How Does Rice Cope with High-Temperature Stress During Its Growth and Development, Especially at the Grain-Filling Stage?
Abstract
1. Introduction
2. Effects of High-Temperature Stress on Rice
2.1. Effects of High Temperature During the Vegetative Growth Stage
2.2. Effects of High Temperature During the Reproductive Growth Stage
2.3. Effects of High Temperature During the Grain-Filling Stage
3. Molecular Mechanisms of Rice Grain-Filling Stage Under Heat Stress
3.1. Molecular Mechanisms for Maintaining Starch Synthesis-Related Enzyme Activity in the Endosperm Under High Temperature
3.2. Molecular Mechanisms for Maintaining Reactive Oxygen Species and Phytohormone Balance in the Endosperm Under High Temperature
3.3. Molecular Mechanisms for Maintaining Protein Homeostasis in the Endosperm Under High Temperature
4. Strategies for Defending Against High-Temperature Effects in Rice
4.1. Identification of Heat-Tolerant Genes and Varieties
4.2. Rational Agronomic Management Practices
5. Conclusions and Future Perspectives
- (1)
- Heat-Tolerant Genetic Breeding:
- (2)
- Regulatory Mechanisms:
- (3)
- Environmental Interactions and Ecological Adaptability:
- (4)
- Intelligent Technologies and Interdisciplinary Integration:
- (5)
- Socio-Economic and Policy Support:
Author Contributions
Funding
Conflicts of Interest
References
- Paul, P.; Dhatt, B.K.; Sandhu, J.; Hussain, W.; Irvin, L.; Morota, G.; Staswick, P.; Walia, H. Divergent phenotypic response of rice accessions to transient heat stress during early seed development. Plant Direct. 2020, 4, e00196. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, W.; Miguel, S.; Assefa, S.; Amri, A.; Bishaw, Z.; Francis, C.; Michael, B. Genetic gains in wheat breeding and its role in feeding the world. Crop Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar]
- Xu, Y.F.; Chu, C.C.; Yao, S.G. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Redfern, S.K.; Azzu, N.; Binamira, J.S. Rice in Southeast Asia: Facing risks and vulnerabilities to respond to climate change. Building resilience for adaptation to climate change in the agriculture sector. In Proceedings of the a Joint FAO/OECD WORKSHOP, Rome, Italy, 23–24 April 2012; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012. [Google Scholar]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.; Khetarpal, S.; Jagadish, K.S. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol. Plant. 2015, 154, 543–559. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathi, S.; Singh, S.P.; Prasad, A.; Akter, F.; Syed, M.A.; Badri, J.; Das, S.P.; Bhattarai, R.; Natividad, M.A.; et al. Rice breeding for yield under drought has selected for longer flag leaves and lower stomatal density. J. Exp. Bot. 2021, 72, 4981–4992. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, J.X. TT3.1: A journey to protect chloroplasts upon heat stress. Stress Biol. 2022, 2, 27. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Murty, M.V.R.; Quick, W.P. Rice responses to rising temperatures-challenges, perspectives and future directions. Plant Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef]
- Xie, Y.J.; Shen, Q.P.; Li, F.F.; Ni, S.; Yu, J.S. Chapter Three-Temperature response of plants and heat tolerance in Rice: A review. Adv. Agron. 2023, 179, 135–203. [Google Scholar]
- Matsui, T.; Omasa, K.; Horie, T. The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Product. Sci. 2015, 4, 90–93. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, L.; Zhou, J.X.; Hu, S.B.; Chen, H.Z. Research progress on heat stress of rice at flowering stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y.S. The molecular basis of heat stress responses in plants. Mol. Plant. 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Gu, Z.D.; Ding, Y.F.; Wang, K.; Jiang, Q.; Zhu, C. Effect of high temperature stress on physiological characteristics of spikelet of rice during flowering stage. Chin. J. Rice Sci. 2016, 30, 637–646. [Google Scholar]
- Guo, J.M.; Wu, Y.; Yang, S.B.; Jiang, X.D.; Xie, X.Y.; Wang, J.J.; Shen, S.H. Yield different and its causes for one season rice under different sowing dates in typical high temperature year. Chin. J. Agrometeorol. 2017, 38, 121–130. [Google Scholar]
- Wei, J.L.; Pan, X.H.; Deng, Q.H. Effects of nighttime temperature increase at different growth stages on double season rice grain yield. Chin. J. Appl. Ecol. 2010, 21, 331–337. [Google Scholar]
- Wu, C.; Cui, K.H.; Wang, W.C.; Li, Q.; Fahad, S.; Hu, Q.Q.; Huang, J.L.; Nie, L.X.; Peng, S.B. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, 34978. [Google Scholar] [CrossRef]
- Gong, Z.Z.; Xiong, L.M.; Shi, H.Z.; Yang, S.H.; Herrera-Estrella, L.R.; Xu, G.H.; Chao, D.Y.; Li, J.R.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Folsom, J.J.; Begcy, K.; Hao, X.J.; Wang, D.; Walia, H. Rice Fertilization-Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol. 2014, 165, 238–248. [Google Scholar] [CrossRef]
- Chen, C.; Begcy, K.; Kiu, K.; Folsom, J.J.; Wang, Z.; Zhang, C.; Walia, H. Heat stress yields a unique MADS box transcription factor in determining seed size and thermal sensitivity. Plant Physiol. 2016, 171, 606–622. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Muthurajan, R.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef]
- Ren, H.M.; Bao, J.P.; Gao, Z.X.; Sun, D.Y.; Zheng, S.Z.; Bai, J.T. How rice adapts to high temperatures. Front. Plant Sci. 2023, 14, 1137923. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Butardo Jr, V.M.; Misra, G.; Cuevas, R.P.; Anacleto, R.; Kishor, P.B.K. Designing climate-resilient rice with ideal grain quality suited for high-temperature stress. J. Exp. Bot. 2015, 66, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, H.X.; Li, L.; Liu, X.; Chen, L.; Chen, W.Z.; Ding, Y.F. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct. Plant Biol. 2018, 45, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Sasaki, M.; Kuribayashi, N.; Susuki, H.; Sasuga, Y.; Shiraya, T.; Inomata, T.; Itoh, K.; Baslam, M.; Mitsui, T. Proteomic and glycomic characterization of rice chalky grains produced under moderate and high-temperature conditions in field system. Rice 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, T.; Parween, S.; Saito, Y.; Shigemitsu, T.; Yamakawa, H.; Nakazono, M.; Masumura, T.; Nishizawa, N.K.; Kondo, M.; Screenivasulu, N. Laser Microdissection-Based Tissue-Specific Transcriptome Analysis Reveals a Novel Regulatory Network of Genes Involved in Heat-Induced Grain Chalk in Rice Endosperm. Plant Cell Physiol. 2019, 60, 626–642. [Google Scholar] [CrossRef]
- Shi, W.J.; Yin, X.Y.; Struik, P.C.; Solis, C.; Xie, F.M.; Schimidt, R.C.; Huang, M.; Zou, Y.B.; Ye, C.R.; Jagadish, S.V.K. High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J. Exp. Bot. 2017, 68, 5233–5245. [Google Scholar] [CrossRef]
- Begcy, K.; Sandhu, J.; Walia, H. Transient Heat Stress During Early Seed Development Primes Germination and Seedling Establishment in Rice. Front. Plant Sci. 2018, 9, 1768. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hakata, M. Atlas of rice grain filling-related metabolism under high temperature: Joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010, 51, 795–809. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Jiang, Y.Y.; Zhang, H.; Wang, S.Y.; Wang, F.L.; Zhu, Y. Genetic Control and High Temperature Effects on Starch Biosynthesis and Grain Quality in Rice. Front. Plant Sci. 2021, 12, 757997. [Google Scholar] [CrossRef]
- Shirdelmoghanloo, H.; Chen, K.F.; Paynter, B.H.; Angessa, T.T.; Westcott, S.; Khan, H.A.; Hill, C.B.; Li, C.D. Grain-Filling Rate Improves Physical Grain Quality in Barley Under Heat Stress Conditions During the Grain-Filling Period. Front. Plant Sci. 2022, 13, 858652. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 5, 100990. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Yang, C.; Xu, J.M.; Lu, H.P.; Liu, J.X. The hot science in rice research: How rice plants cope with heat stress. Plant. Cell Environ. 2023, 46, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.M.; Lu, S.; Li, Z.; Cheng, J.W.; Hu, P.; Zhu, T.Q.; Wang, X.; Jin, M.; Wang, X.X.; Li, L.Q.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhang, Q.; Liu, D.L.; Wang, H.Q.; Yin, J.Y.; Wang, R.; He, M.L.; Cui, M.; Shang, Z.L.; Wang, D.K.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain-filling problem in ‘super’ rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef]
- Tonosaki, K.; Kinoshita, T. Possible roles for polycomb repressive complex 2 in cereal endosperm. Front. Plant Sci. 2015, 6, 144. [Google Scholar] [CrossRef]
- Dhatt, B.K.; Paul, P.; Sandhu, J.; Hussain, W.; Irvin, L.; Zhu, F.Y.; Adviento-Borbe, M.A.; Lorence, A.; Staswick, P.; Yu, H.F.; et al. Allelic variation in rice Fertilization Independent Endosperm 1 contributes to grain width under high night temperature stress. New Phytol. 2021, 229, 335–350. [Google Scholar] [CrossRef]
- Paul, P.; Dhatt, B.K.; Miller, M.; Folsom, J.J.; Wang, Z.; Krassovskaya, I.; Liu, K.; Sandhu, J.; Yu, H.H.; Zhang, C.; et al. MADS78 and MADS79 are essential regulators of early seed development in rice. Plant Physiol. 2020, 182, 933–948. [Google Scholar] [CrossRef]
- Zhu, X.P.; Teng, X.; Wang, Y.L.; Hao, Y.Y.; Jing, R.N.; Wang, Y.F.; Liu, Y.; Zhu, J.P.; Wu, M.M.; Zhong, M.S.; et al. FLOURYENDOSPERM11 encoding a plastid heat shock protein 70 is essential for amyloplast development in rice. Plant Sci. 2018, 277, 89–99. [Google Scholar] [CrossRef]
- Tabassum, R.; Dosaka, T.; Ichida, H.; Morita, R.; Ding, Y.F.; Abe, T.; Katsube-Tanaka, T. FLOURY ENDOSPERM11-2 encodes plastid HSP70-2 involved with the temperature-dependent chalkiness of rice (Oryza sativa L.) grains. Plant J. 2020, 103, 604–616. [Google Scholar] [CrossRef]
- Hakata, M.; Kuroda, M.; Miyashita, T.; Yamaguchi, T.; Kojima, M.; Sakakibara, H.; Mitsui, T.; Yamakawa, H. Suppression of α-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol. J. 2012, 10, 1110–1117. [Google Scholar] [CrossRef]
- Nakata, M.; Fukamatsu, Y.; Miyashita, T.; Hakata, M.; Kimura, R.; Nakata, Y.; Kuroda, M.; Yamaguchi, T.; Yamakawa, H. High Temperature-Induced Expression of Rice α-Amylases in Developing Endosperm Produces Chalky Grains. Front. Plant Sci. 2017, 8, 2089. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.F.; Zhang, H.; Wang, L.C.; Zhu, Z.G.; Gao, J.P.; Li, C.S.; Zhu, Y. High temperature inhibits the accumulation of storage materials by inducing alternative splicing of OsbZIP58 during filling stage in rice. Plant Cell Environ. 2020, 43, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Xu, H.; Zhu, Y.; Liu, Q.Q.; Cai, X.L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013, 64, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, L.; Dai, J.S.; Zhang, C.Q.; Li, J.; Gu, M.H.; Liu, Q.Q.; Zhu, Y. Major QTLs reduce the deleterious effects of high temperature on rice amylose content by increasing splicing efficiency of Wx pre-mRNA. Theor. Appl. Genet. 2014, 127, 273–282. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Feng, M.J.; Zhu, Y. Suppression of OsMADS7 in ice endosperm stabilizes amylose content under high temperature tress. Plant Biotechnol. J. 2018, 16, 18–26. [Google Scholar] [CrossRef]
- Huang, L.C.; Tan, H.Y.; Zhang, C.Q.; Li, Q.F.; Liu, Q.Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef]
- Kato, K.; Suzuki, Y.; Hosaka, Y.; Takahashi, R.; Kodama, I.; Sato, K.; Kawamoto, T.; Kumamaru, T.; Fujita, N. Effect of high temperature on starch biosynthetic enzymes and starch structure in japonica rice cultivar ‘Akitakomachi’ (Oryza sativa L.) endosperm and palatability of cooked rice. J. Cereal Sci. 2019, 87, 209–214. [Google Scholar] [CrossRef]
- Wu, H.M.; Ren, Y.L.; Dong, H.; Xie, C.; Zhao, L.; Wang, X.; Zhang, F.L.; Zhang, B.L.; Jiang, X.K.; Huang, Y.S.; et al. FLOURY ENDOSPERM24, a heat shock protein 101 (HSP101), is required for starch biosynthesis and endosperm development in rice. New Phytol. 2024, 242, 2635–2651. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, Z.Q.; Jiang, H.; Wang, Z.; Wu, F.S.; Xiong, Y.F.; Yao, J.L. A heat stress responsive NAC transcription factor heterodimer plays key roles in rice grain filling. J. Exp. Bot. 2021, 72, 2947–2964. [Google Scholar] [CrossRef]
- Yao, D.P.; Wu, J.; Luo, Q.H.; Li, J.W.; Zhuang, W.; Xiao, G.; Deng, Q.Y.; Lei, D.Y.; Bai, B. Influence of high natural field temperature during grain filling stage on the morphological structure and physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2020, 310, 125817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Zhou, L.H.; Zhu, Z.B.; Lu, H.W.; Zhou, X.Z.; Qian, Y.T.; Li, Q.F.; Gu, M.H.; Liu, Q.Q. Characterization of Grain Quality and Starch Fine Structure of Two Japonica Rice (Oryza sativa) Cultivars with Good Sensory Properties at Different Temperatures during the Filling Stage. J. Agric. Food Chem. 2016, 64, 4048–4057. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, F.; Zhong, L.; Sun, Z. Different of RVA profile among different early indica rice varieties and effect of temperature at grain filling stage on it. Chin. J. Rice Sci. 2003, 17, 39–43. [Google Scholar]
- Suriyasak, C.; Harano, K.; Tanamachi, K.; Matsuo, K.; Tamada, A.; Iwaya-Inoue, M.; Ishibashi, Y. Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness. J. Plant Physiol. 2017, 216, 52–57. [Google Scholar] [CrossRef]
- Wu, B.; Yun, P.; Zhou, H.; Xia, D.; Gu, Y.; Li, P.B.; Yao, J.L.; Zhou, Z.Q.; Chen, J.X.; Liu, R.J.; et al. Natural variation in WHITE-CORE RATE 1 regulates redox homeostasis in rice endosperm to affect grain quality. Plant Cell 2022, 34, 1912–1932. [Google Scholar] [CrossRef]
- Pang, Y.H.; Ying, Y.N.; Yu, F.F.; Bao, J.S. Integrated analysis of phosphoproteome and ubiquitinated proteome in rice endosperm under high temperature stress. J. Zhejiang Univ. Sci. (Agric. Life Sci. Ed.) 2024, 50, 382–392. [Google Scholar]
- Zhang, X.F.; Tong, J.H.; Bai, A.N.; Liu, C.M.; Xiao, L.T.; Xue, H.W. Phytohormone dynamics in developing endosperm influence rice grain shape and quality. J. Integr. Plant Biol. 2020, 62, 1625–1637. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, G.H.; Hu, B.H.; Wu, J.; Chen, W.L.; Ren, Z.J.; Liu, Y.L.; Xie, J.; Yuan, H.; Tu, B.; et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 2021, 7, eabc8873. [Google Scholar] [CrossRef]
- Liu, J.P.; Zhang, C.C.; Wei, C.C.; Wang, M.G.; Yu, F.F.; Xie, Q.; Tu, J.M. The RING Finger Ubiquitin E3 Ligase OsHTAS Enhances Heat Tolerance by Promoting H2O2-Induced Stomatal Closure in Rice. Plant Physiol. 2016, 170, 429–443. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhong, X.; Liao, J.P.; Ji, P.; Yang, J.S.; Cao, Z.R.; Duan, X.M.; Xiong, J.R.; Wang, Y.; Xu, C.; et al. Exogenous abscisic acid improves grain filling capacity under heat stress by enhancing antioxidative defense capability in rice. BMC Plant Biol. 2023, 23, 619. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhou, W.; Guo, X.Y.; Ling, S.; Li, W.; Wang, X.; Yao, J.L. Heat Stress Responsive Aux/IAA Protein, OsIAA29 Regulates Grain Filling Through OsARF17 Mediated Auxin Signaling Pathway. Rice 2024, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Mesihovic, A.; Hu, Y.J.; Schleiff, E. Unfolded protein response in pollen development and heat stress tolerance. Plant Reprod. 2016, 29, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.; Irvin, L.; Liu, K.; Staswick, P.; Zhang, C.; Walia, H. Endoplasmic reticulum stress pathway mediates the early heat stress response of developing rice seeds. Plant Cell Environ. 2021, 44, 2604–2624. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.J.; Zhao, S.L.; Jiao, G.A.; Duan, Y.Q.; Ma, L.Y.; Dong, N.N.; Lu, F.F.; Zhu, M.D.; Shao, G.N.; Hu, S.K.; et al. OPAQUE3, encoding a transmembrane bZIP transcription factor, regulates endosperm storage protein and starch biosynthesis in rice. Plant Commun. 2022, 3, 100463. [Google Scholar] [CrossRef]
- Yang, W.P.; Xu, P.K.; Zhang, J.C.; Zhang, S.; Li, Z.W.; Yang, K.; Chang, X.Y.; Li, Y.B. OsbZIP60-mediated unfolded protein response regulates grain chalkiness in rice. J. Genet. Genom. 2022, 49, 414–426. [Google Scholar] [CrossRef]
- Lu, S.J.; Yang, Z.T.; Sun, L.; Sun, L.; Song, Z.T.; Liu, J.X. Conservation of IRE1-regulated bZIP74 mRNA unconventional splicing in rice (Oryza sativa L.) involved in ER stress responses. Mol. Plant 2012, 5, 504–514. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Qing, T.; Shu, X.L.; Liu, J.X. Unfolded protein response and storage product accumulation in rice grains. Seed Biol. 2022, 1, 33–37. [Google Scholar] [CrossRef]
- Berka, M.; Kopecka, R.; Berkova, V.; Brzobohaty, B.; Cerny, M. Regulation of heat shock proteins 70 and their role in plant immunity. J. Exp. Bot. 2022, 73, 1894–1909. [Google Scholar] [CrossRef]
- De-Jong, W.W.; Caspers, G.J.; Leunissen, J.A. Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int. J. Biol. Macromol. 1998, 22, 151–162. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Lu, F.F.; Jiao, G.A.; Qiu, J.H.; Zhao, S.L.; Zhao, F.L.; Wang, P.; Chen, L.N.; Chen, P.F.; Li, X.W.; Don, N.N.; et al. A molecular module improves rice grain quality and yield at high temperature. Nat. Sci. Rev. 2025, 12, nwae416. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, X.; Wang, M.; Zhu, Q.; Lv, Y.; Xu, J.; Liu, J. The NAT1-bHLH110-CER1/CER1L module regulates heat stress tolerance in rice. Nat. Genet. 2025, 57, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Chao, D.Y.; Wu, Y.; Huang, X.H.; Chen, K.; Cui, L.G.; Su, L.; Ye, W.W.; Chen, H.; Chen, H.C.; et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.R.; Zhang, H.; Gao, J.; Shan, J.X.; Ye, W.W.; Lin, H.X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plant 2022, 8, 53–67. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.F.; Kan, Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Guo, T.; Xiang, Y.H.; Yang, Y.B.; Li, Y.C.; et al. A gen module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef]
- Kan, Y.; Lin, H.X. A research progress of thermo-perception and thermo-responses in rice. Chin. J. Nat. 2022, 44, 411–421. [Google Scholar]
- Mir, M.S.; Raja, W.; Kanth, R.H.; Dar, E.A.; Shah, Z.A.; Bhat, M.A.; Mir, A.H.; Wani, F.J.; Bhat, T.A.; Bhat, J.A.; et al. Optimizing irrigation and nitrogen levels to achieve sustainable rice productivity and profitability. Sci. Rep. 2025, 15, 6675. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, H.; Zhang, F.; Wang, H.; Zhang, F.S. Effects of silicon on anther dehiscence and pollen shedding in rice under high-temperature stress. Acta Agron. Sin. 2005, 31, 134–136. [Google Scholar]
- Zhao, J. Effect of application quantity of N, P and K on resistant capability of rice against hot disaster of high temperature. Soils Fertil. 2005, 5, 13–16. [Google Scholar]
- Chen, T.T.; Li, G.Y.; Islam, M.R.; Fu, W.M.; Feng, B.H.; Tao, L.X.; Fu, G.F. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol. 2019, 19, 525. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, P.H.; Fu, W.M.; Li, G.Y.; Feng, B.H.; Chen, T.T.; Li, H.B.; Tao, L.X.; Fu, G.F. Acid invertase confers heat tolerance in rice plants by maintaining energy homoeostasis of spikelets. Plant Cell Environ. 2020, 43, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of proline function in higher plants under extreme temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
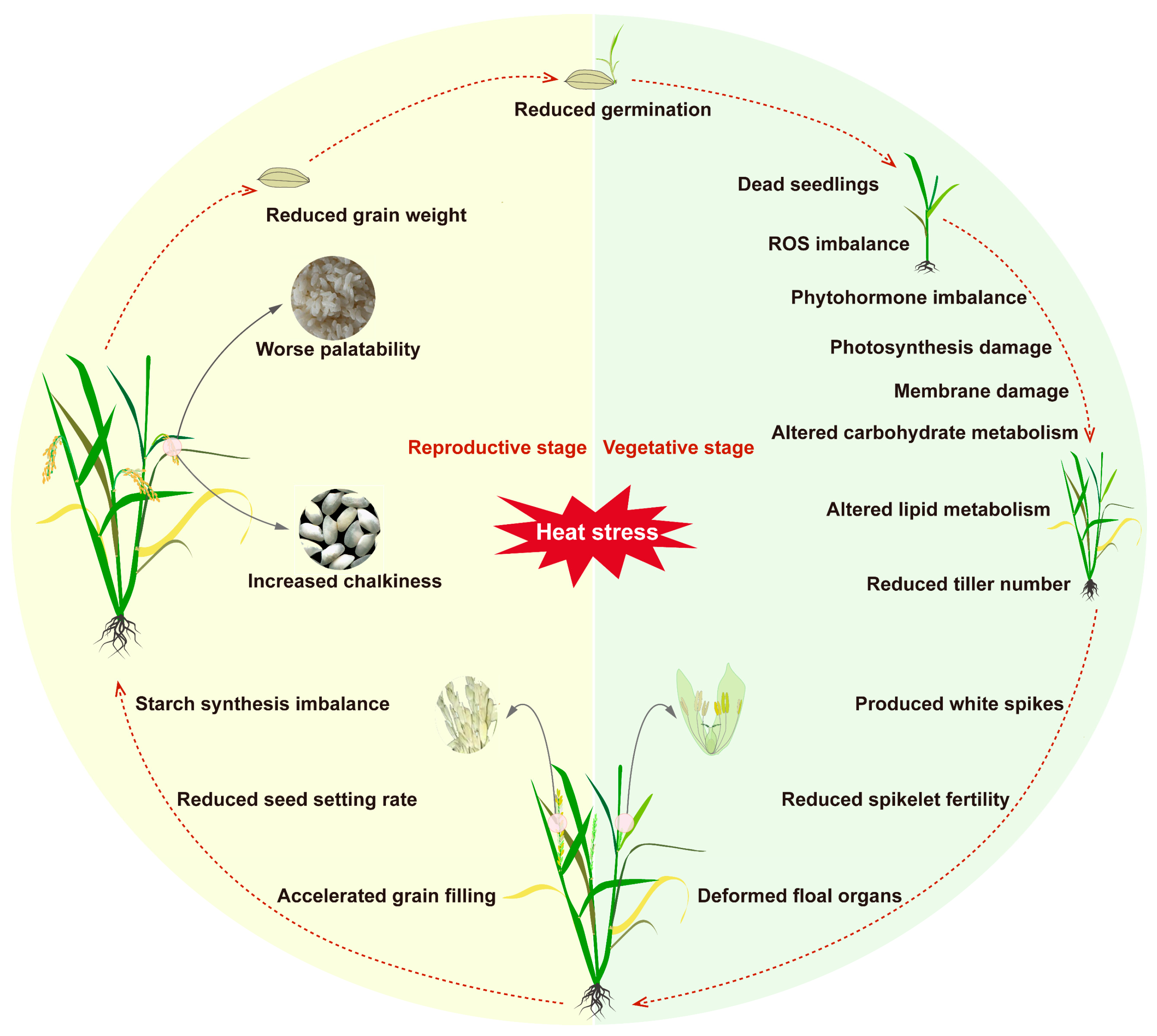
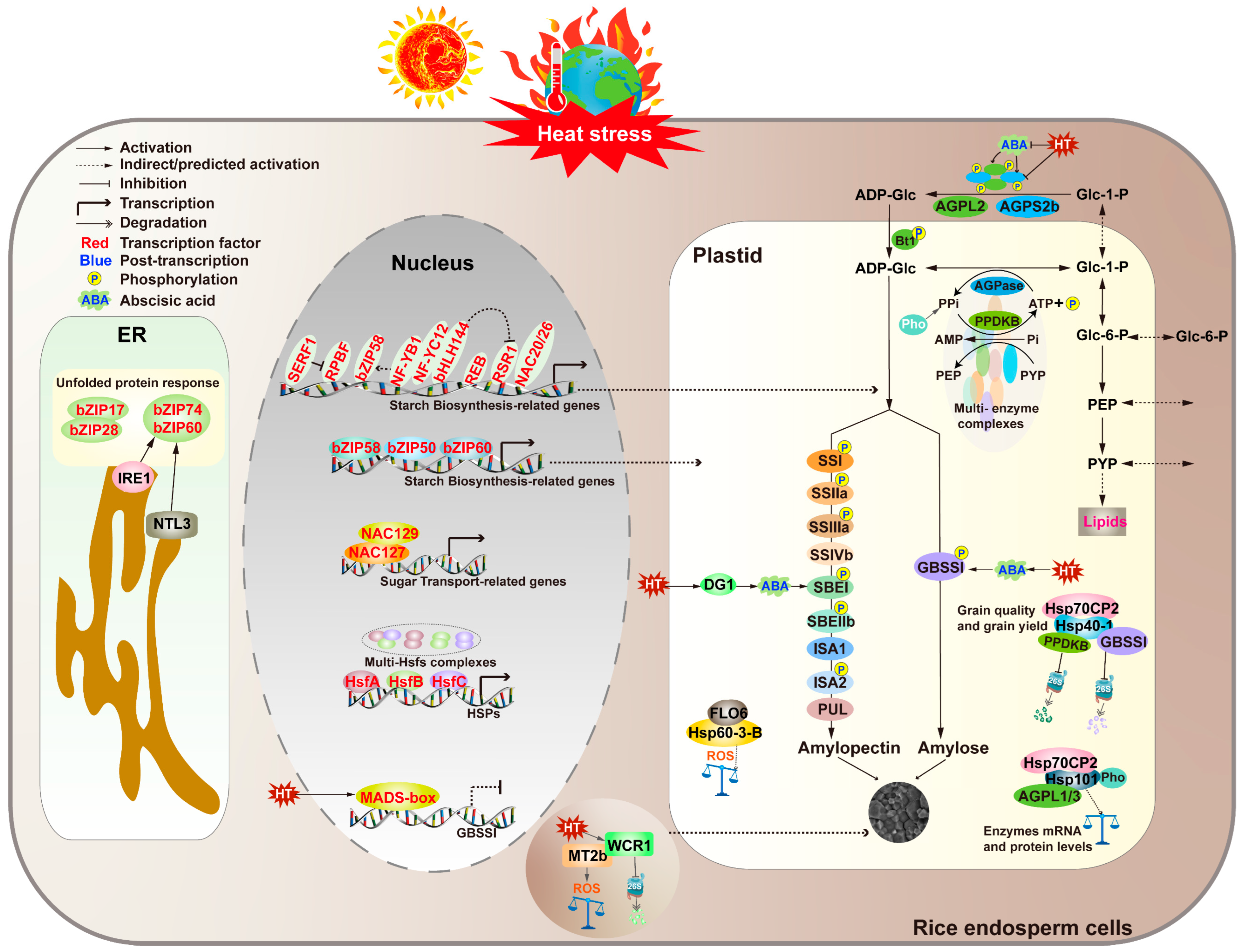
| Growth Stage | High-Temperature Threshold (°C) | Key Effects |
|---|---|---|
| Germination | >35 °C | Dealy germination, reduced rates |
| Vegetative | >33 °C | Reduced tillering, leaf chlorosis |
| Reproductive | >35 °C (critical at flowering) | Pollen sterility, spikelet sterility |
| Grain-filling | >34 °C | Accelerated filling, increased chalkiness |
| Source: FAO, 2012 [4] |
| Types | Gene | Growth Period | Functions Associated with Heat Stress |
|---|---|---|---|
| HSFs | OsHsfA2a | Seedling | Involved in heat tolerance by alternative splicing |
| OsHsfA2c | Seedling | Induced by heat stress | |
| OsHsfA2d | Seedling Tillering | help cells establish protein folding equilibrium | |
| OsHsfA2e | Seedling | Overexpression can enhance the heat tolerance | |
| OsHsfA4b/A4d/A7 | Seedling | Expression was induced by high temperature | |
| OsHsfB1/Bb/B4d | Seedling | Expression was induced by high temperatures | |
| OsHsfC1b | Seedling | Expression was induced by high temperatures | |
| TFs | OsbZIP58 | Filling | High temperature induced alternative splicing to inhibit the accumulation of storage materials |
| OsbZIP60 | Filling | Activated the expression of UPR-related genes by heat stress | |
| OsbZIP50 | Filling | Affected rice endosperm chalky formation | |
| OsbZIP74 | Filling | Its expression was regulated by OsNTL3 under high temperature | |
| HYR | Seedling Filling | Increased yeld by activating photosynthesis-related genes under high temperature | |
| OsDREB2B2 | Seedling | Overexpression improved the heat tolerance of rice | |
| SNAC3 | Seedling Flowering | Overexpression enhanced the ability of rice to resist high temperature | |
| OsNTL3 | Seedling | Loss of function improved the heat tolerance of rice seedlings | |
| OsNAC127/129 | Filling | They form heterodimers to participate in grain filling of rice under high temperature stress | |
| OsMYB48-1 | Seedling | High temperature tolerance was enhanced by ABA biosynthesis | |
| OsMYB55 | Germination Seedling | Increased high temperature tolerance by improved the content of amino acids | |
| OsWRKY11 | Seedling | Improved the heat tolerance by inhibiting expression | |
| OsWRKY10 | Seedling | By regulating the homeostasis of ROS under heat stress | |
| DST | Seedling | Determined heat resistance by regulating the expression of redox homeostasis-related genes | |
| HSPs | OsHsp101 | Seedling | Overexpression enhanced the heat tolerance of rice |
| OsHsp90 | Seedling | Protein abundance increased significantly after heat exposure | |
| OsHsp70CP1 | Seedling | Required for early chloroplast development under high temperature stress | |
| OsHsp70CP2 | Filling | At high temperatures, it exhibits a chalky phenotype; Overexpression improved rice quality and yield at both room temperature and high temperature | |
| OsHsp60-3B | Flowering Filling | Regulated starch grain biosynthesis in rice pollen at high temperature | |
| OsHsp40 | Seedling | Regulates programmed cell death in suspension cells at high temperatures | |
| OsHsp40-1 | Filling | Overexpression improved rice quality and yield at both room temperature and high temperature | |
| OsHsp17.7 | Seedling | Overexpression conferred heat and UV resistance to rice | |
| OsHsp26.7 | Seedling | Protects chloroplasts from oxidative damage caused by high temperatures and ultraviolet rays | |
| OsHsp17.9 | Seedling | Expression of this gene in tobacco showed better heat tolerance | |
| OsHsp1 | Seedling | Enhance the heat tolerance by overexpression | |
| Enzyme | OsHCI1 | Seedling | Enhance plant high-temperature tolerance by overexpression |
| TT3.1 | Seedling | Positive regulation of heat tolerance of rice | |
| OsHTAS | Seedling | Regulated the heat tolerance by adjusting the opening and closing of the stomata | |
| GAD3 | Seedling | Promote the synthesis of stress-related amino acids and enhance high temperature tolerance | |
| OsAPX2 | Seedling | Expression of ascorbate peroxidase was heat-induced | |
| OsGIRL1 | Seedling | Overexpression improved the survival rate of seedlings at high temperature | |
| TT1 | Seedling | Removed the denatured proteins under heat stress | |
| TOGR1 | Seedling | Protects the processing of rRNA precursors at high temperatures and increased heat resistance | |
| PGL1 | Seedling | Sensitivity to heat stress after loss of function | |
| TCM5 | Seedling | Involved in the development of chloroplasts at high temperatures | |
| EG1 | Flowering | Maintained the stable development of floral organs at high temperatures | |
| AET1 | Seedling | Maintained protein stability at high temperatures | |
| OsNSUN2 | Seedling | Regulated the mRNA translation efficiency to maintain normal development at high temperature | |
| WLP2/OsFLN | Seedling | Maintain intracellular redox balance at high temperature to protect chloroplasts from damage | |
| GSA1 | Seedling | Regulates the synthesis of flavonoid metabolites to improve resistance to high temperature | |
| HSE1 | Seedling | Loss of function leaded to ROS outbreak and chloroplast degradation under heat stres | |
| Others | OsGSK1 | Seedling | Sensitivity to heat stress after loss of function |
| OsLEA4/LEA5 | Exhibited significant high-temperature tolerance in Escherichia coli | ||
| OsRZFP34 | Seedling | Decreased temperature tolerance after loss of function | |
| ERECTA | Filling | Overexpression confers heat tolerance in rice | |
| OsANN1 | Seedling | The accumulation of antioxidants under heat stress was regulated to confer heat stress tolerance | |
| HSA1/OsFLN2 | Seedling | Protected chloroplasts from heat stress | |
| LS1 | Seedling | Prevented chloroplast degradation and apoptosis at high temperatures | |
| SLG1 | Seedling | Maintained normal tRNA thiolation levels under heat stress to ensure protein translation | |
| D1 | Seedling | Enhanced photosynthesis efficiency and improved high-temperature tolerance | |
| RBG1 | Seedling | Enhanced heat tolerance through auxin and cytokinin pathways | |
| OsCNGC14/16 | Seedling | Sensitivity to heat stress after loss of function | |
| TT2 | Seedling | Maintained normal wax content to help rice withstand high temperatures | |
| OsGR-RBP4 | Seedling | Overexpression improved yeast tolerance to high-temperature stress | |
| OsGRP3-GRP162 | Seedling Filling | Involved in the splicing of mRNA and positively regulated the heat tolerance of rice | |
| OsEDS1 | Seedling | Excess ROS were removed at high temperatures by interacting with OsCATB/C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Feng, B.; Chen, L.; Qiu, J.; Wei, X. How Does Rice Cope with High-Temperature Stress During Its Growth and Development, Especially at the Grain-Filling Stage? Agronomy 2025, 15, 623. https://doi.org/10.3390/agronomy15030623
Lu F, Feng B, Chen L, Qiu J, Wei X. How Does Rice Cope with High-Temperature Stress During Its Growth and Development, Especially at the Grain-Filling Stage? Agronomy. 2025; 15(3):623. https://doi.org/10.3390/agronomy15030623
Chicago/Turabian StyleLu, Feifei, Baohua Feng, Long Chen, Jiehua Qiu, and Xiangjin Wei. 2025. "How Does Rice Cope with High-Temperature Stress During Its Growth and Development, Especially at the Grain-Filling Stage?" Agronomy 15, no. 3: 623. https://doi.org/10.3390/agronomy15030623
APA StyleLu, F., Feng, B., Chen, L., Qiu, J., & Wei, X. (2025). How Does Rice Cope with High-Temperature Stress During Its Growth and Development, Especially at the Grain-Filling Stage? Agronomy, 15(3), 623. https://doi.org/10.3390/agronomy15030623









