Rapeseed Supports Hairy Vetch in Intercropping, Enhancing Root and Stem Morphology, Nitrogen Metabolism, Photosynthesis, and Forage Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Experimental Design and Crop Management
2.3. Forage Biomass and Crude Protein Yield
2.4. Root Architecture and Biomass
2.5. Stem Diameter and Anatomical Structure
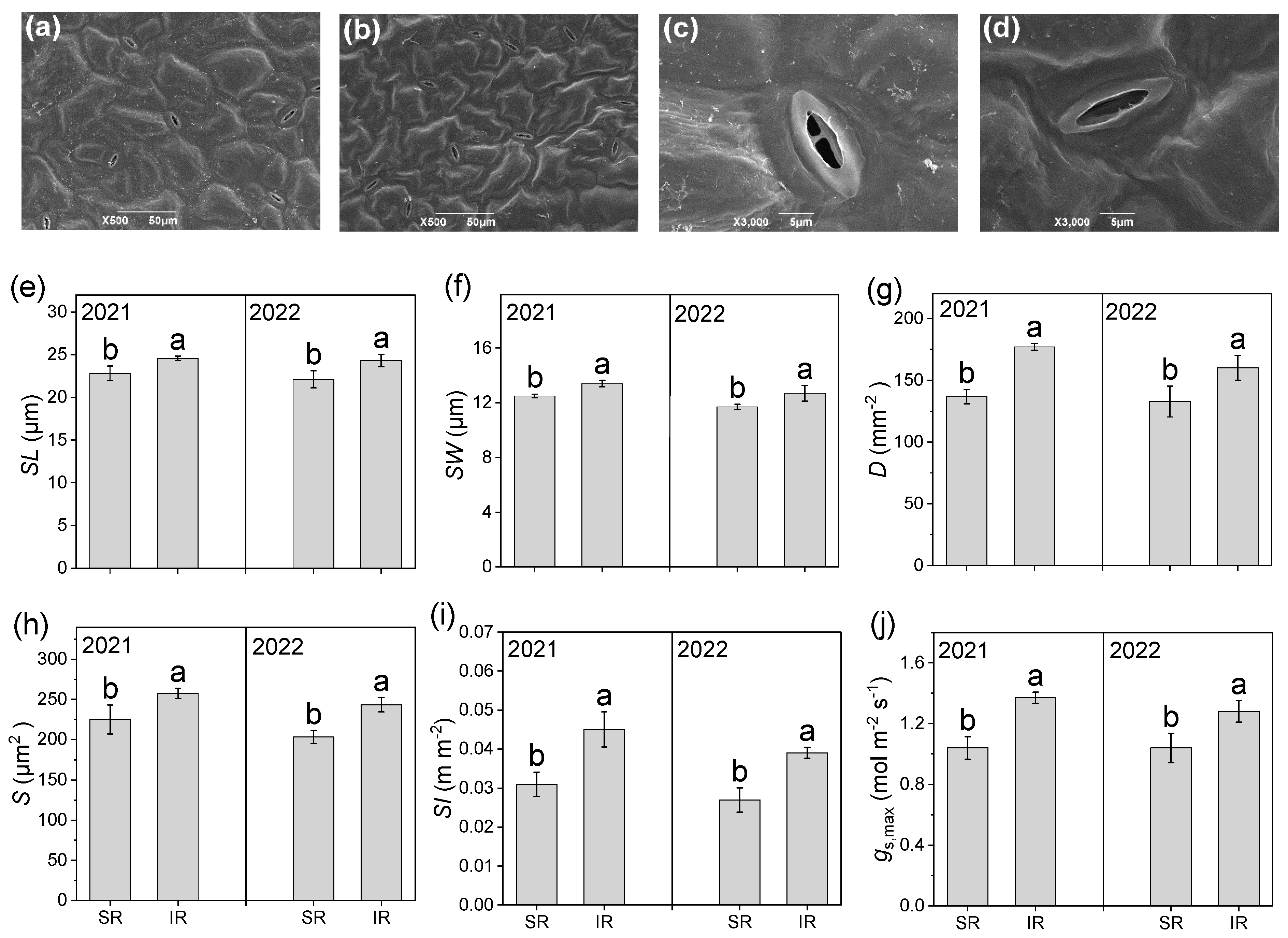
2.6. Light Interception, Gas Exchange, and Stomatal Structure
2.6.1. Light Interception Rate
2.6.2. Photosynthetic Gas Exchange
2.6.3. Stomatal Structure
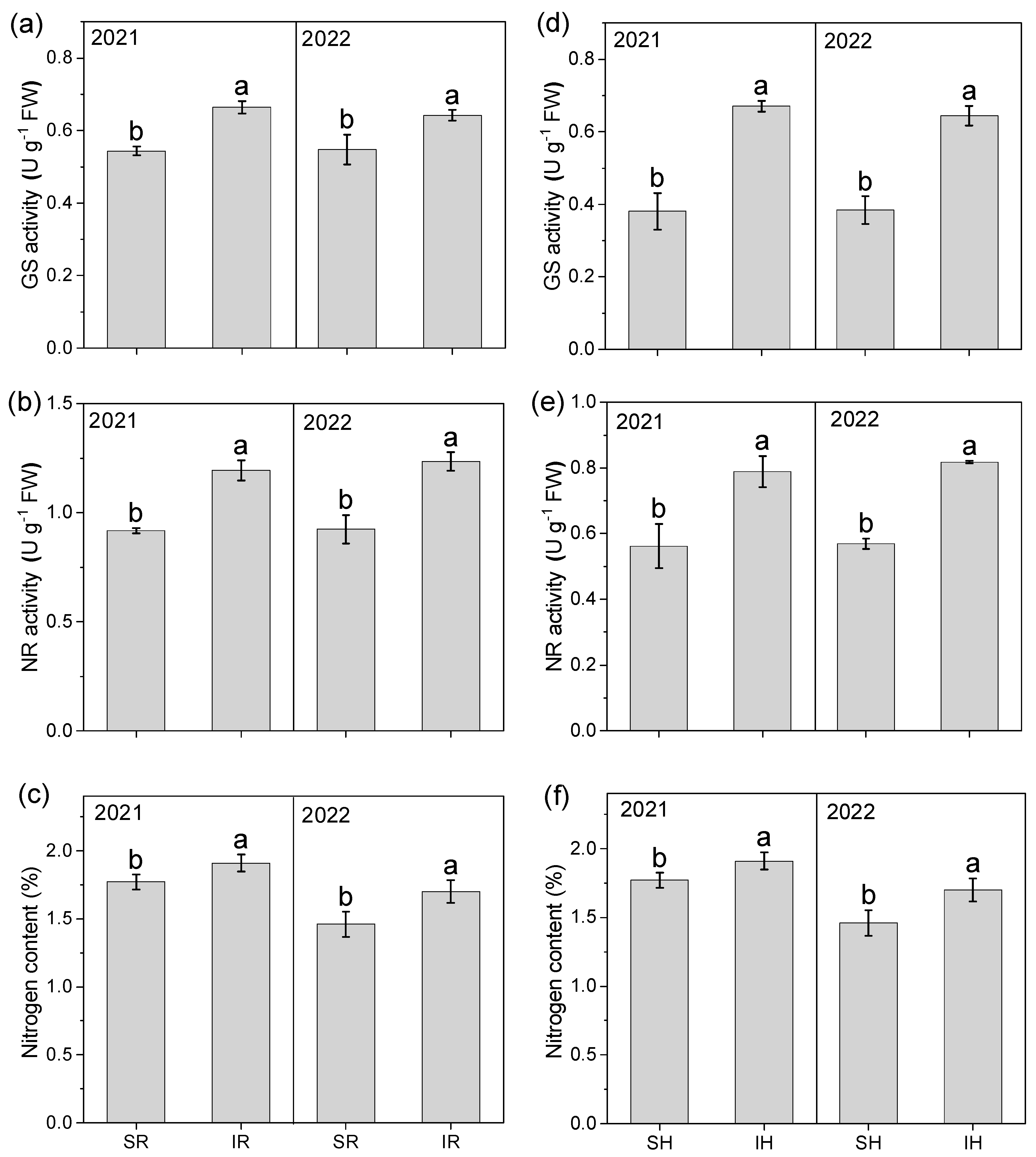
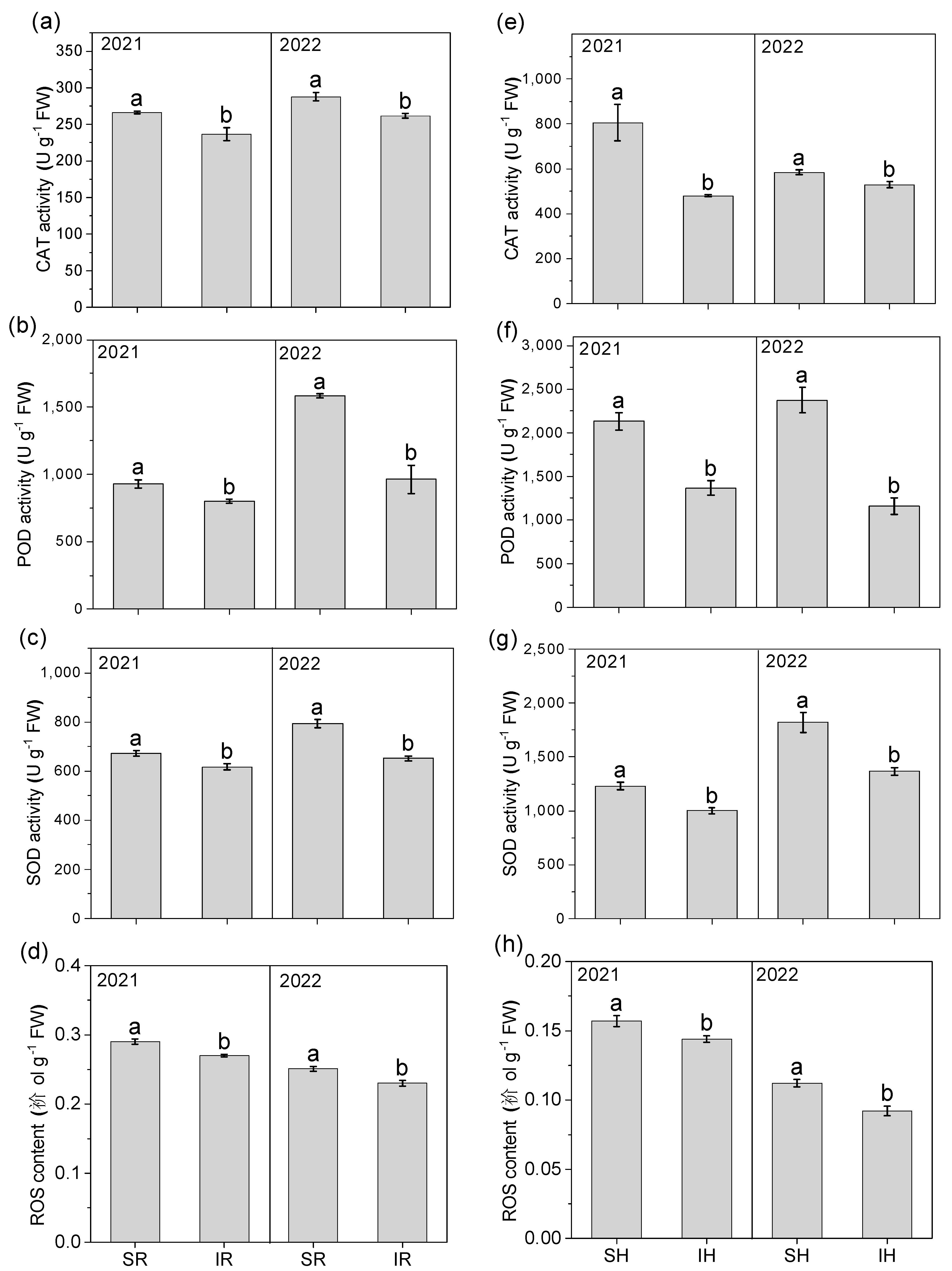
2.7. Enzyme Activity and ROS (Reactive Oxygen Species) Content
2.8. Data Analysis
3. Results and Analysis
3.1. Forage Biomass and Crude Protein Yield
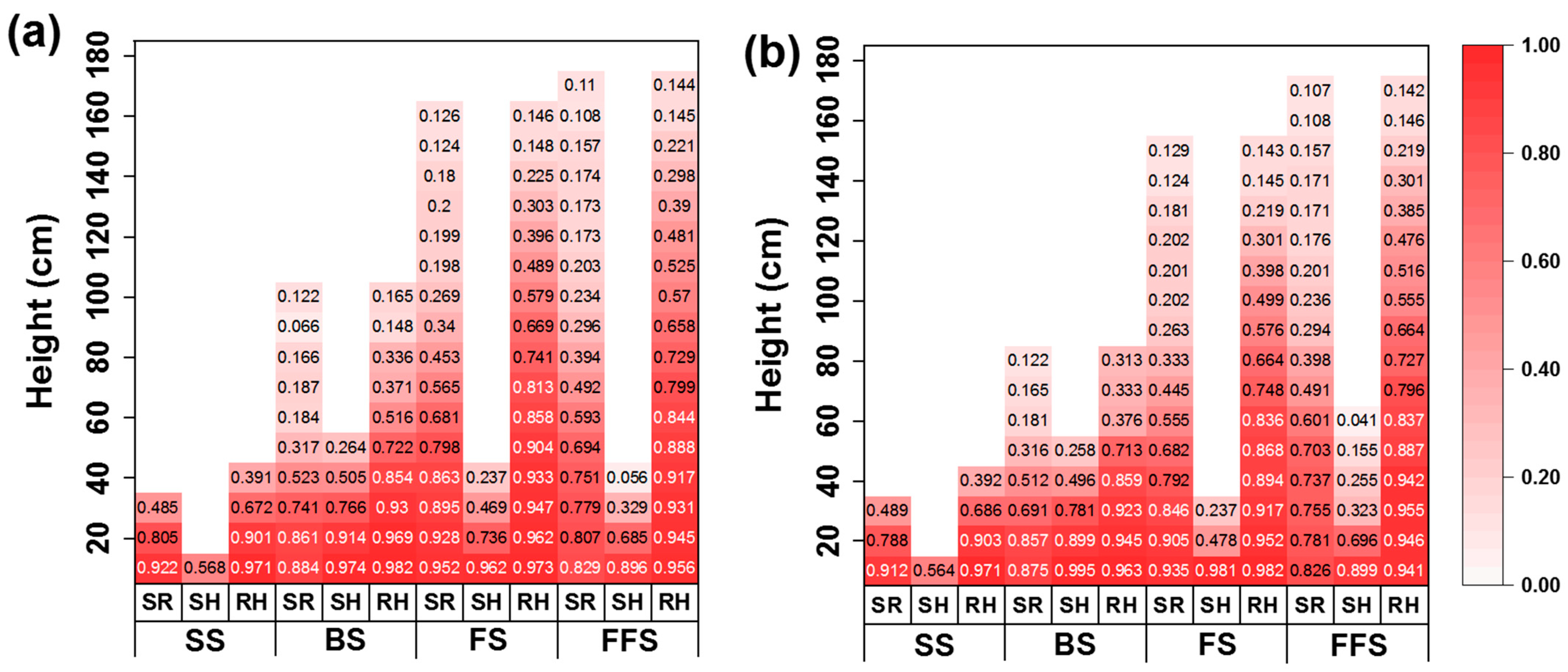
3.2. Light Interception in the Vertical Direction and Canopy Height of the Population
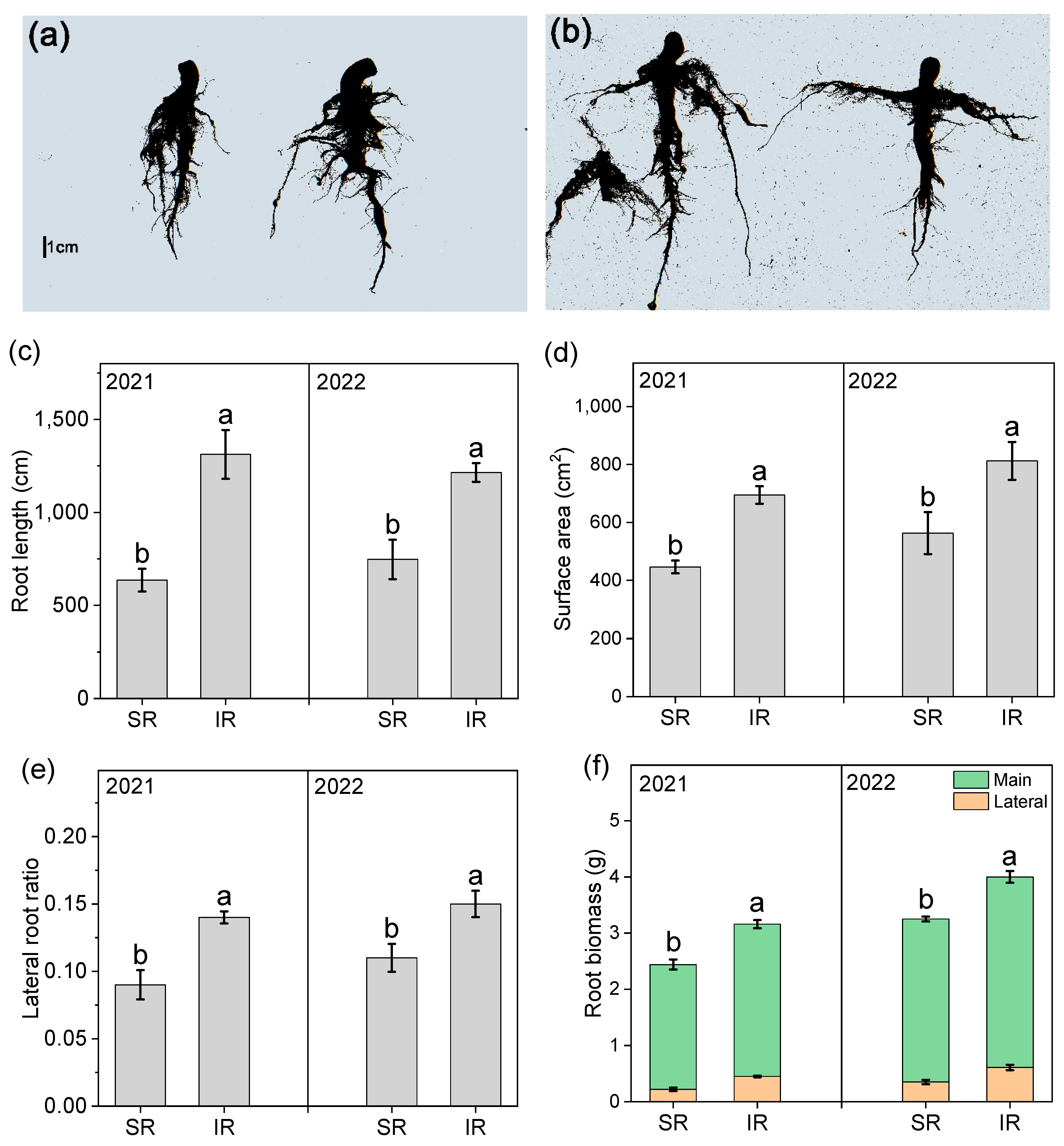
3.3. Root Architecture and Biomass
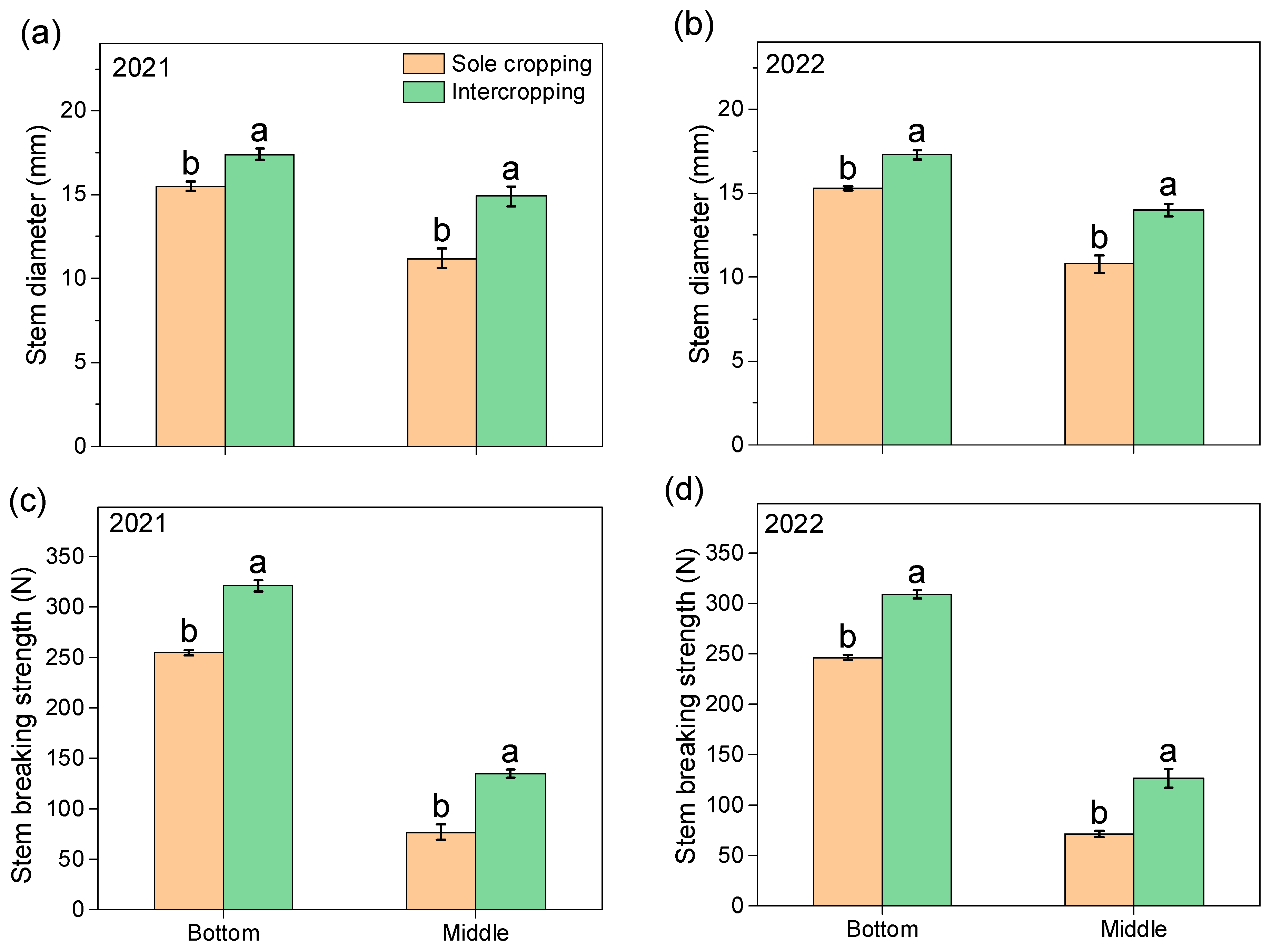
3.4. Rapeseed Stem Diameter and Breaking Strength
3.5. Stem Anatomical Structure
3.6. Gas Exchange
3.7. Stomatal Structure
3.8. Glutamine Synthetase and Nitrate Reductase Activity
3.9. CAT, POD, SOD Enzyme Activity and ROS Content
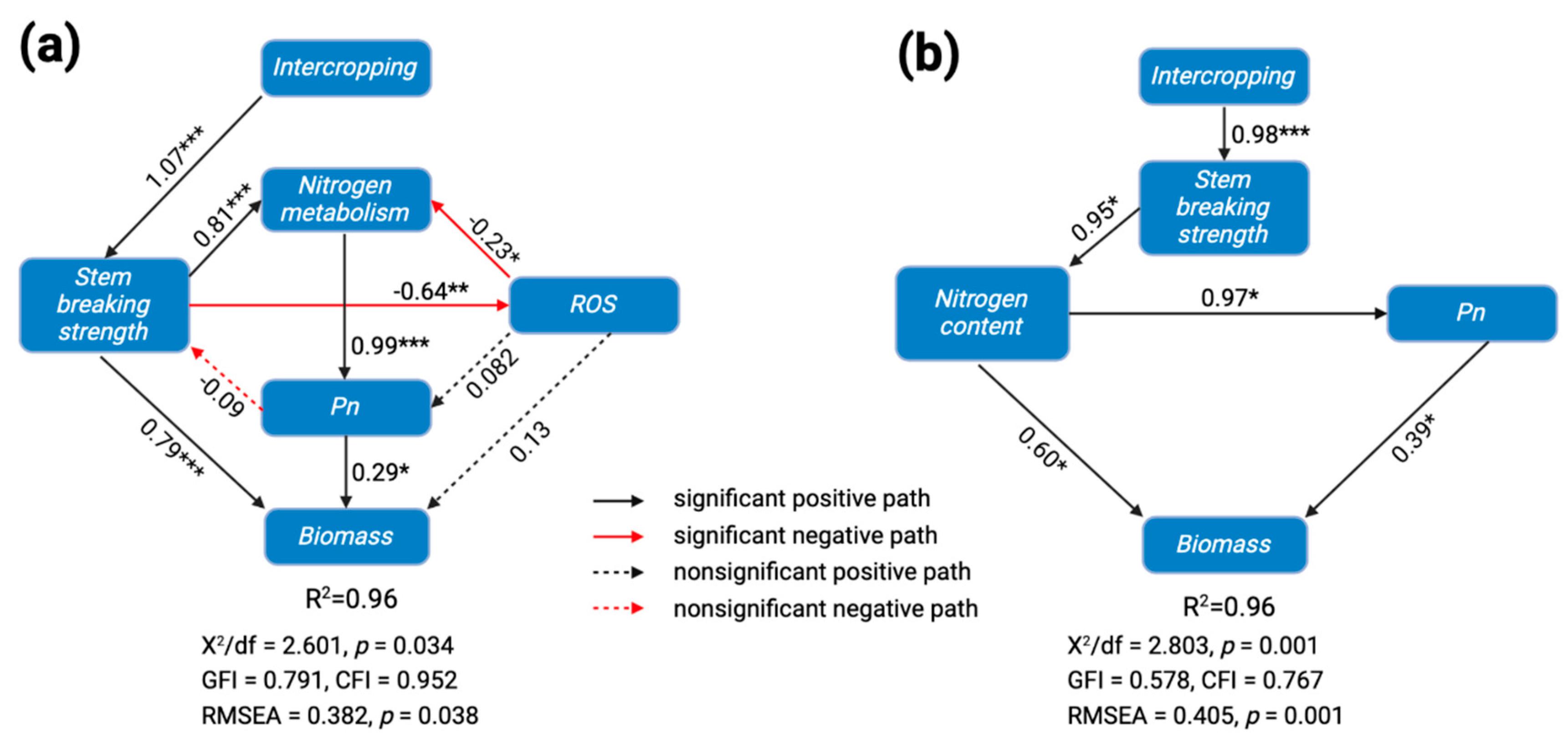
4. Discussion
4.1. Intercropping Improves Population Lodging Resistance and Light Interception
4.2. Intercropping Improved Leaf Gas Exchange and Stomatal Structure
4.3. Effect of Intercropping on Lodging and Forage Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Shah, F.; Ma, B. Understanding of crop lodging and agronomic strategies to improve the resilience of rapeseed production to climate change. Crop Environ. 2022, 1, 133–144. [Google Scholar] [CrossRef]
- Kendall, S.L.; Holmes, H.; White, C.A.; Clarke, S.M.; Berry, P.M. Quantifying lodging-induced yield losses in oilseed rape. Field Crops Res. 2017, 211, 106–113. [Google Scholar] [CrossRef]
- Liu, L.; Liang, G.; Liu, W. Differences in physicochemical properties of stems in oat (Avena sativa L.) varieties with distinct lodging resistance and their regulation of lodging at different planting densities. Plants 2024, 13, 2739. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Tan, X.; Gao, G.; El-Badri, A.M.; Batool, M.; Li, Z.; Ai, X.; Kuai, J.; Wang, J.; et al. Diversified spatial configuration of rapeseed-vetch intercropping benefits soil quality, radiation utilization, and forage production in the Yangtze River Basin. Field Crops Res. 2024, 318, 109587. [Google Scholar] [CrossRef]
- Zeki, A.; Erdem, G.; Ozlem, A.O.; Ugur, B.; Hanife, M.; Ilknur, A. Effects of sowing ratio and harvest periods on hay yields, quality and competitive characteristics of Hungarian vetch—Cereal mixtures. Legume Res. 2017, 40, 677–683. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S.; Hussain, S.; Kataria, S.; Yang, X.; Brestic, M. Effect of shading on leaf anatomical structure, photosynthesis characteristics and chlorophyll fluorescence of soybean (Glycine max). J. Agron. Crop Sci. 2024, 210, e12783. [Google Scholar] [CrossRef]
- Cai, T.; Peng, D.; Wang, R.; Jia, X.; Ren, X. Can intercropping or mixed cropping of two genotypes enhance wheat lodging resistance? Field Crops Res. 2019, 239, 10–18. [Google Scholar] [CrossRef]
- Iqbal, N.; Hussain, S.; Ahmed, Z.; Yang, F.; Wang, X.; Liu, W.; Yong, T.; Du, J.; Shu, K.; Yang, W.; et al. Comparative analysis of maize–soybean strip intercropping systems: A review. Plant Prod. Sci. 2019, 22, 131–142. [Google Scholar] [CrossRef]
- Udumann, S.S.; Ranasinghe, C.S.; Karunarathna, L.K.N.G.; Kaliyadasa, P.E.; Nuwarapaksha, T.D.; Premathilaka, U.G.A.T.; Atapattu, A.J. Optimizing intercropping selection for coconut plantations based on PAR and agro-climatic zones. Agrofor. Syst. 2024, 98, 2847–2859. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, F.; Xie, Z.; Guo, Y.; Li, B.; Ma, Y. Quantification of light interception within image-based 3-D reconstruction of sole and intercropped canopies over the entire growth season. Ann. Bot. 2020, 126, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Abou Khater, L.; Maalouf, F.; Balech, R.; He, Y.; Zong, X.; Rubiales, D.; Kumar, S. Improvement of cool-season food legumes for adaptation to intercropping systems: Breeding faba bean for intercropping with durum wheat as a case study. Front. Plant Sci. 2024, 15, 1368509. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Hussain, J.; Yanwisetpakdee, B.; Iqbal, I.; Chen, X. The effects of monoculture and intercropping on photosynthesis performance correlated with growth of garlic and perennial ryegrass response to different heavy metals. BMC Plant Biol. 2024, 24, 659. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Yang, H.; Chai, Q.; Yin, W.; Hu, F.; Qin, A.; Fan, Z.; Yu, A.; Zhao, C.; Fan, H. Yield photosynthesis and leaf anatomy of maize in inter- and mono-cropping systems at varying plant densities. Crop J. 2022, 10, 893–903. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Pang, T.; Naeem Khan, M.; Liu, W.-G.; Yang, W.-Y. Weak stem under shade reveals the lignin reduction behavior. J. Integr. Agric. 2019, 18, 496–505. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Wang, C.; Guo, M.; El-Badri, A.M.; Batool, M.; Kuai, J.; Wang, J.; Wang, B.; Zhou, G. Rapeseed-maize double-cropping with high biomass and high economic benefits is a soil environment-friendly forage production mode in the Yangtze River Basin. Eur. J. Agron. 2023, 142, 126675. [Google Scholar] [CrossRef]
- Thomas, R.; Sheard, R.; Moyer, J. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Lou, H.; Zhao, B.; Peng, Y.; El-Badri, A.M.; Batool, M.; Wang, C.; Wang, Z.; Huang, W.; Wang, T.; Li, Z.; et al. Auxin plays a key role in nitrogen and plant density-modulated root growth and yield in different plant types of rapeseed. Field Crops Res. 2023, 302, 109066. [Google Scholar] [CrossRef]
- Salah, A.; Nwafor, C.C.; Han, Y.; Liu, L.; Rashid, M.; Batool, M.; El-Badri, A.M.; Cao, C.; Zhan, M. Spermidine and brassinosteroid regulate root anatomical structure, photosynthetic traits and antioxidant defense systems to alleviate waterlogging stress in maize seedlings. S. Afr. J. Bot. 2022, 144, 389–402. [Google Scholar] [CrossRef]
- Jin, F.; Wang, Z.; Zhang, H.; Huang, S.; Chen, M.; Kwame, T.J.; Yong, T.; Wang, X.; Yang, F.; Liu, J.; et al. Quantification of spatial-temporal light interception of crops in different configurations of soybean-maize strip intercropping. Front. Plant Sci. 2024, 15, 1376687. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lu, Z.; Meng, F.; Li, X.; Cong, R.; Ren, T.; Sharkey, T.D.; Lu, J. The reduction in leaf area precedes that in photosynthesis under potassium deficiency: The importance of leaf anatomy. New Phytol. 2020, 227, 1749–1763. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef]
- Mohamed, I.A.A.; Shalby, N.; El-Badri, A.M.; Batool, M.; Wang, C.; Wang, Z.; Salah, A.; Rady, M.M.; Jie, K.; Wang, B.; et al. RNA-seq analysis revealed key genes associated with salt tolerance in rapeseed germination through carbohydrate metabolism, hormone, and MAPK signaling pathways. Ind. Crop Prod. 2022, 176, 114262. [Google Scholar] [CrossRef]
- Baxevanos, D.; Tsialtas, I.T.; Vlachostergios, D.Ν.; Hadjigeorgiou, I.; Dordas, C.; Lithourgidis, A. Cultivar competitiveness in pea-oat intercrops under Mediterranean conditions. Field Crops Res. 2017, 214, 94–103. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, T.; Iqbal, N.; Brestic, M.; Pang, T.; Mumtaz, M.; Shafiq, I.; Li, S.; Wang, L.; Gao, Y.; et al. Effects of lignin, cellulose, hemicellulose, sucrose and monosaccharide carbohydrates on soybean physical stem strength and yield in intercropping. Photochem. Photobiol. Sci. 2020, 19, 462–472. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A.; et al. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Raza, A.; Asghar, M.A.; Javed, H.H.; Ullah, A.; Cheng, B.; Xu, M.; Wang, W.; Liu, C.; Rahman, A.; Iqbal, T.; et al. Optimum nitrogen improved stem breaking resistance of intercropped soybean by modifying the stem anatomical structure and lignin metabolism. Plant Physiol. Biochem. 2023, 199, 107720. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Tang, W.; Huang, Y.; Nan, Z. Effect of row configuration on yield and radiation use of common vetch-oat strip intercropping on the Qinghai-Tibetan plateau. Eur. J. Agron. 2021, 128, 126290. [Google Scholar] [CrossRef]
- de Mattos, E.M.; Binkley, D.; Campoe, O.C.; Alvares, C.A.; Stape, J.L. Variation in canopy structure, leaf area, light interception and light use efficiency among Eucalyptus clones. For. Ecol. Manag. 2020, 463, 118038. [Google Scholar] [CrossRef]
- Yavas, I.; Unay, A. Evaluation of physiological growth parametwers of maize in maize-legume intercroppiung system. J. Anim. Plant Sci. 2016, 26, 1680–1687. [Google Scholar]
- Jannoura, R.; Joergensen, R.G.; Bruns, C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur. J. Agron. 2014, 52, 259–270. [Google Scholar] [CrossRef]
- Pathare, V.S.; Koteyeva, N.; Cousins, A.B. Increased adaxial stomatal density is associated with greater mesophyll surface area exposed to intercellular air spaces and mesophyll conductance in diverse C4 grasses. New Phytol. 2020, 225, 169–182. [Google Scholar] [CrossRef]
- Zeiditoolabi, N.; Khammari, I.; Sirousmehr, A.; Daneshvar, M.; Galavi, M.; Dahmardeh, M. Evaluation of stomata in vetch-barley intercropping and its relationship with forage production in rainfed conditions, under the influence of biofertilizer and superabsorbent. Gesunde Pflanz. 2023, 75, 2045–2073. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Tang, W.; Huang, Y.; Coulter, J.A.; Nan, Z. Common vetch cultivars improve yield of oat row intercropping on the Qinghai-Tibetan plateau by optimizing photosynthetic performance. Eur. J. Agron. 2020, 117, 126088. [Google Scholar] [CrossRef]
- Al-Salman, Y.; Ghannoum, O.; Cano, F.J. Midday water use efficiency in sorghum is linked to faster stomatal closure rate, lower stomatal aperture and higher stomatal density. Plant J. 2023, 115, 1661–1676. [Google Scholar] [CrossRef]
- dos Reis, C.H.G.; da Silva, P.N.; de Castro, E.M.; Pereira, F.J. Tolerance to mild shading levels in cattail as related to increased photosynthesis and changes in its leaf area and anatomy. J. Plant Res. 2024, 137, 627–640. [Google Scholar] [CrossRef]
- Huang, S.-R.; Ai, Y.; Du, J.-B.; Yu, L.; Wang, X.-C.; Yang, W.-Y.; Sun, X. Photosynthetic compensation of maize in heterogeneous light is impaired by restricted photosynthate export. Plant Physiol. Biochem. 2022, 192, 50–56. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kapoor, B.; Kapoor, D.; Thakur, U.; Dolma, Y.; Raza, A. Manifold roles of potassium in mediating drought tolerance in plants and its underlying mechanisms. Plant Sci. 2025, 351, 112337. [Google Scholar] [CrossRef]
- Shah, I.H.; Jinhui, W.; Li, X.; Hameed, M.K.; Manzoor, M.A.; Li, P.; Zhang, Y.; Niu, Q.; Chang, L. Exploring the role of nitrogen and potassium in photosynthesis implications for sugar: Accumulation and translocation in horticultural crops. Sci. Hortic. 2024, 327, 112832. [Google Scholar] [CrossRef]
- Israel, W.K.; Watson-Lazowski, A.; Chen, Z.-H.; Ghannoum, O. High intrinsic water use efficiency is underpinned by high stomatal aperture and guard cell potassium flux in C3 and C4 grasses grown at glacial CO2 and low light. J. Exp. Bot. 2022, 73, 1546–1565. [Google Scholar] [CrossRef] [PubMed]
- Azab, E.S.; Alshallash, K.S.; Alqahtani, M.M.; Safhi, F.A.; Alshamrani, S.M.; Ali, M.A.M.; El-Mageed, T.A.A.; El-Taher, A.M. Physiological, anatomical, and agronomic responses of Cucurbita pepo to exogenously sprayed potassium silicate at different concentrations under varying water regimes. Agronomy 2022, 12, 2155. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Wang, Z.; Khatab, A.; Sherif, A.; Ahmad, H.; Khan, M.N.; Hassan, H.M.; Elrewainy, I.M.; et al. Antioxidative and metabolic contribution to salinity stress responses in two rapeseed cultivars during the early seedling stage. Antioxidants 2021, 10, 1227. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.-Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Khalid, M.H.B.; Zhou, X.-B.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Krämer, K.; Brock, J.; Heyer, A.G. Interaction of nitrate assimilation and photorespiration at elevated CO2. Front. Plant Sci. 2022, 13, 897924. [Google Scholar] [CrossRef]
- Asad, M.A.U.; Guan, X.; Zhou, L.; Qian, Z.; Yan, Z.; Cheng, F. Involvement of plant signaling network and cell metabolic homeostasis in nitrogen deficiency-induced early leaf senescence. Plant Sci. 2023, 336, 111855. [Google Scholar] [CrossRef]
- Kuai, J.; Sun, Y.; Zuo, Q.; Huang, H.; Liao, Q.; Wu, C.; Lu, J.; Wu, J.; Zhou, G. The yield of mechanically harvested rapeseed (Brassica napus L.) can be increased by optimum plant density and row spacing. Sci. Rep. 2015, 5, 18835. [Google Scholar] [CrossRef]
- Cai, Q.; Ji, C.; Yan, Z.; Jiang, X.; Fang, J. Anatomical responses of leaf and stem of Arabidopsis thaliana to nitrogen and phosphorus addition. J. Plant Res. 2017, 130, 1035–1045. [Google Scholar] [CrossRef]
- Kemper, R.; Döring, T.F.; Legner, N.; Meinen, C.; Athmann, M. Oil radish, winter rye and crimson clover: Root and shoot performance in cover crop mixtures. Plant Soil 2023. [Google Scholar] [CrossRef]
- Esnarriaga, D.N.; Mariotti, M.; Cardelli, R.; Arduini, I. The importance of root interactions in field bean/triticale intercrops. Plants 2020, 9, 1474. [Google Scholar] [CrossRef] [PubMed]
- Pélissier, P.-M.; Motte, H.; Beeckman, T. Lateral root formation and nutrients: Nitrogen in the spotlight. Plant Physiol. 2021, 187, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Bull, D.J.; Smethurst, J.A.; Meijer, G.J.; Sinclair, I.; Pierron, F.; Roose, T.; Powrie, W.; Bengough, A.G. Modelling of stress transfer in root-reinforced soils informed by four-dimensional X-ray computed tomography and digital volume correlation data. Proc. R. Soc. A Math. Phys. Eng. Sci. 2022, 478, 20210210. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, C.; Yu, Y.; Shen, J.; van der Werf, W.; Zhang, F. Intercropping legumes and cereals increases phosphorus use efficiency; a meta-analysis. Plant Soil 2021, 460, 89–104. [Google Scholar] [CrossRef]
- Bargaz, A.; Noyce, G.L.; Fulthorpe, R.; Carlsson, G.; Furze, J.R.; Jensen, E.S.; Dhiba, D.; Isaac, M.E. Species interactions enhance root allocation, microbial diversity and P acquisition in intercropped wheat and soybean under P deficiency. Appl. Soil Ecol. 2017, 120, 179–188. [Google Scholar] [CrossRef]
- Borden, K.A.; Thomas, S.C.; Isaac, M.E. Variation in fine root traits reveals nutrient-specific acquisition strategies in agroforestry systems. Plant Soil 2020, 453, 139–151. [Google Scholar] [CrossRef]
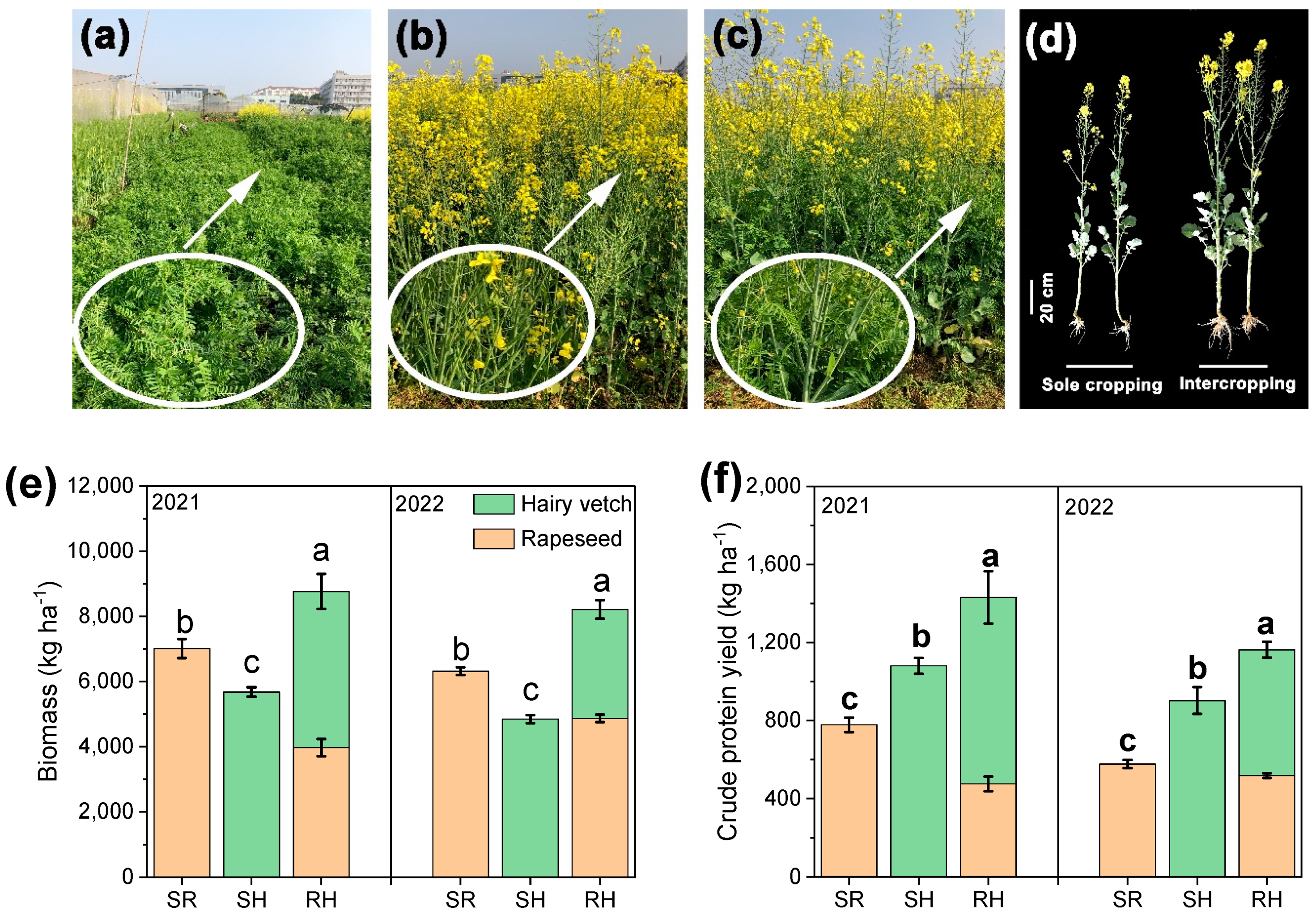

| Year | Stem Position | Treat-ments | CA (μm2) | CT (μm) | VN | VL (μm) | VC (μm) | VA (μm2) |
|---|---|---|---|---|---|---|---|---|
| 2021 | Bottom | SR | 6.99 b | 0.55 b | 34.1 b | 0.82 b | 1.98 b | 0.18 b |
| IR | 11.35 a | 0.75 a | 41.3 a | 0.96 a | 2.35 a | 0.30 a | ||
| Middle | SR | 2.25 b | 0.29 b | 18.1 b | 0.64 b | 1.82 b | 0.13 b | |
| IR | 4.08 a | 0.38 a | 25.7 a | 0.78 a | 1.96 a | 0.18 a | ||
| 2022 | Bottom | SR | 7.45 b | 0.52 b | 34.1 b | 0.81 b | 2.03 b | 0.18 b |
| IR | 11.58 a | 0.73 a | 39.2 a | 0.97 a | 2.41 a | 0.29 a | ||
| Middle | SR | 2.17 b | 0.31 b | 18.3 b | 0.65 b | 1.91 b | 0.13 b | |
| IR | 3.91 a | 0.37 a | 24.3 a | 0.74 a | 2.02 a | 0.17 a |
| Year | Crop | Treatments | E (mol m⁻2 s⁻1) | A (µmol m⁻2 s⁻1) | Ci (µmol mol⁻1) | gs (mol m⁻2 s⁻1) |
|---|---|---|---|---|---|---|
| 2021 | Rapeseed | SR | 0.0043 b | 26.1 b | 252.0 a | 0.311 b |
| IR | 0.0053 a | 29.9 a | 261.8 a | 0.440 a | ||
| Hairy vetch | SH | 0.0023 b | 12.1 b | 234.2 a | 0.130 b | |
| IH | 0.0027 a | 19.1 a | 217.6 a | 0.188 a | ||
| 2022 | Rapeseed | SR | 0.0042 b | 25.1 b | 235.7 b | 0.298 b |
| IR | 0.0050 a | 29.1 a | 260.3 a | 0.428 a | ||
| Hairy vetch | SH | 0.0021 b | 11.6 b | 228.2 a | 0.128 b | |
| IH | 0.0026 a | 18.6 a | 211.5 a | 0.181 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Wang, Z.; Gao, P.; Tan, X.; Wang, X.; Kuai, J.; Wang, J.; Xu, Z.; Wang, B.; Zhou, G.; et al. Rapeseed Supports Hairy Vetch in Intercropping, Enhancing Root and Stem Morphology, Nitrogen Metabolism, Photosynthesis, and Forage Yield. Agronomy 2025, 15, 220. https://doi.org/10.3390/agronomy15010220
Ji J, Wang Z, Gao P, Tan X, Wang X, Kuai J, Wang J, Xu Z, Wang B, Zhou G, et al. Rapeseed Supports Hairy Vetch in Intercropping, Enhancing Root and Stem Morphology, Nitrogen Metabolism, Photosynthesis, and Forage Yield. Agronomy. 2025; 15(1):220. https://doi.org/10.3390/agronomy15010220
Chicago/Turabian StyleJi, Jianli, Zongkai Wang, Pan Gao, Xiaoqiang Tan, Xianling Wang, Jie Kuai, Jing Wang, Zhenghua Xu, Bo Wang, Guangsheng Zhou, and et al. 2025. "Rapeseed Supports Hairy Vetch in Intercropping, Enhancing Root and Stem Morphology, Nitrogen Metabolism, Photosynthesis, and Forage Yield" Agronomy 15, no. 1: 220. https://doi.org/10.3390/agronomy15010220
APA StyleJi, J., Wang, Z., Gao, P., Tan, X., Wang, X., Kuai, J., Wang, J., Xu, Z., Wang, B., Zhou, G., & Zhao, J. (2025). Rapeseed Supports Hairy Vetch in Intercropping, Enhancing Root and Stem Morphology, Nitrogen Metabolism, Photosynthesis, and Forage Yield. Agronomy, 15(1), 220. https://doi.org/10.3390/agronomy15010220








