Facing Heavy Metal Stress, What Are the Positive Responses of Melatonin in Plants: A Review
Abstract
1. Introduction
2. Current Situation of Soil Heavy Metal Pollution and Its Harm to Plants
3. Current Research Status of Melatonin in Stress Resistance
4. What Positive Responses Has Melatonin Had in Plants under Heavy Metal Stress?
4.1. From the Perspective of Plant Phenotype, How Does Melatonin Alleviate Plant Damage under Heavy Metal Stress?
4.1.1. Seed Germination
4.1.2. Growth and Development
4.1.3. Maturity and Aging
4.2. From the Perspective of Physiology, How Does Melatonin Alleviate Heavy Metal Stress in Plants?
4.2.1. Regulatory Effect of Melatonin on Plant Antioxidant System
4.2.2. Regulatory Effect of Melatonin on Plant Photosynthetic System
4.3. From the Perspective of Molecular Biology, How Does Melatonin Alleviate Heavy Metal Stress in Plants?
4.4. From the Perspective of Nutrient Absorption, How Does Melatonin Alleviate Heavy Metal Stress in Plants?
5. Conclusions and Future Research Directions
- (1)
- The research and application of melatonin related to crops should be strengthened. In cultivated soil, crops are mainly planted. With the increasing trend of heavy metal content in cultivated soil, melatonin can improve the tolerance of plants to heavy metals. Therefore, it is necessary to strengthen the research and application of melatonin in the absorption and accumulation of nutrients, yield, and the quality of crops (especially staple grains) so as to truly apply melatonin in production to solve practical problems.
- (2)
- The mechanism of the interaction between melatonin and other plant hormones should be make clear. Melatonin may participate in other plant reactions after being directly perceived by receptors. For example, JA and SA, which are closely related to the defense response, may interact with melatonin to participate in the regulation of plant growth and development, but whether there is a cross between JA, SA, and the MAPK cascade reaction of melatonin remains to be further studied.
- (3)
- Melatonin-hormone signal perception and transmission should be explored. Although researchers have cloned a melatonin plant receptor, CAND2/PMTR1, in recent years, there have been few studies on the direct downstream signals activated after the receptor binds melatonin. The question of whether there are other melatonin receptors is also a scientific problem that needs to be solved urgently.
- (4)
- The selection of crop varieties with high melatonin contents and screening of stress resistance genes should be carried out. These constitute an attractive and challenging research direction to select crop varieties with high melatonin contents and develop crops with resistance to reversion genes by using the existing research results on melatonin and combining the traditional breeding theory and experience.
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Yu, T.; Jiang, T.; Liu, X.; Ma, X.; Yang, Z.; Hou, Q.; Xia, X.; Li, F. Research progress of detection and analysis technology of heavy metal pollution in soil. Geol. China 2021, 48, 460–476. [Google Scholar] [CrossRef]
- Oladoye, P.; Olowe, O.; Asemoloye, M. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, L.; Ma, J.; Wu, X.; Li, Y. Progress in the analysis of soil heavy metal pollution sources in China in recent ten years. J. Agric. Environ. Sci. 2019, 38, 2219–2238. [Google Scholar]
- Sun, Q.; Yang, J.; Sui, F.; Qin, S.; Li, C.; Zhang, W.; Xu, J.; Wang, L.; Zhao, P. Technology and effect analysis of heavy metal pollution remediation in farmland soil based on documentation. Chin. J. Soil Sci. 2023, 54, 998–1008. [Google Scholar]
- Cai, M.; Li, K.; Xie, D.; Wu, R. Research on the status and countermeasures of heavy metal pollution in cultivated soil in China. Environ. Sci. Technol. 2014, 37, 223–230. [Google Scholar]
- Khan, K.; Mohsin, A.; Sharif, H.; Maryam, A.; Ali, J.; Li, X.; Ibrahim, S.; Ayaz, M.; Zhou, Y.; Younas, M. Heavy metal pollution in the soil of a riverine basin: Distribution, source, and potential hazards. Environ. Monit. Assess. 2022, 194, 618. [Google Scholar] [CrossRef]
- Swartjes, F.; Carlon, C.; De Wit, N. The possibilities for the EU-wide use of similar ecological risk-based soil contamination assessment tools. Sci. Total Environ. 2008, 406, 523–529. [Google Scholar] [CrossRef]
- Qian, Y.; Gallagher, F.; Deng, Y.; Wu, M.; Feng, H. Risk assessment and interpretation of heavy metal contaminated soils on an urban brownfield site in New York metropolitan area. Environ. Sci. Pollut Res. 2017, 24, 23549–23558. [Google Scholar] [CrossRef]
- Jones, D.; Yu, X.; Guo, Q.; Duan, X.; Jia, C. Racial disparities in the heavy metal Contamination of Urban Soil in the Southeastern United States. Int. J. Environ. Res. 2022, 19, 1105. [Google Scholar] [CrossRef]
- Fan, T.; Ye, W.; Chen, H.; Lu, H.; Zhang, Y.; Li, D.; Tang, Z.; Ma, Y. Research on farmland soil heavy metal pollution and remediation technology. Ecol. Environ. Sci. 2022, 22, 1727–1736. [Google Scholar] [CrossRef]
- Pouresmaieli, M.; Ataei, M.; Forouzandeh, P.; Azizollahi, P.; Mahmoudifard, M. Recent progress on sustainable phytoremediation of heavy metals from soil. J. Environ. Chem. Eng. 2022, 10, 108482. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef] [PubMed]
- Cocârţă, D.; Neamţu, S.; Reşetar Deac, A. Carcinogenic risk evaluation for human health risk assessment from soils contaminated with heavy metals. Int. J. Environ. Sci. Technol. 2022, 13, 2025–2036. [Google Scholar] [CrossRef]
- Xiang, M.; Li, Y.; Yang, J.; Lei, K.; Li, Y.; Li, F.; Zheng, D.; Fang, X.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2022, 278, 116911. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Ji, Y.; Wu, X.; Xu, X.; Wu, P. Melatonin promotes seed germination via regulation of ABA signaling under low temperature stress in cucumber. J. Plant Growth Regul. 2023, 42, 2232–2245. [Google Scholar] [CrossRef]
- Lerner, A.; Case, J.; Takahashi, Y.; Lee, T.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytesl. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Song, L.; Tan, Z.; Zhang, W.; Li, Q.; Jiang, Z.; Shen, S.; Luo, S.; Chen, X. Exogenous melatonin improves the chilling tolerance and preharvest fruit shelf life in eggplant by affecting ROS- and senescence-related processes. Hortic. Plant J. 2023, 9, 523–540. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous melatonin activates antioxidant systems to increase the ability of rice seeds to germinate under high temperature conditions. Plants 2022, 11, 886. [Google Scholar] [CrossRef]
- He, M.; Mei, S.; Zhai, Y.; Geng, G.; Yu, L.; Wang, Y. Effects of melatonin on the growth of sugar beet (Beta vulgaris L.) seedlings under drought stress. J. Plant Growth Regul. 2022, 42, 5116–5130. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kazerooni, E.; Kang, S.M.; Al-Sadi, A.; Lee, I.J. Melatonin enhances the tolerance and recovery mechanisms in Brassica juncea (L.) Czern. under saline conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.; Alam, P.; Hayat, S. Perspective of melatonin-mediated stress resilience and Cu remediation efficiency of Brassica juncea in Cu-contaminated soils. Front. Plant Sci. 2022, 13, 910714. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, J.; Gao, Y.; Han, K.; Liu, J.; Wang, Y. Exogenous melatonin improved the growth and development of naked oat seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2022, 29, 88109–88118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Study of the Mechanism of Enhancing Salt and Cadmium Stress in Pigless Gland Cotton Triggered by Melatonin Seed. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2022. [Google Scholar]
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 2019, 139, 630–641. [Google Scholar] [CrossRef]
- Shang, E.; Xu, E.; Zhang, H.; Huang, C. Spatial and temporal changes of heavy metals and pollution sources in major grain producing areas in China. Environ. Sci. 2018, 39, 4670–4683. [Google Scholar] [CrossRef]
- The National Soil Pollution Survey Bulletin. 2014. Available online: https://www.gov.cn/foot/site1/20140417/782bcb88840814ba158d01 (accessed on 12 October 2023).
- Chen, H.; Zheng, C.; Tu, C.; Zhu, Y. Status and control countermeasures of Heavy metal pollution in China. J. Hum. Environ. 1999, 28, 5. [Google Scholar]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.; Frost, R.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Shen, L.; Xu, B.; Jiang, W.; Guang, Z. Current situation of heavy metal pollution in China and prevention and control countermeasures. Mod. Food Prod. 2017, 11, 289–290. [Google Scholar]
- Bae, J.; Benoit, D.; Watson, A. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotox. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fu, Q.; Zheng, J.; Zhang, A.; Wang, H. Transcriptomic and metabolomic analyses reveal that melatonin promotes melon root development under copper stress by inhibiting jasmonic acid biosynthesis. Hortic. Res. 2020, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Ma, X.; Ouyang, Z.; Deng, L.; Shen, S.; Dong, X.; Du, N.; Dong, H.; Guo, Z.; et al. Melatonin alleviates copper toxicity via improving ROS metabolism and antioxidant defense response in tomato seedlings. Antioxidants 2022, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Q.; Shah, F.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Sirohi, P.; Yadav, A.; Singh, M.; Kumar, A.; Singh, N. A comparative screening of abiotic stress tolerance in early flowering rice mutants. J. Biotechnol. 2019, 302, 112–122. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Xu, T.; Ma, H.; Xia, T. The combined application of γ-PGA-producing bacteria and biochar reduced the content of heavy metals and improved the quality of tomato (Solanum lycopersicum L.). Environ. Sci. Pollut. Res. 2022, 29, 88938–88950. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.; Reiter, R.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.; Chu, Y.; Reiter, R.; Yu, X.; Zhu, D.; Zhang, W.; Ma, B.; Lin, Q.; Zhang, J.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2014, 66, 695–707. [Google Scholar] [CrossRef]
- Bychkov, I.; Andreeva, A.; Kudryakova, N.; Kusnetsov, V. Cytokinin modulates responses to phytomelatonin in Arabidopsis thaliana under high light stress. Int. J. Mol. Sci. 2023, 24, 738. [Google Scholar] [CrossRef]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Chen, Z.; Guo, Z.; Xu, N.; Sui, X.; Roy, M.; Kareem, H.; Hassan, M.; Cui, J.; Wang, Q. Exogenous melatonin promotes the growth of alfalfa (Medicago sativa L.) under NaCl stress through multiple pathways. Ecotox. Environ. Saf. 2022, 242, 113938. [Google Scholar] [CrossRef]
- Wei, J.; Liang, J.; Liu, D.; Liu, Y.; Liu, G.; Wei, S. Melatonin-induced physiology and transcriptome changes in banana seedlings under salt stress conditions. Front. Plant Sci. 2022, 13, 938262. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Posmyk, M.; Kuran, H.; Marciniak, K.; Janas, K. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.P.; Liu, X.; Zhou, Z.Z.; Li, D.Y.; Zhao, X.F.; Zhu, L.H.; Luo, Y.F.; Hu, S.N. Identification of a G2-like transcription factor, OsPHL3, functions as a negative regulator of flowering in rice by co-expression and reverse genetic analysis. BMC Plant Biol. 2018, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Sakouhi, L.; Kadri, O.; Werghi, S.; Massoud, M.; Kharbech, O.; Murata, Y.; Chaoui, A. Seed pretreatment with melatonin confers cadmium tolerance to chickpea seedlings through cellular redox homeostasis and antioxidant gene expression improvement. Environ. Sci. Pollut. Res. 2023, 30, 73612–73627. [Google Scholar] [CrossRef]
- Ou, C.; Cheng, W.; Wang, Z.; Yao, X.; Yang, S. Exogenous melatonin enhances Cd stress tolerance in Platycladus orientalis seedlings by improving mineral nutrient uptake and oxidative stress. Ecotox. Environ. Saf. 2023, 252, 114619. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. J. 2020, 22, 679–690. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Karahan, H.; Kucukoduk, M.; Turkan, I. Ferulic acid confers tolerance against excess boron by regulating ROS levels and inducing antioxidant system in wheat leaves (Triticum aestivum). Environ. Exp. Bot. 2019, 161, 193–202. [Google Scholar] [CrossRef]
- Ayyaz, A.; Farooq, M.; Dawood, M.; Majid, A.; Javed, M.; Athar, H.R.; Bano, H.; Zafar, Z. Exogenous melatonin regulates chromium stress-induced feedback inhibition of photosynthesis and antioxidative protection in Brassica napus cultivars. Plant Cell Rep. 2021, 40, 2063–2080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, H.; Xie, Y.; Li, M.; Wang, Y.; Liang, D. Oxidation resistance of exogenous melatonin on leaves of kiwifruit seedlings under copper stress. Int. Conf. Civ. Transp. Environ. Eng. 2017, 135, 216–219. [Google Scholar]
- Awan, S.; Khan, I.; Rizwan, M.; Irshad, M.; Wang, X.; Zhang, X.; Huang, L. Reduction in the cadmium (Cd) accumulation and toxicity in pearl millet (Pennisetum glaucum L.) by regulating physio-biochemical and antioxidant defense system via soil and foliar application of melatonin. Environ. Pollut. 2023, 328, 121658. [Google Scholar] [CrossRef]
- Araújo, J.D.O.; Dias, D.C.F.D.S.; Nascimento, W.M.; Martins, A.O.; Limão, M.A.R. Accelerated aging test and antioxidant enzyme activity to assess chickpea seed vigor. J. Seed Sci. 2021, 43, e202143038. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Qian, J.; Si, W.; Tan, Q.; Xu, J.; Zhao, Y. Melatonin enhances the cadmium tolerance of mushrooms through antioxidant-related metabolites and enzymes. Food Chem. 2020, 330, 127263. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, S.; Ma, L.; Kong, L.; Pan, S.; Tang, X.; Tian, H.; Duan, M.; Mo, Z. Effect of exogenous melatonin application on the grain yield and antioxidant capacity in aromatic rice under combined lead-cadmium stress. Antioxidants 2022, 11, 776. [Google Scholar] [CrossRef]
- He, J. Effect of Melatonin on the Antioxidant Capacity of Naked Oat Seedlings under Cadmium and Lead Stress. Master’s Thesis, Northwest University, Xi’an, China, 2021. [Google Scholar]
- Jahan, M.S.; Guo, S.R.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Mohamed, H.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Li, H. Effects of exogenous melatonin on photosynthetic characteristics of eggplant (Solanum melongena L.) under cadmium stress. In Proceedings of the 2015 6th International Conference on Manufacturing Science and Engineering, Guangzhou, China, 28–29 November 2015. [Google Scholar] [CrossRef]
- Yang, X.L.; Xu, H.; Li, D.; Gao, X.; Li, T.L.; Wang, R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 2018, 56, 884–892. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Wang, Y.; Dong, J.; Zhang, X.; Wang, K.; Ying, J.; Li, C.; Liu, L. Melatonin-induced DNA demethylation of metal transporters and antioxidant genes alleviates lead stress in radish plants. Hortic. Res. 2021, 8, 124. [Google Scholar] [CrossRef]
- Yan, Q.; Duan, Z.; Mao, J.; Li, X.; Dong, F. Effects of root-zone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. Soil Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.; Balabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Wang, Q.; Gao, J.; Tan, X.; Yang, F. Pre-treatment of melatonin enhances the seed germination responses and physiological mechanisms of soybean (Glycine max L.) under abiotic stresses. Front. Plant Sci 2023, 14, 1149873. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.; Helton, P.; Reiter, R. Phytoremediative capacity of plants enriched with melatonin. Plant Signal Behav. 2007, 2, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Wang, H.; Chen, S.; Reiter, R.; Zhao, D. Molecular mechanisms and evolutionary history of phytomelatonin in flowering. J. Exp. Bot. 2022, 73, 5840–5850. [Google Scholar] [CrossRef]
- Abbas, F.; Zhou, Y.; He, J.; Ke, Y.; Qin, W.; Yu, R.; Fan, Y. Metabolite and transcriptome profiling analysis revealed that melatonin positively regulates floral scent production in Hedychium coronarium. Front. Plant Sci. 2021, 12, 808899. [Google Scholar] [CrossRef]
- Ye, J.; Yang, W.; Li, Y.; Wang, S.; Yin, L.; Deng, X. Seed pre-soaking with melatonin improves wheat yield by delaying leaf senescence and promoting root development. Agronomy 2020, 10, 84. [Google Scholar] [CrossRef]
- Ibrahim, M.; Elbar, O.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.; Alkahtani, J.; El-Gawad, H. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality pro-perties of tomato plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, J.; Deng, M. Plant leaf antioxidant system and its response to stress stress. Chin. Agric. Sci. Bull. 2007, 1, 105–110. [Google Scholar] [CrossRef]
- Malecka, A.; Jarmuszkiewicz, W.; Tomaszewska, B. Antioxidative defense to lead stress in subcellular components of peer root cells. Acta Biochim. Pol. 2001, 48, 687. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2014, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Pieri, C.; Moroni, F.; Marra, M.; Marcheselli, F.; Recchioni, R. Melatonin is an efficient antioxidant. Arch. Gerontol. Geriatr. 1995, 20, 159–165. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.; Weeda, S.; Yang, C.; Yang, Z.; Ren, S.; Guo, Y. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Zoufan, P.; Zare Bavani, M.R.; Tousi, S.; Rahnama, A. Effect of exogenous melatonin on improvement of chlorophyll content and photochemical efficiency of PSII in mallow plants (Malva parviflora L.) treated with cadmium. Physiol. Mol. Biol. Plants 2023, 29, 145–157. [Google Scholar] [CrossRef]
- Gago, J.; Carriquí, M.; Nadal, M.; Clemente-Moreno, M.; Coopman, R.; Fernie, A.; Flexas, J. Photosynthesis optimized across land plant phylogeny. Trends Plant Sci. 2019, 24, 947–958. [Google Scholar] [CrossRef]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Li, R. Effect of Cd, Pb and their composite contamination on the submicrostructure of physiological ocytes in flue-cured tobacco leaves. Chin. J. Plant Ecol. 2000, 24, 238–242. [Google Scholar] [CrossRef]
- Yang, D.; Xu, C.; Zhao, F.; Dai, Y. Effect of cadmium ions on the spinach chloroplast photosystem. J. Int. Plant Biol. 1989, 52–57. [Google Scholar]
- Debnath, B.; Hussain, M.; Irshad, M.; Mitra, S.; Li, M.; Liu, S.; Qiu, D. Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 2018, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Chen, Y.; Ding, C.; Yuan, S.; Reiter, R.; Yuan, M. Melatonin: A potential agent in delaying leaf senescence. Crit. Rev. Plant Sci. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Głowacka, K.; Sokolnik, A.; Okorski, A.; Najdzion, J. The effect of cadmium on the activity of stress-related enzymes and the ultrastructure of pea roots. Plants 2019, 8, 413. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Khan, D.; Cai, N.; Zhu, W.; Li, L.; Guan, M.; Pu, X.; Chen, Q. The role of phytomelatonin receptor 1-mediated signaling in plant growth and stress response. Front. Plant Sci. 2023, 14, 1142753. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.; Zhang, J.; Shan, C.; Rengel, Z.; Song, Z.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Zhang, Y.; Guo, J.; Lu, K.; Liu, W. CAND2/PMTR1 is required for melatonin-conferred osmotic stress tolerance in arabidopsis. Int. J. Mol. Sci. 2021, 22, 4014. [Google Scholar] [CrossRef]
- Wang, L.; Lu, K.; Li, T.; Zhang, Y.; Guo, J.; Song, R.; Liu, W. Maize Phytomelatonin Receptor1 functions in plant osmotic and drought stress tolerance. J. Exp. Bot. 2021, 73, 5961–5973. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, Z.; Ma, W.; Zhang, S.; Hou, S.; Wei, J.; Dong, S.; Yu, X.; Song, Y.; Gao, W.; et al. Melatonin functions in priming of stomatal immunity in panax notoginseng and Arabidopsis thaliana. Plant Physiol. 2021, 187, 2837–2851. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wei, Y.; Yin, H.; Hu, W.; Cheng, X.; Guo, J.; Dong, Y.; Zheng, L.; Xie, H.; Zeng, H.; et al. PP2C1 fine-tunes melatonin biosynthesis and phytomelatonin receptor PMTR1 binding to melatonin in cassava. J. Pineal Res. 2022, 73, e12804. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gong, J. Vacuolar sequestration capacity and long-distance metal transport in plants. Front. Plant Sci. 2014, 5, 19. [Google Scholar] [CrossRef]
- Arif, N.; Sharma, N.; Yadav, V.; Ramawat, N.; Dubey, N.; Tripathi, D.; Chauhan, D.; Sahi, S. Understanding heavy metal stress in a rice crop: Toxicity, tolerance mechanisms, and amelioration strategies. J. Plant Biol. 2019, 62, 239–253. [Google Scholar] [CrossRef]
- Kovtun, Y.; Chiu, W.; Tena, G.; Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Rentel, M.; Knight, M. Oxidative stress-induced calcium signaling in arabidopsis. Plant Physiol. 2004, 135, 1471–1479. [Google Scholar] [CrossRef]
- Asai, S.; Ohta, K.; Yoshioka, H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 2008, 20, 1390–1406. [Google Scholar] [CrossRef]
- Xie, K.; Chen, J.; Wang, Q.; Yang, Y. Direct phosphorylation and activation of a mitogen-activated protein kinase by a calcium-dependent protein kinase in rice. Plant Cell 2014, 26, 3077–3089. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Mrabet, M.; Mhadhbi, H.; Brini, F. Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ. Sci. Pollut. Res. 2021, 28, 64967–64986. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.; Nicole, M.; Sritubtim, S.; Morency, M.; Ellis, M.; Ehlting, J.; Beaudoin, N.; Barbazuk, B.; Klessig, D.; Lee, J.; et al. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006, 11, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; He, J.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Paithankar, J.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2020, 20, 128350. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Gaillard, S.; Jacquet, H.; Vavasseur, A.; Leonhardt, N.; Forestier, C. AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana. BMC Plant Biol. 2008, 8, 22. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, S.; Yu, G.; Chen, N.; Li, X.; Liu, H. Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses. PLoS ONE 2013, 8, e82264. [Google Scholar] [CrossRef]
- Das, N.; Bhattacharya, S.; Maiti, M. Enhanced cadmium accumulation and tolerance in transgenic tobacco overexpressing rice metal tolerance protein gene OsMTP1 is promising for phytore-mediation. Plant Physiol. Biochem. 2016, 105, 297–309. [Google Scholar] [CrossRef]
- Jalmi, S.; Bhagat, P.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A. Traversing the link between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Cong, W.; Miao, Y.; Xu, L.; Zhang, Y.; Yuan, C.; Wang, J.; Zhuang, T.; Lin, X.; Jiang, L.; Wang, N.; et al. Transgenerational memory of gene expression changes induced by heavy metal stress in rice (Oryza sativa L.). BMC Plant Biol. 2019, 19, 282. [Google Scholar] [CrossRef]
- Liu, X.; Feng, S.; Zhang, B.; Wang, M.; Cao, H.; Rono, J.; Chen, X.; Yang, Z. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, S.; Feki, K.; Kamoun, Y.; Saïdi, M.; Jemli, S.; Ghorbel, M.; Alcon, C.; Brini, F. Highlight on the expression and the function of a novel MnSOD from diploid wheat (T. monococcum) in response to abiotic stress and heavy metal toxicity. Plant Physiol. Biochem. 2019, 142, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Baxter, I.; Tchieu, J.; Sussman, M.; Boutry, M.; Palmgren, M.; Gribskov, M.; Harper, J.; Axelsen, K. Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 2003, 132, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qian, Q.; Wu, K.; Luo, J.; Wang, S.; Zhang, C.; Ma, Y.; Liu, Q.; Huang, X.; Yuan, Q.; et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 2014, 46, 652–656. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Wang, F.; Yang, J.; Gao, M.; Li, C.; Liu, Y.; Liu, Y.; Yamaji, N.; Ma, J.; et al. The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels. Plant Cell 2015, 27, 711–723. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y.; et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47, 834–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, L.; Zhu, Z.; Yuan, L.; Xie, D.; Sun, C. TOND1 confers tolerance to nitrogen deficiency in rice. Plant J. 2015, 81, 367–376. [Google Scholar] [CrossRef]
- Fan, X.; Tang, Z.; Tan, Y.; Zhang, Y.; Luo, B.; Yang, M.; Lian, X.; Shen, Q.; Miller, A.; Xu, G. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. USA 2016, 113, 7118–7123. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, M.; Ma, T.; Wang, Y. Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell 2016, 28, 3005–3019. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Y.; Xu, Y.; Qi, Z.; Li, M.; Ahammed, G.; Xia, X.; Shi, K.; Zhou, Y.; Reiter, R.; et al. HsfA1a up-regulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef]
- Lee, K.; Choi, G.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferas. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Liu, Q.; Li, Z.; Cui, Y.; Shen, Z.; Zheng, L. RNA-Seq analysis of rice roots reveals the involvement of post-transcriptional regulation in response to cadmium stress. Front. Plant Sci. 2016, 6, 1136. [Google Scholar] [CrossRef]
- Hu, S.; Yu, Y.; Chen, Q.; Mu, G.; Shen, Z.; Zheng, L. OsMYB45 plays an important role in rice resistance to cadmium stress. Plant Sci. 2017, 264, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, F.; Tang, M.; Wang, Y.; Dong, J.; Ying, J.; Chen, Y.; Hu, B.; Li, C.; Liu, L. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Bai, S.; Han, X.; Feng, D. Shoot-root signal circuit: Phytoremediation of heavy metal contaminated soil. Front. Plant Sci. 2023, 14, 1139744. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J. Effect of heavy metal compound pollution in soil environment on wheat. Chin. Environ. Sci. 1993, 13, 367–371. [Google Scholar] [CrossRef]
- Lidon, F.; Henriques, R. Effect of copper toxicity on growth and uptake and translocation metals in rice plants. Plant Nutr. 1993, 16, 1449–1464. [Google Scholar] [CrossRef]
- Jahan, M.; Guo, S.; Baloch, A.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotox. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Gamuyao, R.; Chin, J.; Pariasca-Tanaka, J.; Pesaresi, P.; Catausan, S.; Dalid, C.; Slamet-Loedin, I.; Tecson-Mendoza, E.; Wissuwa, M.; Heuer, S. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 2012, 488, 535–539. [Google Scholar] [CrossRef]
- Puga, M.; Mateos, I.; Charukesi, R.; Wang, Z.; Franco-Zorrilla, J.; de Lorenzo, L.; Irigoyen, M.; Masier, S.; Bustos, R.; Rodríguez, J.; et al. SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14947–14952. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Deng, F.; Yamaji, N.; Pinson, S.; Fujii-Kashino, M.; Danku, J.; Douglas, A.; Guerinot, M.; Salt, D.; Ma, J. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun. 2016, 7, 12138. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, G.; Sánchez-Bermejo, E.; de Lorenzo, L.; Crevillén, P.; Fraile-Es-canciano, A.; Mohan, T.; Mouriz, A.; Catarecha, P.; Sobrino-Plata, J.; Olsson, S.; et al. WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 2013, 25, 2944–2957. [Google Scholar] [CrossRef]
- Zhai, Z.; Gayomba, S.; Jung, H.; Vimalakumari, N.; Piñeros, M.; Craft, E.; Rutzke, M.; Danku, J.; Lahner, B.; Punshon, T.; et al. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell 2014, 26, 2249–2264. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, L.; Jeffrey, P.; Shi, Y.; Morel, F. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 2008, 452, 56–61. [Google Scholar] [CrossRef]
- Schachtman, D.; Schroeder, J. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658. [Google Scholar] [CrossRef]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.; Eide, D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.; Von Wiren, N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 1999, 11, 937–947. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.; Blake-Kalff, M.; Hawkesford, M.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [CrossRef]
- Shin, H.; Shin, H.; Dewbre, G.; Harrison, M. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006, 140, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Rea, P. Plant ATP-Binding cassette transporters. Annu. Rev. Plant Biol. 2007, 58, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Conte, S.; Walker, E. Genetic and biochemical approaches for studying the yellow stripe-like transporter family in plants. Curr. Top. Membr. 2012, 69, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qi, C.; Li, S.; Wang, Z.; Wang, X.; Wang, J.; Ren, S.; Li, X.; Zhang, N.; Guo, Y. Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in cucumber. Plant Cell Physiol. 2018, 60, 562–574. [Google Scholar] [CrossRef]
- Gill, S.; Khan, N.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Parnika, J.; Krishan, K.; Navneet, K.; Shalu, G.; Akbar, A.; Naeem, M. Melatonin: Discovery, biosynthesis, phytohormones crosstalk, and roles in agricultural crops under abiotic stress conditions. Environ. Exp. Bot. 2024, 226, 105942. [Google Scholar] [CrossRef]
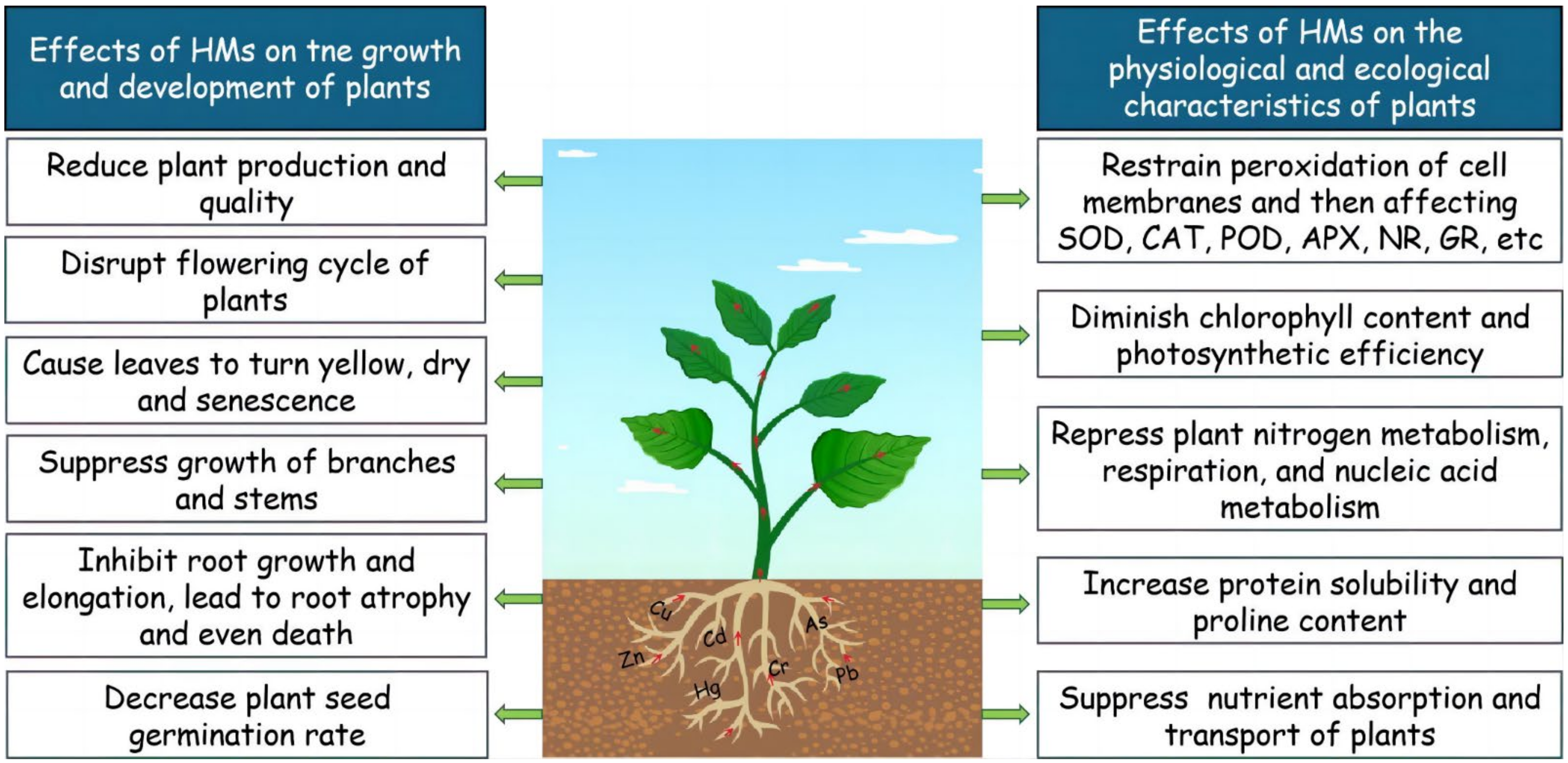
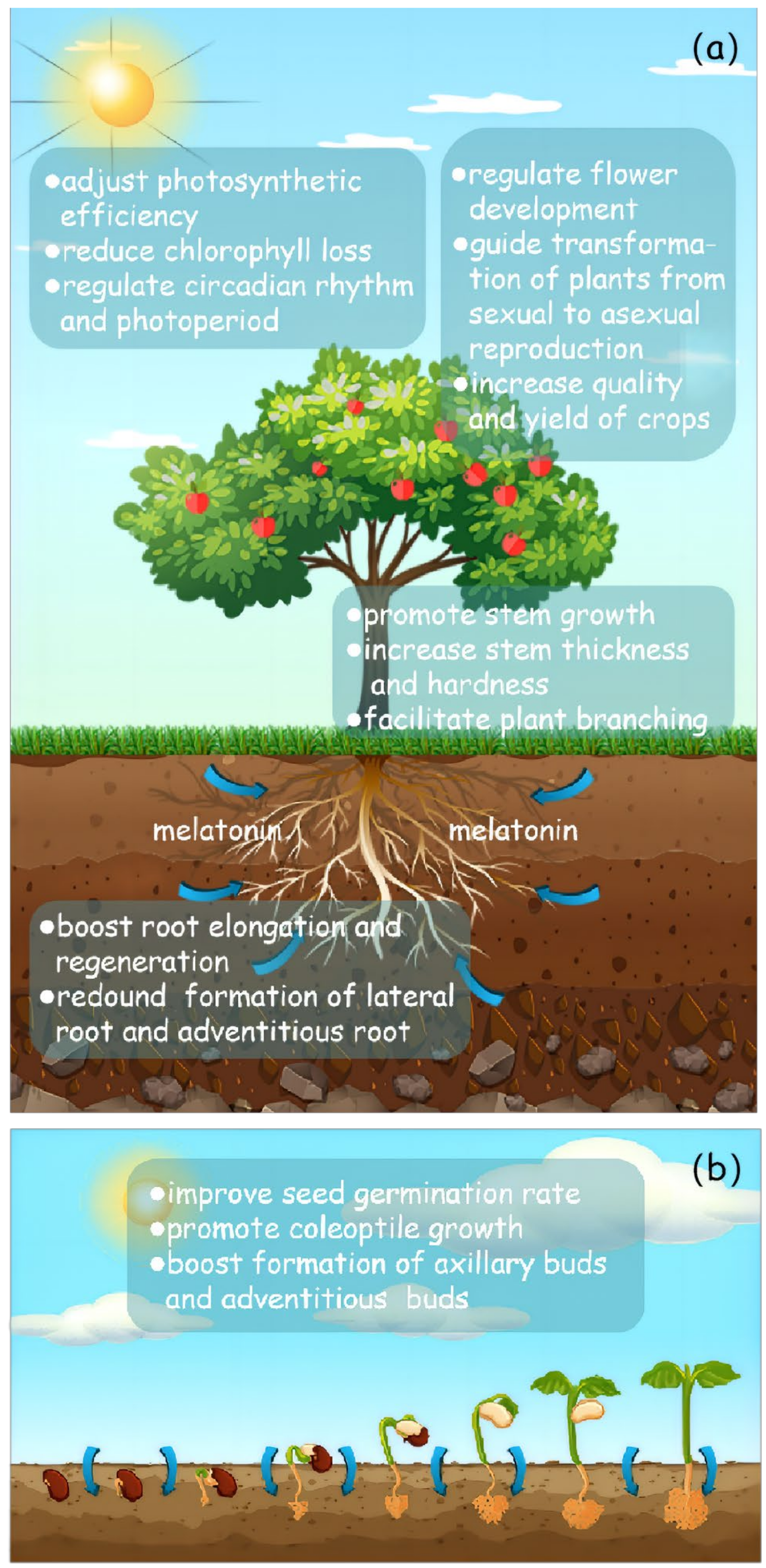
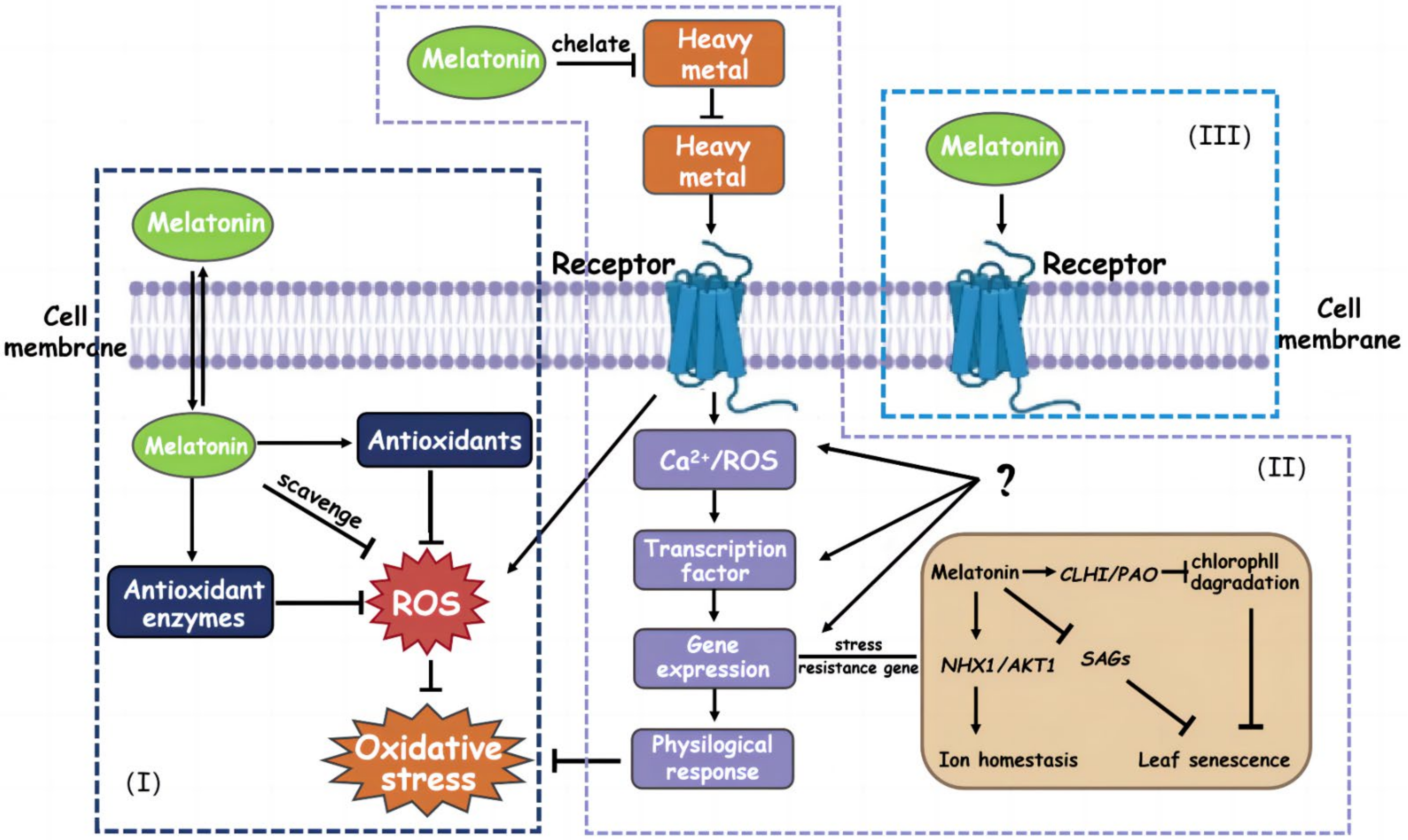

| Pollutant Type | Point Exceeding the Standard Rate (%) | Proportion of Pollution Points of Different Degrees (%) | |||
|---|---|---|---|---|---|
| Light | Mild | Moderate | Serious | ||
| Cd | 7.00 | 5.20 | 0.80 | 0.50 | 0.50 |
| Hg | 1.60 | 1.20 | 0.20 | 0.10 | 0.10 |
| As | 2.70 | 2.00 | 0.40 | 0.20 | 0.10 |
| Cu | 2.10 | 1.60 | 0.30 | 0.15 | 0.05 |
| Pb | 1.50 | 1.10 | 0.20 | 0.10 | 0.10 |
| Cr | 1.10 | 0.90 | 0.15 | 0.04 | 0.01 |
| Zn | 0.90 | 0.75 | 0.08 | 0.05 | 0.02 |
| Ni | 4.80 | 3.90 | 0.50 | 0.30 | 0.10 |
| Physiological Level | Heavy Metal | Crops | Application Ways | Melatonin Concentration | Effect | Specific Index | References |
|---|---|---|---|---|---|---|---|
| Phenotype | Cr | Wheat | soaking seed | 100 μM (50 grains) | ↑ | germination rate | [33] |
| Cu | Brassica oleracea rubrum | soaking seed | 1 μM; 10 μM (100 grains) | ↑ | germination rate | [49] | |
| Cu | Melon seedlings | soaking seed | 300 μmol/L (1000 grains) | ↑ | root growth inhibition, root structure | [34] | |
| Cu | Tomato | spraying leaf | 100 µM (6 plants) | ↑ | tomato branch and leaf growth | [35] | |
| Cd | Wheat seedlings | soaking seed | 100 µM (150 grains) | ↑ | primary root growth | [36] | |
| Cd | Rice | spraying leaf | 100 μM (9 plants) | ↑ | repression of Cd in flowering rice | [50] | |
| Cd | Naked oat | root irrigation | 50 μM; 100 μM (32 plants) | ↑ | plant height, fresh weight, and dry weight | [24] | |
| Cd | Chickpea | soaking seed | 10 μM | ↑ | seed germination and root elongation | [51] | |
| Cd | Platycladus orientalis seedlings | spraying leaf | 200 μmol/L (4 plants) | ↑ | seedling height, leaf length, leaf width, and stem width | [52] | |
| Al; Cd | Kale-shaped rape | soaking seed | 50 μM; 100 μM (100 grains) | ↑ | germination rate of seeds, bud length, and root length | [53] | |
| Antioxidant system | Al | Wheat | hydroponics | 10 μM (21 plants) | ↑ | SOD, POD, CAT, AsA, GSH, Proline, DPPH, FRAP, GSH/GSSG, AsA/DAH, H2O2, and MDA | [54] |
| Cr | Swede type rape | soaking seed | 10 μM (10 grains) | ↑ | SOD, POD, CAT, APX, H2O2, and MDA | [55] | |
| Cr | Wheat | soaking seed | 100 μM (50 grains) | ↑ | SOD, POD, CAT, APX, the pro-oxidant NADPH-oxidase, H2O2, O2− | [33] | |
| Cu | Monkey peach | root irrigation | 0.1 μmol/L (3 plants) | ↑ | proline, TPC, TFC, TFAC, AsA, DPPH, and FRAP | [56] | |
| Cu | Tomato | spraying leaf | 100 μM (6 plants) | ↑ | SOD, POD, CAT, APX, FRAP, GSH/GSSG, and ASA/DHA | [35] | |
| Cd | Pearl mille | spraying leaf | 100 μmol/L; 200 μmol/L | ↑ | proline, SOD, POD, CAT, APX, H2O2, and MDA | [57] | |
| Cd | Chickpea | soaking seed | 10 μM | ↑ | APX, MDHAR, DHAR, GSH, GSH-PX, the pro-oxidant NADPH-oxidase, NADH-oxidase activities, and H2O2 | [58] | |
| Cd | Mushrooms | hydroponics | 100 μM | ↑ | proline, SOD, POD, CAT, APX, GR, H2O2, and O2− | [59] | |
| Al; Cd | Kale-shaped rape | soaking seed | 50 μM; 100 μM (100 grains) | ↑ | SOD, POD, CAT, APX, H2O2, and MDA | [53] | |
| Pb-Cd compound | Aromatic Rice | spraying leaf; root irrigation | 50 μmol/L; 300 μmol/L (25 plants) | ↑ | AsA, GSH, SOD, POD, CAT, H2O2, and MDA | [60] | |
| Cd; Pb | Oat wheat seedlings | root irrigation | 100 μM | ↑ | proline, SOD, POD, CAT, H2O2, O2−, and MDA | [61] | |
| Photosynthetic system | Cr | Swede type rape | soaking seed | 10 μM (10 grains) | ↑ | improving the PSI and PSII efficiency | [55] |
| Cd | Tomato | spraying leaf | 100 μM (6 plants) | ↑ | chlorophyll content and photosynthetic rate | [62] | |
| Cd | Eggplant | spraying leaf | 150 μmol/L (2 plants) | ↑ | LUE, Pn, Tr, Gs, and Ci | [63] | |
| Cd | Naked oat seedlings | hydroponics | 50 μM; 100 μM (32 plants) | ↑ | chlorophyll content | [24] | |
| Cd | Platycladus orientalis seedlings | spraying leaf | 200 μmol/L (4 plants) | ↑ | chlorophyll content | [52] | |
| Al; Cd | Kale-shaped rape | soaking seed | 50 μM; 100 μM (100 grains) | ↑ | photosynthetic rate | [53] | |
| Pb-Cd compound | Aromatic Rice | spraying leaf; root irrigation | 50 μmol/L; 300 μmol/L (25 plants) | ↑ | chlorophyll content | [60] | |
| Ni | Tomato | spraying leaf | 100 μM (10 plants) | ↑ | chlorophyll content, photosynthetic rate, and blade gas-exchange parameters | [64] | |
| Gene expression | Cu | Melon seedlings | soaking seed | 300 μmol/L (1000 grains) | ↑ | AP2/ERF, BBR/BP, HD-ZIP, and transcription factor family | [34] |
| Cu | Tomato | spraying leaf | 100 μM (6 plants) | ↑ | CAT, APX, GR, MDHAR relative gene expression, TDC, SNAT, and COMT | [35] | |
| Cd | Mushrooms | hydroponics | 100 μM (8 plants) | ↑ | CAT, SOD, POD, GR, and APX relative gene expression | [59] | |
| Cd | Naked oat seedlings | hydroponics | 50 μM; 100 μM (32 plants) | ↑ | LOX, POX, Asmap1, NAC, and WRKY1 | [24] | |
| Cd | Platycladus orientalis seedlings | spraying leaf | 200 μmol/L (4 plants) | ↑ | POD, GST, APX, and ADH | [52] | |
| Cd | Chickpea | soaking seed | 10 μM | ↑ | G6PDH and RBOHF | [58] | |
| Pb | Radish | spraying leaf | 50 μM | ↑ | RsWRKY41, RsMYB2, RsAPX2, RsPOD52, and RsGST | [65] | |
| Cd; Pb | Oat wheat seedlings | root irrigation | 100 μM | ↑ | LOX, POX, and Asmap1 | [61] | |
| Al; Cd | Kale-shaped rape | soaking seed | 50 μM; 100 μM (100 grains) | ↑ | BnCOMT-1, BnCOMT-5, BnCOMT-8, BnCOMT-4, and BnCOMT-6 | [53] | |
| Nutrient absorption | Cu | Cucumber | hydroponics | 0.01 μmol/L (15 plants) | ↑ | K, Mg, Ca, P, and Mn | [66] |
| Cd | Cotton | spraying leaf | 50 μM | ↑ | Ca, Mg, Fe, Zn, N, Mn K, P, and S | [25] | |
| Ni | Tomato | spraying leaf | 100 μM (10 plants) | ↑ | N, P, Ca, Mg, S, Fe, Mn, and Zn | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Liu, X.; Liu, F.; Yang, Y.; Kou, T. Facing Heavy Metal Stress, What Are the Positive Responses of Melatonin in Plants: A Review. Agronomy 2024, 14, 2094. https://doi.org/10.3390/agronomy14092094
Cheng X, Liu X, Liu F, Yang Y, Kou T. Facing Heavy Metal Stress, What Are the Positive Responses of Melatonin in Plants: A Review. Agronomy. 2024; 14(9):2094. https://doi.org/10.3390/agronomy14092094
Chicago/Turabian StyleCheng, Xianghan, Xiaolei Liu, Feifei Liu, Yuantong Yang, and Taiji Kou. 2024. "Facing Heavy Metal Stress, What Are the Positive Responses of Melatonin in Plants: A Review" Agronomy 14, no. 9: 2094. https://doi.org/10.3390/agronomy14092094
APA StyleCheng, X., Liu, X., Liu, F., Yang, Y., & Kou, T. (2024). Facing Heavy Metal Stress, What Are the Positive Responses of Melatonin in Plants: A Review. Agronomy, 14(9), 2094. https://doi.org/10.3390/agronomy14092094





