Engineering Soil Quality and Water Productivity Through Optimal Phosphogypsum Application Rates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soil Infiltration with Phosphogypsum
2.3. Infiltration Models
2.3.1. Wetting Front Propulsion Model [39]
2.3.2. Cumulative Infiltration Model
- (1)
- Lewis equation:
- (2)
- Philip infiltration model:
- (3)
- Horton infiltration model:
2.3.3. Model Parameters Calculation
2.3.4. Evaluating Indices of Models
2.4. Field Experiment
2.5. Comprehensive Evaluation
2.6. Statistical Analysis
3. Results
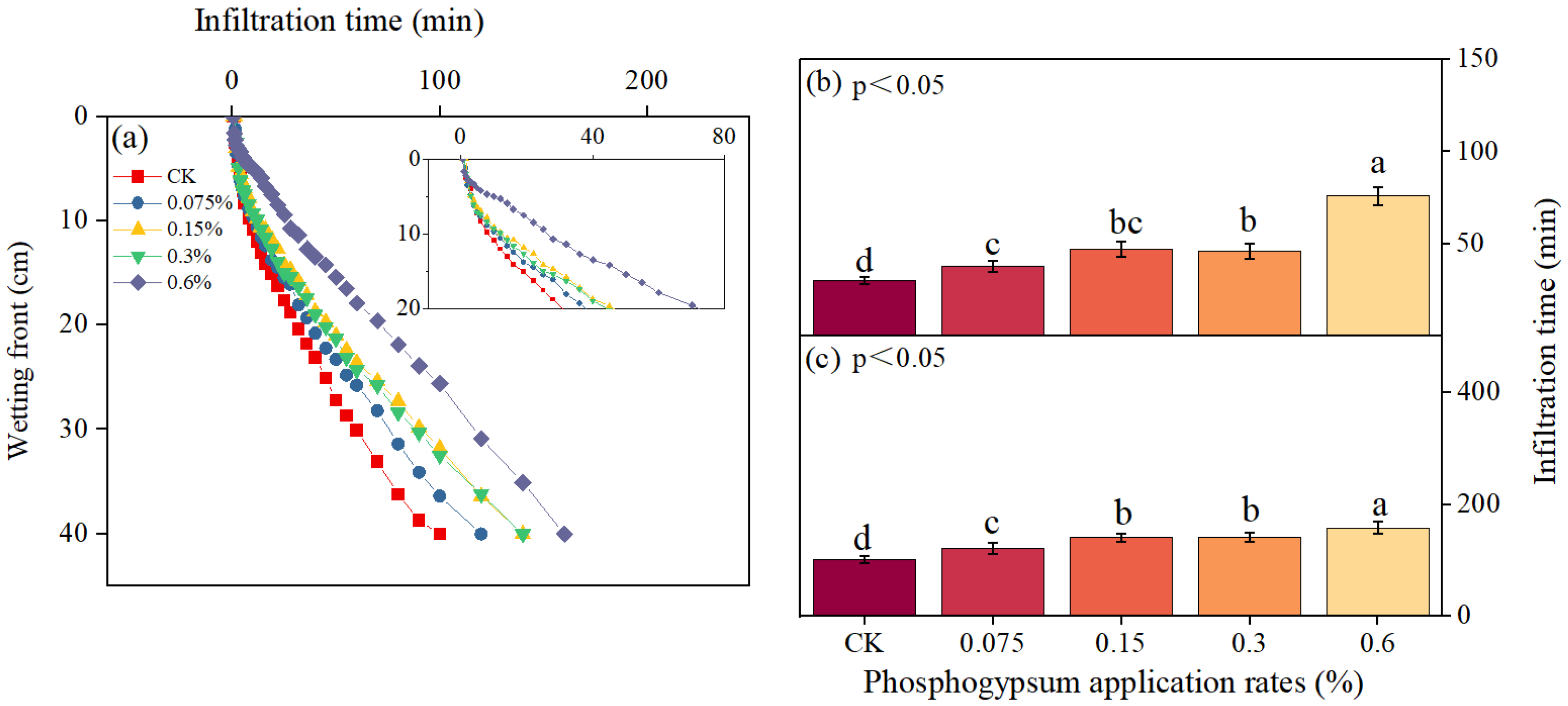
3.1. Dynamic Changes of Wetting Front
3.2. Soil Water Cumulative Infiltration and Infiltration Rate
3.3. Soil Water Infiltration Models
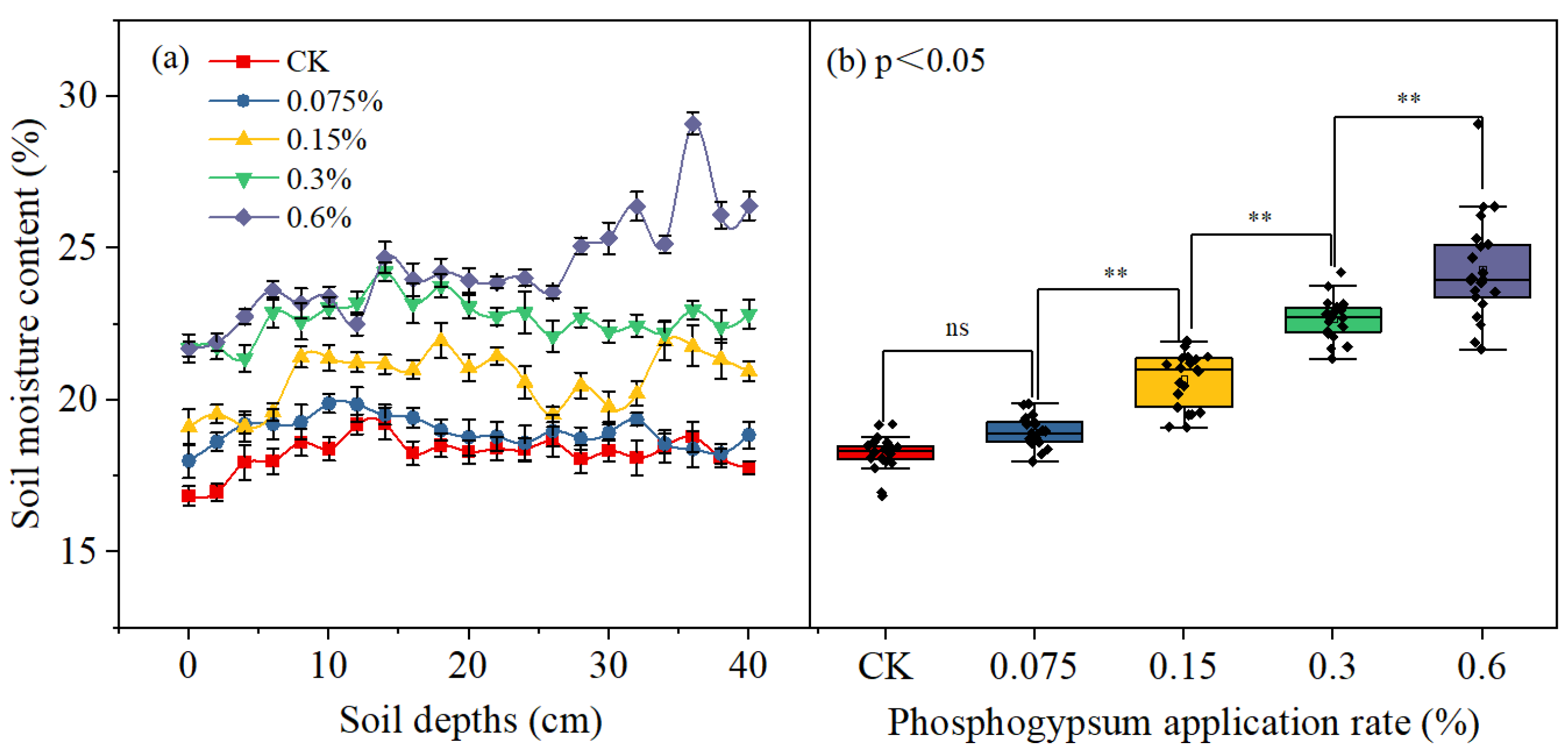
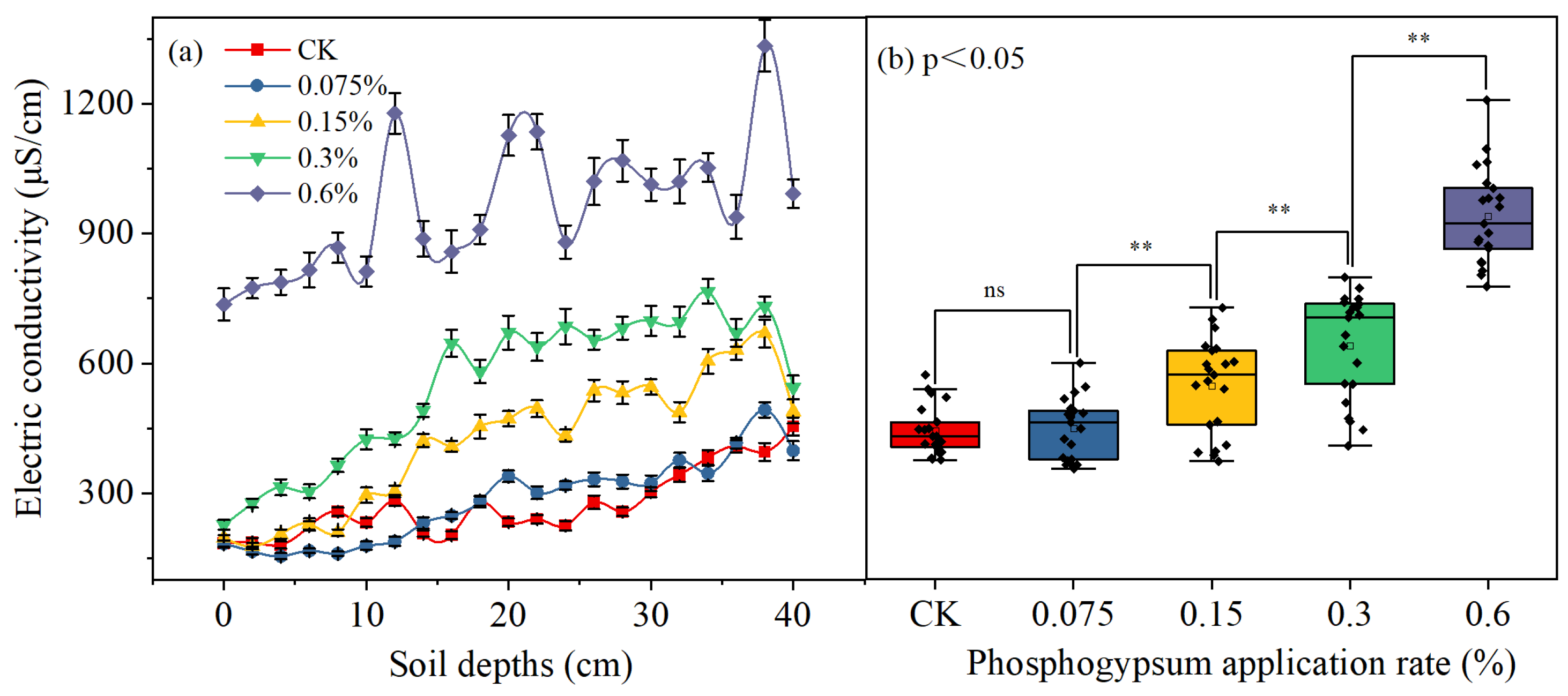
3.4. Water Retention Capacity and Soil Salt Content
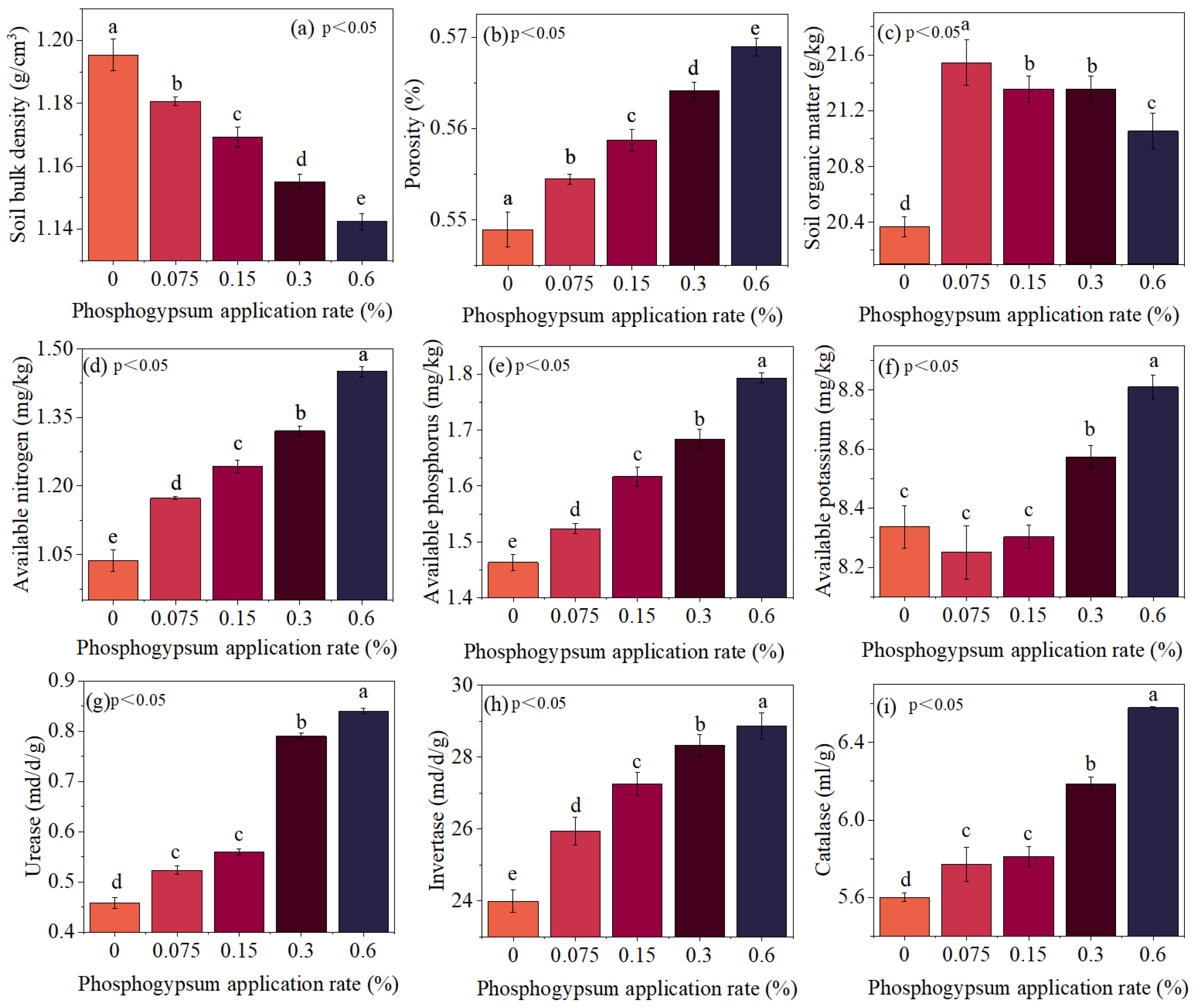
3.5. Soil Quality
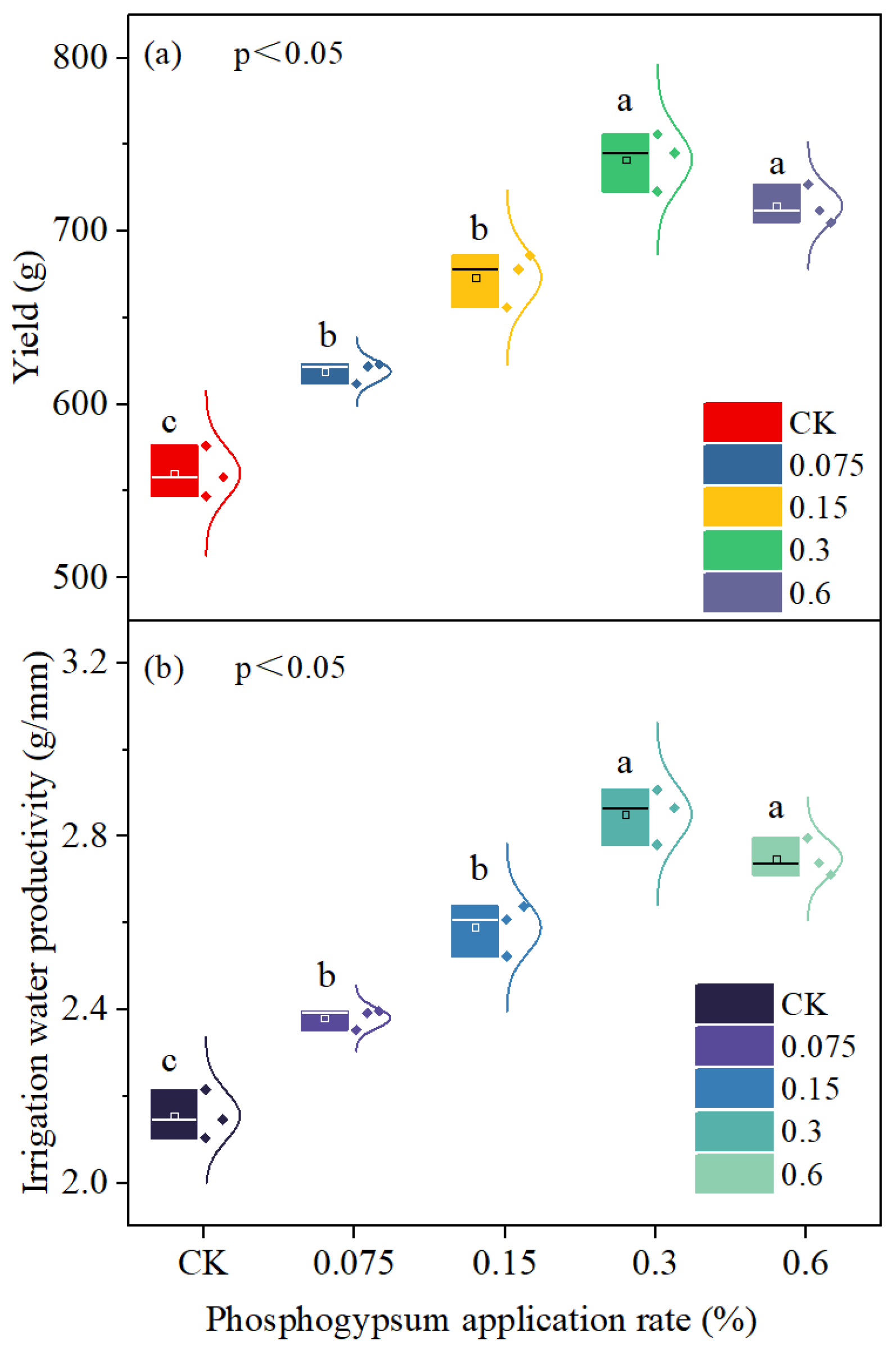
3.6. Yield and Irrigation Water Productivity
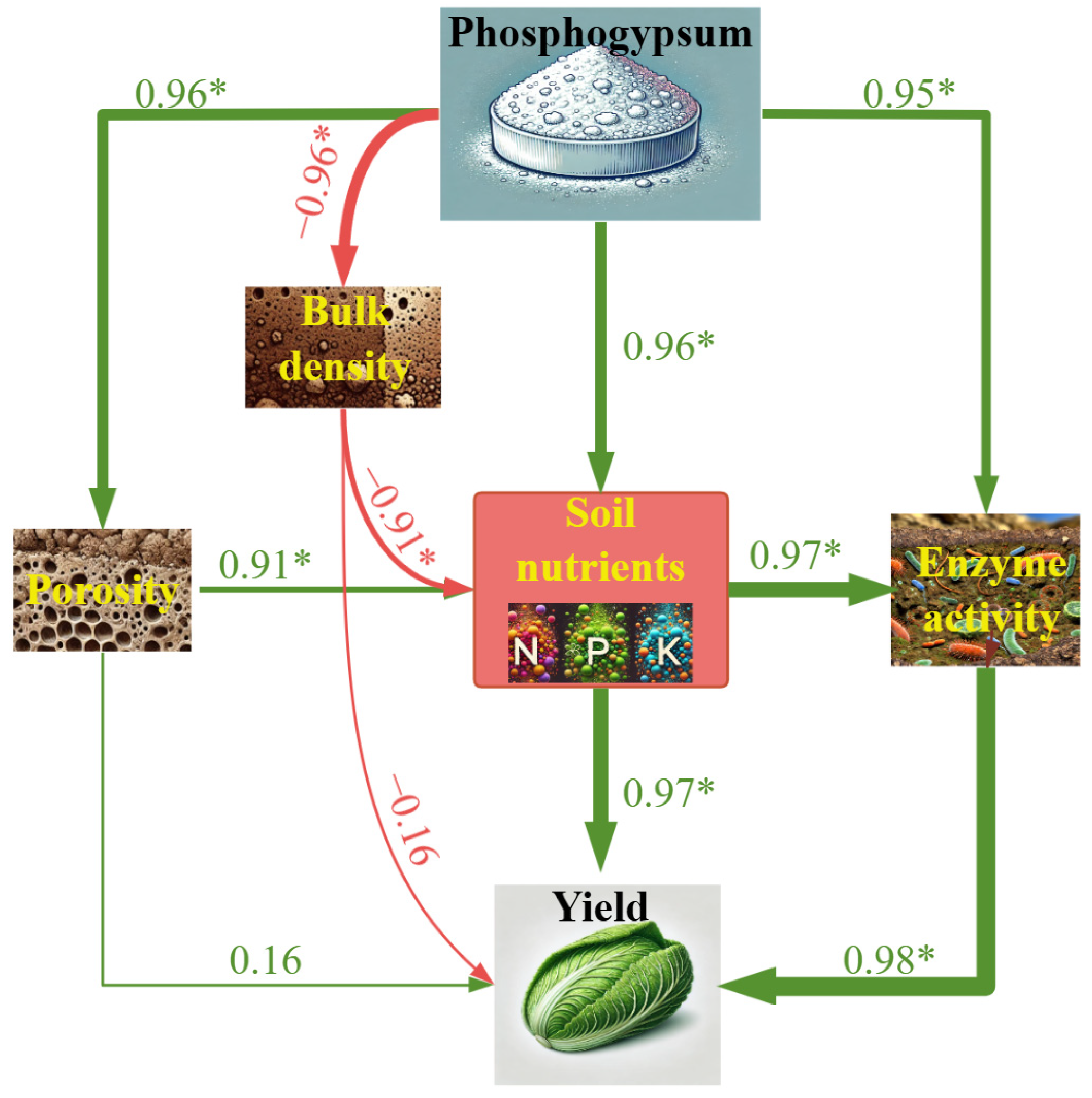
3.7. Partial Least Squares Path Model
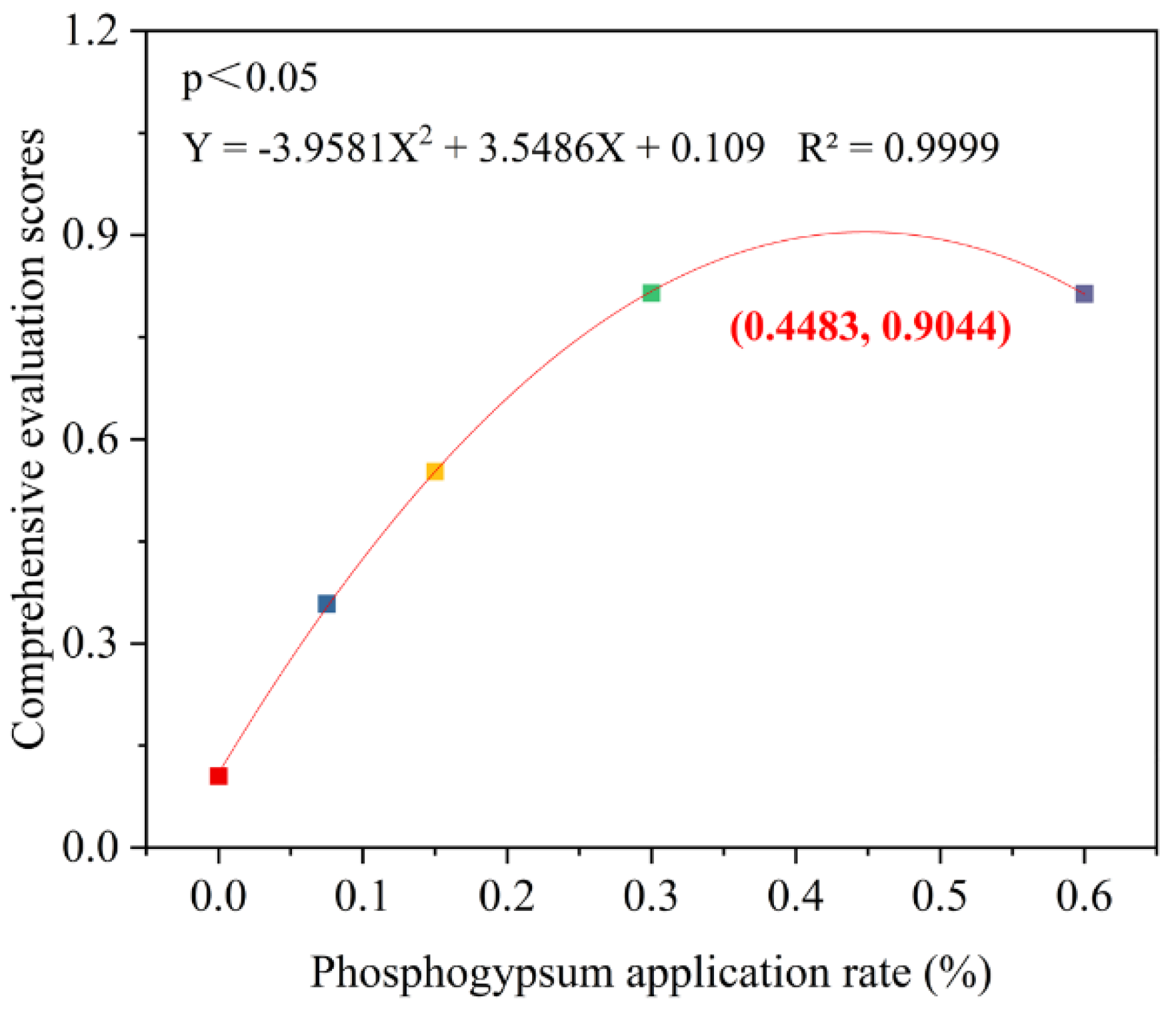
3.8. Optimal Phosphogypsum Application Rate Based on Comprehensive Evaluations
4. Discussion
4.1. Effects of Phosphogypsum on Soil Water Infiltration, Water Retention Capacity and Soil Salt Content
4.2. Effects of Phosphogypsum on Soil Quality, Crop Yield and Irrigation Water Productivity
4.3. Effects of Phosphogypsum Management on Overall Benefits
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FAO | Food and Agriculture Organization | MAE | Mean absolute error |
| WUE | Water use efficiency | RRMSE | Relative root mean square error |
| PAM | Polyacrylamide | CRM | Coefficient of residual mass |
| CaSO4·2H2O | Calcium sulfate dihydrate | CE | Coefficient of efficiency |
| Ca2⁺ | Calcium ion | Yiobs | ith observed value |
| SO42⁻ | Sulfate ion | Yisim | ith simulated value |
| Na⁺ | Sodium ion | SOM | Soil organic matter |
| N | Nitrogen | AN | Available nitrogen |
| P | Phosphorus | AP | Available phosphorus |
| S | Sulfur | AK | Available potassium |
| Ca3(PO4)2 | Calcium phosphate | Y | Cabbage yield |
| IWP | Irrigation water productivity | IA | Total irrigation amount |
| SiO2 | Silicon dioxide | CK | No phosphogypsum |
| P2O5 | Phosphorus pentoxide | TOPSIS | Technique for Order of Preference by Similarity to Ideal Solution |
| F | Fluorine | ahj | jth indicator in the hth evaluation objects |
| Fe2O3 | Iron oxide | o | Number of object |
| Al2O3 | Aluminum oxide | m | Number of indicator |
| A | Initial condition of the wetting front propagation rate | Vhj | Weighted normalized value of the jth indicator for the ith evaluation object |
| B | Time dependency of wetting front advancement | Wh | Weight |
| I | Cumulative infiltration | A+ | Positive ideal solution values |
| t | Infiltration time | A− | Negative ideal solution values |
| K | Initial soil water conductivity | D+ | Distance to Positive Ideal Solution |
| α | Non-linear relationship between cumulative infiltration and time | D− | Distance to Negative Ideal Solution |
| S | Sorptivity | n | Total number of data points |
| a | Final infiltration rate | CI | Closeness index |
| b | Initial infiltration rate | PLS-PM | Partial least squares path modeling |
| c | Decay constant | Ws | Weight of the wet soil |
| Ds | Weight of the dry soil | KCl | Potassium chloride |
| Rn | Radon | As | Arsenic |
| Ra | Radium | Pb | Lead |
| Th | Thorium | Hg | Mercury |
| U | Uranium | Cd | Cadmium |
| Cr | Chromium | Ni | Nickel |
| HCl | Hydrochloric Acid |
References
- Falk, D.; Winowiecki, L.A.; Vågen, T.-G.; Lohbeck, M.; Ilstedt, U.; Muriuki, J.; Mwaniki, A.; Bargués Tobella, A. Drivers of field-saturated soil hydraulic conductivity: Implications for restoring degraded tropical landscapes. Sci. Total Environ. 2024, 907, 168038. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point. Main Report; FAO: Rome, Italy, 2022. [Google Scholar]
- Mwafulirwa, L.; Sizmur, T.; Daymond, A.; Atuah, L.; Quaye, A.K.; Coole, S.; Robinson, S.; Hammond, J.; Awudzi, G.; Awunyo-Vitor, D.; et al. Cocoa pod husk-derived organic soil amendments differentially affect soil fertility, nutrient leaching, and greenhouse gas emissions in cocoa soils. J. Clean. Prod. 2024, 479, 144065. [Google Scholar] [CrossRef]
- Yang, F.; Wang, W.; Wu, Z.; Peng, J.; Xu, H.; Ge, M.; Lin, S.; Zeng, Y.; Sardans, J.; Wang, C.; et al. Fertilizer reduction and biochar amendment promote soil mineral-associated organic carbon, bacterial activity, and enzyme activity in a jasmine garden in southeast China. Sci. Total Environ. 2024, 954, 176300. [Google Scholar] [CrossRef]
- Saraugi, S.S.; Routray, W. Advances in sustainable production and applications of nano-biochar. Sci. Total Environ. 2024, 955, 176883. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Hao, S.; Chen, J.; Chen, S.; Liu, J.; Liu, H.; Zhu, X.; Li, X.; Zhang, A. Effects of composted straw, biochar, and polyacrylamide addition on soil permeability and dynamic leaching characteristics of pollutants in loessial soil in urban greenbelts according to indoor simulation experiments. Agronomy 2024, 14, 1958. [Google Scholar] [CrossRef]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Hamdan, E.H.; Gharaibeh, M.A.; Hanandeh, A.E. Improving aggregate stability and hydraulic properties of Sandy loam soil by applying polyacrylamide polymer. Soil Tillage Res. 2021, 206, 104821. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, S.; Shi, Z.; Zhou, H.; Zheng, H.; Hu, J.; Mei, J.; Bai, L.; Jia, J. Effects of mixed-based biochar on water infiltration and evaporation in aeolian sand soil. J. Arid Land 2022, 14, 374–389. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Chen, P.; Li, H.; Li, W.; Liu, J.; Zong, R.; Wang, D.; Liang, Y.; Wang, Z. Biochar improves water and nitrogen use efficiency of cotton under mulched drip irrigation in arid regions. Ind. Crops Prod. 2024, 222, 119830. [Google Scholar] [CrossRef]
- Patel, M.R.; Panwar, N.L. Evaluating the agronomic and economic viability of biochar in sustainable crop production. Biomass Bioenergy 2024, 188, 107328. [Google Scholar] [CrossRef]
- Ullah, M.S.; Malekian, R.; Randhawa, G.S.; Gill, Y.S.; Singh, S.; Esau, T.J.; Zaman, Q.U.; Afzaal, H.; Du, D.L.; Farooque, A.A. The potential of biochar incorporation into agricultural soils to promote sustainable agriculture: Insights from soil health, crop productivity, greenhouse gas emission mitigation and feasibility perspectives—A critical review. Rev. Environ. Sci. Biotechnol. 2024, 23, 1105–1130. [Google Scholar] [CrossRef]
- Pang, Y.; Li, N.; Fan, M.; Jiang, P.; Zhou, A.; Wang, W. Soil utilization of solid waste: Small-strain dynamic properties and microscopic mechanism of phosphogypsum-modified coastal cement soil. J. Mater. Res. Technol. 2024, 33, 4265–4279. [Google Scholar] [CrossRef]
- Chen, T.; Wang, L.; He, B.; Peng, X.; Nie, X.; Ma, F.; Han, P.; Bai, X. Study on the solidification/stabilization of cadmium-contaminated soil by red mud-assisted blast furnace slag under excitation conditions. J. Clean. Prod. 2024, 435, 140505. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; Liu, N.; Jiang, Z. Utilization of waste phosphogypsum in high-strength geopolymer concrete: Performance optimization and mechanistic exploration. J. Build. Eng. 2024, 98, 111253. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, H.; Zheng, L.; Wang, K.; Li, H.; Wang, Y.; Feng, H. Utilization of waste phosphogypsum to prepare hydroxyapatite nanoparticles and its application towards removal of fluoride from aqueous solution. J. Hazard. Mater. 2012, 241–242, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shui, Z.; Sun, T.; Li, X.; Zhang, M. Recycling utilization of phosphogypsum in eco excess-sulphate cement: Synergistic effects of metakaolin and slag additives on hydration, strength and microstructure. J. Clean. Prod. 2022, 358, 131901. [Google Scholar] [CrossRef]
- Reijnders, L. Cleaner phosphogypsum, coal combustion ashes and waste incineration ashes for application in building materials: A review. Build. Environ. 2007, 42, 1036–1042. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Mantero, J.; Bolívar, J.P.; García-Tenorio, R.; Vaca, F.; Lozano, R.L. Physico-chemical and radioactive characterization of TiO2 undissolved mud for its valorization. J. Hazard. Mater. 2011, 191, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Oliveira, M.L.S.; Crissien, T.J.; Santosh, M.; Bolivar, J.; Shao, L.; Dotto, G.L.; Gasparotto, J.; Schindler, M. A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere 2022, 286, 131513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Xue, B.; Chen, L.; Wang, G.; Wang, J.; Wan, H.; Lin, X.; Zhu, G. Simulation of red mud/phosphogypsum-based artificial soil engineering applications in vegetation restoration and ecological reconstruction. Sci. Total Environ. 2024, 951, 175656. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Huang, D.; Huang, Q.; Chen, Y.; Zhang, H.; Shui, Z. Recovery and recycling core of phosphogypsum: Characteristic hazardous elements risk assessment and analysis. Process Saf. Environ. Prot. 2023, 170, 738–756. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, A.; Duan, H.; Zhang, H.; Yang, J. Status and development trends of phosphogypsum utilization in China. Circ. Econ. 2024, 3, 100116. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Yi, Y.; Tian, J.; Xuan, C.; Wang, Y.; Wen, Y.; Cao, J. Effects of phosphogypsum on enzyme activity and microbial community in acid soil. Sci. Rep. 2023, 13, 6189. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Bossolani, J.W.; Crusciol, C.A.C.; Mariano, E.; Fonseca, M.; Moretti, L.G.; Momesso, L.; Portugal, J.R.; Costa, N.R.; Calonego, J.C.; Kuramae, E.E. Long term co-application of lime and phosphogypsum increases 15 N recovery and reduces 15 N losses by modulating soil nutrient availability, crop growth and N cycle genes. Eur. J. Agron. 2023, 149, 126907. [Google Scholar] [CrossRef]
- Michalovicz, L.; Tormena, C.A.; Lopes Müller, M.M.; Dick, W.A.; Cervi, E.C. Residual effects of phosphogypsum rates and machinery traffic on soil attributes and common-bean (Phaseolus vulgaris) yield in a no-tillage system. Soil Tillage Res. 2021, 213, 105152. [Google Scholar] [CrossRef]
- Costa, R.F.; Firmano, R.F.; Colzato, M.; Crusciol, C.A.C.; Alleoni, L.R.F. Sulfur speciation in a tropical soil amended with lime and phosphogypsum under long-term no-tillage system. Geoderma 2022, 406, 115461. [Google Scholar] [CrossRef]
- Turner, B.L.; Haygarth, P.M. Phosphatase activity in temperate pasture soils: Potential regulation of labile organic phosphorus turnover by phosphodiesterase activity. Sci. Total Environ. 2005, 344, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Basset, C.; Abou Najm, M.; Ghezzehei, T.; Hao, X.; Daccache, A. How does soil structure affect water infiltration? A meta-data systematic review. Soil Tillage Res. 2023, 226, 105577. [Google Scholar] [CrossRef]
- Hasana, H.; Beyene, S.; Kifilu, A.; Kidanu, S. Effect of phosphogypsum amendment on chemical properties of sodic soils at different incubation periods. Appl. Environ. Soil Sci. 2022, 2022, 9097994. [Google Scholar] [CrossRef]
- Duan, G.; Liu, M.; Liang, Z.; Wang, M.; Yang, H.; Xu, Y.; Yu, T.; Jin, Y.; Hu, J.; Liu, J. Amendments of severe saline-sodic paddy land: Optimal combination of phosphogypsum, farmyard fertilizer, and wood peat. Agronomy 2023, 13, 1364. [Google Scholar] [CrossRef]
- Lin, R.; Huang, G.; Ma, F.; Pan, T.; Wang, X.; Han, Y.; Liao, Y. Investigation of phosphogypsum-based cementitious materials: The effect of lime modification. Dev. Built Environ. 2024, 18, 100477. [Google Scholar] [CrossRef]
- GB15618; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Liu, Z.; Cai, L.; Dong, Q.; Zhao, X.; Han, J. Effects of microplastics on water infiltration in agricultural soil on the Loess Plateau, China. Agric. Water Manag. 2022, 271, 107818. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Zhen, Q.; Zheng, J. Petroleum induces soil water repellency and impedes the infiltration and evaporation processes in sandy soil. J. Hydrol. 2024, 643, 131990. [Google Scholar] [CrossRef]
- Nepal, J.; Xin, X.; Maltais-Landry, G.; Barra Netto-Ferreira, J.; Wright, A.L.; He, Z. Water dispersible carbon nanomaterials reduced N, P, and K leaching potential in sandy soils: A column leaching study. Sci. Total Environ. 2024, 954, 176755. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Wu, Q.; Zong, T.; Xin, X.; Xie, J.; Yang, J. Use of rice straw nano-biochar to slow down water infiltration and reduce nitrogen leaching in a clayey soil. Sci. Total Environ. 2024, 948, 174956. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fei, L.; Zhu, H.; Hao, K.; Jie, F. Study on the influence of fertilizer solution concentration on soil water and nitrogen transport characteristics under film hole irrigation. J. Mater. Res. Technol. 2021, 21, 1653–1665. [Google Scholar] [CrossRef]
- Swartzendruber, D. Revised attribution of the power form infiltration equation. Water Resour. Res. 1993, 29, 2455–2456. [Google Scholar] [CrossRef]
- Feng, Z.; Nie, W.; Ma, Y.; Li, Y.; Ma, X.; Zhu, H. Effects of urea solution concentration on soil hydraulic properties and water infiltration capacity. Sci. Total Environ. 2023, 898, 165471. [Google Scholar] [CrossRef]
- Sasal, M.C.; Andriulo, A.E.; Taboada, M.A. Soil porosity characteristics and water movement under zero tillage in silty soils in Argentinian Pampas. Soil Tillage Res. 2006, 87, 9–18. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, W.; Goodwin, P.H.; Wang, Y.; Zheng, S.-J.; Li, X. Effect of cover crop on soil fertility and bacterial diversity in a banana plantation in southwestern China. Soil Tillage Res. 2024, 240, 106092. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Meyer, A.H.; Olatunji, O.S.; Mulidzi, R.; Lewu, F.B. Soil Ph, nitrogen, phosphatase and urease activities in response to cover crop species, termination stage and termination method. Heliyon 2021, 7, e05980. [Google Scholar] [CrossRef] [PubMed]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, P.; Kong, C.H. Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 2009, 45, 436–441. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Yang, Q.; Wang, X.; Cui, N. Determining optimal deficit irrigation and fertilization to increase mango yield, quality, and WUE in a dry hot environment based on TOPSIS. Agric. Water Manag. 2021, 245, 106650. [Google Scholar] [CrossRef]
- Pushpalatha, K.; Sherubha, P.; Sasirekha, S.P.; Kumar Anguraj, D. A constructive delay-aware model for opportunistic routing protocol in MANET. Expert Syst. Appl. 2024, 255, 124527. [Google Scholar] [CrossRef]
- Holatko, J.; Brtnicky, M.; Kintl, A.; Baltazar, T.; Malicek, O.; Mustafa, A.; Skladanka, J.; Kucerik, J.; Alamri, S.; Lochman, J.; et al. Effect of alfalfa-grass mixed culture and inoculation with Azotobacter and Rhizobium on soil biological properties and nutrient transformation activities. Eur. J. Soil Biol. 2024, 122, 103651. [Google Scholar] [CrossRef]
- Hu, D.; Zhou, X.; Ma, G.; Pan, J.; Ma, H.; Chai, Y.; Li, Y.; Yue, M. Increased soil bacteria-fungus interactions promote soil nutrient availability, plant growth, and coexistence. Sci. Total Environ. 2024, 955, 176919. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Song, X.; Li, S.; Liu, Z.; Liu, X.; Han, Z.; Gao, H.; Gao, Q.; Zha, Y.; Liu, Y.; et al. Biochar enhances soil hydrological function by improving the pore structure of saline soil. Agric. Water Manag. 2024, 306, 109170. [Google Scholar] [CrossRef]
- Fouladidorhani, M.; Shayannejad, M.; Mosaddeghi, M.R.; Shariatmadari, H.; Arthur, E. Biochar, manure and superabsorbent improve the physical quality of saline-sodic soil under greenhouse conditions. Soil Sci. Soc. Am. J. 2023, 87, 1003–1017. [Google Scholar] [CrossRef]
- He, Z.; Liang, J.; Lu, Y.; Yang, Q.; Lu, C.; Wu, D. Enhanced soil moisture management using waste green algae-derived polymers: Optimization of application rate and mixing depth. Agronomy 2023, 13, 2335. [Google Scholar] [CrossRef]
- Qi, J.; Zhu, H.; Zhou, P.; Wang, X.; Wang, Z.; Yang, S.; Yang, D.; Li, B. Application of phosphogypsum in soilization: A review. Int. J. Environ. Sci. Technol. 2023, 20, 10449–10464. [Google Scholar] [CrossRef]
- Kong, L.; Fan, X.; Li, X.; Zhai, B.; Tian, Z.; Hong, Y.; Jiang, P.; Zhang, J.; Wang, J. Water infiltration and evaporation process with ATP addition in newly reclaimed soil. Agronomy 2024, 14, 2628. [Google Scholar] [CrossRef]
- Wang, J.; Shi, D.; Li, Y.; Chen, A.; Feng, S.; Liu, C. A study on infiltration characteristics and one-dimensional algebraic model simulation in reclaimed soil with biochar. Water 2023, 15, 2985. [Google Scholar] [CrossRef]
- Sun, X.; Ding, Z.; Bi, Y.; Wang, X. Water-holding properties of clinoptilolite/sodium polyacrylate-modified compacted clay cover of Tailing Pond. Int. J. Environ. Res. Public Health 2022, 19, 15554. [Google Scholar] [CrossRef]
- Brevik, E.C.; Fenton, T.E.; Lazari, A. Soil electrical conductivity as a function of soil water content and implications for soil mapping. Precis. Agric. 2006, 7, 393–404. [Google Scholar] [CrossRef]
- Ankit; Prakash, D.; Sheoran, S.; Yadav, P.K.; Raj, D.; Rachna; Gupta, R.K.; El-Hendawy, S.; Mattar, M.A. Sustainable cropping sequences to improve soil fertility and microbiological properties. Sustainability 2024, 16, 9821. [Google Scholar] [CrossRef]
- Khan, M.A.; Basir, A.; Shah, S.T.; Rehman, M.U.; Hassan, M.u.; Zheng, H.; Basit, A.; Székely, Á.; Jamal, A.; Radicetti, E.; et al. Sustainable soil management in alkaline soils: The role of biochar and organic nitrogen in enhancing soil fertility. Land 2024, 13, 1869. [Google Scholar] [CrossRef]
- Tao, W.-Q.; Wu, Q.-Q.; Zhang, J.; Chang, T.-T.; Liu, X.-N. Effects of applying organic amendments on soil aggregate structure and tomato yield in facility agriculture. Plants 2024, 13, 3064. [Google Scholar] [CrossRef] [PubMed]
- Outbakat, M.B.; El Mejahed, K.; El Gharous, M.; El Omari, K.; Beniaich, A. Effect of phosphogypsum on soil physical properties in moroccan salt-affected soils. Sustainability 2022, 14, 13087. [Google Scholar] [CrossRef]
- Guenet, B.; Lenhart, K.; Leloup, J.; Giusti-Miller, S.; Pouteau, V.; Mora, P.; Nunan, N.; Abbadie, L. The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma 2012, 170, 331–336. [Google Scholar] [CrossRef]
- Ren, Y.; Su, L.; Hou, X.; Shao, J.; Liu, K.; Shen, Q.; Zhang, R.; Xun, W. Rhizospheric compensation of nutrient cycling functions dominates crop productivity and nutrient use efficiency. Appl. Soil Ecol. 2023, 182, 104722. [Google Scholar] [CrossRef]
- Lu, L.; Tong, C.; Liu, Y.; Yang, W. Analysis of physicochemical properties, enzyme activity, microbial diversity in rhizosphere soil of coconut (Cocos nucifera L.) under organic and chemical fertilizers, irrigation conditions. Agriculture 2024, 14, 1937. [Google Scholar] [CrossRef]
- Zhang, J.; Amonette, J.E.; Flury, M. Effect of biochar and biochar particle size on plant-available water of sand, silt loam, and clay soil. Soil Tillage Res. 2021, 212, 104992. [Google Scholar] [CrossRef]
- Niu, M.; Chen, X.; Pan, Y.; Wang, S.; Xue, L.; Duan, Y.; Ahmad, S.; Zhou, Y.; Zhao, K.; Peng, D. Biochar effectively promoted growth of ardisia crenata by affecting the soil physicochemical properties. Plants 2024, 13, 1736. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Antony, E.; Singandhupe, R.B. Impact of drip and surface irrigation on growth, yield and WUE of capsicum (Capsicum annum L.). Agric. Water Manag. 2004, 65, 121–132. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Xi, J.; Li, S.; Li, Z. Optimization of soil hydrological properties in degraded grasslands by soil amendments. J. Hydrol. 2024, 643, 131946. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. CATENA 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, H.; Li, T.; Hou, R.; Liu, D.; Ji, Y.; Zhou, Z.; Yang, L. Effects of biochar addition on soil hydraulic properties before and after freezing-thawing. CATENA 2019, 176, 112–124. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Feng, H.; Yu, Q.; Fan, X.; Liu, C.; Chen, J.; Yang, Z.; Biswas, A. Combining biochar with cotton-sugarbeet intercropping increased water-fertilizer productivity and economic benefits under plastic mulched drip irrigation in Xinjiang, China. Ind. Crops Prod. 2023, 192, 116060. [Google Scholar] [CrossRef]
- Guan, R.; Li, Y.; Jia, Y.; Jiang, F.; Li, L. Acidified biochar one-off application for saline-alkali soil improvement: A three-year field trial evaluating the persistence of effects. Ind. Crops Prod. 2024, 222, 119972. [Google Scholar] [CrossRef]
- Bachani, J.; Mahanty, A.; Aftab, T.; Kumar, K. Insight into calcium signalling in salt stress response. S. Afr. J. Bot. 2022, 151, 1–8. [Google Scholar] [CrossRef]



| Chemical Composition (%) | Heavy Metals Content (mg/kg) | Heavy Metals Standard Value (mg/kg) | pH | ||
|---|---|---|---|---|---|
| Calcium sulfate | 62.72 | Arsenic | 6.89 | 30 | 4.2 |
| Silicon dioxide | 14.55 | Lead | 1.81 | 70 | |
| Phosphorus pentoxide | 0.81 | Cadmium | 0.16 | 0.30 | |
| Aluminum oxide | 0.49 | Mercury | 0.13 | 0.50 | |
| Soluble fluorine | 0.42 | Chromium | 11.21 | 150 | |
| Iron oxide | 0.175 | Nickel | 2.65 | 200 | |
| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Performance Parameters | CK | 0.075 | 0.150 | 0.3 | 0.600 | ||
| Wetting front propulsion model | A | 2.537 | 2.419 | 2.343 | 2.058 | 0.819 | |
| B | 0.604 | 0.592 | 0.596 | 0.561 | 0.758 | ||
| MAE | 0.553 | 0.581 | 0.620 | 0.616 | 0.594 | ||
| RRMSE | 0.044 | 0.049 | 0.056 | 0.050 | 0.061 | ||
| CRM | 0.007 | 0.004 | 0.004 | 0.005 | −0.015 | ||
| CE | 0.996 | 0.995 | 0.994 | 0.995 | 0.995 | ||
| Cumulative infiltration model | Lewis | K | 2.571 | 2.027 | 1.492 | 0.954 | 0.427 |
| α | 0.431 | 0.488 | 0.566 | 0.657 | 0.820 | ||
| MAE | 0.461 | 0.418 | 0.383 | 0.482 | 0.239 | ||
| RRMSE | 0.074 | 0.059 | 0.048 | 0.057 | 0.034 | ||
| CRM | −0.088 | −0.052 | −0.011 | 0.037 | 0.046 | ||
| CE | 0.990 | 0.993 | 0.995 | 0.994 | 0.999 | ||
| Philip | S | 3.800 | 3.380 | 3.061 | 3.134 | 2.475 | |
| MAE | 1.509 | 1.248 | 1.229 | 0.995 | 2.340 | ||
| RRMSE | 0.099 | 0.094 | 0.103 | 0.076 | 0.231 | ||
| CRM | 0.045 | 0.040 | 0.043 | 0.031 | 0.087 | ||
| CE | 0.978 | 0.981 | 0.978 | 0.987 | 0.921 | ||
| Horton | a | 0.310 | 0.265 | 0.227 | 0.199 | 0.228 | |
| b | 1.651 | 1.650 | 1.426 | 1.374 | 1.361 | ||
| c | 0.123 | 0.143 | 0.132 | 0.104 | 0.315 | ||
| MAE | 0.520 | 0.570 | 0.575 | 0.815 | 0.426 | ||
| RRMSE | 0.038 | 0.042 | 0.048 | 0.062 | 0.041 | ||
| CRM | 0.002 | 0.003 | 0.003 | −0.002 | −0.001 | ||
| CE | 0.997 | 0.996 | 0.995 | 0.992 | 0.997 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, A.; Li, J.; Xiao, Y.; He, Z.; Liang, J. Engineering Soil Quality and Water Productivity Through Optimal Phosphogypsum Application Rates. Agronomy 2025, 15, 35. https://doi.org/10.3390/agronomy15010035
Luo A, Li J, Xiao Y, He Z, Liang J. Engineering Soil Quality and Water Productivity Through Optimal Phosphogypsum Application Rates. Agronomy. 2025; 15(1):35. https://doi.org/10.3390/agronomy15010035
Chicago/Turabian StyleLuo, Anrong, Jun Li, Yanan Xiao, Zijian He, and Jiaping Liang. 2025. "Engineering Soil Quality and Water Productivity Through Optimal Phosphogypsum Application Rates" Agronomy 15, no. 1: 35. https://doi.org/10.3390/agronomy15010035
APA StyleLuo, A., Li, J., Xiao, Y., He, Z., & Liang, J. (2025). Engineering Soil Quality and Water Productivity Through Optimal Phosphogypsum Application Rates. Agronomy, 15(1), 35. https://doi.org/10.3390/agronomy15010035






