Oat Nutrition, Traits, and Yield as Affected by the Interaction of Nitrogen Rates and Plant Density in Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design
2.3. Sampling and Measurement
2.4. Data Analysis
3. Results
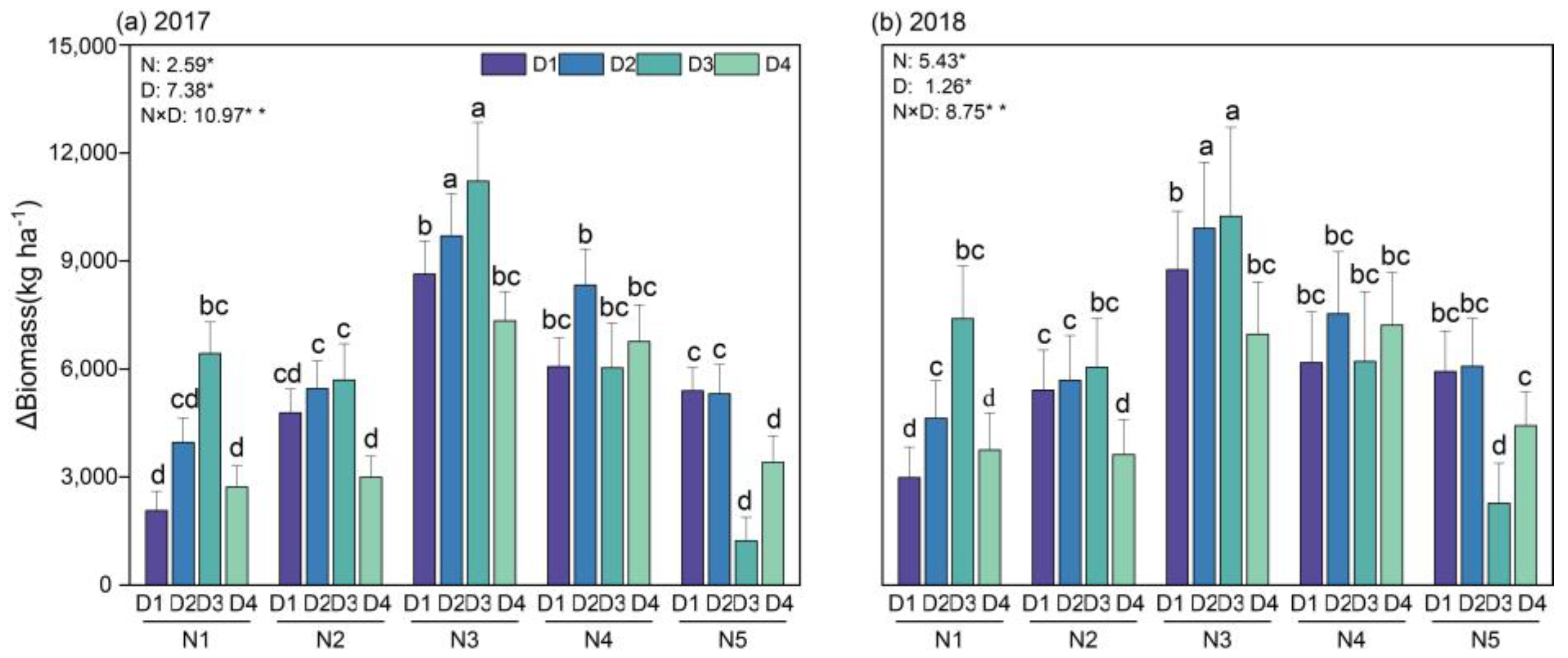
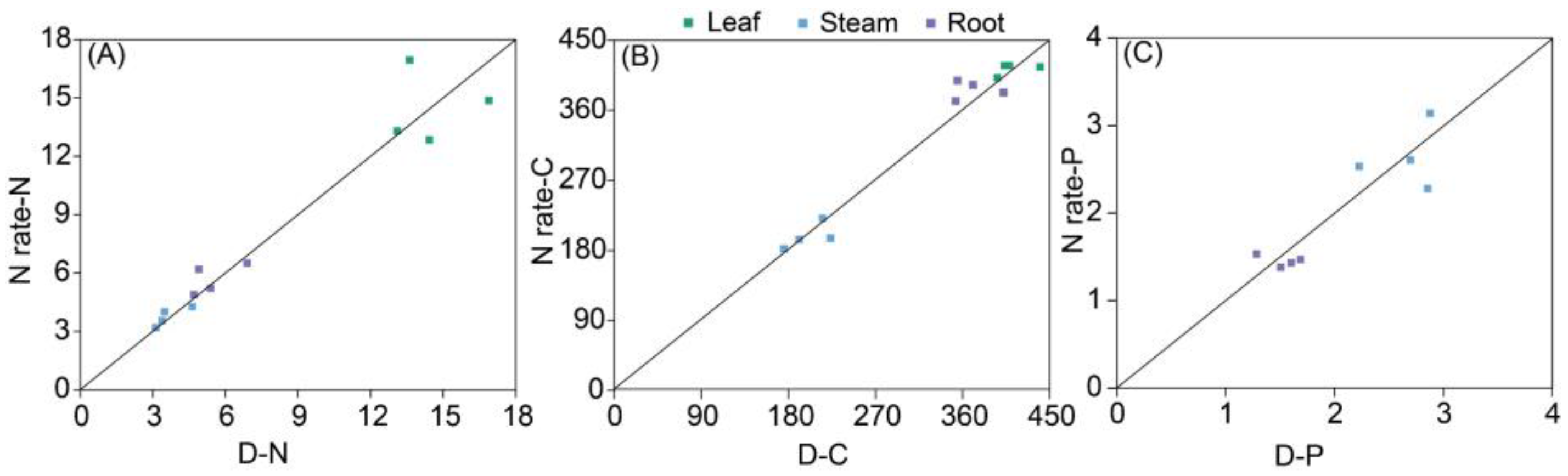
3.1. Biomass Discrepancy
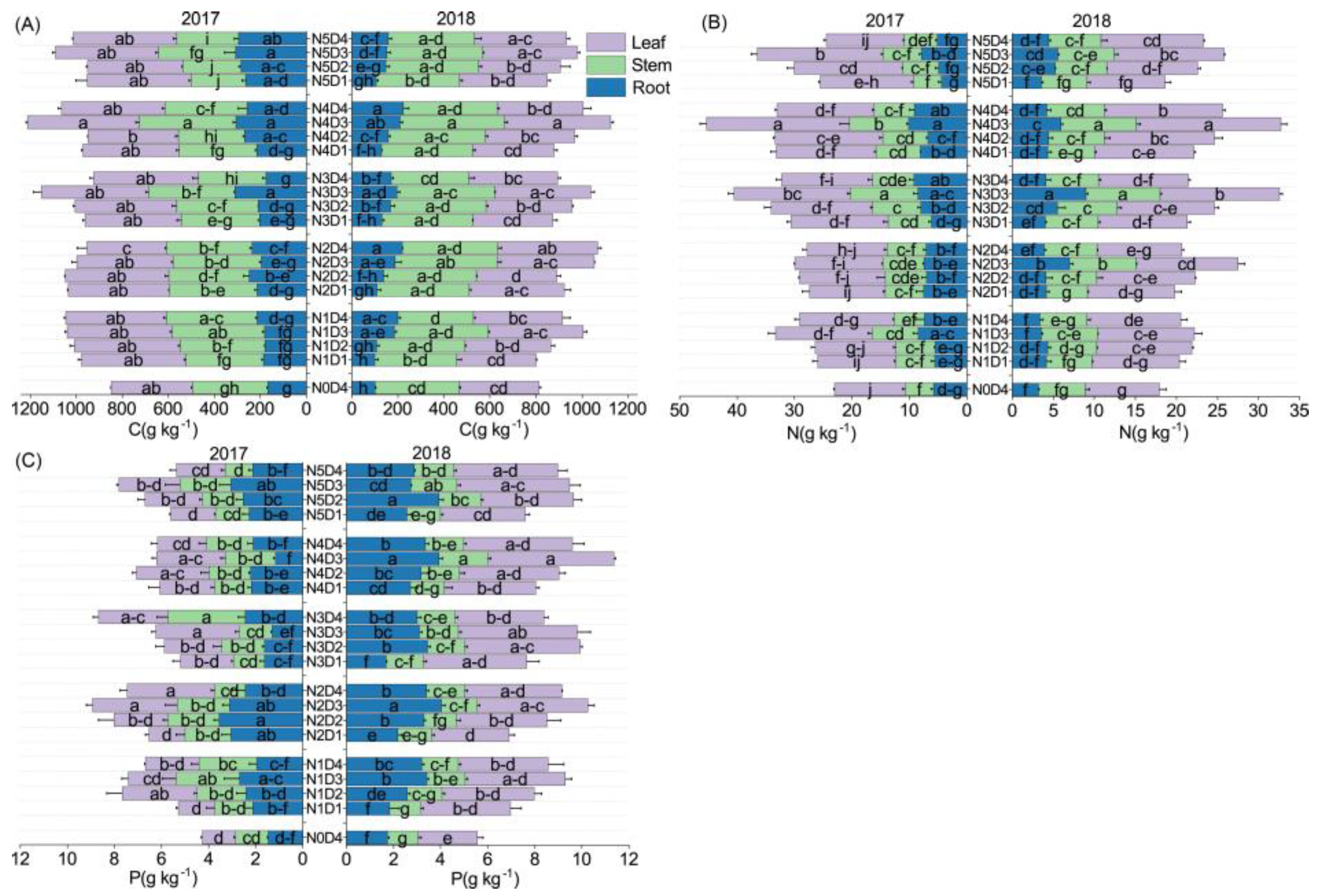
3.2. The C, N, and P Contents in the Different Organs of Oats
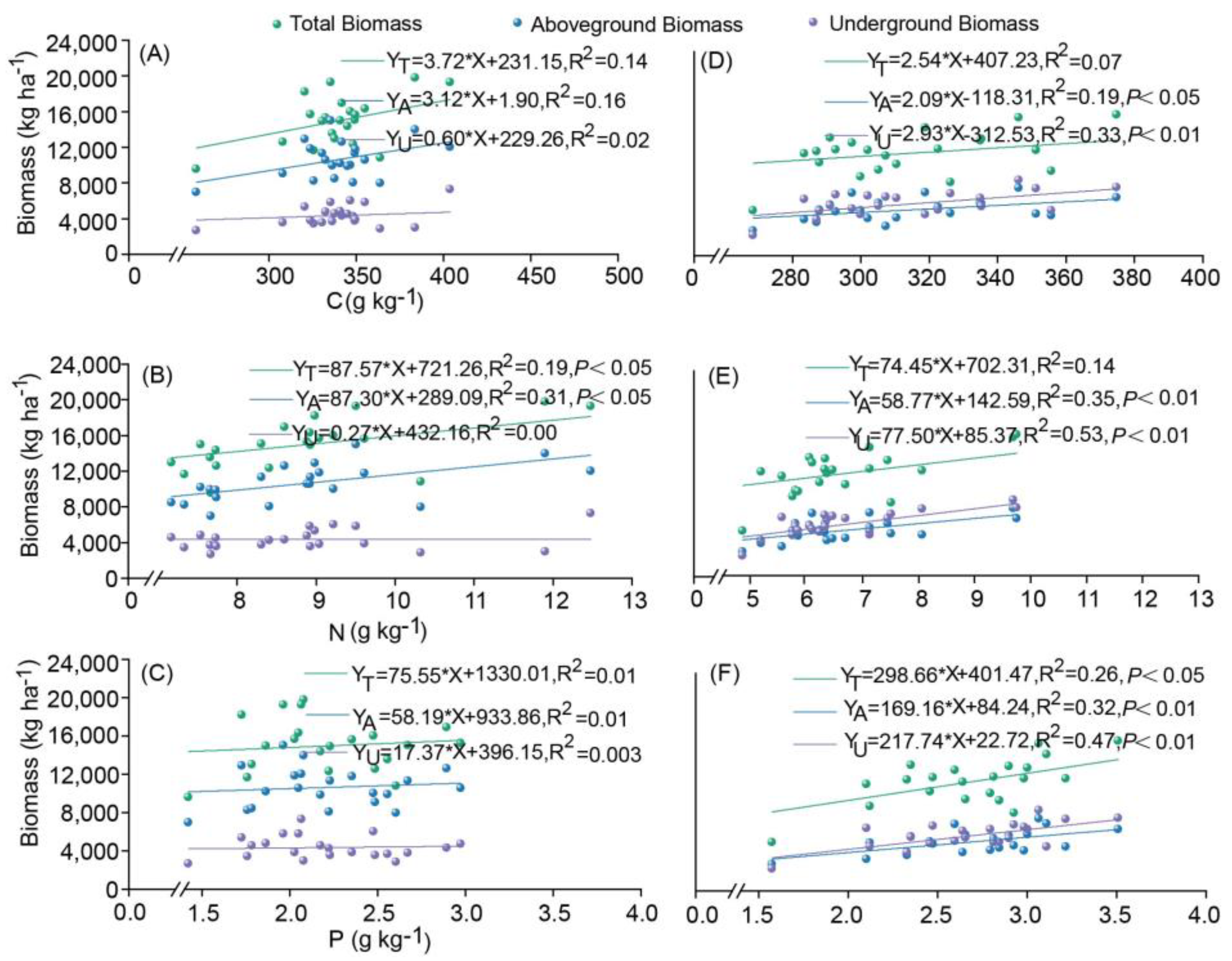
3.3. Relationships Between Plant Biomass and C, N, and P Contents of Plants
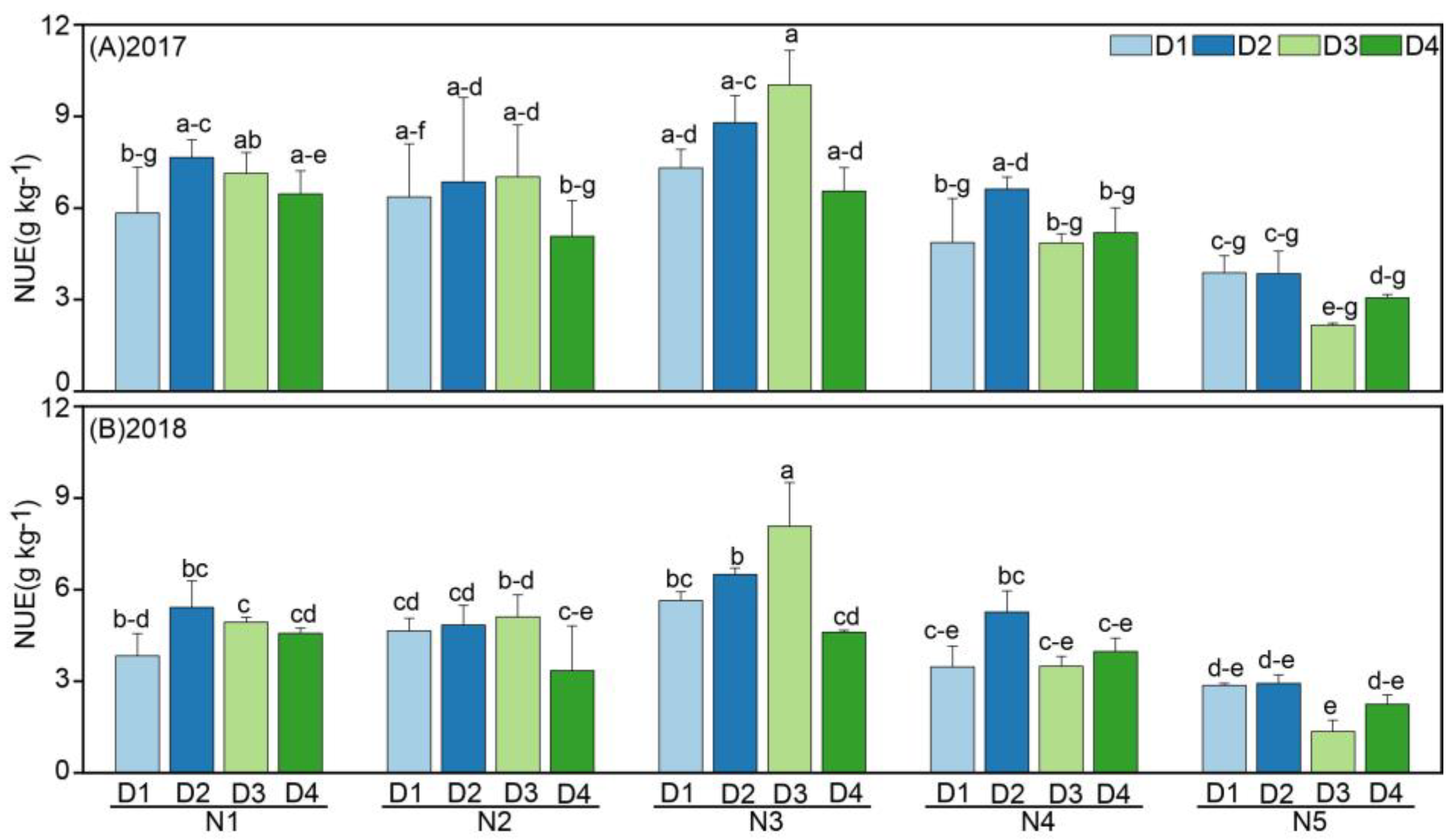
3.4. Effects of Planting Density and N Rate on the NUE of Oats
3.5. Effect of N Application and Planting Density on C, N, and P Content and Stoichiometric Ratio of the 0–30 cm Soil Layer
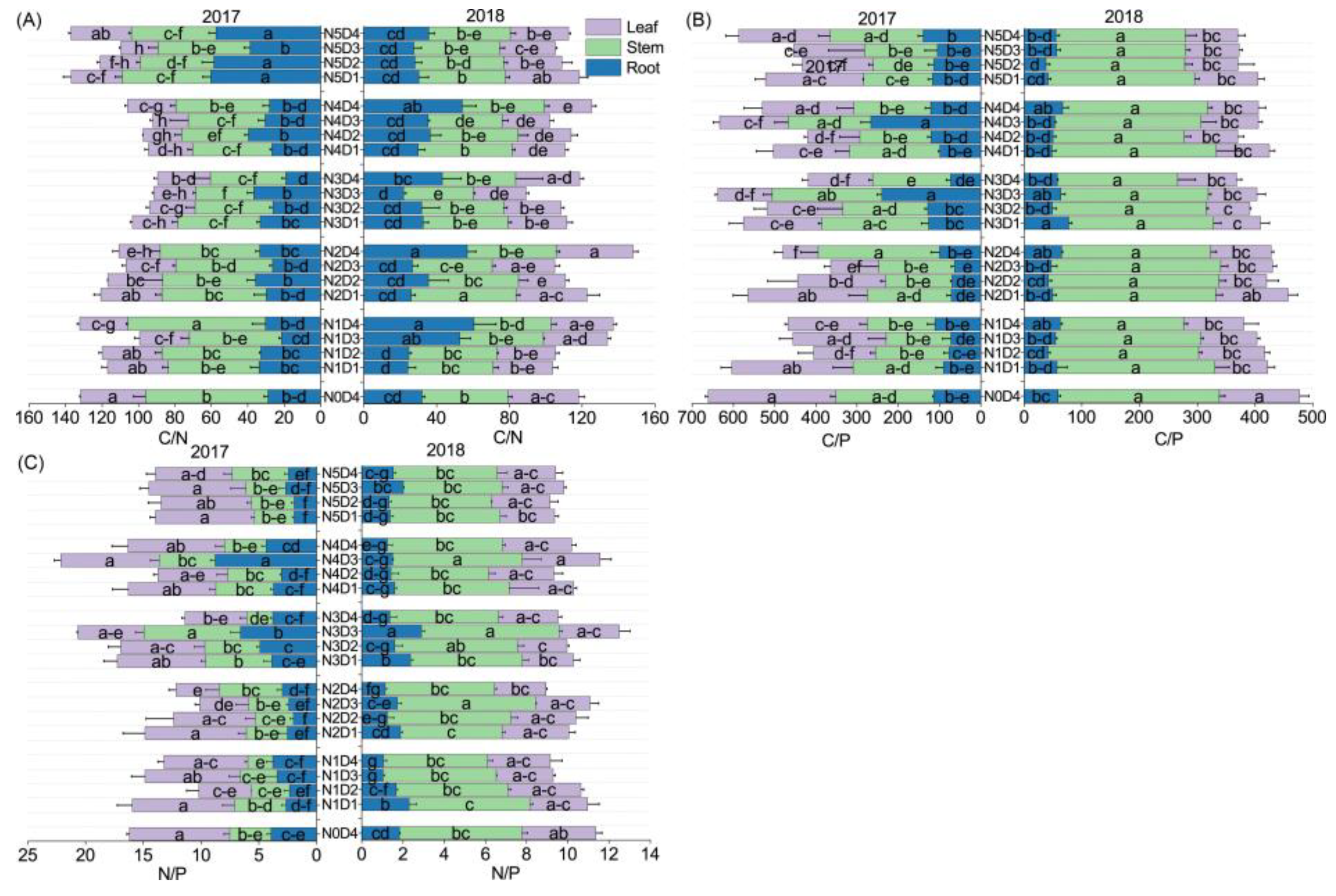
3.6. Regulation of N Rate and Planting Density on Oat Stoichiometry
4. Discussion
4.1. Effect of N Rate and Planting Density on the C:N:P Stoichiometry in Different Plant Organs
4.2. Changes in Soil Nutrient Conditions Affect Plant C:N:P Stoichiometry
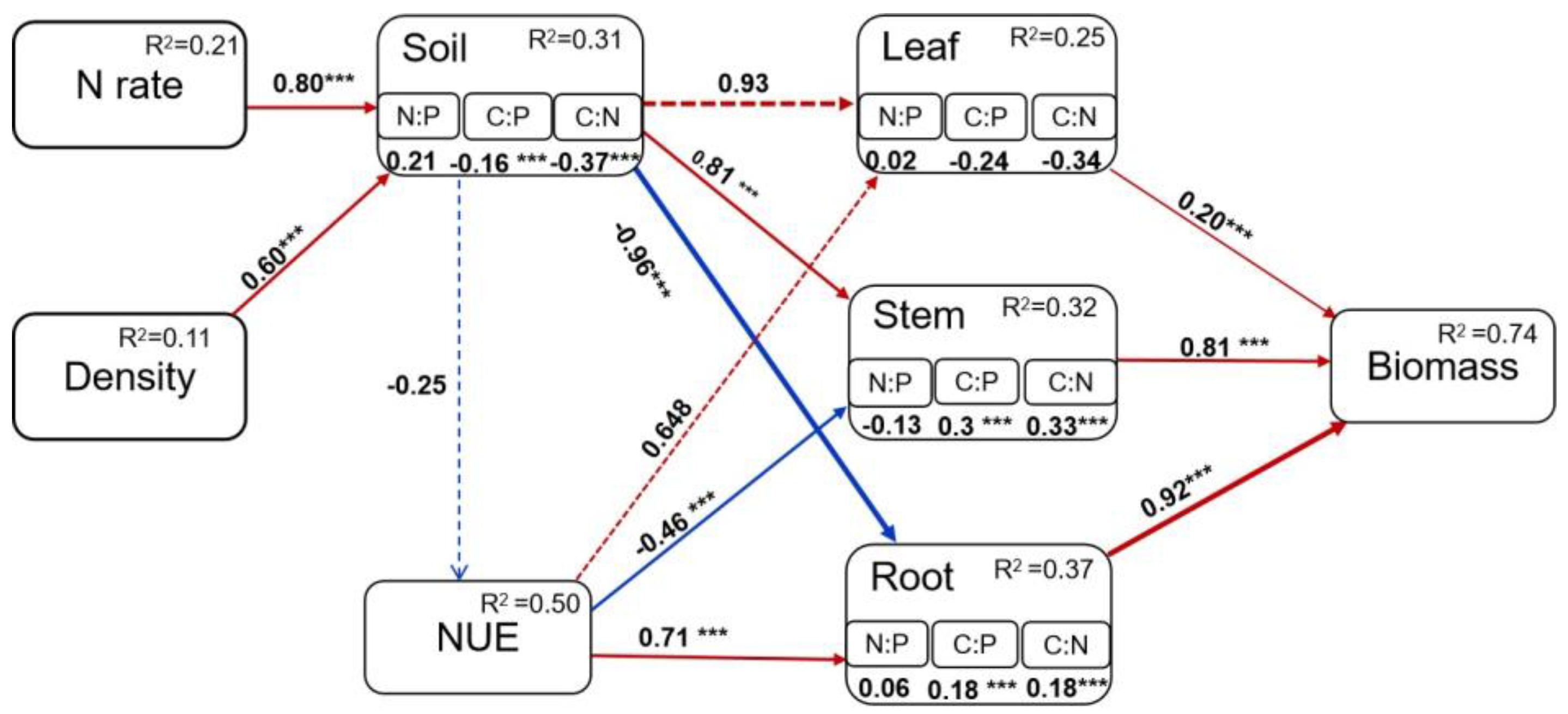
4.3. Regulatory Pathways of Nitrogen Rate and Density on Oat Stoichiometry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, X.; Liu, T.; Hao, L.; Duan, L.; Wang, G.; Singh, V.P.; Zhang, M.; Wu, R.; Ma, K. Carbon dynamics of the plant-soil system during vegetation succession in dune-meadow cascade ecosystems in Horqin Sandy Land, China. Ecol. Indic. 2023, 155, 110916. [Google Scholar] [CrossRef]
- Sui, Y.; Yuan, H. Agroecological and economic approach of prevention and curing of sandstorms in China. Ecol. Econ. 2007, 61, 129–133. [Google Scholar] [CrossRef]
- Kairis, O.; Karavitis, C.; Kounalaki, A.; Salvati, L.; Kosmas, C. The effect of land management practices on soil erosion and land desertification in an olive grove. Soil Use Manag. 2013, 29, 597–606. [Google Scholar] [CrossRef]
- Verón, S.R.; Paruelo, J.M.; Oesterheld, M. Grazing-induced losses of biodiversity affect the transpiration of an arid ecosystem. Oecologia 2011, 165, 501–510. [Google Scholar] [CrossRef]
- Hayatu, N.G.; Liu, Y.-R.; Han, T.-F.; Daba, N.A.; Zhang, L.; Shen, Z.; Li, J.-W.; Muazu, H.; Lamlom, S.F.; Zhang, H.-M. Carbon sequestration rate, nitrogen use efficiency and rice yield responses to long-term substitution of chemical fertilizer by organic manure in a rice–rice cropping system. J. Integr. Agric. 2023, 22, 2848–2864. [Google Scholar] [CrossRef]
- Li, Y.; Tian, L.; Zhou, H.; Wang, H.; He, X.; Jin, Y.; Zhang, H. Comparison of water use efficiency of sand-binding species along revegetation chronosequence in an alpine desert. Ecol. Indic. 2023, 153, 110475. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Wang, X.-P.; Ma, X.-Z.; Zhang, Y.-F.; Hu, R. The stable isotopic composition variation characteristics of desert plants and water sources in an artificial revegetation ecosystem in Northwest China. CATENA 2020, 189, 104499. [Google Scholar] [CrossRef]
- Tamburini, G.; Aguilera, G.; Öckinger, E. Grasslands enhance ecosystem service multifunctionality above and below-ground in agricultural landscapes. J. Appl. Ecol. 2022, 59, 3061–3071. [Google Scholar] [CrossRef]
- Ibrahimi, K.; Alghamdi, A.G. Available water capacity of sandy soils as affected by biochar application: A meta-analysis. CATENA 2022, 214, 106281. [Google Scholar] [CrossRef]
- Hayatu, N.G.; Noma, S.; Nabayi, A.; Abdelsattar, M.; Haruna, F.; Amadou, A.; Sani, I.; Sharu, M.B.; Anka, A.B.; Abubakar, S.D. Characterization And Classification Of Soils On Grazing Lands In Kwallatawa Village, Sokoto State, Nigeria. SVU-Int. J. Agric. Sci. 2020, 2, 326–339. [Google Scholar] [CrossRef]
- Dal Molin, S.J.; Ernani, P.R.; Gerber, J.M. Soil acidification and nitrogen release following application of nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2020, 51, 2551–2558. [Google Scholar] [CrossRef]
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant nutrient stress adaptation: A prospect for fertilizer limited agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- Da Silva, J.M.; Fontes, P.C.R.; Milagres, C.d.C.; de Abreu, J.A.A. Yield and nitrogen use efficiency of bell pepper grown in SLAB fertigated with different nitrogen rates. J. Plant Nutr. 2020, 43, 2833–2843. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Li, W.; Xue, X.; Yang, L.; Xu, J.; Yang, B.; Ding, R.; Jia, Z.; Zhang, X.; et al. Reducing fertilization with high planting density increases maize yield stability and nitrogen use efficiency in semi-arid areas. Eur. J. Agron. 2024, 159, 127223. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, Z.; Zhao, X.; Guo, H.; Yan, L.; Zhou, M.; Zhang, L.; Ye, Y.; Liu, W.; Xu, Y.; et al. Comparison of Carbon Footprint Differences in Nitrogen Reduction and Density Increase in Double Cropping Rice under Two Evaluation Methods. Agronomy 2024, 14, 803. [Google Scholar] [CrossRef]
- Luo, Z.; Song, H.; Huang, M.; Zhang, Z.; Peng, Z.; Zi, T.; Tian, C.; Eissa, M.A. Nitrogen-Reduction in Intensive Cultivation Improved Nitrogen Fertilizer Utilization Efficiency and Soil Nitrogen Mineralization of Double-Cropped Rice. Agronomy 2022, 12, 1103. [Google Scholar] [CrossRef]
- Evers, B.J.; Blanco-Canqui, H.; Staggenborg, S.A.; Tatarko, J. Dedicated Bioenergy Crop Impacts on Soil Wind Erodibility and Organic Carbon in Kansas. Agron. J. 2013, 105, 1271–1276. [Google Scholar] [CrossRef]
- Yan, Z.B.; Tian, D.; Huang, H.Y.; Sun, Y.F.; Hou, X.H.; Han, W.X.; Guo, Y.L.; Fang, J.Y. Interactive effects of plant density and nitrogen availability on the biomass production and leaf stoichiometry of Arabidopsis thaliana. J. Plant Ecol. 2023, 16, 101. [Google Scholar] [CrossRef]
- Musa, A.; Zhang, Y.; Cao, J.; Wang, Y.; Liu, Y. Relationship between root distribution characteristics of Mongolian pine and the soil water content and groundwater table in Horqin Sandy Land, China. Trees 2019, 33, 1203–1211. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Wang, G.; Liu, Z.; Sun, W.; Zhang, Y.; Zhang, X. Different Responses in Root Water Uptake of Summer Maize to Planting Density and Nitrogen Fertilization. Front. Plant Sci. 2022, 13, 918043. [Google Scholar] [CrossRef]
- Hu, B.; Bourennane, H.; Arrouays, D.; Denoroy, P.; Lemercier, B.; Saby, N.P.A. Developing pedotransfer functions to harmonize extractable soil phosphorus content measured with different methods: A case study across the mainland of France. Geoderma 2021, 381, 114645. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Xu, L.; Zhu, J.; Dai, G.; He, N. C:N:P stoichiometry in terrestrial ecosystems in China. Sci. Total Environ. 2021, 795, 148849. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A. Marschner’s Mineral Nutrition of Higher Plants. 3rd edition. Edited by P. Marschner. Amsterdam, Netherlands: Elsevier/Academic Press (2011), pp. 684, US$124.95. ISBN 978-0-12-384905-2. Exp. Agric. 2012, 48, 305. [Google Scholar] [CrossRef]
- Verzeaux, J.; Alahmad, A.; Habbib, H.; Nivelle, E.; Roger, D.; Lacoux, J.; Decocq, G.; Hirel, B.; Catterou, M.; Spicher, F.; et al. Cover crops prevent the deleterious effect of nitrogen fertilisation on bacterial diversity by maintaining the carbon content of ploughed soil. Geoderma 2016, 281, 49–57. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, Y.; Guo, L.; Yang, L.; Wang, B.; Wang, X.; Liu, W.; Su, Y.; Wu, J.; Liu, L. Foliar nutrient resorption stoichiometry and microbial phosphatase catalytic efficiency together alleviate the relative phosphorus limitation in forest ecosystems. New Phytol. 2023, 238, 1033–1044. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Zhang, L.; Song, Z.; Zhou, S. The allometry of plant height explains species loss under nitrogen addition. Ecol. Lett. 2021, 24, 553–562. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Optimization of Growing Medium and Preservation Methods for Plant Beneficial Bacteria, and Formulating a Microbial Biopreparation for Raspberry Naturalization. Agronomy 2021, 11, 2521. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plant Properties; Instytut Ochrony Srodowiska: Warszawa, Poland, 1991. [Google Scholar]
- Ma, Z.; Zeng, Y.; Wu, J.; Zhou, Q.; Hou, F. Plant litter influences the temporal stability of plant community biomass in an alpine meadow by altering the stability and asynchrony of plant functional groups. Funct. Ecol. 2022, 36, 148–158. [Google Scholar] [CrossRef]
- Shipley, B. The AIC model selection method applied to path analytic models compared using a d-separation test. Ecology 2013, 94, 560–564. [Google Scholar] [CrossRef]
- Sardans, J.; Alonso, R.; Janssens, I.A.; Carnicer, J.; Vereseglou, S.; Rillig, M.C.; Fernández-Martínez, M.; Sanders, T.G.M.; Peñuelas, J. Foliar and soil concentrations and stoichiometry of nitrogen and phosphorous across European Pinus sylvestris forests: Relationships with climate, N deposition and tree growth. Funct. Ecol. 2016, 30, 676–689. [Google Scholar] [CrossRef]
- Sattelmacher, B.; Klotz, F.; Marschner, H. Influence of the nitrogen level on root growth and morphology of two potato varieties differing in nitrogen acquisition. Plant Soil 1990, 123, 131–137. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Lyu, M.; Xiong, D.; Wang, J.; Chen, Y.; Li, Y.; Wang, M.; Yang, Y. Short-term effects of soil warming and nitrogen addition on the N:P stoichiometry of Cunninghamia lanceolata in subtropical regions. Plant Soil 2017, 411, 395–407. [Google Scholar] [CrossRef]
- Hedwall, P.-O.; Bergh, J.; Brunet, J. Phosphorus and nitrogen co-limitation of forest ground vegetation under elevated anthropogenic nitrogen deposition. Oecologia 2017, 185, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Ciais, P.; Gasser, T.; Smith, P.; Herrero, M.; Havlík, P.; Obersteiner, M.; Guenet, B.; Goll, D.S.; Li, W.; et al. Climate warming from managed grasslands cancels the cooling effect of carbon sinks in sparsely grazed and natural grasslands. Nat. Commun. 2021, 12, 118. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Shangguan, Z.-P. Interaction of soil water storage and stoichiometrical characteristics in the long-term natural vegetation restoration on the Loess Plateau. Ecol. Eng. 2018, 116, 7–13. [Google Scholar] [CrossRef]
- Liu, X.; Lamb, E.G.; Zhang, S. Nitrogen addition impacts on soil microbial stoichiometry are driven by changes in plant resource stoichiometry not by the composition of main microbial groups in an alpine meadow. Biol. Fertil. Soils 2020, 56, 261–271. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob. Change Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Bin, Y.; Zhu, X.; Ma, S.; Xie, E.; Zeng, H.; Li, C.; Wu, J. An increase in intercropped species richness improves plant water use but weakens the nutrient status of both intercropped plants and soil in rubber–tea agroforestry systems. Agric. Water Manag. 2023, 284, 108353. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Env. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Shafiq, F.; Anwar, S.; Khan, S. Editorial: Advances in plant-soil nitrogen management strategies. Front. Plant Sci. 2024, 15, 1380284. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Gutiérrez Castorena, E.V.; Gutiérrez-Castorena, M.d.C.; González Vargas, T.; Cajuste Bontemps, L.; Delgadillo Martínez, J.; Suástegui Méndez, E.; Ortiz Solorio, C.A. Micromapping of microbial hotspots and biofilms from different crops using digital image mosaics of soil thin sections. Geoderma 2016, 279, 11–21. [Google Scholar] [CrossRef]
- Adhikary, P.P.; Mohanty, S.; Rautaray, S.K.; Manikandan, N.; Mishra, A. Alternate wetting and drying water management can reduce phosphorus availability under lowland rice cultivation irrespective of nitrogen level. Environ. Monit. Assess. 2023, 195, 1420. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.-R.; An, S.-S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. CATENA 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Boudjabi, S.; Chenchouni, H. Soil fertility indicators and soil stoichiometry in semi-arid steppe rangelands. CATENA 2022, 210, 105910. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, H.; Zhao, X.; Drake, S. Facilitative effects of shrubs in shifting sand on soil macro-faunal community in Horqin Sand Land of Inner Mongolia, Northern China. Eur. J. Soil Biol. 2011, 47, 316–321. [Google Scholar] [CrossRef]
- Nendel, C.; Melzer, D.; Thorburn, P.J. The nitrogen nutrition potential of arable soils. Sci. Rep. 2019, 9, 5851. [Google Scholar] [CrossRef]



| Nitrogen | Density | C | N | P | C/N | C/P | N/P |
|---|---|---|---|---|---|---|---|
| N0 | D2 | 5.33 ± 0.49 b–d | 0.25 ± 0.02 b | 0.18 ± 0.01 c | 21.55 ± 2.06 ab | 28.99 ± 2.06 a–c | 1.36 ± 0.19 a |
| N1 | D1 | 5.74 ± 0.32 a–d | 0.32 ± 0.01 ab | 0.21 ± 0.01 b | 15.95 ± 0.49 ab | 27.31 ± 1.33 a–c | 0.9 ± 0.13 a |
| D2 | 4.68 ± 0.89 cd | 0.3 ± 0.03 ab | 0.23 ± 0.03 ab | 16.25 ± 4.46 ab | 20.03 ± 2.51 d | 1.3 ± 0.27 a | |
| D3 | 5.49 ± 0.44 a–d | 0.29 ± 0.03 b | 0.23 ± 0.01 ab | 17.51 ± 2.57 ab | 24.13 ± 2.34 b–d | 1.4 ± 0.16 a | |
| D4 | 5.89 ± 0.08 a–c | 0.27 ± 0.05 b | 0.19 ± 0.03 bc | 23.17 ± 5.13 a | 32.57 ± 5.23 a | 1.50 ± 0.44 a | |
| N2 | D1 | 5.42 ± 0.44 a–d | 0.32 ± 0.03 ab | 0.2 ± 0.02 bc | 16.77 ± 0.12 ab | 26.85 ± 3.05 a–c | 1.6 ± 0.19 a |
| D2 | 5.72 ± 0.82 a–d | 0.35 ± 0.02 a | 0.28 ± 0.03 a | 16.49 ± 2.68 ab | 20.62 ± 1.88 d | 1.27 ± 0.13 a | |
| D3 | 6.74 ± 0.79 ab | 0.35 ± 0.05 a | 0.28 ± 0.04 a | 19.67 ± 5.12 ab | 24.45 ± 4.39 b–d | 1.31 ± 0.36 a | |
| D4 | 5.85 ± 0.55 a–c | 0.29 ± 0.01 b | 0.17 ± 0.01 c | 20.48 ± 2.12 ab | 34.36 ± 5.72 a | 1.67 ± 0.13 a | |
| N3 | D1 | 4.89 ± 0.3 cd | 0.3 ± 0.02 ab | 0.19 ± 0.04 bc | 16.54 ± 2.04 ab | 26.85 ± 4.61 a–c | 1.65 ± 0.34 a |
| D2 | 5.51 ± 0.04 a–d | 0.32 ± 0.03 ab | 0.25 ± 0.03 ab | 21.78 ± 1.78 ab | 22.04 ± 1.46 cd | 1.36 ± 0.21 a | |
| D3 | 7.02 ± 0.26 a | 0.22 ± 0.02 b | 0.24 ± 0.02 ab | 19.56 ± 0.74 ab | 29.36 ± 3.05 a–c | 1.50 ± 0.11 a | |
| D4 | 4.02 ± 0.26 d | 0.34 ± 0.04 ab | 0.2 ± 0.03 bc | 12.01 ± 0.9 b | 21.27 ± 4.37 cd | 1.81 ± 0.48 a | |
| N4 | D1 | 4.89 ± 0.42 cd | 0.31 ± 0.03 ab | 0.18 ± 0.01 c | 15.88 ± 2.11 ab | 26.54 ± 2.49 a–c | 1.68 ± 0.07 a |
| D2 | 4.96 ± 0.38 cd | 0.36 ± 0.03 a | 0.18 ± 0.02 c | 17.59 ± 3.07 ab | 28.24 ± 4.1 a–c | 1.63 ± 0.28 a | |
| D3 | 5.17 ± 0.32 b–d | 0.34 ± 0.05 ab | 0.19 ± 0 bc | 15.57 ± 1.43 ab | 27.34 ± 1.92 a–c | 1.78 ± 0.25 a | |
| D4 | 4.73 ± 0.63 cd | 0.33 ± 0.05 ab | 0.2 ± 0.01 bc | 14.24 ± 0.97 ab | 23.83 ± 2.98 b–d | 1.68 ± 0.22 a | |
| N5 | D1 | 5.21 ± 0.12 b–d | 0.29 ± 0.02 b | 0.19 ± 0.04 bc | 17.78 ± 0.86 ab | 28.64 ± 6.7 a–c | 1.6 ± 0.31 a |
| D2 | 4.52 ± 0.34 cd | 0.26 ± 0.05 b | 0.21 ± 0.02 b | 17.38 ± 2.11 ab | 21.47 ± 2.04 cd | 1.24 ± 0.09 a | |
| D3 | 4.42 ± 0.12 cd | 0.27 ± 0.01 b | 0.23 ± 0.01 ab | 16.16 ± 0.73 ab | 19.59 ± 0.49 d | 1.22 ± 0.09 a | |
| D4 | 5.04 ± 0.95 cd | 0.32 ± 0.02 ab | 0.2 ± 0.03 bc | 15.92 ± 3.96 ab | 24.43 ± 1.55 b–d | 1.6 ± 0.28 a | |
| F value | N | 2.1 | 1.6 | 2.2 | 0.6 | 0.3 | 2.2 |
| D | 2.2 | 0.3 | 4.8 * | 0.3 | 1.5 | 5.4 * | |
| N×D | 1.2 | 1.9 | 1.8 | 1.8 | 3.4 ** | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Deng, J.; Gao, K.; Zhang, Z. Oat Nutrition, Traits, and Yield as Affected by the Interaction of Nitrogen Rates and Plant Density in Sandy Soil. Agronomy 2025, 15, 150. https://doi.org/10.3390/agronomy15010150
Lin Z, Deng J, Gao K, Zhang Z. Oat Nutrition, Traits, and Yield as Affected by the Interaction of Nitrogen Rates and Plant Density in Sandy Soil. Agronomy. 2025; 15(1):150. https://doi.org/10.3390/agronomy15010150
Chicago/Turabian StyleLin, Zhiling, Jianqiang Deng, Kai Gao, and Zhixin Zhang. 2025. "Oat Nutrition, Traits, and Yield as Affected by the Interaction of Nitrogen Rates and Plant Density in Sandy Soil" Agronomy 15, no. 1: 150. https://doi.org/10.3390/agronomy15010150
APA StyleLin, Z., Deng, J., Gao, K., & Zhang, Z. (2025). Oat Nutrition, Traits, and Yield as Affected by the Interaction of Nitrogen Rates and Plant Density in Sandy Soil. Agronomy, 15(1), 150. https://doi.org/10.3390/agronomy15010150






