. korshinskyi characteristics. The plot shows the eigenvalues of the principal components. The first five principal components were selected as they have eigenvalues greater than 1, as indicated by the dashed line.

Table 7.
Correlation coefficients between G. korshinskyi characteristics and principal components.
Cluster analysis was conducted based on principal component scores, and the data were grouped into three clusters using an average distance of 1.1 as the criterion. Group I was the largest cluster with 34 lines, representing 68% of the total. Group II was the smallest cluster with seven lines, representing 14% of the total. Group III contained nine lines, representing 18% of the total resources (Figure 10).
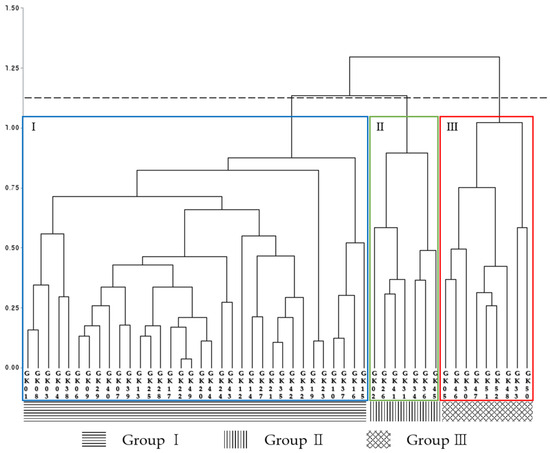
Figure 10.
Dendrogram of Glycyrrhiza korshinskyi lines classified into three clusters using a distance criterion of 1.1. Group I contained 34 lines, Group II contained 7 lines, and Group III contained 9 lines. Group III was evaluated for high growth characteristics and glycyrrhizin content characteristics.
Group I had lower plant height, leaf size, root size, stolon length, and glycyrrhizin content than other clusters. Group II had higher stolon length, stolon diameter, and stolon weight than other clusters. Group III had higher plant height, leaf length, leaf width, root diameter, root weight, and glycyrrhizin content than other clusters (Table 8).

Table 8.
Mean value of characteristics of each cluster classified by average linkage cluster in G. korshinskyi.
We grouped that each cluster exhibited different characteristics. Group I was the largest but had lower quantitative and qualitative traits. Group II was characterized by superior stolon traits, making it useful for propagation and functional studies. Group III had high growth characteristics and glycyrrhizin content, making it suitable for development of high-yield and high-glycyrrhizin cultivars.
4. Conclusions
In this research, G. korshinskyi lines were evaluated using growth characteristics, correlation analysis, principal component analysis, and cluster analysis. Growth characteristics were evaluated based on the emergence rate of G. korshinskyi resources, plant height, stem diameter, node number, leaf length, leaf width, root length, root diameter, root weight, stolon length, stolon diameter, stolon number, stolon weight, and glycyrrhizin content. Correlation analysis showed that plant height had strong positive correlations with node number, root diameter, and root weight. Root diameter had strong positive correlations with root weight and stolon diameter. We propose using these characteristics as effective indicators for breeding research. Principal component analysis explained approximately 76.05% of the total variance up to PC5. Plant height, root diameter, root weight, stolon diameter, glycyrrhizin content, stolon length, stolon number, and stolon weight were useful characteristics to review G. korshinskyi. Cluster analysis revealed three groups. Group III contained nine lines with high plant height, leaf length, leaf width, root diameter, root weight, and glycyrrhizin content. These lines contributed to the selection of high-yield and high-quality cultivars. In conclusion, this research evaluated the agronomic characteristics of G. korshinskyi resources through growth traits, correlation analysis, and principal component analysis. This research established a foundation for future breeding programs and functional studies.
Author Contributions
Conceptualization, J.L.; methodology D.S. and J.L.; investigation, D.S.; data curation, D.S. and J.P.; writing—original draft preparation, D.S.; writing—review and editing, S.W. and J.L.; supervision, J.L.; project administration, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01732206), the Rural Development Administration (RDA) Fellowship Program of the National Institute of Horticultural and Herbal Science, and the Collaborative Research Program between the university and the RDA, Republic of Korea.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cui, Y.M.; Li, P.Q. Flora of China; Science Press: Beijing, China, 1998; Volume 42, pp. 168–174. [Google Scholar]
- Ding, Y.; Brand, E.; Wang, W.; Zhao, Z. A Resource Over the Millennia: Licorice Applications in Ancient and Modern Times. J. Ethnopharmacol. 2022, 298, 115594. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine: Expanded Commission E Monographs; American Botanical Council: Austin, TX, USA, 2000; pp. 233–236. [Google Scholar]
- Wang, Y.Q.; Zhu, W. Investigation and analysis of licorice resources in China. J. Shanxi Agric. Univ. 2002, 4, 366–369. [Google Scholar]
- Çetin, Ö.; Duran, A.; Marthin, E.; Küçüködük, M. Karyological studies in some Glycyrrhiza (Fabaceae) taxa from Turkey. Caryologia 2015, 68, 254–264. [Google Scholar] [CrossRef]
- Hosseini, M.; Ebrahimi, M.; Abadía, J.; Kadkhodaei, S.; Amirian, R. Growth, phytochemical parameters, and glycyrrhizin production in licorice (Glycyrrhiza glabra L.) grown in the field with saline water irrigation. Ind. Crops Prod. 2022, 177, 114444. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, B.K.; Yan, M.; Fang, P.F.; Li, H.D.; Hu, C.P.; Yang, Y.; Cao, P.; Jiang, P.; Fan, X.R. A protective mechanism of licorice (Glycyrrhiza uralensis): Isoliquiritigenin stimulates detoxification system via Nrf2 activation. J. Ethnopharmacol. 2015, 162, 134–139. [Google Scholar] [CrossRef]
- Shin, S.W.; Yoon, E.K.; Jo, S.H.; Hwang, J.H. A study on the ‘Harmonizing all medicinal properties of Gancao’. J. Korean Med. Class. 2020, 33, 179–196. [Google Scholar]
- Mohammed, E.A.H.; Peng, Y.; Wang, Z.; Qiang, X.; Zhao, Q. Synthesis, Antiviral, and Antibacterial Activity of the Glycyrrhizic Acid and Glycyrrhetinic Acid Derivatives. Russ. J. Bioorg. Chem. 2022, 48, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Shi, J.-J.; Liu, Y.-J.; Yu, J.; Li, C.-Y.; Tao, F.; Cao, J.-F.; Yang, G.-J.; Chen, J. The State-of-the-Art Antibacterial Activities of Glycyrrhizin: A Comprehensive Review. Microorganisms 2024, 12, 1155. [Google Scholar] [CrossRef]
- Richard, S.A. Exploring the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediat. Inflamm. 2021, 2021, 6699560. [Google Scholar] [CrossRef]
- Si, L.; Yan, X.; Hao, W.; Ma, X.; Ren, H.; Ren, B.; Li, D.; Dong, Z.; Zheng, Q. Licochalcone D induces apoptosis and inhibits migration and invasion in human melanoma A375 cells. Oncol. Rep. 2018, 39, 2160–2170. [Google Scholar]
- Wang, J.; Zhang, Y.S.; Thakur, K.; Hussain, S.S.; Zhang, J.G.; Xiao, G.R.; Wei, Z.J. Licochalcone A form licorice root, an inhibitor of human hepatoma cell growth via induction of cell apoptosis and cell cycle arrest. Food Chem. Toxicol. 2018, 120, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Bioactivity and potential health benefits of licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef]
- Lee, K.L. The indigenization of licorice and its meaning during the early days of the Joseon Dynasty. Korean J. Med. Hist. 2015, 24, 423–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, M.W.; Lee, S.H.; Park, C.G.; Jeong, J.T.; Han, J.W.; Ma, K.H.; Chang, J.K. ‘Wongam’, a licorice interspecific hybrid cultivar with high yield. Korean J. Breed. Sci. 2020, 52, 454–459. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, M.W.; Seo, K.H.; Park, C.G.; Jeong, J.T.; Han, J.W.; Ma, K.H.; Chang, J.K. Cultivation and quality characterization of ‘Dagam’, a licorice interspecific hybrid cultivar. Korean J. Med. Crop Sci. 2021, 29, 110–116. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety. The Korean Pharmacopoeia, 12th ed.; Monographs, Part II; Ministry of Food and Drug Safety: Seoul, Republic of Korea, 2019; Volume 2, pp. 4–6. [Google Scholar]
- Kim, S.; Cho, Y. Development of Licorice Quality Improvement and Seed Production Technology; Ginseng & Medicinal Plant Research Institute: Gangwon, Republic of Korea, 2013. [Google Scholar]
- Lim, J.T.; Lee, H.J.; Cho, K.S.; Song, D.S. Analysis of lodging-related characteristics in rice plants. Korean J. Crop Sci. 1992, 37, 78–85. [Google Scholar]
- Ko, J.Y.; Song, S.B.; Choe, M.E.; Kwak, D.Y.; Kim, J.I.; Park, C.H.; Choi, J.M.; Woo, K.S.; Jung, T.W. Short culm, lodging tolerance, non-glutinous foxtail millet (Setaria italica Beauv.) variety ‘Daname’. Korean J. Breed. Sci. 2017, 49, 396–402. [Google Scholar] [CrossRef]
- Choe, M.E.; Kim, J.I.; Jung, T.W.; Kwak, D.Y.; Kim, K.Y.; Ko, J.Y.; Woo, K.S.; Song, S.B.; Jung, K.Y.; Oh, I.S. Waxy sorghum (Sorghum bicolor L.) variety ‘Nampungchal’ with lodging resistance and high yield. Korean J. Breed. Sci. 2016, 48, 192–197. [Google Scholar]
- Yan, B.; Hou, J.; Li, W.; Luo, L.; Ye, M.; Zhao, Z.; Wang, W. A review on the plant resources of important medicinal licorice. J. Ethnopharmacol. 2023, 301, 115823. [Google Scholar] [CrossRef]
- Öztürk, M.; Altay, V.; Hakeem, K.R.; Akçiçek, E. Liquorice: From Botany to Phytochemistry; Springer: Cham, Switzerland, 2018; pp. 12–13. [Google Scholar]
- Tucker, S.C. Floral development in legumes. Plant Physiol. 2003, 131, 911–926. [Google Scholar] [CrossRef]
- Reaume, T. Wild licorice Glycyrrhiza lepidota Fabaceae–Pea family. Nat. Manit. 2010, 1–3. Available online: https://www.naturemanitoba.ca/sites/default/files/WILD%20LICORICE.pdf (accessed on 1 September 2023).
- Flora of China. Glycyrrhiza linnaeus sp. 2015, 10, 509–510. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200012145 (accessed on 1 September 2023).
- Hayashi, H.; Hattori, S.; Inoue, K.; Sarsenbaev, K.; Ito, M.; Honda, G. Field survey of Glycyrrhiza plants in Central Asia (1). Characterization of Glycyrrhiza uralensis Fisch., Glycyrrhiza glabra and the putative intermediate collected in Kazakhstan. Biol. Pharm. Bull. 2003, 26, 867–871. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, C.G.; Choi, G.; Chang, J.K.; Lee, J.H.; Ju, Y.S. Comparative study of external-internal morphological shape in origins and hybrids for Glycyrrhizae Radix et Rhizome. Korean J. Herbol. 2019, 34, 1–12. [Google Scholar]
- Fu, K. On the Transforming of Chinese Licorice from Wild Growing into Domestication; Northeast Forestry University Press: Harbin, China, 1989; pp. 1–73. [Google Scholar]
- Buwalda, F.; Kim, K.S.; Frenck, R.; Locker, B.; Berg-De Vos, B.V.D. Effects of irrigation frequency and type of root medium on the growth of chrysanthemum cuttings. Acta Hort. 1995, 401, 193–200. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Park, J.S.; Isobe, T.; Kusakari, S.; Fujiwara, K. Promotion of adventitious roots in Chrysanthemum cuttings by soaking in ozonated water. Ozone Sci. Eng. 2009, 31, 15–20. [Google Scholar] [CrossRef]
- Son, D.K.; Oh, M.Y.; Hwang, H.S.; Han, J.W.; Jung, J.T.; Ma, K.H.; Woo, S.H.; Lee, J.H. Growth and Yield Characteristics of Glycyrrhiza spp. Korea J. Med. Crop Sci. 2021, 29, 242–252. [Google Scholar] [CrossRef]
- Oh, M.W.; Son, D.K.; Jeong, J.T.; Han, J.W.; Ma, K.H.; Lee, J.H. Study on Propagation Method of Vegetative Organ Using Licorice Rhizome (Stolon). Korea J. Med. Crop Sci. 2022, 30, 25–30. [Google Scholar] [CrossRef]
- Graebin, C.S. The pharmacological activities of glycyrrhizinic acid (“glycyrrhizin”) and glycyrrhietinic acid. Sweeteners 2017, 34, 245–261. [Google Scholar]
- Kojoma, M.; Hayashi, S.; Shibata, T.; Yamamoto, Y.; Sekizaki, H. Variation of glycyrrhizin and liquiritin contents within a population of 5-year-old licorice (Glycyrrhiza uralensis) plants cultivated under the same conditions. Biol. Pharm. Bull. 2011, 34, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Q.; Wei, S.; Wang, D.; Fang, Y.; Lin, F.; Zhao, Z.; Hou, J.; Wang, W. Effect of genotype and environment on five bioactive components of cultivated licorice (Glycyrrhiza uralensis Fisch.) population in northern China. Biol. Pharm. Bull. 2015, 38, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Oloumi, H.; Hassibi, N. Study the correlation between some climate parameters and the content of phenolic compounds in roots of Glycyrrhiza glabra. J. Med. Plants Res. 2011, 5, 6011–6016. [Google Scholar]
- Zhang, J.T.; Xu, B.; Li, M. Relationships between the bioactive compound content and environmental variables in Glycyrrhiza uralensis populations in different habitats of North China. Phyton 2011, 80, 161–166. [Google Scholar]
- Janmohammadi, M.; Movahedi, Z.; Sabaghnia, N. Multivariate statistical analysis of some characteristics of bread wheat for breeding under rainfed conditions. J. Agric. Sci. Belgrade 2014, 59, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).