Mechanistic Insights into Phosphorus Efficiency and Shoot P Concentration in Chinese Cabbage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material and Sowing Plan
2.2. Soil Collection and Chinese Cabbage Seed
2.3. Harvest and Measurements
2.4. Calculation and Data Analyses
3. Results
3.1. Phosphorus Supply and Efficiency Impact on Chinese Cabbage Traits
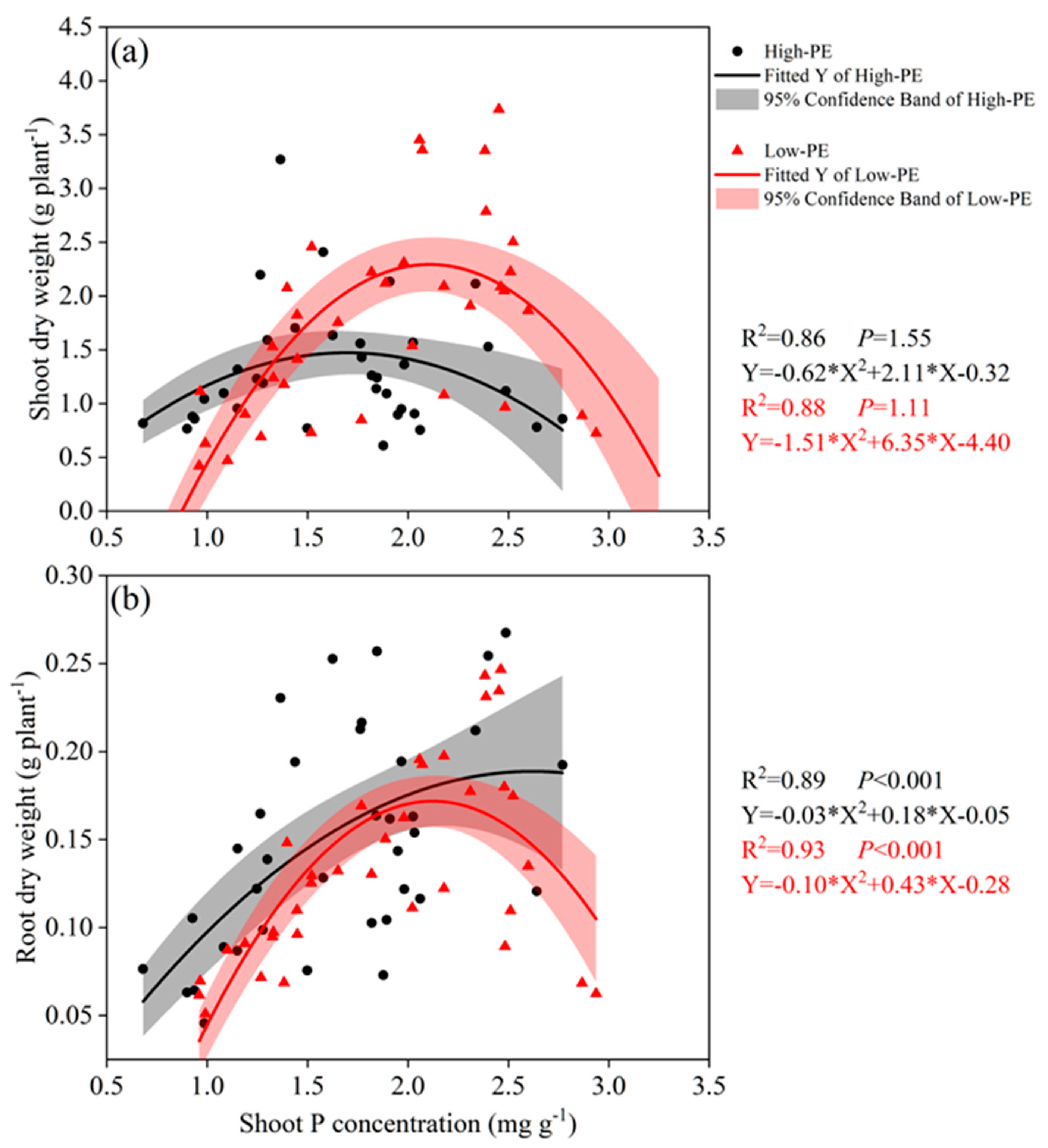
3.2. Shoot/Root Dry Weight, P Concentration and P Content
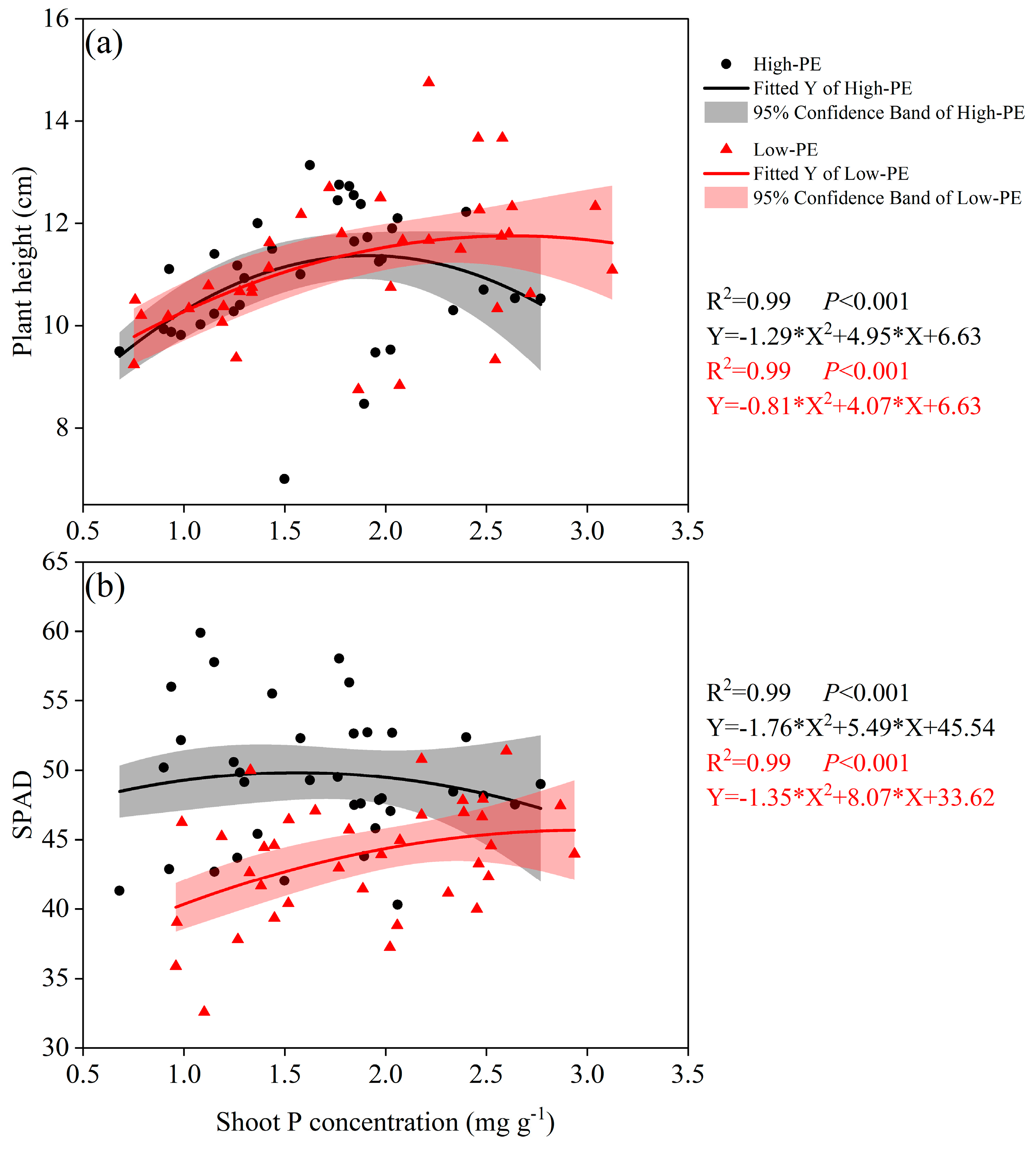
3.3. Plant Height and SPAD
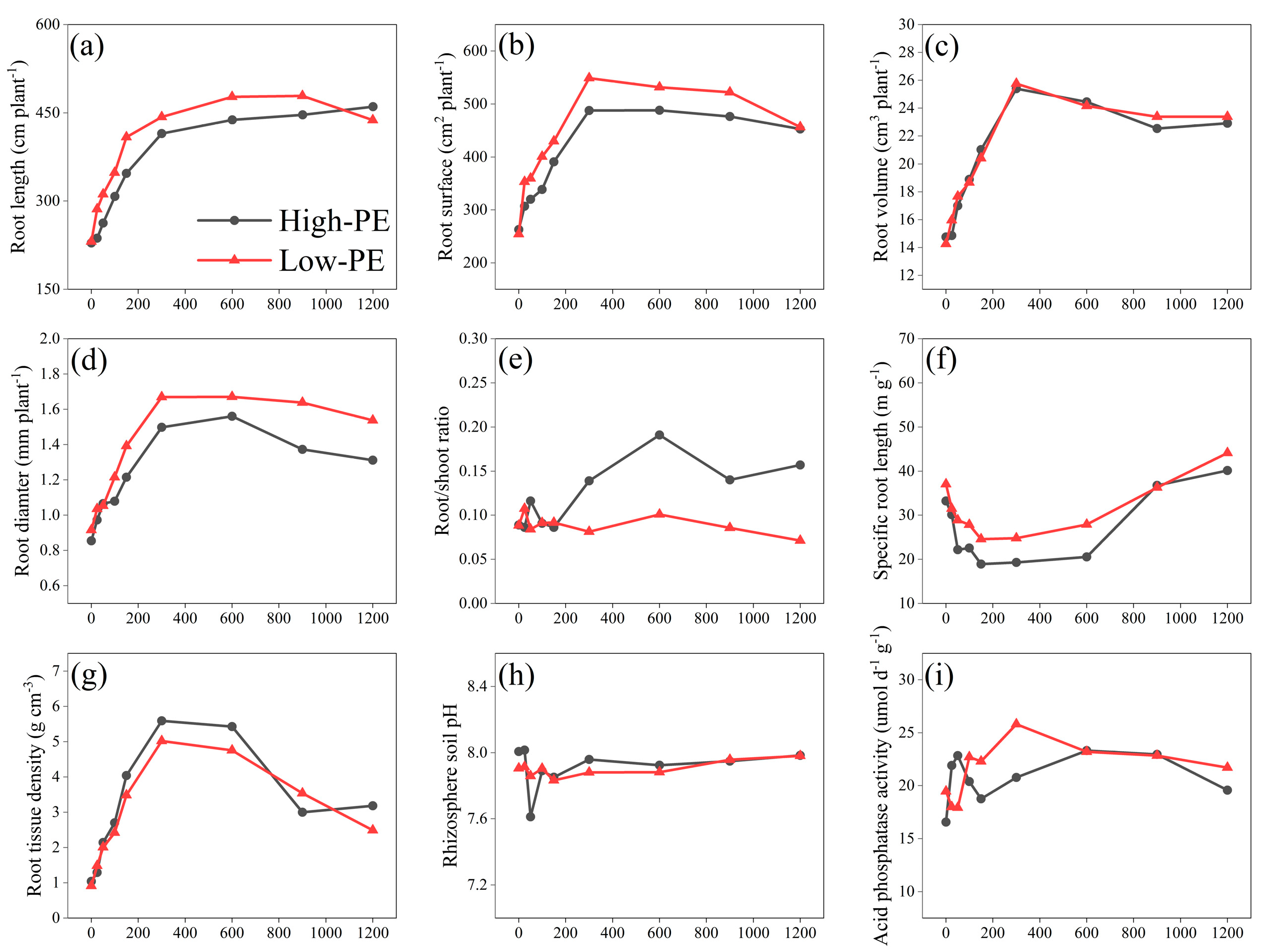
3.4. Root Traits
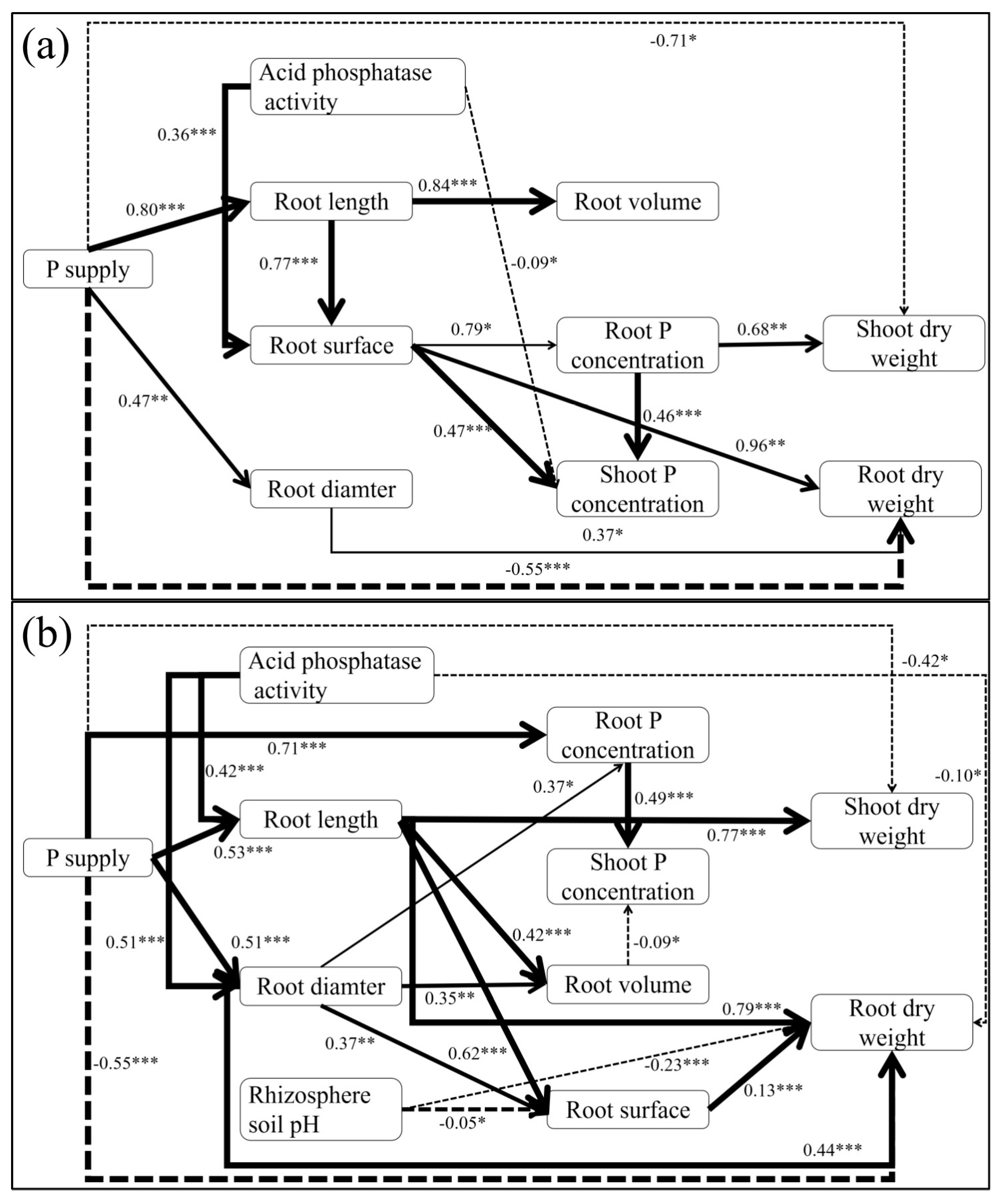
3.5. Path Analysis of Root Traits on Shoot and Root Dry Weight
4. Discussion
4.1. The Relationship Between Chinese Cabbage Traits and P Supply Levels
4.2. The Relationship Between Chinese Cabbage Traits and Shoot P Concentration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.L.; Rengel, Z.; Palta, J.; Siddique, K.H.M. Efficient root systems for enhancing tolerance of crops to water and phosphorus limitation. Indian Soc. Plant Physiol. 2018, 23, 689–696. [Google Scholar] [CrossRef]
- Riskin, S.H.; Porder, S.; NeillC Figueira, A.M.E.S.; Tubbesing, C.; Mahowald, N. The fate of phosphorus fertilizer in Amazon soya bean fields. Philosophical transactions of the Royal Society of London. Ser. B Biol. Sci. 2013, 368, 20120154. [Google Scholar]
- Nierves Mary, C.P.; Salas Felix, M. Assessment of soil phosphorus and phosphorus fixing capacity of three vegetable farms at cabintan, Ormoc City, Leyte. World J. Agric. Res. 2015, 3, 70–73. [Google Scholar]
- Reddy, V.R.P.; Aski, M.S.; Mishra, G.P.; Dikshit, H.K.; Singh, A.; Pandey, R.; Singh, M.P.; Gayacharan, R.V.; Priti, R.N.; Nair, R.M. Genetic variation for root architectural traits in response to phosphorus deficiency in mungbean at the seedling stage. PLoS ONE 2020, 15, 0221008. [Google Scholar] [CrossRef] [PubMed]
- Soumya, P.R.; Sharma, S.; Meena, M.K.; Pandey, R. Response of diverse bread wheat genotypes in terms of root architectural traits at seedling stage in response to low phosphorus stress. Plant Physiol. Rep. 2020, 26, 152–161. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Zhu, L.X.; Li, D.X.; Wang, N.; Sun, H.C.; Zhang, Y.J.; Zhang, K.; Li, A.C.; Bai, Z.Y.; Li, C.D.; et al. In situ root phenotypes of cotton seedlings under phosphorus stress revealed through rhizopot. Front. Plant Sci. 2021, 12, 716691. [Google Scholar] [CrossRef] [PubMed]
- Gerke, J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, H.; Shan, Y.Z.; Ma, H.Y.; Wang, H.Y.; Xie, F.T.; Ao, X. Physiological response of phosphorus-efficient and inefficient soybean genotypes under phosphorus-deficiency. Russ. J. Plant Physiol. 2020, 67, 175–184. [Google Scholar] [CrossRef]
- Sun, H.W.; Guo, X.L.; Xu, F.G.; Wu, D.X.; Zhang, X.H.; Lou, M.M.; Luo, F.F.; Xu, G.H.; Zhang, Y.L. Overexpression of OsPIN2 regulates root growth and formation in response to phosphate deficiency in rice. Int. J. Mol. Sci. 2019, 20, 5144. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Chen, X.Y.; Gao, Y.J.; Hong, L.J.; Chen, Y.L. Alteration in root morphological and physiological traits of two maize cultivars in response to phosphorus deficiency. Rhizosphere 2020, 14, 100201. [Google Scholar] [CrossRef]
- Becquer, A.; Haling, R.E.; Warren, A.; Hull, R.A.; Stefanski, A.; Richardson, A.E.; Ryan, M.H.; Kidd, D.R.; Lambers, H.; Sandral, G.A.; et al. Critical phosphorus requirements of Trifolium species: The importance of root morphology and root acclimation in response to phosphorus stress. Physiol. Plant. 2021, 173, 1030–1047. [Google Scholar] [CrossRef]
- Wen, Z.H.; Pang, J.Y.; Tueux, G.; Liu, Y.F.; Shen, J.B.; Ryan, M.H.; Lambers, H.; Siddique, K.H.M. Contrasting patterns in biomass allocation, root morphology and mycorrhizal symbiosis for phosphorus acquisition among 20 chickpea genotypes with different amounts of rhizosheath carboxylates. Funct. Ecol. 2020, 34, 1365–2435. [Google Scholar] [CrossRef]
- Gu, J.C.; Yu, S.Q.; Sun, Y.; Wang, Z.Q.; Guo, D.L. Influence of root structure on root survivorship: An analysis of 18 tree species using a minirhizotron method. Ecol. Res. 2011, 26, 755–762. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Q.; Pan, J.J.; Sun, L.J.; Sun, Y.F.; Xue, Y.; Song, K. Transcriptome analysis in roots and leaves of wheat seedlings in response to low-phosphorus stress. Sci. Rep. 2019, 9, 19802. [Google Scholar] [CrossRef]
- Du, Q.G.; Wang, K.; Xu, C.; Zou, C.; Xie, C.X.; Xu, Y.B.; Li, W.X. Strand-specific RNA-Seq transcriptome analysis of genotypes with and without low-phosphorus tolerance provides novel insights into phosphorus-use efficiency in maize. BMC Plant Biol. 2016, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Deng, Y.; Chen, X.P.; Xu, X.F.; Chen, R.Y.; Lv, Y.; Zhao, Y.Y.; Zhao, X.Q.; He, X.; Li, B.; et al. Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J. Exp. Bot. 2013, 64, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Richardson, A.E.; Nichols, S.N.; Crush, J.R. Pasture plants and soil fertility management to improve the efficiency of phosphorus fertiliser use in temperate grassland systems. Crop Pasture Sci. 2014, 65, 556–575. [Google Scholar] [CrossRef]
- Jeffery, R.P.; Simpson, R.J.; Lambers, H.; Kidd, D.R.; Ryan, M.H. Root morphology acclimation to phosphorus supply by six cultivars of Trifolium subterraneum L. Plant Soil 2017, 412, 21–34. [Google Scholar] [CrossRef]
- Shen, J.; Li, H.; Neumann, G.; Zhang, F. Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Sci. 2005, 168, 837–845. [Google Scholar] [CrossRef]
- Wen, Z.H.; Li, H.G.; Shen, J.B.; Rengel, Z. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 2017, 416, 377–389. [Google Scholar] [CrossRef]
- Hou, S.S.; Pu, Z.T.; Zhang, R.F.; Zhang, C.; Wang, H.; Wang, X.X.; Zhao, J.J. Shoot and root traits are associated with varying soil phosphorus supply in Chinese cabbage. J. Soil Sci. Plant Nutr. 2024, 24, 4280–4293. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Li, H.Y.; Xu, L.T.; Li, J.X.; Lyu, X.C.; Li, S.; Wang, C.; Wang, X.L.; Ma, C.M.; Yan, C. Multi-omics analysis of the regulatory effects of low-phosphorus stress on phosphorus transport in soybean roots. Front. Plant Sci. 2022, 13, 992036. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zhang, J.Q.; Wang, H.; Rengel, Z.; Li, H.B. Plasticity and co-variation of root traits govern differential phosphorus acquisition among 20 wheat genotypes. Oikos 2023, 2023, 08606. [Google Scholar] [CrossRef]
- Luo, B.W.; Ma, P.; Nie, Z.; Zhang, X.; He, X.; Ding, X.; Feng, X.; Lu, Q.X.; Ren, Z.Y.; Lin, H.J.; et al. Metabolite profiling and genome-wide association studies reveal response mechanisms of phosphorus deficiency in maize seedling. Plant J. 2019, 97, 947–969. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.J.; Price, A.; Steele, K.A. Evidence from nearisogenic lines that root penetration increases with root diameter and bending stiffness in rice. Funct. Plant Biol. 2008, 35, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D.M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, P.; Peng, Y.F.; Li, X.X.; Chen, F.J.; Li, C.J. Fine root patterning and balanced inorganic phosphorus distribution in the soil indicate distinctive adaptation of maize plants to phosphorus deficiency. Pedosphere 2012, 22, 870–877. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, K.R.; Teng, W.; Zhan, A.; Tong, Y.P.; Feng, G.; Cui, Z.P.; Zhang, F.S.; Chen, X.P. Is the inherent potential of maize roots efficient for soil phosphorus acquisition? PLoS ONE 2014, 9, 90287. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; Rubio, G. Root morphological traits related to phosphorus-uptake efficiency of soybean, sunflower, and maize. J. Plant Nutr. Soil Sci. 2015, 178, 807–815. [Google Scholar] [CrossRef]
- Barry, D.; Miller, M. Phosphorus nutritional requirement of maize seedlings for maximum yield. Agron. J. 1989, 81, 95–99. [Google Scholar] [CrossRef]
- Bollons, H.M.; Barraclough, P.B. Assessing the phosphorus status of winter wheat crops: Inorganic orthophosphate in whole shoots. J. Agric. Sci. 1999, 133, 285–295. [Google Scholar] [CrossRef]
- Ciereszko, I.; Szczygla, A.; Zebrowska, E. Phosphate deficiency affects acid phosphatase activity and growth of two wheat varieties. J. Plant Nutr. 2011, 34, 815–829. [Google Scholar] [CrossRef]



| Phosphorus Supply (mg kg−1) | FAC1 | FAC2 | Composite Score | Ranking |
|---|---|---|---|---|
| 0 | −1.44 | −0.85 | −1.31 | 9 |
| 25 | −1.01 | −0.53 | −0.90 | 8 |
| 50 | −0.88 | 0.84 | −0.51 | 7 |
| 100 | −0.49 | 0.42 | −0.29 | 6 |
| 150 | −0.05 | 1.41 | 0.27 | 4 |
| 300 | 1.00 | 0.89 | 0.97 | 2 |
| 600 | 1.21 | 0.29 | 1.01 | 1 |
| 900 | 0.94 | −0.92 | 0.53 | 3 |
| 1200 | 0.73 | −1.55 | 0.23 | 5 |
| Chinese Cabbage Seeds | Genotypes | Shoot Dry Weight at P0/Shoot Dry Weight at P600 | Phosphorus Efficiency |
|---|---|---|---|
| A | Inbred lines | 0.85 | High-PE |
| B | Hybrids | 0.69 | High-PE |
| C | Hybrids | 0.66 | High-PE |
| D | Inbred lines | 0.60 | High-PE |
| E | Hybrids | 0.53 | Low-PE |
| F | Inbred lines | 0.43 | Low-PE |
| G | Hybrids | 0.42 | Low-PE |
| H | Inbred lines | 0.30 | Low-PE |
| Traits | Phosphorus Efficiency (PE) | Phosphorus Supply | PE × Phosphorus Supply | |||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| Shoot dry weight | 38.68 | <0.001 | 25.22 | <0.001 | 3.12 | <0.01 |
| Shoot P concentration | 30.91 | <0.001 | 65.10 | <0.001 | 1.62 | 0.12 |
| Shoot P content | 69.75 | <0.001 | 30.67 | <0.001 | 4.84 | <0.001 |
| Root dry weight | 8.63 | <0.01 | 49.65 | <0.001 | 1.55 | 0.14 |
| Root P concentration | 17.57 | <0.001 | 76.92 | <0.001 | 4.19 | <0.001 |
| Root P content | 28.57 | <0.001 | 65.12 | <0.001 | 3.51 | <0.01 |
| Plant height | 2.17 | 0.14 | 16.42 | <0.001 | 0.74 | 0.66 |
| SPAD | 100.12 | <0.001 | 3.57 | <0.01 | 1.28 | 0.25 |
| Root length | 15.92 | <0.001 | 60.27 | <0.001 | 1.25 | 0.27 |
| Root surface | 18.12 | <0.001 | 50.81 | <0.001 | 0.87 | 0.55 |
| Root volume | 0.34 | 0.56 | 64.40 | <0.001 | 0.39 | 0.92 |
| Root diameter | 22.25 | <0.001 | 40.63 | <0.001 | 1.08 | 0.38 |
| Root/shoot ratio | 47.82 | <0.001 | 6.60 | <0.001 | 8.49 | <0.001 |
| Specific root length | 15.02 | <0.001 | 18.86 | <0.001 | 0.57 | 0.80 |
| Root tissue density | 4.32 | <0.05 | 63.94 | <0.001 | 1.25 | 0.27 |
| Rhizosphere soil pH | 0.04 | 0.83 | 1.36 | 0.22 | 0.64 | 0.75 |
| Acid phosphatase activity | 2.00 | 0.16 | 4.91 | <0.001 | 4.47 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Hou, S.; Zhang, C.; Wang, H.; Wang, X. Mechanistic Insights into Phosphorus Efficiency and Shoot P Concentration in Chinese Cabbage. Agronomy 2025, 15, 130. https://doi.org/10.3390/agronomy15010130
Zhang R, Hou S, Zhang C, Wang H, Wang X. Mechanistic Insights into Phosphorus Efficiency and Shoot P Concentration in Chinese Cabbage. Agronomy. 2025; 15(1):130. https://doi.org/10.3390/agronomy15010130
Chicago/Turabian StyleZhang, Ruifang, Saisai Hou, Chi Zhang, Hong Wang, and Xinxin Wang. 2025. "Mechanistic Insights into Phosphorus Efficiency and Shoot P Concentration in Chinese Cabbage" Agronomy 15, no. 1: 130. https://doi.org/10.3390/agronomy15010130
APA StyleZhang, R., Hou, S., Zhang, C., Wang, H., & Wang, X. (2025). Mechanistic Insights into Phosphorus Efficiency and Shoot P Concentration in Chinese Cabbage. Agronomy, 15(1), 130. https://doi.org/10.3390/agronomy15010130





