CaHY5 Mediates UV-B Induced Anthocyanin Biosynthesis in Purple Pepper
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. CaHY5 TFs Cloning and Bioinformatics Analysis
2.3. Subcellular Localization of CaHY5
2.4. Hormone Treatments
2.5. Light Treatments
2.6. Anthocyanin Content Determination
2.7. Analysis of Relative Gene Expression
2.8. Virus-Induced Gene Silencing
2.9. Transient Expression of CaHY5 in Purple Pepper
2.10. Statistical Analyses
3. Results
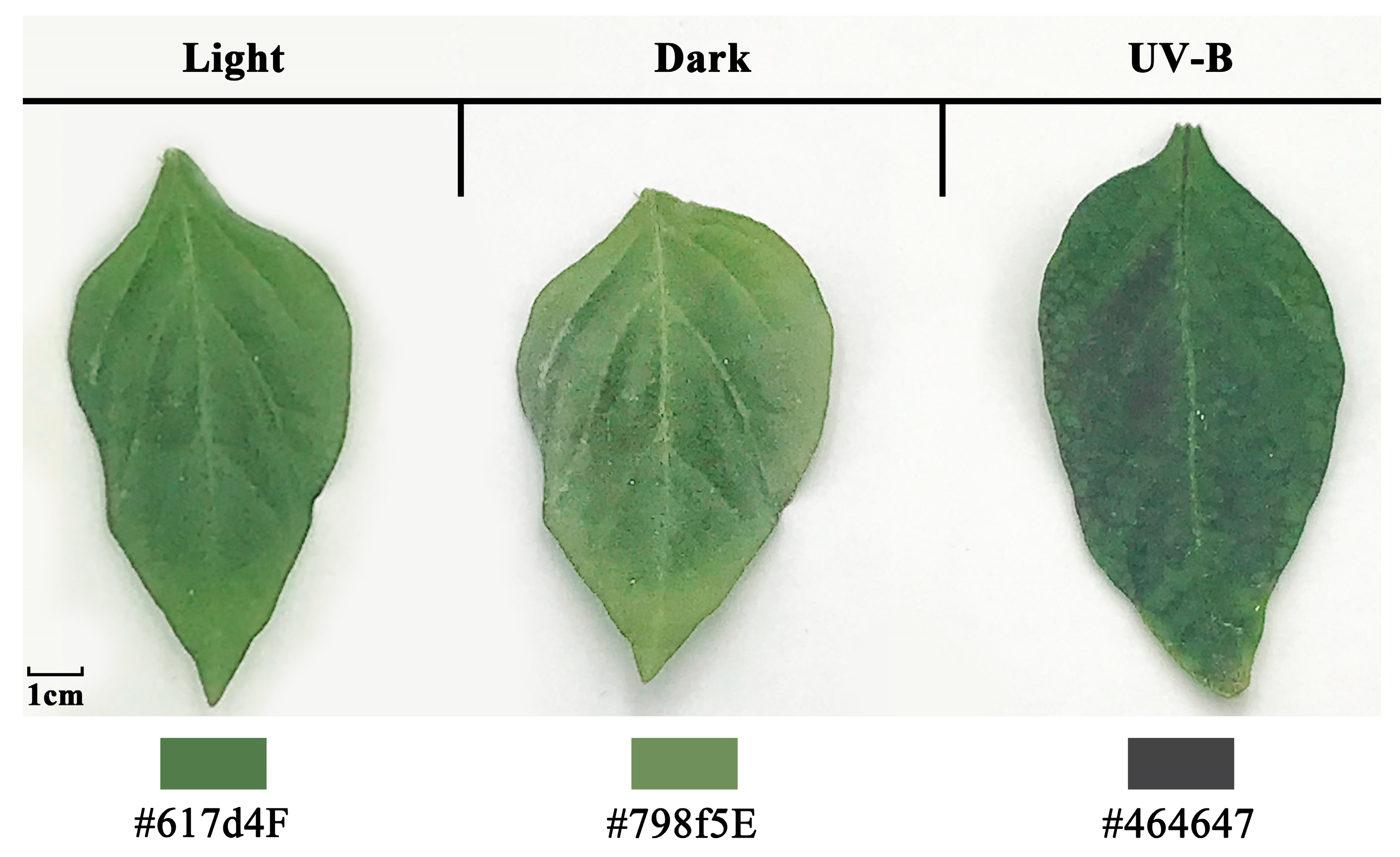
3.1. Anthocyanin Content in Purple Pepper After Exposure to Different Light Treatments
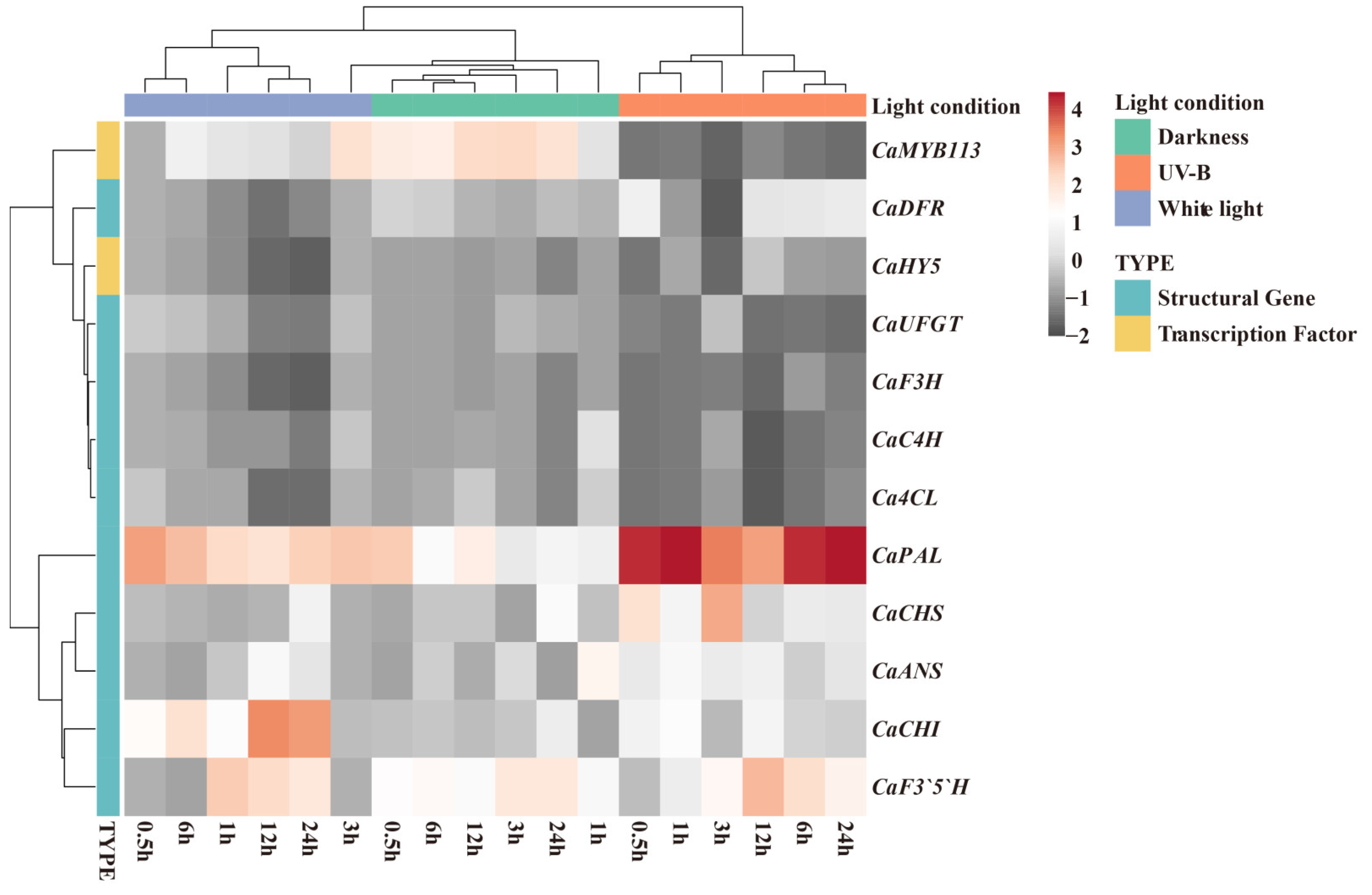
3.2. Expression Levels of Transcription Factors and Anthocyanin Biosynthesis Genes in Purple Pepper Fruits Under Different Light Treatments
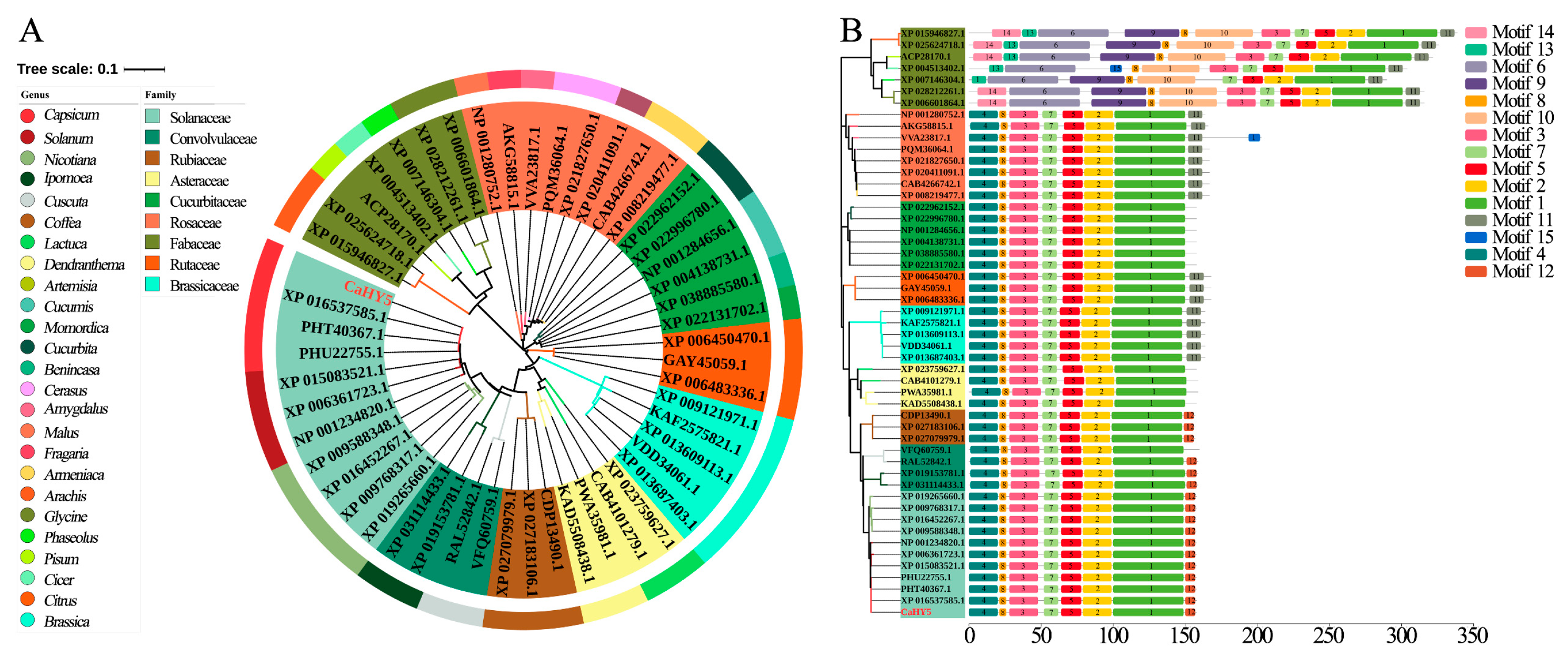
3.3. CaHY5 Cloning and Bioinformatics Analysis
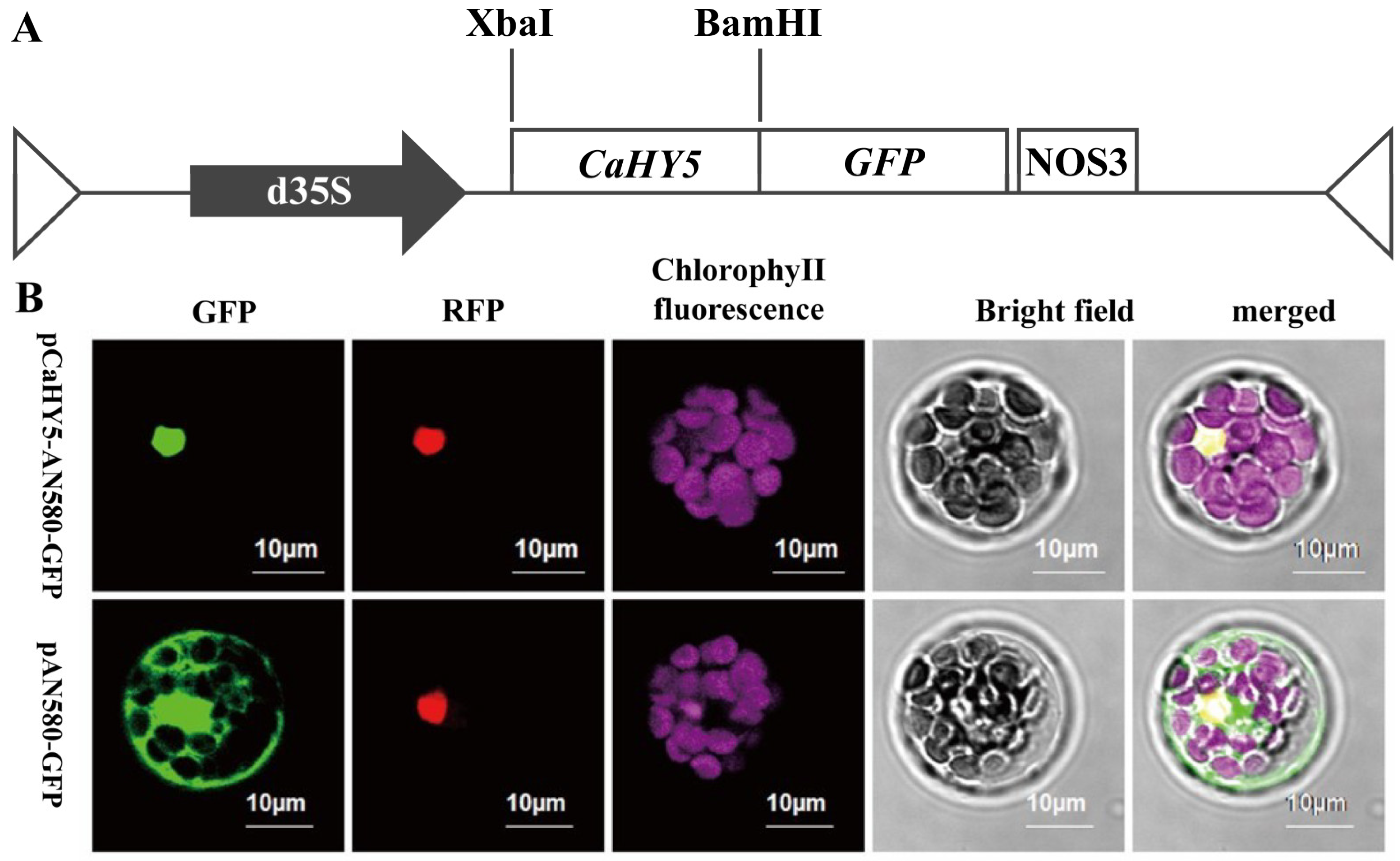
3.4. Subcellular Localization of CaHY5 in Purple Pepper
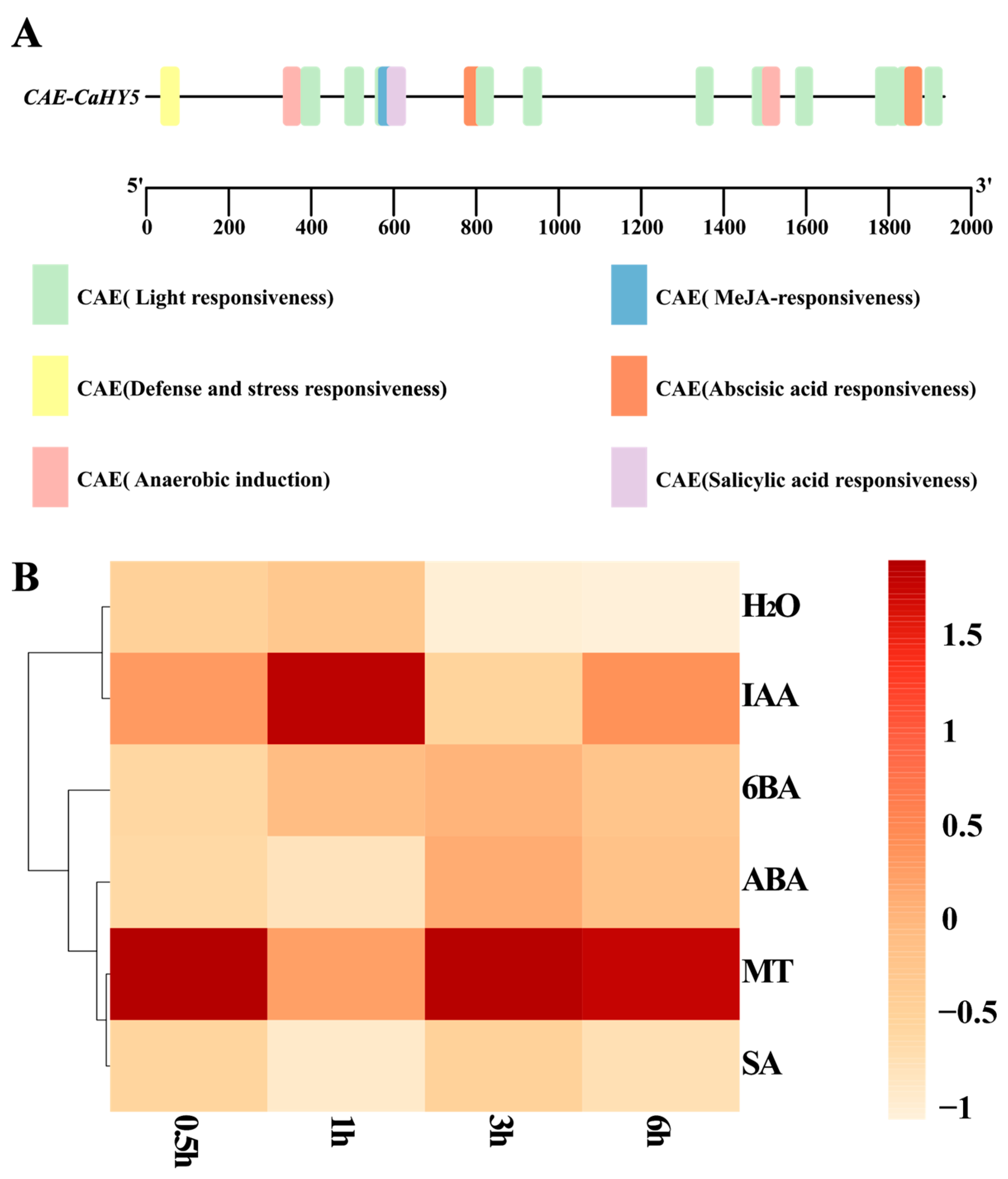
3.5. Upstream Cis-Element Analysis of CaHY5 and Heat Map of CaHY5 Expression Under Different Exogenous Factors
3.6. Downregulation of CaHY5 Decreases Genes of Anthocyanin Biogenesis Pathway Expression
3.7. Transient Overexpression of CaHY5 in Purple Pepper
4. Discussion
4.1. CaHY5 Gene Is Regulated by Several Factors
4.2. Effect of Different Light Conditions on Anthocyanin Biosynthesis in Purple Pepper
4.3. UV-B Induces Expression of Anthocyanin Biosynthesis Genes to Promote Anthocyanin Accumulation
4.4. CaHY5 Directly Regulates CaF3H Expression to Promote Anthocyanin Accumulation in Purple Pepper
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Liu, Y.; Yin, Y.; Gao, S.; Wu, F.; Yu, C.; Wang, F.; Kang, B.C.; Xu, K.; Jiao, C. Identification of CaPs locus involving in purple stripe formation on unripe fruit, reveals allelic variation and alternative splicing of R2R3-MYB transcription factor in pepper (Capsicum annuum L.). Front. Plant Sci. 2023, 14, 1140851. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.L. Purple Pepper Plants, an Anthocyanin Powerhouse: Extraction, Separation and Characterization. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2014. [Google Scholar]
- Chen, T.-T.; Liu, H.; Li, Y.-P.; Yao, X.-H.; Qin, W.; Yan, X.; Wang, X.-Y.; Peng, B.-W.; Zhang, Y.-J.; Shao, J. AaSEPALLATA1 integrates jasmonate and light-regulated glandular secretory trichome initiation in Artemisia annua. Plant Physiol. 2023, 192, 1483–1497. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; De Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- An, J.P.; Xu, R.R.; Wang, X.N.; Zhang, X.W.; You, C.X.; Han, Y. MdbHLH162 connects the gibberellin and jasmonic acid signals to regulate anthocyanin biosynthesis in apple. J. Integr. Plant Biol. 2024, 66, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Xu, X.; Su, Y.; Gao, Y.; Yang, J.; Xie, C.; Ma, J. A MYB family transcription factor TdRCA1 from wild emmer wheat regulates anthocyanin biosynthesis in coleoptile. Theor. Appl. Genet. 2024, 137, 208. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S. Nature′s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. BioMed Res. Int. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.; Visser, R.G.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ni, J.; Wang, S.; Yu, W.; Liao, Y.; Pan, C.; Zhang, M.; Tao, R.; Wei, J.; Gao, Y.; Wang, D. The ethylene-responsive transcription factor PpERF9 represses PpRAP2. 4 and PpMYB114 via histone deacetylation to inhibit anthocyanin biosynthesis in pear. Plant Cell 2023, 35, 2271–2292. [Google Scholar] [CrossRef] [PubMed]
- Menconi, J.; Perata, P.; Gonzali, S. In pursuit of purple: Anthocyanin biosynthesis in fruits of the tomato clade. Trends Plant Sci. 2024, 29, 589–604. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Aza-Gonzalez, C.; Herrera-Isidrón, L.; Núñez-Palenius, H.; Martínez De La Vega, O.; Ochoa-Alejo, N. Anthocyanin accumulation and expression analysis of biosynthesis-related genes during chili pepper fruit development. Biol. Plant. 2013, 57, 49–55. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–342. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, W.; Gao, S.; Jahan, M.S.; Xiao, J.; Guo, T.; Chen, C.; Li, B.; Luo, C.; He, X. Integrated transcriptome and metabolome analyses reveal anthocyanin biosynthesis in red and green mango pericarps under light and shade conditions. Sci. Hortic. 2024, 338, 113617. [Google Scholar] [CrossRef]
- Tang, B.; Li, L.; Hu, Z.; Chen, Y.; Tan, T.; Jia, Y.; Xie, Q.; Chen, G. Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in purple pepper. J. Agric. Food Chem. 2020, 68, 12152–12163. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Shin, D.H.; Choi, S.-B.; Park, Y.-I. Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol. Cells 2012, 34, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lin-Wang, K.; Wang, H.; Gu, C.; Dare, A.P.; Espley, R.V.; He, H.; Allan, A.C.; Han, Y. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J. 2015, 82, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-Y.; Gong, Z.-H. Research Progress of Anthocyanin Biosynthesis and Regulation from Purple Pepper (Capsicum annuum L.). In Proceedings of the 2017 2nd International Conference on Biological Sciences and Technology (BST 2017), F, Zhuhai, China, 17–19 November 2018; Atlantis Press: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhao, Y.; Min, T.; Chen, M.; Wang, H.; Zhu, C.; Jin, R.; Allan, A.C.; Lin-Wang, K.; Xu, C. The photomorphogenic transcription factor PpHY5 regulates anthocyanin accumulation in response to UVA and UVB irradiation. Front. Plant Sci. 2021, 11, 603178. [Google Scholar] [CrossRef]
- Alabd, A.; Ahmad, M.; Zhang, X.; Gao, Y.; Peng, L.; Zhang, L.; Ni, J.; Bai, S.; Teng, Y. Light-responsive transcription factor PpWRKY44 induces anthocyanin accumulation by regulating PpMYB10 expression in pear. Hortic. Res. 2022, 9, uhac199. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Kapoor, P.; Chunduri, V.; Pandey, A.K.; Garg, M. Spotlight on the overlapping routes and partners for anthocyanin transport in plants. Physiol. Plant. 2021, 171, 868–881. [Google Scholar] [CrossRef]
- Ni, J.; Liao, Y.; Zhang, M.; Pan, C.; Yang, Q.; Bai, S.; Teng, Y. Blue light simultaneously induces peel anthocyanin biosynthesis and flesh carotenoid/sucrose biosynthesis in mango fruit. J. Agric. Food Chem. 2022, 70, 16021–16035. [Google Scholar] [CrossRef] [PubMed]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, B.; Bai, J.; Qian, M.; Shu, Q.; Su, J.; Teng, Y. Effects of high temperatures on UV-B/visible irradiation induced postharvest anthocyanin accumulation in ‘Yunhongli No. 1’(Pyrus pyrifolia Nakai) pears. Sci. Hortic. 2012, 134, 53–59. [Google Scholar] [CrossRef]
- Yue, P.; Jiang, Z.; Sun, Q.; Wei, R.; Yin, Y.; Xie, Z.; Larkin, R.M.; Ye, J.; Chai, L.; Deng, X. Jasmonate activates a CsMPK6-CsMYC2 module that regulates the expression of β-citraurin biosynthetic genes and fruit coloration in orange (Citrus sinensis). Plant Cell 2023, 35, 1167–1185. [Google Scholar] [CrossRef]
- Qian, M.; Wu, H.; Yang, C.; Zhu, W.; Shi, B.; Zheng, B.; Wang, S.; Zhou, K.; Gao, A. RNA-Seq reveals the key pathways and genes involved in the light-regulated flavonoids biosynthesis in mango (Mangifera indica L.) peel. Front. Plant Sci. 2023, 13, 1119384. [Google Scholar] [CrossRef]
- Christie, J.M.; Jenkins, G.I. Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 1996, 8, 1555–1567. [Google Scholar]
- Rius, S.P.; Grotewold, E.; Casati, P. Analysis of the P1 promoter in response to UV-B radiation in allelic variants of high-altitude maize. BMC Plant Biol. 2012, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Avilés, W.; Heuvelink, E.; Marcelis, L.F.; Kappers, I.F. Ménage à trois: Light, terpenoids, and quality of plants. Trends Plant Sci. 2024, 29, 572–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Sun, Y.; Zhang, X.; Du, B.; Turupu, M.; Yao, Q.; Gai, S.; Tong, S.; Huang, J. Two B-box proteins, PavBBX6/9, positively regulate light-induced anthocyanin accumulation in sweet cherry. Plant Physiol. 2023, 192, 2030–2048. [Google Scholar] [CrossRef]
- Li, C.; Pei, J.; Yan, X.; Cui, X.; Tsuruta, M.; Liu, Y.; Lian, C. A poplar B-box protein PtrBBX23 modulates the accumulation of anthocyanins and proanthocyanidins in response to high light. Plant Cell Environ. 2021, 44, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Bursch, K.; Toledo-Ortiz, G.; Pireyre, M.; Lohr, M.; Braatz, C.; Johansson, H. Identification of BBX proteins as rate-limiting cofactors of HY5. Nat. Plants 2020, 6, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Jeong, C.Y.; Kang, G.H.; Yoo, S.D.; Hong, S.W.; Lee, H. MYBD employed by HY5 increases anthocyanin accumulation via repression of MYBL 2 in Arabidopsis. Plant J. 2015, 84, 1192–1205. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Yin, L.; Ni, J.; Yang, Q.; Yan, X.; Yang, F.; Guo, X.; Li, H.; Teng, Y. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019, 100, 1208–1223. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Ge, H.; Shi, S.; Li, D.; Liu, Y.; Chen, H. A light-responsive transcription factor SmMYB35 enhances anthocyanin biosynthesis in eggplant (Solanum melongena L.). Planta 2022, 255, 12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ren, L.; Lian, H.; Liu, Y.; Chen, H. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. 2016, 249, 46–58. [Google Scholar] [CrossRef]
- Shin, J.; Park, E.; Choi, G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007, 49, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lv, J.; Zhao, K.; Zhang, X.; Li, Z.; Zhang, H.; Huo, J.; Wan, H.; Wang, Z.; Zhu, H. Ethylene-inducible AP2/ERF transcription factor involved in the capsaicinoid biosynthesis in Capsicum. Front. Plant Sci. 2022, 13, 832669. [Google Scholar] [CrossRef]
- Liu, F.; Yu, H.; Deng, Y.; Zheng, J.; Liu, M.; Ou, L.; Yang, B.; Dai, X.; Ma, Y.; Feng, S. PepperHub, an informatics hub for the chili pepper research community. Mol. Plant 2017, 10, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Wang, H.; Zhang, Y.; Li, W.; Liu, J.; Cheng, Q.; Sun, L.; Shen, H. Identification of the regulatory genes of UV-B-induced anthocyanin biosynthesis in pepper fruit. Int. J. Mol. Sci. 2022, 23, 1960. [Google Scholar] [CrossRef] [PubMed]
- Lightbourn, G.J.; Griesbach, R.J.; Novotny, J.A.; Clevidence, B.A.; Rao, D.D.; Stommel, J.R. Effects of anthocyanin and carotenoid combinations on foliage and immature fruit color of Capsicum annuum L. J. Hered. 2008, 99, 105–111. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef]
- Xu, D. COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020, 228, 1748–1753. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mumtaz, M.A.; Zhang, Y.; Yang, Z.; Hao, Y.; Shu, H.; Zhu, J.; Bao, W.; Cheng, S.; Zhu, G. Response of anthocyanin biosynthesis to light by strand-specific transcriptome and miRNA analysis in Capsicum annuum. BMC Plant Biol. 2022, 22, 79. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light induced regulation pathway of anthocyanin biosynthesis in plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Bakshi, S.; Yadukrishnan, P.; Lingwan, M.; Dolde, U.; Wenkel, S.; Masakapalli, S.K.; Datta, S. The B-Box-Containing MicroProtein miP1a/BBX31 Regulates Photomorphogenesis and UV-B Protection. Plant Physiol. 2019, 179, 1876–1892. [Google Scholar] [CrossRef]
- Chang, C.-S.-J.; Li, Y.H.; Chen, L.T.; Chen, W.C.; Hsieh, W.P.; Shin, J.; Jane, W.N.; Chou, S.J.; Choi, G.; Hu, J.M. LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J. 2008, 54, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Chi, C.; Jin, L.J.; Zhu, J.; Yu, J.-Q.; Zhou, Y.-H. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef]
- Chen, M.; Gu, H.; Wang, L.; Shao, Y.; Li, R.; Li, W. Exogenous ethylene promotes peel color transformation by regulating the degradation of chlorophyll and synthesis of anthocyanin in postharvest mango fruit. Front. Nutr. 2022, 9, 911542. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-W.; Ma, Z.; Ma, Y.-Q.; Zhu, Y.; Lei, M.-Q.; Hao, C.-Y.; Chen, L.-Y.; Xu, Z.-Q.; Huang, X. Role of melatonin in UV-B signaling pathway and UV-B stress resistance in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 114–129. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Mo, Y.; Zhou, H.; Li, M.; Cheng, H.; Li, P.; Zhang, R.; Huang, Y.; Wang, Y.; Xu, J.; et al. CaHY5 Mediates UV-B Induced Anthocyanin Biosynthesis in Purple Pepper. Agronomy 2025, 15, 28. https://doi.org/10.3390/agronomy15010028
Zhang X, Mo Y, Zhou H, Li M, Cheng H, Li P, Zhang R, Huang Y, Wang Y, Xu J, et al. CaHY5 Mediates UV-B Induced Anthocyanin Biosynthesis in Purple Pepper. Agronomy. 2025; 15(1):28. https://doi.org/10.3390/agronomy15010028
Chicago/Turabian StyleZhang, Xiang, Yunrong Mo, Huidan Zhou, Mengjuan Li, Hong Cheng, Pingping Li, Ruihao Zhang, Yaoyao Huang, Yanyan Wang, Junqiang Xu, and et al. 2025. "CaHY5 Mediates UV-B Induced Anthocyanin Biosynthesis in Purple Pepper" Agronomy 15, no. 1: 28. https://doi.org/10.3390/agronomy15010028
APA StyleZhang, X., Mo, Y., Zhou, H., Li, M., Cheng, H., Li, P., Zhang, R., Huang, Y., Wang, Y., Xu, J., Liao, J., Xie, Q., Zhao, K., Deng, M., & Lv, J. (2025). CaHY5 Mediates UV-B Induced Anthocyanin Biosynthesis in Purple Pepper. Agronomy, 15(1), 28. https://doi.org/10.3390/agronomy15010028




