Potential of Three Plant Extracts in Suppressing Potato Dry Rot Caused by Fusarium incarnatum Under Normal and Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fusarium incarnatum, the Causal Agent of Potato Dry Rot
2.3. Preparation of Plant Extracts
2.4. In Vitro Antifungal Properties of Plant Ethanolic Extracts
2.5. Control of Potato Dry Rot Disease in Tubers Under Storage Conditions
2.6. Biochemical Changes in Treated Potato Tubers
2.6.1. Activities of Defense-Related Enzymes
The Catalase Enzyme Activity
The Peroxidase Activity
The Polyphenol Oxidase Activity
Phenylalanine Ammonia-Lyase
2.6.2. Total Soluble Phenolic Compounds
2.6.3. Total Soluble Flavonoid Compounds
2.6.4. Assessment of Malondialdehyde as an Indication of Lipid Peroxidation
2.7. Determination of Lignin Content (%)
2.8. The Weight Loss (%) of Potato Tubers
2.9. Statistical Analysis
3. Results
3.1. In Vitro Antifungal Activity of Tested Ethanolic Extracts Against F. incarnatum
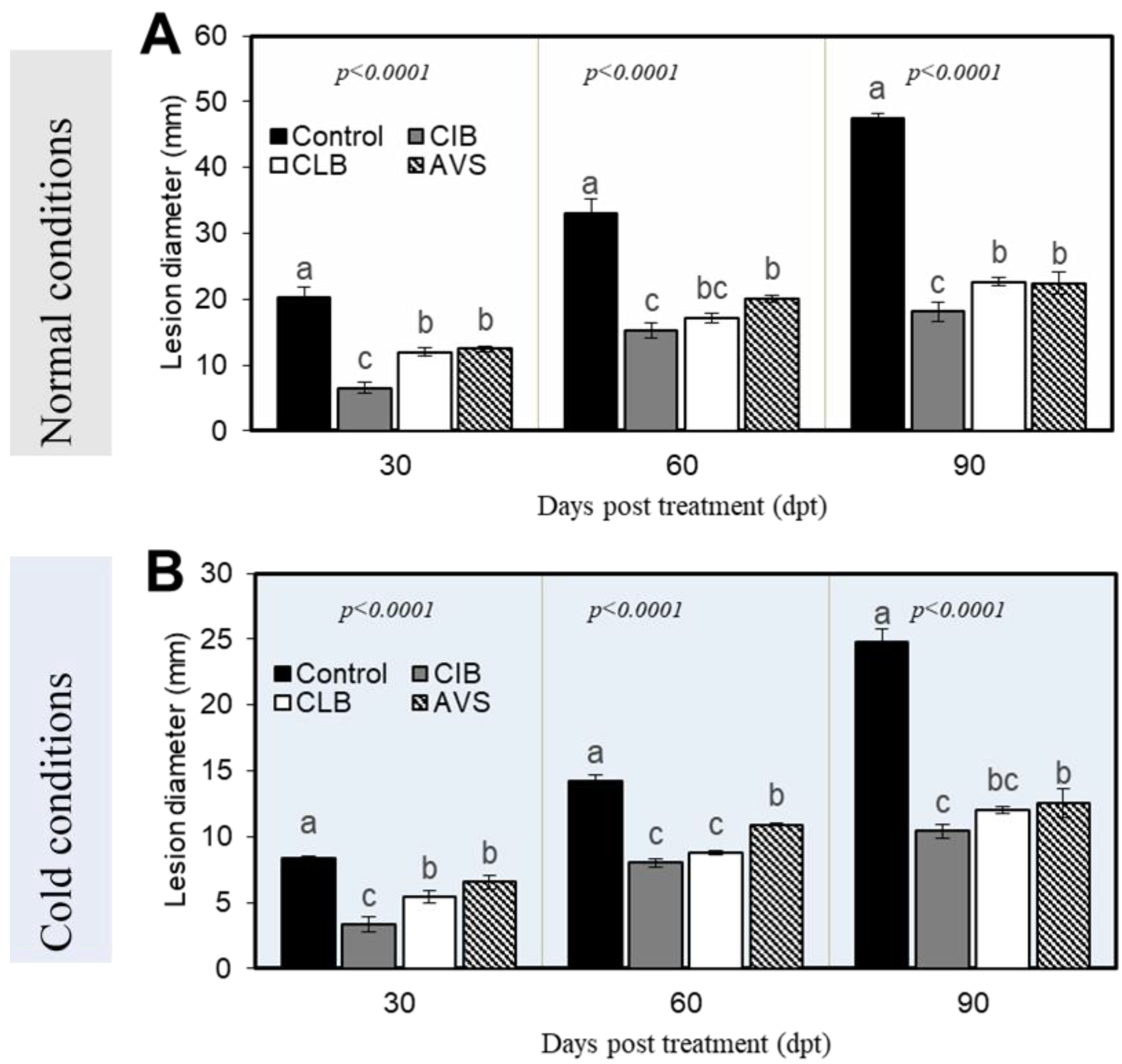
3.2. Control of Potato Dry Rot Using Plant Extracts Under Normal and Cold Conditions
3.3. Tested Extracts Enhanced the Defense-Related Enzymes
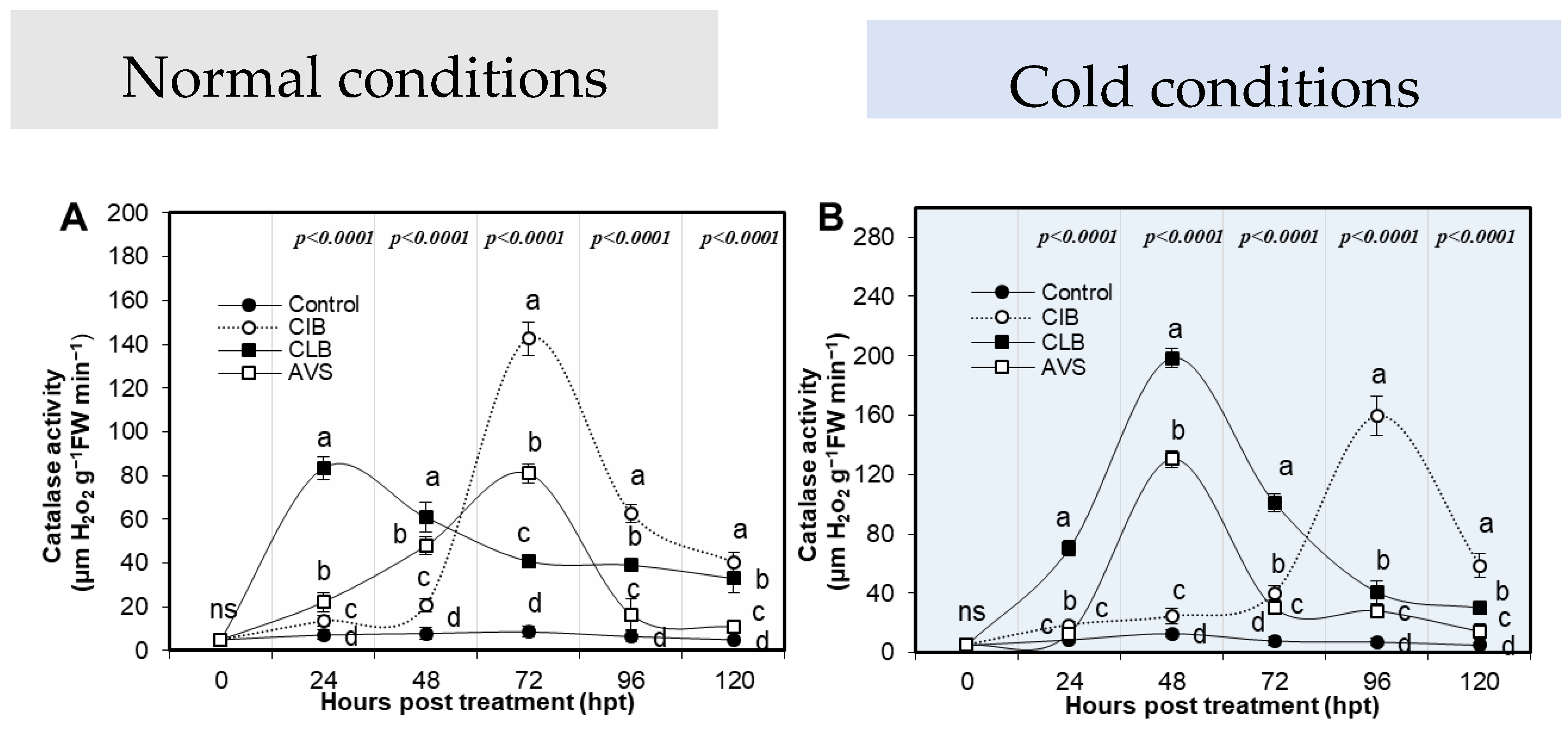
3.3.1. Catalase Activity
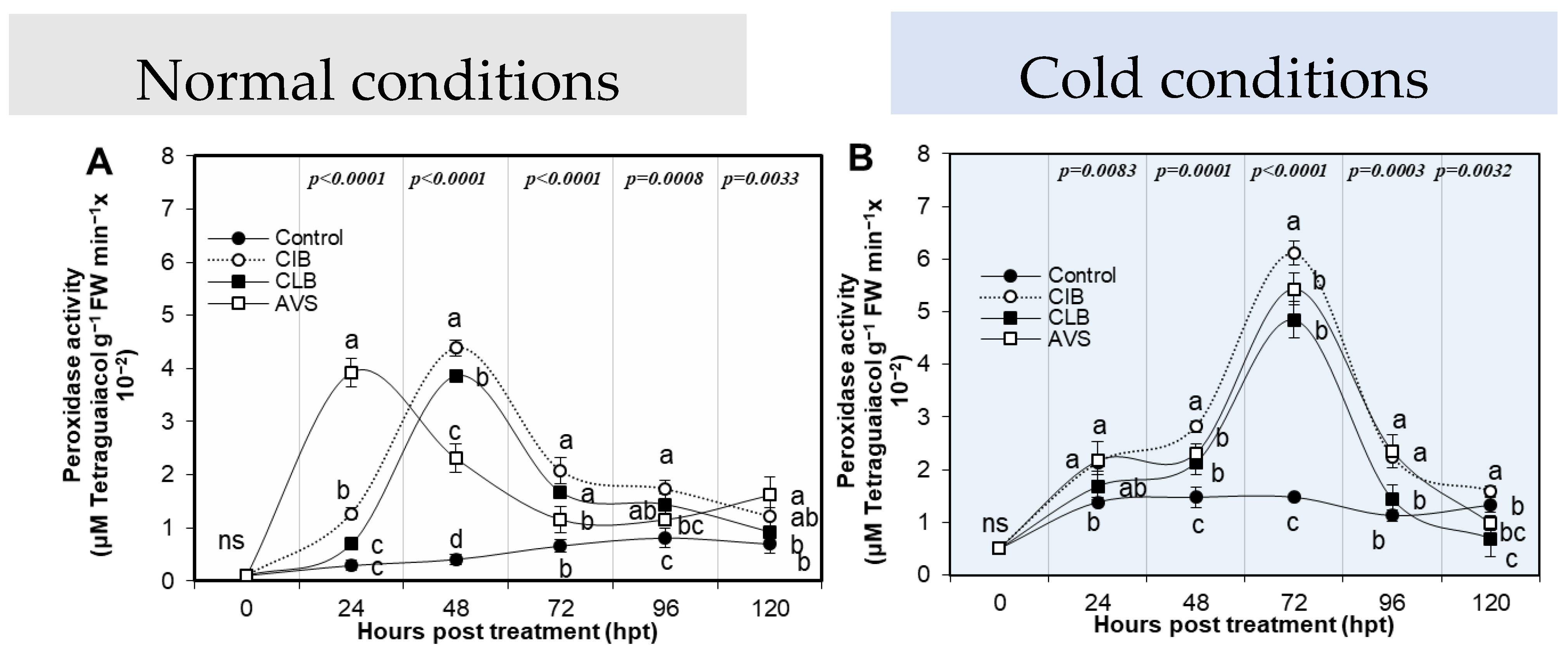
3.3.2. Peroxidase Activity
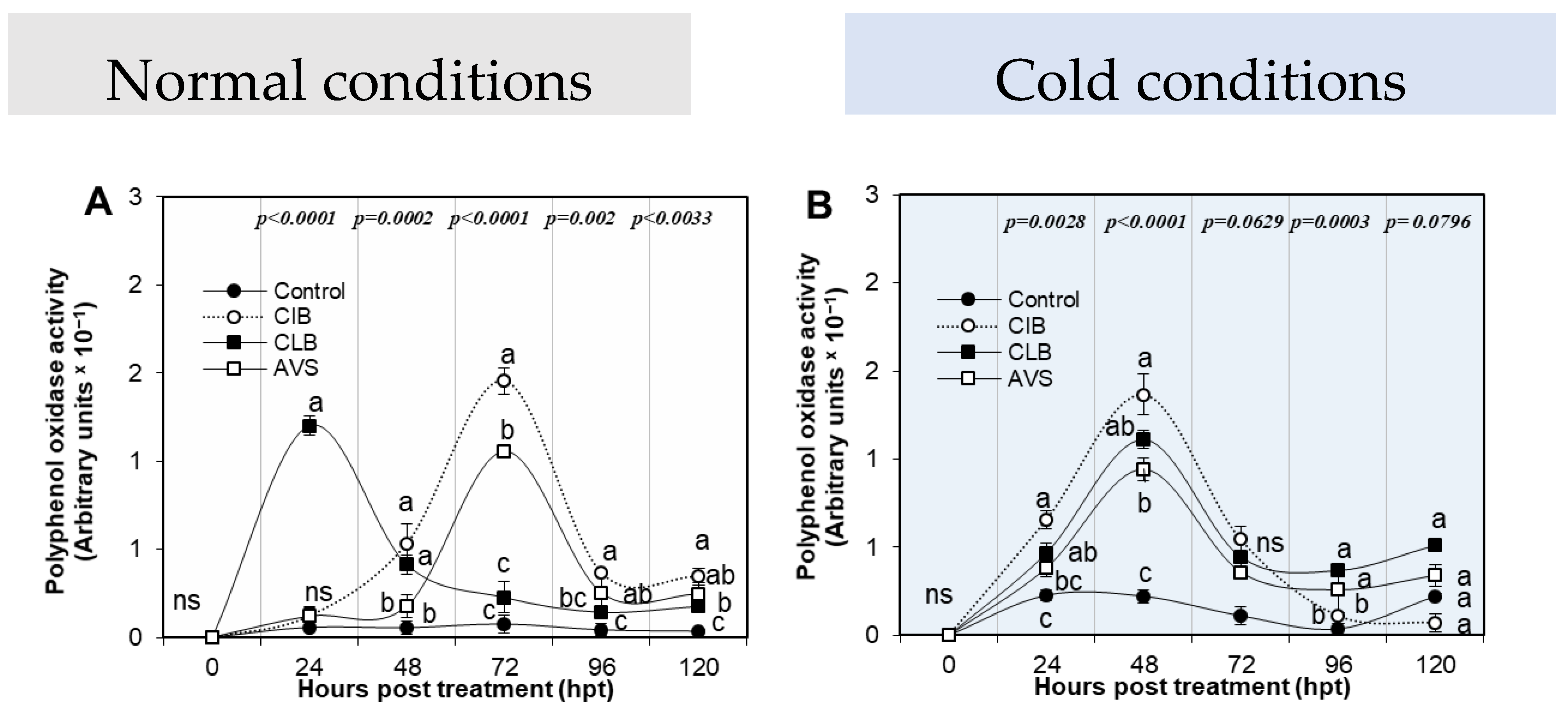
3.3.3. Polyphenol Oxidase Activity
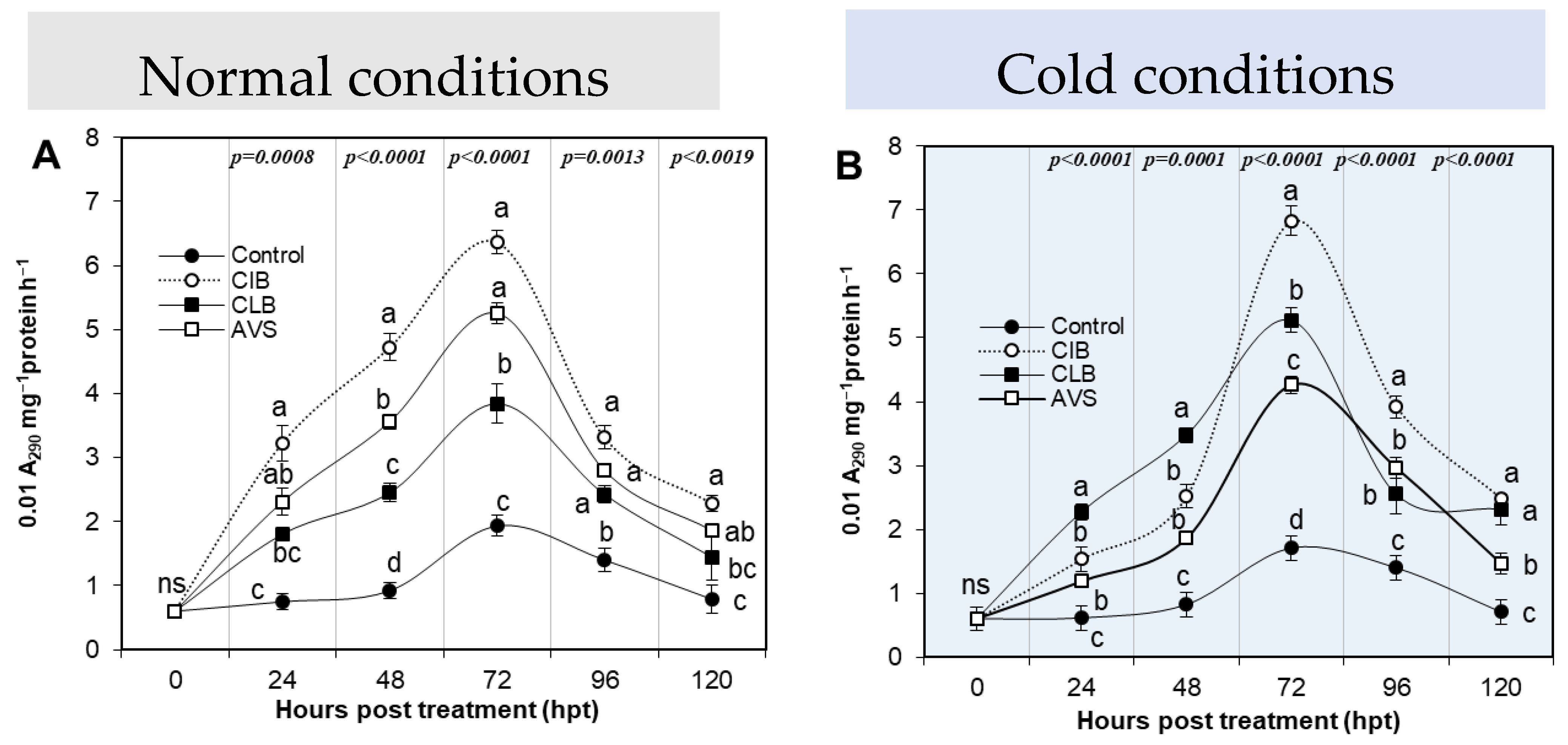
3.3.4. Phenylalanine Ammonia-Lyase Activity
3.4. Tested Extracts Enhanced Total Soluble Phenolic Content
3.5. Tested Extracts Enhanced Total Soluble Flavonoids
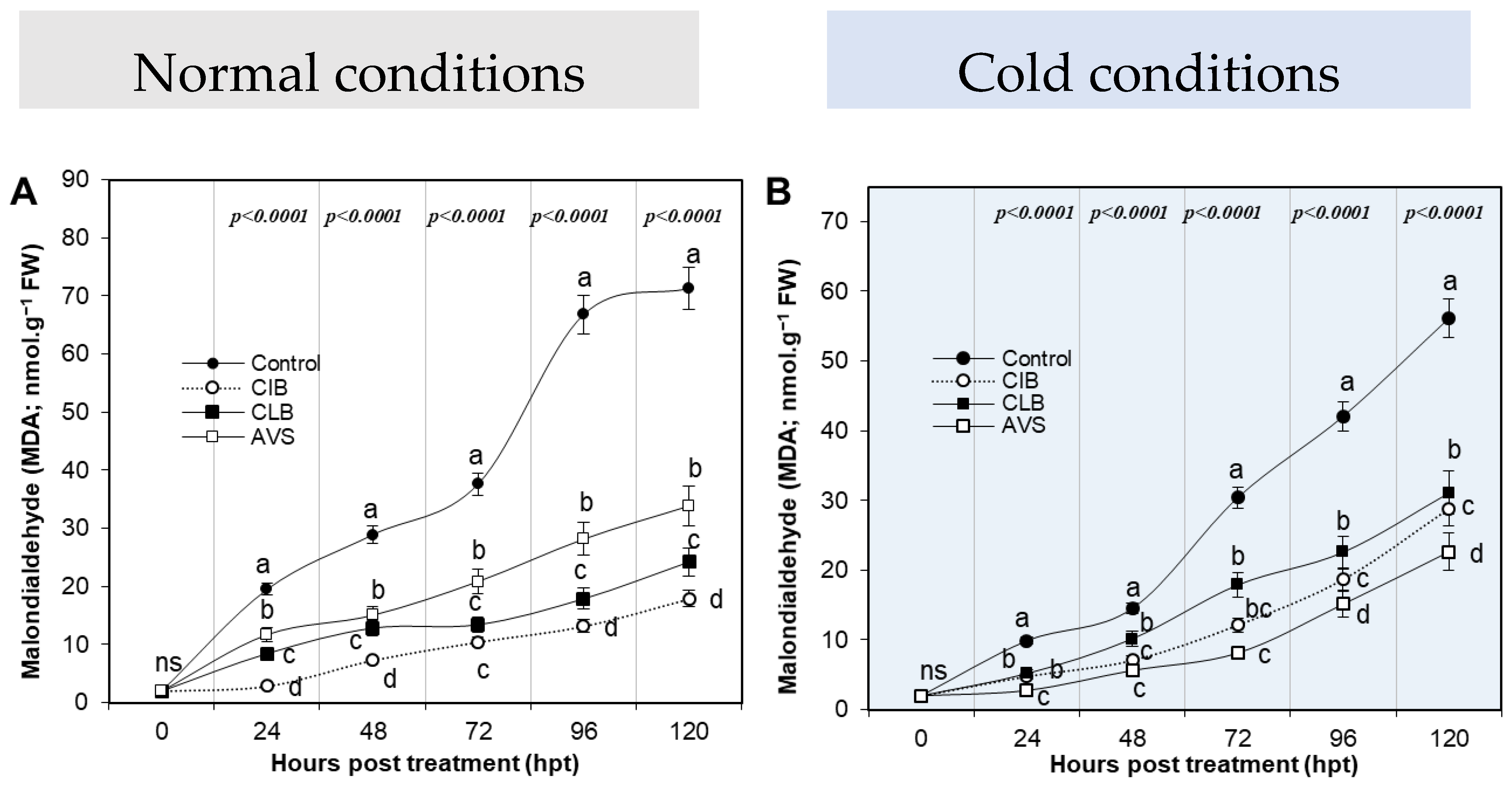
3.6. Tested Extracts Alleviate Lipid Peroxidation
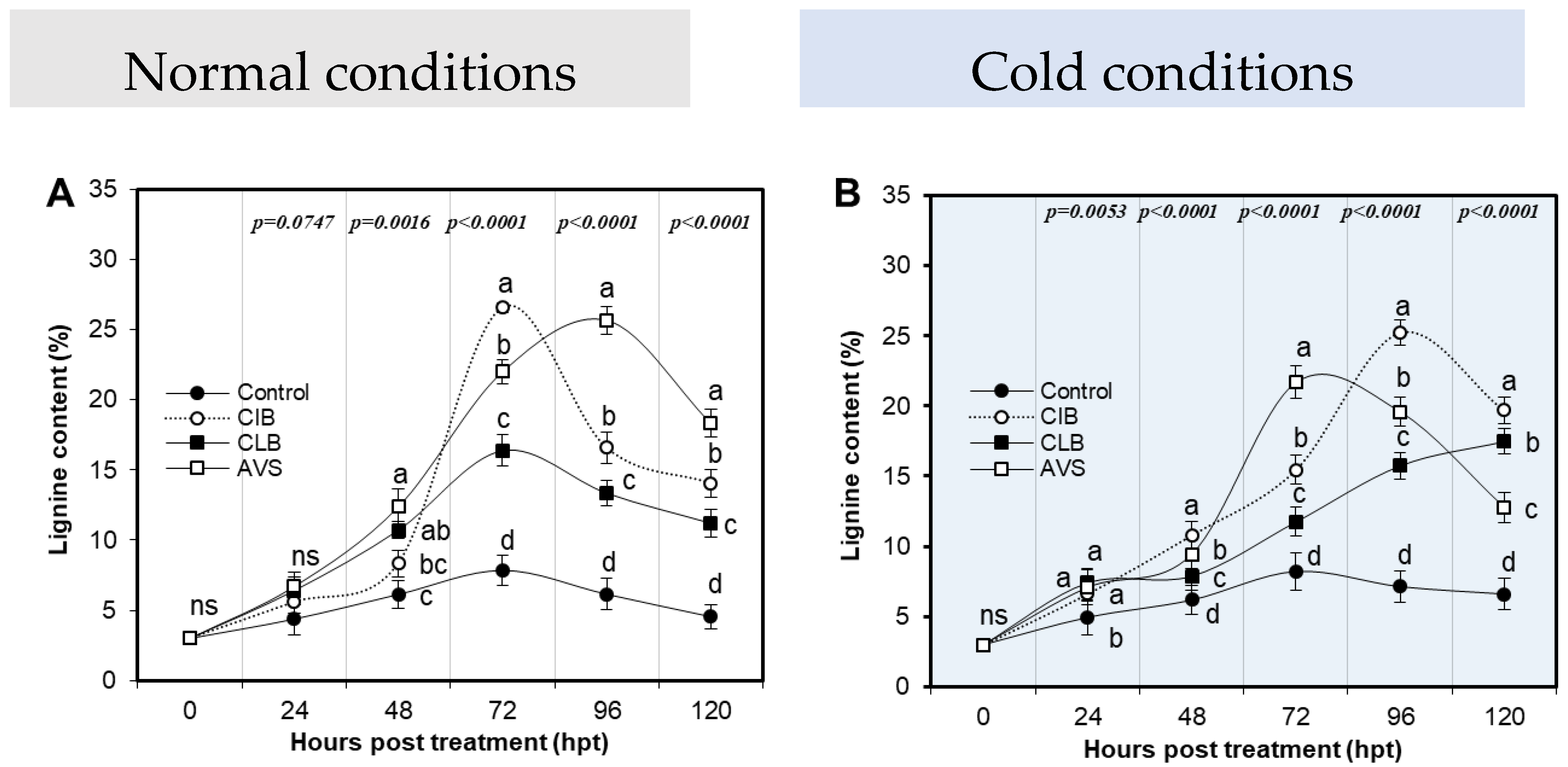
3.7. Tested Extracts Elevated Lignin Content in Potato Tubers
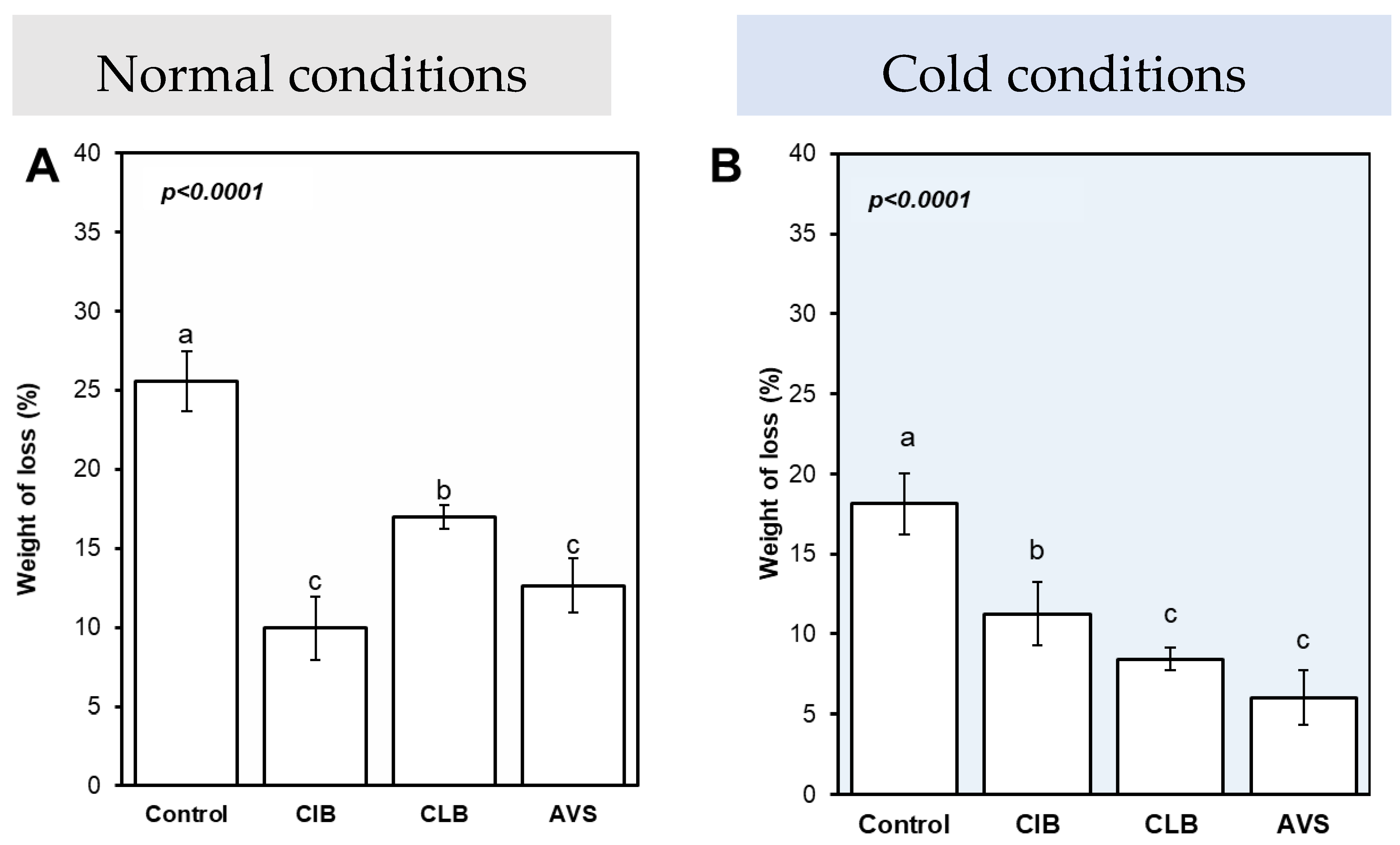
3.8. Tested Extracts Effectively Minimized the Weight Loss of Stored Potato Tubers
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erper, I.; Alkan, M.; Zholdoshbekova, S.; Turkkan, M.; Yildirim, E.; Özer, G. First Report of Dry Rot of Potato Caused by Fusarium sambucinum in Kyrgyzstan. J. Plant Dis. Prot. 2022, 129, 189–191. [Google Scholar] [CrossRef]
- FAOSTAT FAO. Agriculture Organization of the United Nations FAO Statistical Database; FAO: Faro, Portugal, 2023. [Google Scholar]
- Liu, J.; Sun, Z.; Zou, Y.; Li, W.; He, F.; Huang, X.; Lin, C.; Cai, Q.; Wisniewski, M.; Wu, X. Pre- and Postharvest Measures Used to Control Decay and Mycotoxigenic Fungi in Potato (Solanum tuberosum L.) during Storage. Crit. Rev. Food Sci. Nutr. 2022, 62, 415–428. [Google Scholar] [CrossRef]
- Azil, N.; Stefańczyk, E.; Sobkowiak, S.; Chihat, S.; Boureghda, H.; Śliwka, J. Identification and Pathogenicity of Fusarium spp. associated with tuber dry rot and wilt of potato in Algeria. Eur. J. Plant Pathol. 2021, 159, 495–509. [Google Scholar] [CrossRef]
- Batta, Y.A. Efficacy of Two Species of Entomopathogenic Fungi against the Stored-Grain Pest, Sitophilus granarius L. (Curculionidae: Coleoptera), via Oral Ingestion. Egypt. J. Biol. Pest Control 2018, 28, 44. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Bashyal, B.M.; Shanmugam, V.; Lal, M.K.; Kumar, R.; Sharma, S.; Naga, K.C.; Chourasia, K.N.; Aggarwal, R. First Report of Dry Rot of Potato Caused by Fusarium proliferatum in India. J. Plant Dis. Prot. 2022, 129, 173–179. [Google Scholar] [CrossRef]
- Wharton, P.S.; Kirk, W.W. Evaluation of Biological Seed Treatments in Combination with Management Practices for the Control of Fusarium Dry Rot of Potato. Biol. Control 2014, 73, 23–30. [Google Scholar] [CrossRef]
- Hay, W.T.; Fanta, G.F.; Rich, J.O.; Schisler, D.A.; Selling, G.W. Antifungal Activity of a Fatty Ammonium Chloride Amylose Inclusion Complex against Fusarium sambucinum; Control of Dry Rot on Multiple Potato Varieties. Am. J. Potato Res. 2019, 96, 79–85. [Google Scholar] [CrossRef]
- Sobkowiak, S.; Janiszewska, M.; Stefańczyk, E.; Wasilewicz-Flis, I.; Śliwka, J. Quantitative Trait Loci for Resistance to Potato Dry Rot Caused by Fusarium sambucinum. Agronomy 2022, 12, 203. [Google Scholar] [CrossRef]
- Heltoft, P.; Brurberg, M.B.; Skogen, M.; Le, V.H.; Razzaghian, J.; Hermansen, A. Fusarium spp. Causing Dry Rot on Potatoes in Norway and Development of a Real-Time PCR Method for Detection of Fusarium coeruleum. Potato Res. 2016, 59, 67–80. [Google Scholar] [CrossRef]
- Cullen, D.W.; Toth, I.K.; Pitkin, Y.; Boonham, N.; Walsh, K.; Barker, I.; Lees, A.K. Use of Quantitative Molecular Diagnostic Assays to Investigate Fusarium Dry Rot in Potato Stocks and Soil. Phytopathology 2005, 95, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Bojanowski, A.; Avis, T.J.; Pelletier, S.; Tweddell, R.J. Management of Potato Dry Rot. Postharvest Biol. Technol. 2013, 84, 99–109. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Sagar, V.; Aggarwal, R.; Naga, K.C.; Lal, M.K.; Chourasia, K.N.; Kumar, D.; Kumar, M. Potato Dry Rot Disease: Current Status, Pathogenomics and Management. 3 Biotech 2020, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- El-Hassan, K.I.; El-Saman, M.G.; Mosa, A.A.; Mostafa, M.H. Variation among Fusarium spp. the Causal of Potato Tuber Dry Rot in Their Pathogenicity and Mycotoxins Production. Egypt. J. Phytopathol. 2007, 35, 53–68. [Google Scholar]
- Gherbawy, Y.A.; Hussein, M.A.; El-Dawy, E.G.A.; Hassany, N.A.; Alamri, S.A. Identification of Fusarium spp. Associated with Potato Tubers in Upper Egypt by Morphological and Molecular Characters. Asian J. Biochem. Genet. Mol. Biol. 2019, 2, 1–14. [Google Scholar] [CrossRef]
- El-Nagar, A.S.; Abdel Wahab, M.H.; Elzaawely, A.A.; El-Zahaby, H.M. Morphological and Molecular Identification of Fusarium incarnatum as the Causal Agent of Potato Dry Rot Disease. J. Sustain. Agric. Environ. Sci. 2024, 3, 68–73. [Google Scholar] [CrossRef]
- Xue, H.; Liu, Q.; Yang, Z. Pathogenicity, Mycotoxin Production, and Control of Potato Dry Rot Caused by Fusarium spp.: A Review. J. Fungi 2023, 9, 843. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Farhan, M.; Ahmad, M.; Kiran, R.; Shahbaz, M.; Abbas, A.; Hakim, F.; Shabbir, M.; Tan, Y.S.; Sathiya Seelan, J.S. Fungicide Resistance in Fusarium species: Exploring Environmental Impacts and Sustainable Management Strategies. Arch. Microbiol. 2025, 207, 31. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, M.A.; Fernando, W.G.D. The Mechanisms of Developing Fungicide Resistance in Fusarium graminearum Causing Fusarium Head Blight and Fungicide Resistance Management. Pathogens 2024, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of Plant Extracts and Essential Oils in Fighting against Postharvest Fruit Pathogens and Extending Fruit Shelf Life: A Review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Dawood, D.H.; Elsebai, M.F.; Mira, A.; Taher, M.A. Control of Dry Rot and Resistance Induction in Potato Tubers against Fusarium sambucinum Using Red Onion Peel Extract. Postharvest Biol. Technol. 2023, 195, 112119. [Google Scholar] [CrossRef]
- Ramírez-Mejía, J.M.; Aguilera-Galvez, C.; Kema, G.H.J.; Valencia-Riascos, L.M.; Zapata-Henao, S.; Gómez, L.A.; Villegas-Escobar, V. Combining Cyclic Lipopeptides and Cinnamon Extract Enhance Antifungal Activity against Fusarium oxysporum Strains Pathogenic to Banana and Delay Fusarium Wilt under Greenhouse Conditions. Trop. Plant Pathol. 2024, 49, 838–849. [Google Scholar] [CrossRef]
- Carmello, C.R.; Magri, M.M.R.; Cardoso, J.C. Cinnamon Extract and Sodium Hypochlorite in the in vitro Control of Fusarium oxysporum f. sp. lycopersici and Alternaria alternata from Tomato. J. Phytopathol. 2022, 170, 802–810. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Ramadhani, A.M.; Erwa, I.Y.; Ishag, O.A.O.; Saeed, M.B. Phytochemical Screening, Chemical Composition and Antimicrobial Activity of Cinnamon verum Bark. Int. Res. J. Pure Appl. Chem. 2020, 21, 36–43. [Google Scholar] [CrossRef]
- Jahanshir, M.; Nobahar, M.; Ghorbani, R.; Malek, F. Effect of Clove Mouthwash on the Incidence of Ventilator-Associated Pneumonia in Intensive Care Unit Patients: A Comparative Randomized Triple-Blind Clinical Trial. Clin. Oral Investig. 2023, 27, 3589–3600. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The Extracts of Cinnamon and Clove as Potential Biofungicides against Strawberry Grey Mould. Plants 2020, 9, 613. [Google Scholar] [CrossRef]
- Sobhy, S.; Al-Askar, A.A.; Bakhiet, E.K.; Elsharkawy, M.M.; Arishi, A.A.; Behiry, S.I.; Abdelkhalek, A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations 2023, 10, 189. [Google Scholar] [CrossRef]
- de Almeida, E.N.; Moura, G.S.; Franzener, G. Potenciais Alternativas Com Extratos Vegetais No Controle Da Pinta Preta Do Tomateiro. Rev. Verde Agroecol. Desenvolv. Sustentável 2017, 12, 687–694. [Google Scholar] [CrossRef][Green Version]
- Leontopoulos, S.; Skenderidis, P.; Petrotos, K.; Mitsagga, C.; Giavasis, I. Preliminary Studies on Suppression of Important Plant Pathogens by Using Pomegranate and Avocado Residual Peel and Seed Extracts. Horticulturae 2022, 8, 283. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of Quercetin and Quercetin Glycosides from Onion (Allium cepa L.) Solid Waste as an Antioxidant, Urease and Xanthine Oxidase Inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef] [PubMed]
- El-Nagar, A.; Mazrou, Y.S.A.; El-Fawy, M.M.; Abou-Shlell, M.K.; Seleim, M.A.A.; Makhlouf, A.H.; Hegazy, M.G.A. New Trichoderma Strains Suppress Blue Mold in Oranges by Damaging the Cell Membrane of Penicillium italicum and Enhancing Both Enzymatic and Non-Enzymatic Defense Mechanisms in Orange Fruits. Horticulturae 2024, 10, 1076. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Harrach, B.D.; Fodor, J.; Pogány, M.; Preuss, J.; Barna, B. Antioxidant, Ethylene and Membrane Leakage Responses to Powdery Mildew Infection of near-Isogenic Barley Lines with Various Types of Resistance. Eur. J. Plant Pathol. 2008, 121, 21–33. [Google Scholar] [CrossRef]
- El-Nagar, A.; Elzaawely, A.A.; El-Zahaby, H.M.; Xuan, T.D.; Khanh, T.D.; Gaber, M.; El-Wakeil, N.; El-Sayed, Y.; Nehela, Y. Benzimidazole Derivatives Suppress Fusarium Wilt Disease via Interaction with ERG6 of Fusarium equiseti and Activation of the Antioxidant Defense System of Pepper Plants. J. Fungi 2023, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Zhang, Z.; Yang, D.; Yang, B.; Gao, Z.; Li, M.; Jiang, Y.; Hu, M. β-Aminobutyric Acid Induces Resistance of Mango Fruit to Postharvest Anthracnose Caused by Colletotrichum gloeosporioides and Enhances Activity of Fruit Defense Mechanisms. Sci. Hortic. 2013, 160, 78–84. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.I.; Merodio, C. Effect of High Carbon Dioxide Concentration on PAL Activity and Phenolic Contents in Ripening Cherimoya Fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified Thiobarbituric Acid Assay for Measuring Lipid Oxidation in Sugar-Rich Plant Tissue Extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Huang, L.; Wu, G.; Zhang, S.; Kuang, F.-Y.; Chen, F. The Identification and Functional Verification of the Cinnamate 4-Hydroxylase Gene from Wax Apple Fruit and Its Role in Lignin Biosynthesis during Nitric Oxide-Delayed Postharvest Cottony Softening. Postharvest Biol. Technol. 2019, 158, 110964. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant Extracts and Other Natural Compounds as Alternatives for Post-Harvest Management of Fruit Fungal Pathogens: A Review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Shu, C.; Ge, L.; Li, Z.; Chen, B.; Liao, S.; Lu, L.; Wu, Q.; Jiang, X.; An, Y.; Wang, Z.; et al. Antibacterial Activity of Cinnamon Essential Oil and Its Main Component of Cinnamaldehyde and the Underlying Mechanism. Front. Pharmacol. 2024, 15, 1378434. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and Its Derivatives, a Novel Class of Antifungal Agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Martinko, K.; Mioč, E. Antifungal Effect of Cinnamon Bark Extract on the Phytopathogenic Fungus Fusarium sporotrichioides. Food Technol. Biotechnol. 2024, 62, 458–464. [Google Scholar] [CrossRef]
- Morgaan, H.A.; Omar, H.M.G.; Zakaria, A.S.; Mohamed, N.M. Repurposing Carvacrol, Cinnamaldehyde, and Eugenol as Potential Anti-Quorum Sensing Agents against Uropathogenic Escherichia Coli Isolates in Alexandria, Egypt. BMC Microbiol. 2023, 23, 300. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado Fruit and By-Products as Potential Sources of Bioactive Compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef] [PubMed]
- Steglińska, A.; Bekhter, A.; Wawrzyniak, P.; Kunicka-Styczyńska, A.; Jastrząbek, K.; Fidler, M.; Śmigielski, K.; Gutarowska, B. Antimicrobial Activities of Plant Extracts against Solanum tuberosum L. Phytopathogens. Molecules 2022, 27, 1579. [Google Scholar] [CrossRef] [PubMed]
- Carmello, C.R.; Cardoso, J.C. Effects of Plant Extracts and Sodium Hypochlorite on Lettuce Germination and Inhibition of Cercospora longissima in vitro. Sci. Hortic. 2018, 234, 245–249. [Google Scholar] [CrossRef]
- Mvuemba, H.N.; Green, S.E.; Tsopmo, A.; Avis, T.J. Antimicrobial Efficacy of Cinnamon, Ginger, Horseradish and Nutmeg Extracts against Spoilage Pathogens. Phytoprotection 2010, 90, 65–70. [Google Scholar] [CrossRef]
- Venturoso, L.R.; Bacchi, L.M.A.; Gavassoni, W.L.; Conus, L.A.; Pontim, B.C.A.; Bergamin, A.C. Atividade Antifúngica de Extratos Vegetais Sobre o Desenvolvimento de Fitopatógenos. Summa Phytopathol. 2011, 37, 18–23. [Google Scholar] [CrossRef]
- Yang, C.-J.; Gao, Y.; Du, K.-Y.; Luo, X.-Y. Screening of 17 Chinese Medicine Plants against Phytopathogenic Fungi and Active Component in Syzygium aromaticum. J. Plant Dis. Prot. 2020, 127, 237–244. [Google Scholar] [CrossRef]
- Jeewon, R.; Pudaruth, S.B.; Bhoyroo, V.; Aullybux, A.A.; Rajeshkumar, K.C.; Alrefaei, A.F. Antioxidant and Antifungal Properties of Cinnamon, Cloves, Melia azedarach L. and Ocimum gratissimum L. Extracts against Fusarium oxysporum Isolated from Infected Vegetables in Mauritius. Pathogens 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, H.; Chen, X.; Chen, J.; Li, X.; Qiao, G.; Tian, Y.; Yuan, R.; Su, S.; Liu, X.; et al. Aqueous Extract of Clove Inhibits Tumor Growth by Inducing Autophagy through AMPK/ULK Pathway. Phytother. Res. 2019, 33, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; El-Bebany, A.F.; Salem, M.Z.M.; Komeil, D.A. Productivity and Post-Harvest Fungal Resistance of Hot Pepper as Affected by Potassium Silicate, Clove Extract Foliar Spray and Nitrogen Application. Plants 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Kim, Y. Myricetin Disturbs the Cell Wall Integrity and Increases the Membrane Permeability of Candida Albicans. J. Microbiol. Biotechnol. 2022, 32, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Abula, A.; Abulizi, A.; Wang, C.; Dou, Q.; Maimaiti, Y.; Abudouaini, A.; Huo, S.-X.; Aibai, S. Ellagic Acid Inhibits Trichophyton Rubrum Growth via Affecting Ergosterol Biosynthesis and Apoptotic Induction. Evid.-Based Complement. Altern. Med. 2020, 2020, 7305818. [Google Scholar] [CrossRef] [PubMed]
- Kupnik, K.; Primožič, M.; Kokol, V.; Knez, Ž.; Leitgeb, M. Enzymatic, Antioxidant, and Antimicrobial Activities of Bioactive Compounds from Avocado (Persea americana L.) Seeds. Plants 2023, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Lara, R.; Rodriguez-Sánchez, D.G.; Diaz De La Garza, R.I.; Garcia-Cruz, M.I.; Castillo, A.; Pacheco, A.; Hernández-Brenes, C. Purified Avocado Seed Acetogenins: Antimicrobial Spectrum and Complete Inhibition of Listeria Monocytogenes in a Refrigerated Food Matrix. CyTA-J. Food 2019, 17, 228–239. [Google Scholar] [CrossRef]
- Bahru, T.B.; Tadele, Z.H.; Ajebe, E.G. A Review on Avocado Seed: Functionality, Composition, Antioxidant and Antimicrobial Properties. Chem. Sci. Int. J. 2019, 27, 1–10. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Thakral, V.; Sudhakaran, S.; Jadhav, H.; Mahakalkar, B.; Sehra, A.; Dhar, H.; Kumar, S.; Sonah, H.; Sharma, T.R.; Deshmukh, R. Unveiling Silicon-Mediated Cadmium Tolerance Mechanisms in Mungbean (Vigna radiata L.) Wilczek): Integrative Insights from Gene Expression, Antioxidant Responses, and Metabolomics. J. Hazard. Mater. 2024, 474, 134671. [Google Scholar] [CrossRef]
- Song, Y.; Hu, C.; Xue, Y.; Gu, J.; He, J.; Ren, Y. 24-Epibrassinolide Enhances Mango Resistance to Colletotrichum gloeosporioides via Activating Multiple Defense Response. Sci. Hortic. 2022, 303, 111249. [Google Scholar] [CrossRef]
- Ren, Y.; Xue, Y.; Tian, D.; Zhang, L.; Xiao, G.; He, J. Improvement of Postharvest Anthracnose Resistance in Mango Fruit by Nitric Oxide and the Possible Mechanisms Involved. J. Agric. Food Chem. 2020, 68, 15460–15467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhao, L.; Zhang, X.; Foku, J.M.; Li, J.; Hu, W.; Zhang, H. Efficacy of Yarrowia Lipolytica in the Biocontrol of Green Mold and Blue Mold in Citrus Reticulata and the Mechanisms Involved. Biol. Control 2019, 139, 104096. [Google Scholar] [CrossRef]
- Papoutsis, K.; Vuong, Q.V.; Tesoriero, L.; Pristijono, P.; Stathopoulos, C.E.; Gkountina, S.; Lidbetter, F.; Bowyer, M.C.; Scarlett, C.J.; Golding, J.B. Microwave Irradiation Enhances the in Vitro Antifungal Activity of Citrus by-Product Aqueous Extracts against Alternaria alternata. Int. J. Food Sci. Technol. 2018, 53, 1510–1517. [Google Scholar] [CrossRef]
- Patra, K.; Jana, S.; Mandal, D.P.; Bhattacharjee, S. Evaluation of the Antioxidant Activity of Extracts and Active Principles of Commonly Consumed Indian Spices. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 299–315. [Google Scholar] [CrossRef]
- Miramontes-Corona, C.; Torres-Santiago, G.; Rodriguez, M.M.J.; Corona-González, R.I.; Toriz, G. Phenolic Profile, Antioxidant Activity and Antimicrobial Properties of Avocado (Persea americana) Seed Extracts. Chem. Pap. 2024, 78, 5061–5069. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Guo, Y.; Kang, L.; Yu, Y. Carbon Monoxide Enhances the Resistance of Jujube Fruit against Postharvest Alternaria Rot. Postharvest Biol. Technol. 2020, 168, 111268. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Han, Z.; Xue, S.; Bi, Y.; Prusky, D. Effects of Nitric Oxide Treatment on Lignin Biosynthesis and Texture Properties at Wound Sites of Muskmelons. Food Chem. 2021, 362, 130193. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, W.; Lv, H.; Gao, Y.; Kang, Y.; Wu, Y.; Wang, F.; Zhang, W.; Liang, H. Lignin Synthesis Pathway in Response to Rhizoctonia Solani Kühn Infection in Potato (Solanum tuberosum L.). Chem. Biol. Technol. Agric. 2024, 11, 135. [Google Scholar] [CrossRef]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The Role of the Secondary Cell Wall in Plant Resistance to Pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, W.; Kang, Y.; Shi, M.; Yang, X.; Yu, H.; Zhang, R.; Liu, Y.; Qin, S. Physiological and Dynamic Transcriptome Analysis of Two Potato Varieties Reveal Response of Lignin and MAPK Signal to Dry Rot Caused by Fusarium sulphureum. Sci. Hortic. 2021, 289, 110470. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Wang, B.; Zhang, J.; Nie, J. Dynamic Degradation of Penconazole and Its Effect on Antioxidant Enzyme Activity and Malondialdehyde Content in Apple Fruit. Sci. Hortic. 2022, 300, 111053. [Google Scholar] [CrossRef]

| Treatment | Total Soluble Phenolics (mg GAE g−1 FW) | ||||
|---|---|---|---|---|---|
| Hours Post-Treatment (hpt) | |||||
| 24 | 48 | 72 | 96 | 120 | |
| Control | 12.47 ± 0.57 d | 24.81 ± 0.14 d | 22.26 ± 0.96 c | 6.97 ± 0.36 d | 3.56 ± 0.39 d |
| CIB | 15.22 ± 0.02 c | 33.30 ± 0.05 b | 35.54 ± 0.04 a | 23.07 ± 0.04 b | 4.72 ± 0.12 c |
| CLB | 27.46 ± 0.03 a | 35.95 ± 0.17 a | 19.48 ± 0.05 d | 16.23 ± 0.04 c | 14.13 ± 0.02 a |
| AVS | 16.12 ± 0.13 b | 27.23 ± 0.10 c | 33.80 ± 0.14 b | 25.21 ± 0.08 a | 8.08 ± 0.18 b |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Treatment | Total Soluble Phenolics (mg GAE g−1 FW) | ||||
|---|---|---|---|---|---|
| Hours Post-Treatment (hpt) | |||||
| 24 | 48 | 72 | 96 | 120 | |
| Control | 5.38 ± 0.10 d | 12.17 ± 0.08 d | 8.63 ± 0.22 d | 3.14 ± 0.31 c | 1.19 ± 0.12 d |
| CIB | 13.38 ± 0.04 b | 21.28 ± 0.06 b | 23.11 ± 0.03 b | 33.23 ± 0.15 a | 21.47 ± 0.04 a |
| CLB | 24.45 ± 0.11 a | 34.14 ± 0.71 a | 26.55 ± 0.71 a | 23.52 ± 0.08 b | 19.33 ± 0.06 b |
| AVS | 8.59 ± 0.18 c | 15.77 ± 0.07 c | 17.83 ± 0.10 c | 34.39 ± 0.96 a | 12.46 ± 0.36 c |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Treatment | Total Soluble Flavonoids (mg RE g−1 FW) | ||||
|---|---|---|---|---|---|
| Hours Post-Treatment (hpt) | |||||
| 24 | 48 | 72 | 96 | 120 | |
| Control | 1.08 ± 0.49 a | 2.76 ± 0.14 c | 0.84 ± 0.01 a | 0.70 ± 0.21 a | 0.39 ± 0.01 a |
| CIB | 3.95 ± 0.01 a | 8.08 ± 0.96 a | 2.41 ± 0.78 a | 1.27 ± 0.07 a | 0.55 ± 0.23 a |
| CLB | 2.04 ± 0.01 a | 6.93 ± 0.01 ab | 1.49 ± 0.01 a | 1.11 ± 0.02 a | 0.79 ± 0.01 a |
| AVS | 1.75 ± 0.24 a | 5.79 ± 0.28 b | 2.31 ± 1.30 a | 1.47 ± 0.34 a | 0.61 ± 0.22 a |
| p = 0.0862 | p < 0.0001 | p = 0.164 | p = 0.0091 | p = 0.0771 | |
| Treatment | Total Soluble Flavonoids (mg RE g−1 FW) | ||||
|---|---|---|---|---|---|
| Hours Post-Treatment (hpt) | |||||
| 24 | 48 | 72 | 96 | 120 | |
| Control | 1.79 ± 0.04 a | 2.53 ± 0.02 d | 1.89 ± 0.13 c | 1.40 ± 0.03 c | 1.19 ± 0.06 c |
| CIB | 2.16 ± 0.46 a | 5.06 ± 0.19 b | 3.21 ± 0.20 a | 2.53 ± 0.19 a | 2.21 ± 0.12 b |
| CLB | 2.28 ± 0.05 a | 3.93 ± 0.03 c | 2.50 ± 0.04 b | 2.22 ± 0.13 b | 2.36 ± 0.13 ab |
| AVS | 2.24 ± 0.32 a | 8.89 ± 0.18 a | 3.42 ± 0.02 a | 2.71 ± 0.02 a | 2.59 ± 0.03 a |
| p = 0.2089 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Nagar, A.; Mazrou, Y.S.A.; Elzaawely, A.A.; Makhlouf, A.H.; Hassan, M.; El-Zahaby, H.M.; Xuan, T.D. Potential of Three Plant Extracts in Suppressing Potato Dry Rot Caused by Fusarium incarnatum Under Normal and Cold Storage. Agronomy 2025, 15, 593. https://doi.org/10.3390/agronomy15030593
El-Nagar A, Mazrou YSA, Elzaawely AA, Makhlouf AH, Hassan M, El-Zahaby HM, Xuan TD. Potential of Three Plant Extracts in Suppressing Potato Dry Rot Caused by Fusarium incarnatum Under Normal and Cold Storage. Agronomy. 2025; 15(3):593. https://doi.org/10.3390/agronomy15030593
Chicago/Turabian StyleEl-Nagar, Asmaa, Yasser S. A. Mazrou, Abdelnaser A. Elzaawely, Abeer H. Makhlouf, Mohamed Hassan, Hassan M. El-Zahaby, and Tran Dang Xuan. 2025. "Potential of Three Plant Extracts in Suppressing Potato Dry Rot Caused by Fusarium incarnatum Under Normal and Cold Storage" Agronomy 15, no. 3: 593. https://doi.org/10.3390/agronomy15030593
APA StyleEl-Nagar, A., Mazrou, Y. S. A., Elzaawely, A. A., Makhlouf, A. H., Hassan, M., El-Zahaby, H. M., & Xuan, T. D. (2025). Potential of Three Plant Extracts in Suppressing Potato Dry Rot Caused by Fusarium incarnatum Under Normal and Cold Storage. Agronomy, 15(3), 593. https://doi.org/10.3390/agronomy15030593







