Verification of the Introgression of Narenga porphyrocoma Germplasm into Saccharum officinarum Using Molecular Markers and GISH Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
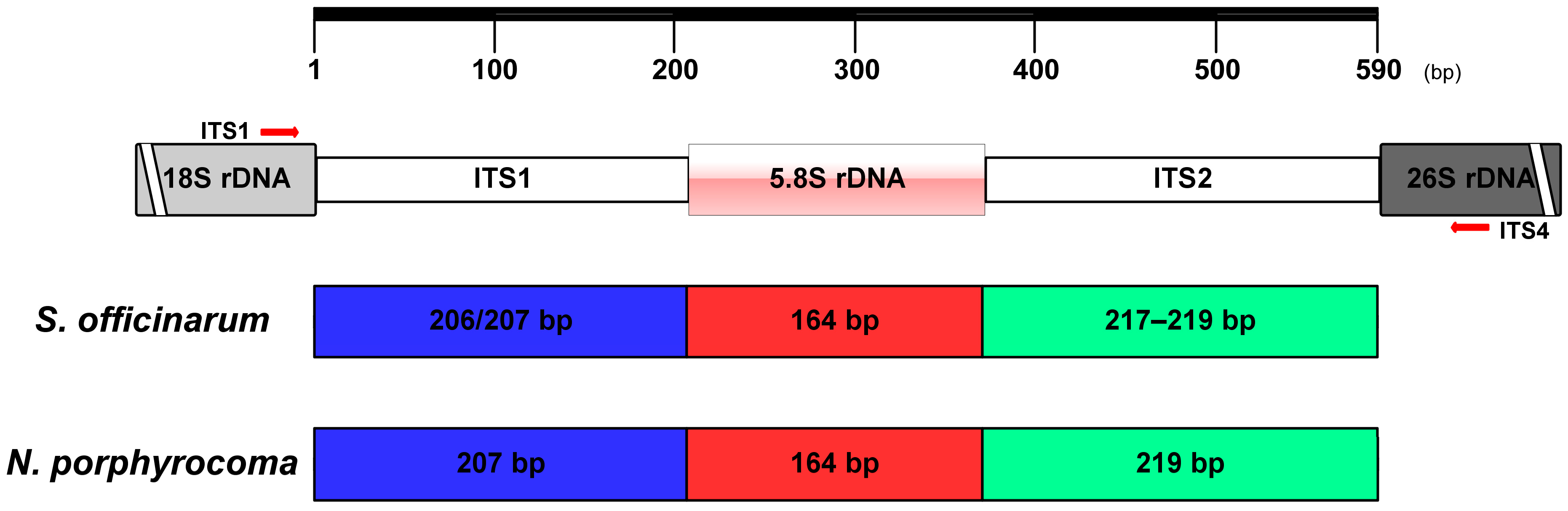
2.2. DNA Extraction and PCR Amplification of nrDNA-ITS Region
2.3. ITS Sequence Analysis and Alignment
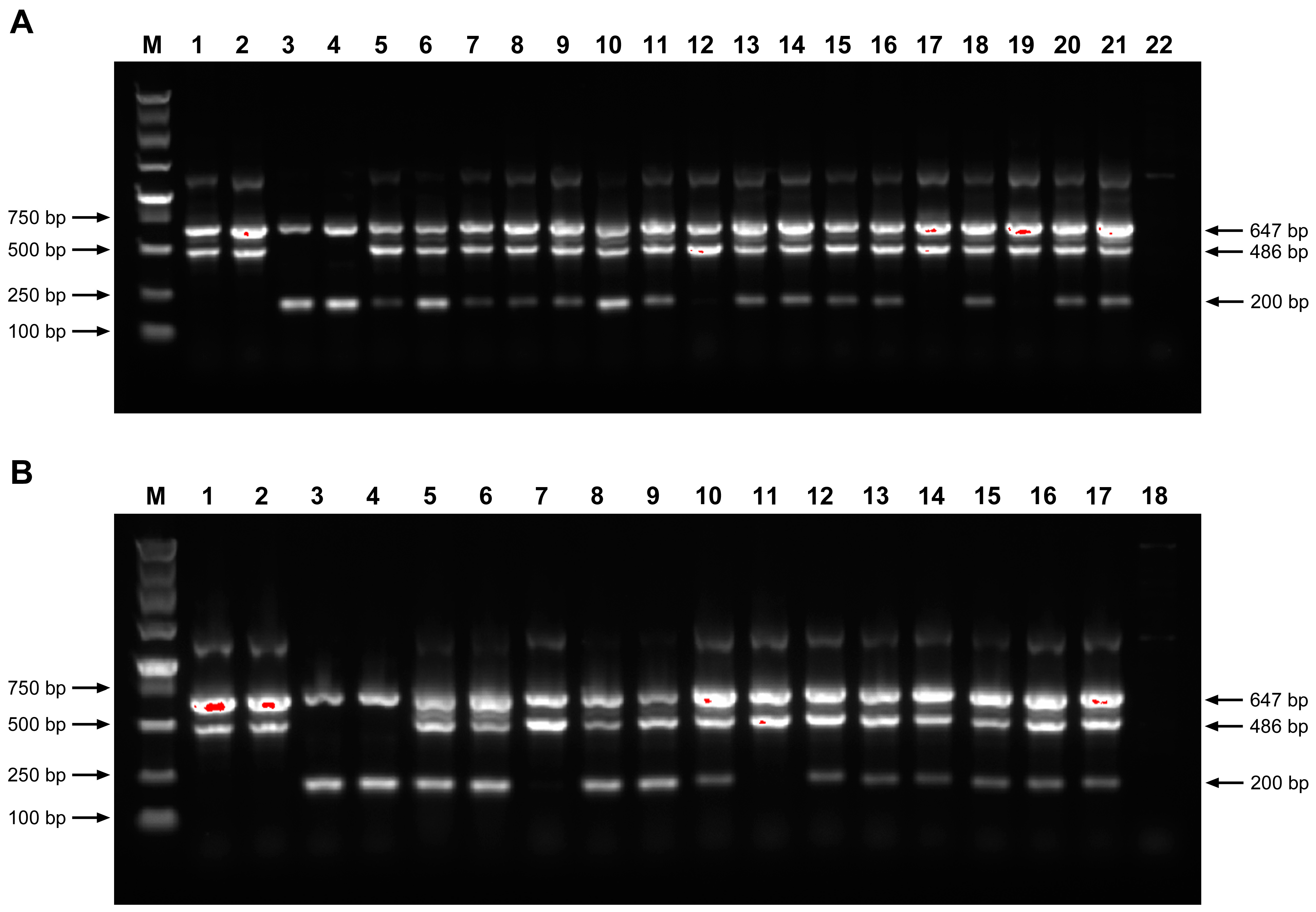
2.4. ARMS-PCR Strategy
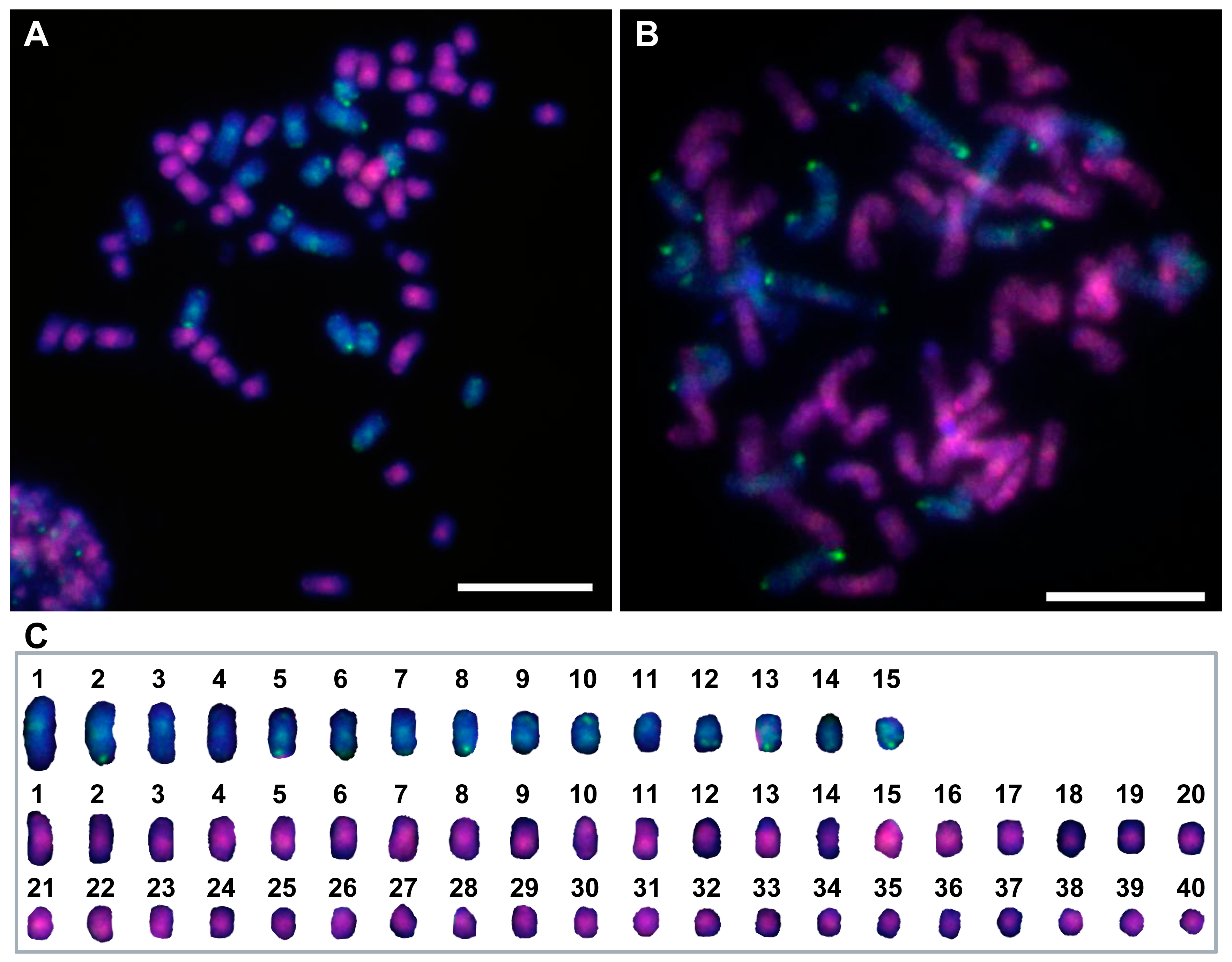
2.5. Genomic In Situ Hybridization Procedure
3. Results
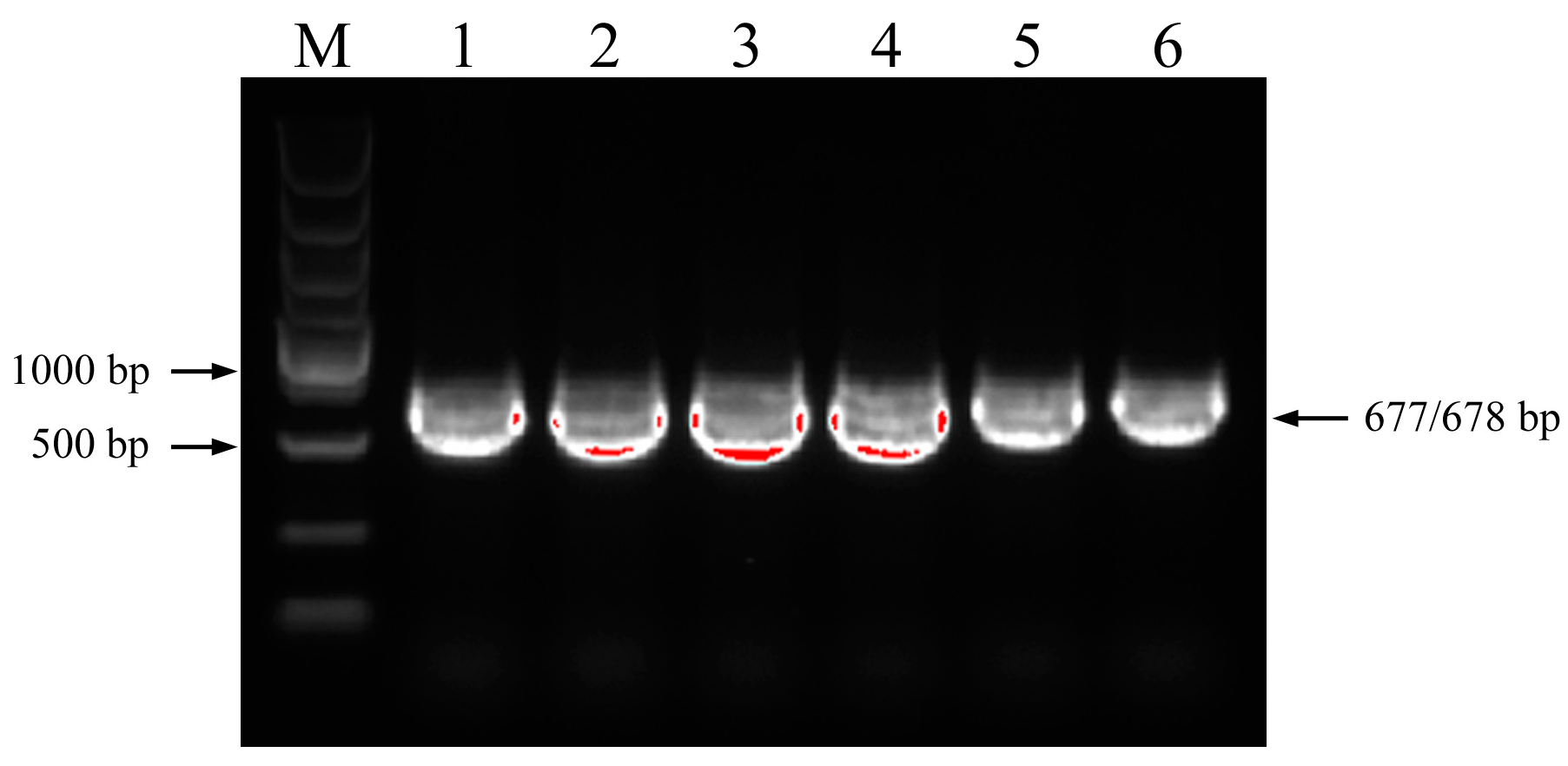
3.1. PCR Amplification of nrDNA-ITS
3.2. Sequence Analysis of ITS1-5.8S-ITS2
3.3. Tetra-Primer ARMS PCR Identification of Genuine Hybrids Between S. officinarum and N. porphyrocoma
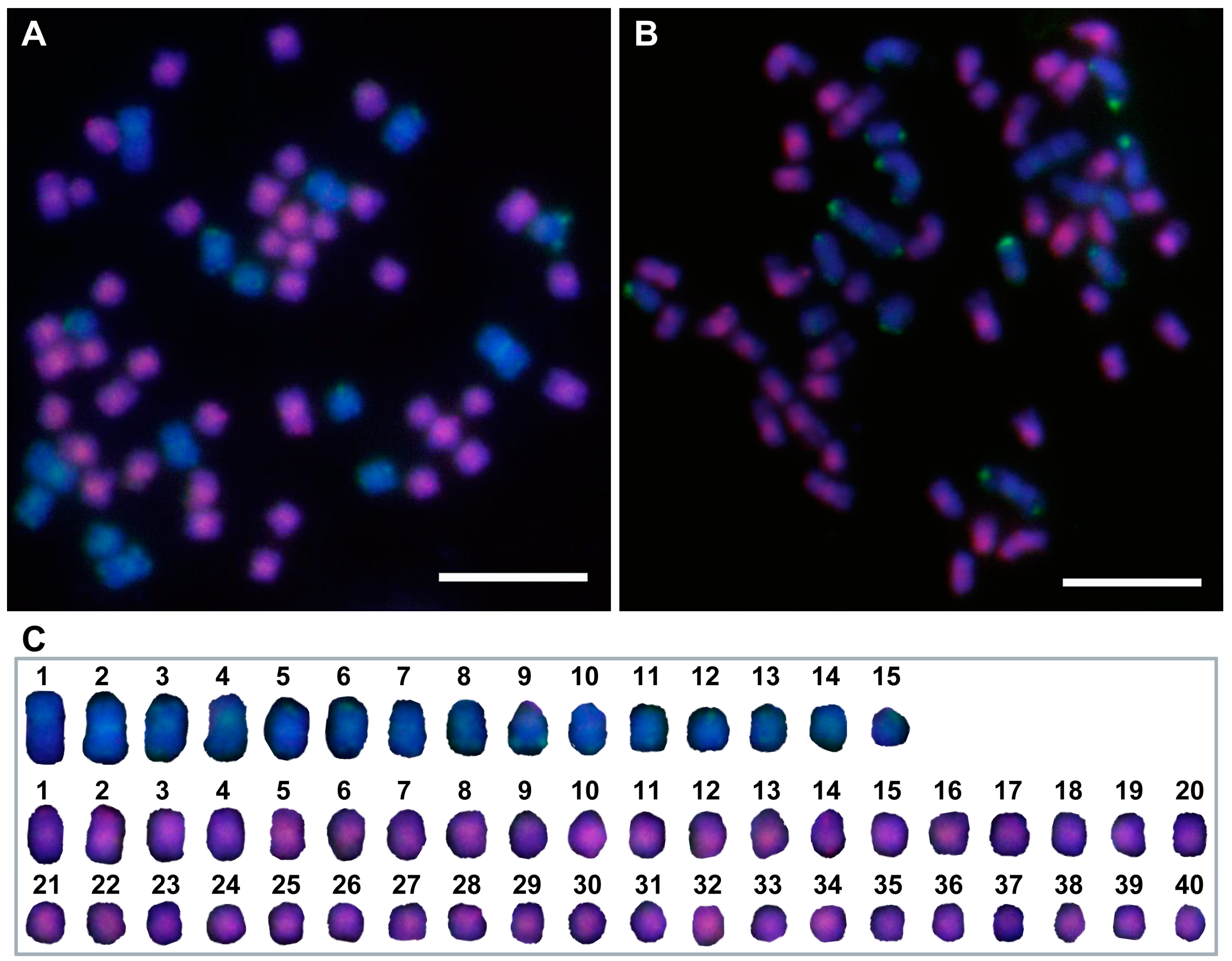
3.4. Chromosome Composition of the F1 Intergeneric Hybrids by GISH
3.5. Main Agronomic Traits of F1 Hybrids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillon, S.L.; Shapter, F.M.; Henry, R.J.; Cordeiro, G.; Izquierdo, L.; Lee, L.S. Domestication to crop improvement: Genetic resources for Sorghum and Saccharum (Andropogoneae). Ann. Bot. 2007, 100, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Waclawovsky, A.J.; Sato, P.M.; Lembke, C.G.; Moore, P.H.; Souza, G.M. Sugarcane for bioenergy production: An assessment of yield and regulation of sucrose content. Plant Biotechnol. J. 2010, 8, 263–276. [Google Scholar] [CrossRef] [PubMed]
- D’Hont, A.; Ison, D.; Alix, K.; Roux, C.; Glaszmann, J.C. Determination of basic chromosome numbers in the genus Saccharum by physical mapping of ribosomal RNA genes. Genome 1998, 41, 221–225. [Google Scholar] [CrossRef]
- Piperidis, N.; D’Hont, A. Sugarcane genome architecture decrypted with chromosome-specific oligo probes. Plant J. 2020, 103, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.W.; Gao, X.N.; Zeng, Q.Y.; Zheng, Z.; Wu, C.W. Sugarcane breeding, germplasm development and related molecular research in China. Sugar Tech 2022, 24, 73–85. [Google Scholar] [CrossRef]
- Li, H.W.; Ma, T.H.; Yao, S.Y.; Shang, K.C. Cytological studies of sugarcane and its relatives. ⅩⅡ. F1 hybrids of POJ2725 × Narenga porphyrocoma (Hance) Bor. Taiwan Sug. 1954, 1, 1–11. [Google Scholar]
- Liu, X.H.; Fang, F.X.; Zhang, R.H.; Song, H.Z.; Yang, R.Z.; Gao, Y.J.; Ou, H.P.; Lei, J.C.; Luo, T.; Duan, W.X.; et al. Identification of progenies from sugarcane × Narenga porphyrocoma (Hance) Bor. by SSR marker. Southwest China J. Agric. Sci. 2012, 25, 38–43. (In Chinese) [Google Scholar]
- Wang, X.H.; Yang, Q.H.; Li, F.S.; He, L.L.; He, S.C. Molecular identification of Saccharum spp. × Erianthus fulvus hybrids using sequence-characterized amplified region markers. Crop Sci. 2009, 49, 864–870. [Google Scholar] [CrossRef]
- Cai, Q.; Aitken, K.; Deng, H.H.; Chen, X.W.; Fu, C.; Jackson, P.A.; Mcintyre, C.L. Verification of the introgression of Erianthus arundinaceus germplasm into sugarcane using molecular markers. Plant Breed. 2005, 124, 322–328. [Google Scholar] [CrossRef]
- Kar, S.; Weng, T.Y.; Nakashima, T.; Villanueva-Morales, A.; Stewart, J.R.; Sacks, E.J.; Terajima, Y.; Yamada, T. Field performance of Saccharum × Miscanthus intergeneric hybrids (miscanes) under cool climatic conditions of northern Japan. Bioenerg. Res. 2020, 13, 132–146. [Google Scholar] [CrossRef]
- Aitken, K.; Li, J.; Wang, L.; Cai, Q.; Fan, Y.H.; Jackson, P. Characterization of intergeneric hybrids of Erianthus rockii and Saccharum using molecular markers. Genet. Resour. Crop Evol. 2007, 54, 1395–1405. [Google Scholar] [CrossRef]
- Duan, W.X.; Zhang, B.Q.; Zhou, S.; Huang, Y.X.; Wang, Z.P.; Gao, Y.J.; Yang, C.F.; Lin, S.H.; Zhang, G.M. Identification and preliminary evaluation of smut resistance in BC1 hybrids derived from Saccharum L. × Narenga porphyrocoma (Hance) Bor. J. China Agric. Univ. 2018, 23, 29–37. (In Chinese) [Google Scholar] [CrossRef]
- Wei, J.J.; Xiu, Z.H.; Ou, H.P.; Chen, J.H.; Tan, H.W.; Liu, X.H. Transcriptome profile analysis of twisted leaf disease response in susceptible sugarcane with Narenga porphyrocoma genetic background. Trop. Plant Biol. 2019, 12, 293–303. [Google Scholar] [CrossRef]
- Koujalagi, D.; Jeena, A.S.; Singh, S.P.; Chourasia, K.; Khan, K.A. Hybridity tests to identify true hybrids in sugarcane. J. Hill Agric. 2017, 8, 10–16. [Google Scholar] [CrossRef]
- Devarumath, R.M.; Kalwade, S.B.; Kawar, P.G.; Sushir, K.V. Assessment of Genetic Diversity in Sugarcane Germplasm Using ISSR and SSR Markers. Sugar Tech 2012, 14, 334–344. [Google Scholar] [CrossRef]
- Brookes, A.J. The essence of SNPs. Gene 1999, 234, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Dhillon, S.; Ke, X.; Collins, A.R.; Day, I.N. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001, 29, E88. [Google Scholar] [CrossRef]
- Medrano, R.F.; de Oliveira, C.A. Guidelines for the tetra-primer ARMS-PCR technique development. Mol. Biotechnol. 2014, 56, 599–608. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.J.; Nie, H.L.; Kim, H.Y.; Lee, J.E. Molecular identification of sweet potato accessions using ARMS-PCR based on SNPs. J. Plant Biotechnol. 2020, 47, 124–130. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Dalhaimer, P.; Zhao, L. Tetra-Primer amplification-refractory mutation system (ARMS)-PCR for genotyping mouse leptin gene mutation. Animals 2022, 12, 2680. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.X.; Chen, X.; Hu, Q.Q.; Yao, Y.A.; Wang, X.M.; Xu, A.C.; Ge, J.; Guan, F. Development of a tetra-primer ARMS-PCR for identification of sika and red deer and their hybrids. Anal. Sci. 2023, 39, 1947–1956. [Google Scholar] [CrossRef]
- Blin, M.; Dametto, S.; Agniwo, P.; Webster, B.L.; Angora, E.; Dabo, A.; Boissier, J. A duplex tetra-primer ARMS-PCR assay to discriminate three species of the Schistosoma haematobium group: Schistosoma curassoni, S. bovis, S. haematobium and their hybrids. Parasit Vectors 2023, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Piperidis, N. GISH: Resolving interspecific and intergeneric hybrids. Methods Mol. Biol. 2021, 2222, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Huang, Y.J.; Deng, Z.H.; Li, Q.W.; Huang, Z.X.; Chen, R.K.; Zhang, M.Q. Unexpected inheritance pattern of Erianthus arundinaceus chromosomes in the intergeneric progeny between Saccharum spp. and Erianthus arundinaceus. PLoS ONE 2014, 9, e110390. [Google Scholar] [CrossRef]
- Yu, F.; Wang, P.; Deng, Z.H.; Wu, J.Y.; Yang, Y.Q.; Chen, R.K.; Zhang, M.Q.; Xu, L.N. Characterization of chromosome composition of sugarcane in nobilization by using genomic in situ hybridization. Mol. Cytogenet. 2018, 11, 35. [Google Scholar] [CrossRef]
- Yang, C.F.; Yang, L.T.; Li, Y.R.; Zhang, G.M.; Zhang, C.Y.; Wang, W.Z. Sequence characteristics and phylogenetic implications of the nrDNA internal transcribed spacers (ITS) in protospecies and landraces of sugarcane (Saccharum officinarum L.). Sugar Tech 2016, 18, 8–15. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, X.J.; Liu, H.B.; Xu, C.H.; Lin, X.Q.; Li, C.J.; Deng, Z.H. Phylogenetic analysis of different ploidy Saccharum spontaneum based on rDNA-ITS sequences. PLoS ONE 2016, 11, e0151524. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.W.; He, Z.H.; Xiao, Y.; Ouyang, K.X.; Wang, X.; Hu, X.S. Population structure and genetic diversity in the natural distribution of Neolamarckia cadamba in China. Genes 2023, 14, 855. [Google Scholar] [CrossRef]
- Kim, W.J.; Yang, S.; Choi, G.; Park, I.; Noh, P.; Kim, M.J.; Moon, B.C. Accurate and rapid identification of Longan Arillus and Litchi Semen by a multiplex PCR assay. Plants 2020, 9, 948. [Google Scholar] [CrossRef]
- Sun, Y.W.; Liao, Y.J.; Hung, Y.S.; Chang, J.C.; Sung, J.M. Development of ITS sequence based SCAR markers for discrimination of Paphiopedilum armeniacum, Paphiopedilum micranthum, Paphiopedilum delenatii and their hybrids. Sci. Hortic. 2011, 127, 405–410. [Google Scholar] [CrossRef]
- Muto, T.; Kuronuma, T.; Ando, M.; Katsuoka, H.; Inaba, Z.; Watanabe, H. Development of the sequence-characterized amplified region (SCAR) marker for distinction of intergeneric hybrids between Argyranthemum frutescens (L.) Sch. Bip. and Rhodanthemum gayanum (Cross. & Durieu) B.H. Wilcox, K. Bremer & Humphries. Plant Biotechnol. 2020, 37, 77–81. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.T.; Huang, F.; Deng, Z.H.; Chen, R.K.; Zhang, M.Q. A new method based on SNP of nrDNA-ITS to identify Saccharum spontaneum and its progeny in the genus Saccharum. PLoS ONE 2018, 13, e0197458. [Google Scholar] [CrossRef]
- Li, X.T.; Guo, Y.R.; Huang, F.; Wu, J.Y.; Zhang, M.Q.; Deng, Z.H. Authenticity identification of Saccharum officinarum and Saccharum spontaneum germplasm materials. Agronomy 2022, 12, 819. [Google Scholar] [CrossRef]
- Lin, P.P.; Hu, X.G.; Xue, L.; Zhang, M.Q.; Deng, Z.H.; Yu, F. Identification of sugarcane S. spontaneum (Poaceae) germplasm: Evidence from rDNA-ITS and rDNA locus analyses. Agronomy 2022, 12, 3167. [Google Scholar] [CrossRef]
- Doyle, J.J.T.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 39–40. [Google Scholar]
- D’Hont, A.; Grivet, L.; Feldmann, P.; Rao, S.; Berding, N.; Glaszmann, J.C. Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Mol. Gen. Genet. 1996, 250, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Wang, Q.N.; Qiu, Y.S.; Qin, Y.X.; Li, X.T.; Fang, N. Production, identification and characterization of Erianthus rockii × Narenga porphyrocoma intergeneric hybrids as a new germplasm for sugarcane breeding and genetic research. Sugar Tech 2020, 22, 389–395. [Google Scholar] [CrossRef]
- Meena, M.R.; Govindaraj, P.; Kumar, R.A.; Elayaraja, K.; Appunu, C.; Kumar, R.; Chhabra, M.L.; Kulshreshtha, N.; Hemaprabha, G. Biomass and energy potential of Erianthus arundinaceus and Saccharum spontaneum-derived novel sugarcane hybrids in rainfed environments. BMC Plant Biol. 2024, 24, 198. [Google Scholar] [CrossRef]
- Hodkinson, T.R.; Chase, M.W.; Lledó, M.D.; Salamin, N.; Renvoize, S.A. Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. J. Plant Res. 2002, 115, 381–392. [Google Scholar] [CrossRef]
- Wang, H.T.; Kim, M.K.; Kim, Y.J.; Lee, H.N.; Jin, H.Z.; Chen, J.Y.; Yang, D.C. Molecular authentication of the Oriental medicines Pericarpium citri reticulatae and Citri unshius pericarpium using SNP markers. Gene 2012, 494, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Pachakkil, B.; Terajima, Y.; Ohmido, N.; Ebina, M.; Irei, S.; Hayashi, H.; Takagi, H. Cytogenetic and agronomic characterization of intergeneric hybrids between Saccharum spp. hybrid and Erianthus arundinaceus. Sci. Rep. 2019, 9, 1748. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, K.; Deng, Z.H.; Huang, Y.J.; Huang, F.; Chen, R.K.; Zhang, M.Q. Chromosome transmission in BC4 progenies of intergeneric hybrids between Saccharum spp. and Erianthus arundinaceus (Retz.) Jeswiet. Sci. Rep. 2019, 9, 2528. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhang, R.H.; Li, H.B.; Zhang, G.M.; Song, H.Z.; Li, Y.R. The chromosome transmission of the hybrid progeny of S. officinarum and Narenga porphyrocoma and the inheritance of Narenga porphyrocoma blood relationship in the offspring. Mol. Plant Breed. 2019, 17, 411–418. (In Chinese) [Google Scholar]



| Species Name | Haplotype of ITS Sequence | FI-So (5′-3′) | RI-Np (5′-3′) | GenBank Accession No. | |||
|---|---|---|---|---|---|---|---|
| GGAACACCTAAATTGCCTTGCG | GGCCGACCGCCCCGCTGA | ||||||
| S. officinarum | SoITS-1 | GGAACACCTAAATTGCCTTGCG | GGCCGACCGCTCCACCGC | AB250691 SRR528718 | |||
| SoITS-2 | GGAACACCTATATTGCCTTGCG | GGCCGACCGCTCCACCGC | AB250692 AY116284 | ||||
| SoITS-3 | GGAACACCTATATTGCCTTGCG | GGCCGATCGCTCCACCGC | AF345229 AF345230 AF345231 | ||||
| SoITS-4 | GGAGCACCTATATTGCCCTGCG | GGCCGACCGCTCCACCGC | AB250693 | ||||
| N. porphyrocoma | NpITS-1 | GGAACACTCATATTGCCTTGCT | GGCCGACCGCTCCGCCGA | AF345233 | AF345234 | AF345235 | AF345236 |
| NpITS-2 | GGAACACTCATATTGCCCTGCT | GGCCGACCGCTCCGCCGA | JX156343 SRR3399436 | ||||
| NpITS-3 | GGAACACTTATATTGCCTTGCT | GGCCGACCGCTCCGCCGA | EF211957 | ||||
| Material Name | Tiller Number (no./stool) | Stalk Length (cm) | Internode Number | Leaf Length (cm) | Leaf Width (cm) | Stalk Diameter (mm) | Juice Brix % |
|---|---|---|---|---|---|---|---|
| Badila | 5.08 ± 0.67 | 207.20 ± 13.15 | 20.25 ± 1.14 | 136.33 ± 6.87 | 5.46 ± 0.27 | 39.29 ± 2.48 | 20.98 ± 0.62 |
| Zhanjiang Qingpi | 5.25 ± 0.62 | 213.53 ± 16.57 | 16.08 ± 1.16 | 136.47 ± 5.63 | 7.10 ± 0.49 | 40.39 ± 2.44 | 15.16 ± 0.68 |
| Guangdong 32 | 64.33 ± 11.39 | 163.87 ± 15.36 | 5.67 ± 0.65 | 124.80 ± 6.88 | 1.74 ± 0.17 | 6.77 ± 0.65 | 4.34 ± 1.08 |
| Badila × Guangdong 32 | 20.93 ± 2.65 ** | 174.08 ± 7.32 ** | 15.73 ± 1.03 ** | 166.20 ± 11.45 ** | 4.80 ± 0.27 ** | 17.48 ± 1.93 ** | 11.11 ± 1.54 ** |
| Zhanjiang Qingpi × Guangdong 32 | 22.73 ± 3.03 ** | 183.67 ± 12.89 ** | 13.67 ± 1.11 ** | 165.47 ± 7.41 ** | 6.81 ± 0.28 | 19.12 ± 1.82 ** | 12.33 ± 1.53 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Zhang, W.; Qin, Y.; Wu, Q.; Liang, Q.; Wu, J.; Sun, S.; Wang, Z.; An, Y.; Wang, J.; et al. Verification of the Introgression of Narenga porphyrocoma Germplasm into Saccharum officinarum Using Molecular Markers and GISH Analysis. Agronomy 2025, 15, 121. https://doi.org/10.3390/agronomy15010121
Wang G, Zhang W, Qin Y, Wu Q, Liang Q, Wu J, Sun S, Wang Z, An Y, Wang J, et al. Verification of the Introgression of Narenga porphyrocoma Germplasm into Saccharum officinarum Using Molecular Markers and GISH Analysis. Agronomy. 2025; 15(1):121. https://doi.org/10.3390/agronomy15010121
Chicago/Turabian StyleWang, Gang, Wei Zhang, Yuanxia Qin, Qingdan Wu, Qinggan Liang, Jiantao Wu, Shengren Sun, Zhuqing Wang, Yuxing An, Jianqiang Wang, and et al. 2025. "Verification of the Introgression of Narenga porphyrocoma Germplasm into Saccharum officinarum Using Molecular Markers and GISH Analysis" Agronomy 15, no. 1: 121. https://doi.org/10.3390/agronomy15010121
APA StyleWang, G., Zhang, W., Qin, Y., Wu, Q., Liang, Q., Wu, J., Sun, S., Wang, Z., An, Y., Wang, J., Wang, Q., & Chang, H. (2025). Verification of the Introgression of Narenga porphyrocoma Germplasm into Saccharum officinarum Using Molecular Markers and GISH Analysis. Agronomy, 15(1), 121. https://doi.org/10.3390/agronomy15010121





