Abstract
Improving the productivity of medicinal and aromatic species via eco-friendly approaches is imperative worldwide because of their therapeutic impacts. Biostimulants have been recognized among the best cultural practices in the last few decades. Among them, bee honey (BH) and ginger extract (GE) are new sources of multifunctional biostimulants that positively influence plant growth and development. However, there are currently no detailed reports on the impacts of BH or GE as promising growth and yield enhancers for medicinal and aromatic species. Also, the mechanism involved in stimulating growth and essential oil content with BH or GE is still unidentified. This work was, therefore, undertaken to analyze the impact of BH and GE on the growth, productivity, and essential oil content of sage plants (Salvia officinalis L.). Sage plants were sprayed monthly for three months with the same volume of BH (0, 5, 10, and 15 g L−1), GE (0, 5, 10, and 15 mg L−1), or tap water, which was used as a control. BH or GE application improved the plant height, branch number, herb yield, total chlorophyll content, total phenolics, and antioxidant capacity relative to the controls, more so with GE. Intriguingly, the essential oil percentage, oil yield, and oil constituents were enhanced by BH and GE. In this respect, the highest levels of biostimulants, particularly GE, were more effective. On a percentage basis, the essential oil yield per hectare was largely increased by 127.91 and 138.89% with GE (10 g L−1) in both seasons relative to the controls. The contents of IAA, GA3, and CK in THE sage leaves were substantially increased by BH and GE, and higher levels of both biostimulants and GE were more effective. The nutrient levels of N, P, K, Fe, Zn, and Mg were also elevated by BH and GE compared with the untreated plants. These results suggest that BH or GE application could be a promising biostimulant for improving the productivity of sage and provide a new understanding of their mechanisms in this aspect.
1. Introduction
Medicinal and aromatic species have been recognized as essential components of healthcare worldwide [1]. Recently, the demand for such plants has increased due to their active ingredient contents that have been widely used in several industries [2]. Sage (Salvia officinalis L.), also known as common sage, is a perennial aromatic woody herb native to the Mediterranean area and extensively cultivated worldwide [3]. The Salvia genus includes about 900 species, and it is the main genus of the Lamiaceae family [4,5]. Sage leaves are mainly used for food flavoring and for herbal tea in the food industry [6,7]. Further, sage is used in the perfumery, cosmetic, and pharmaceutical industries [3,8]. Moreover, sage leaves show antibacterial [9], antioxidant [10], antidiabetic [11], and antitumoral [12] activities. These properties of sage originate from its essential oil content, which contains several bioactive compounds, such as terpenes, flavonoids, phenolics, and others [13]. Due to climate change and decreasing cultivated land, competition between industrial crops has been observed, hence, maximizing the productivity of aromatic species, including sage, has become a big challenge [14].
It is known that chemical fertilization is the main factor affecting the productivity of aromatic species. However, the long-term use of chemical fertilizers leads to several concerns, such as environmental contamination, the destruction of soil microbial communities, and high costs [15,16]. Therefore, safe alternatives to these chemical fertilizers that can play a crucial role in enhancing the production of sage plants should be found. Among these safe alternative treatments, biostimulants are promising sustainable effectors for promoting growth and productivity [17]. Hence, they could be a successful alternative strategy to substitute or reduce chemical fertilizers [18]. Also, biostimulants are good for both organic and sustainable production at quite a low cost [19]. The direct impacts beyond these natural biostimulants are ascribed to the fact that they contain several plant-growth-promoting substances such as osmoprotectants, antioxidants, phytohormones, and mineral nutrients, which trigger biochemical and physiological changes, collectively improving productivity [20,21,22,23,24].
Bee honey (BH) could be a good candidate to enhance plant growth and productivity, as it is a natural substance containing mono, di-, and polysaccharides, minerals, proteins, lipids, organic and inorganic acids, phenolics compounds, and enzymes [25]. BH is rich in vitamins, antioxidants, nutrients including iodine and selenium, and osmotically active molecules, all of which make it an effective eco-friendly strategy that can be applied to enhance growth and plant productivity [20]. Several works have indicated that BH application resulted in a higher water content, photosynthetic pigments, biomass, and yield production in several plant species under normal and stress conditions [20,26,27,28]. However, no research has investigated the possible beneficial impact of BH on enhancing the growth and productivity of medicinal plants, including sage, at the field level.
Ginger extract (Zingiber officinale Roscoe, GE) as a biostimulant could be an alternate strategy for enhancing plant growth and yield. Ginger belongs to the Zingiberaceae family, and its rhizomes are widely used as dietary supplements, medicinal supplements, and spices [29]. The main components of GE are zingiberene, sabinene, camphene, β-sesquiphellandrene, α-farnesene, geranial, α-curcumene, and neral [29]. GE also contains 6-gingerol, and its derivatives have a high antioxidant capacity [30]. Moreover, GE also contains flavonoids, phenolics, carbohydrates, carotenoids, citric acid, ascorbic acid, organic acids, and nutrient elements such as phosphorus, calcium, potassium, iron, zinc, copper, magnesium, and manganese [31]. Indeed, the foliar application of GE as an eco-friendly biostimulant enhanced the growth and productivity of Origanum majorana [32] and damask rose [31]. Reports concerning the impact of GE on aromatic herbs, including sage, are, however, scarce, and information about regulations for essential oil biosynthesis and phytochemical accumulation in response to GE application in sage is yet unavailable. Accordingly, BH and GE applications were proposed to improve sage productivity and its essential oil content.
To our knowledge, the application of BH or GE as natural biostimulants to enhance sage productivity and essential oil content has not been investigated before. The current research was, therefore, conducted to assess the possibility of BH or GE foliar application as a promising biostimulant to improve the productivity and essential oil content in sage. Changes in growth, yield attributes, essential oil composition, chlorophyll content, phenolics, antioxidant capacity, phytohormones, and nutrients under control conditions and BH or GE supply were analyzed to understand the stimulatory mechanisms of both biostimulants.
2. Materials and Methods
2.1. Experimental Site and Soil Attributes
This study was conducted at the farm of the Horticulture Department, Faculty of Agriculture, Tanta University, Egypt (Lat 30°47′18.00″ N, Lon 30°59′54.61″ E) during the 2022 and 2023 seasons. Homogenous seedlings of sage (Salvia officinalis L.) were obtained from the nursery and planted on March 15th in both seasons. The soil was prepared and divided into plots (2.10 × 2 m each) that contained three rows (70 cm spacing), with 8 plants in each row (25 cm apart), resulting in 51,300 plants per hectare (10,000 m2). The experimental soil was clay, and its properties are presented in Table 1.

Table 1.
The physical and chemical properties of used soil.
2.2. Experimental Design and Treatments
Clove bee honey was used in this investigation. Bee honey (BH) was firstly diluted using distilled water, as previously described [28], and applied via foliar spraying at three concentrations of 5, 10, and 15 g L−1, denoted as BH5, BH10, and BH15, respectively. Ginger extract (GE) was prepared according to Shabana et al. [32]. Air-dried ginger rhizomes were ground into a fine powder, and a sample of 100 g was extracted using 80% ethanol. Next, the extraction was filtered using Whatman No. 1 paper (3 times), and then, a rotary evaporator was used to evaporate the alcohol and dry the extract at 40 °C under reduced pressure. Three concentrations were finally prepared at 5, 10, and 15 mg L−1 using distilled water, denoted as GE5, GE10, and GE15, respectively, and were applied via foliar spraying. The main components of BH and GE are presented in Table 2.

Table 2.
The main components of bee honey (BH) and ginger extract (GE).
One month after the sage seedling cultivation, BH or GE foliar spraying was applied and repeated monthly for three months, while tap water was used to spray the control plants. Tween 20 (Polysorbate 20, C58H114O26, 0.1%, v/v) was used as a surfactant to ensure the best penetration into leaves, and the spraying was performed until reaching the run-off point using a manual pump. The experiment was designed in a complete randomized block design (CRBD) according to Snedecor and Cochran [33], and each treatment was replicated three times. All plants received constant fertilizer doses of 100 kg/fed (calcium super phosphate, 15.5% P2O5), 75 kg/fed (ammonium nitrate, 33.5% N), and 50 kg/fed (potassium sulfate, 48% K2O) following the recommendations of the Ministry of Agriculture, Egypt.
2.3. Growth and Herb Yield Characters
For each season and after the last biostimulant spray (on 1 July in both seasons), the growth and herb yield were determined, and samples were collected for subsequent analysis in each season. Eight plants were randomly chosen to measure the plant height (cm) and fresh weight of the herb (g). To measure the dry weight of the herb (g), it was oven-dried at 70 °C until achieving a constant weight.
2.4. Determination of Essential Oil Percentage
Each fresh sample (50 g) of sage herb was extracted using hydro distillation units in a Clevenger apparatus for 3 h [19], and the percentage of essential oil was calculated using the following equation:
Essential oil percentage = (oil volume/sample FW) × 100
The essential oil yield per plant and hectare was then calculated based on the fresh herb weight and number of plants per hectare. After the essential oil collection, anhydrous sodium sulfate was used to dry the oil, and it was then stored at −4 °C in the dark until the GC-MS analysis.
2.5. Essential Oil Constituents
Identifying the components of the sage essential oil was performed by a GC (Varian, CP-3800, Inc. 2700 Mitchell Drive, Walnut Creek, CA, USA) and MS (Saturn 2200, Varian, CP-3800, Inc. 2700 Mitchell Drive, Walnut Creek, CA, USA) with a VF-5MS column (0.25 µm film thickness and ID 30 × 0.25 mm). A system of electron ionization with energy of 70 eV was utilized to detect the GC-MS. The carrier gas was helium, and it was used at a flow rate of 1 mL/m. The injector and detector temperatures of the MS transfer line were set at 130 and 280 °C, respectively. The injection of an essential oil sample (1 μL, spilt ratio 1:30) was manually performed. The constituents of the sage essential oil were identified by linking the components’ mass spectrum and retention indices with standards and also with the NIST library detected in the GC-MS system.
2.6. Determination of Chlorophyll Content
For the chlorophyll content determination, three samples from each treatment were collected at the beginning of the flowering stage from the third leaf of the main stem. Each sample (0.2 g) of sage leaves was used to extract chlorophyll using acetone solvent (80%) according to Metzner et al. [34]. The obtained extracts were then centrifuged for 10 min at 15,000× g and investigated at 663 and 647 nm by a spectrophotometer (ST150SA Model 7205, Cole-Parmer Ltd. Stone, Staffs, UK). To calculate the chlorophyll a and b contents, the following equations were used, as per Lichtenthaler [35]:
where A647 and A663 are the absorbances at wavelengths of 663 and 647 nm, respectively. Both values were then combined to calculate the total chlorophyll and are presented as mg g−1 FW.
Chl a = 12.25·A663 − 2.79·A647
Chl b = 21.50·A647 − 5.10·A663
Total Chl = Chl a + Chl b
2.7. Determination of Total Phenol Content
For the total phenol content estimation, three samples from each treatment were collected at the beginning of the flowering stage from the third leaf of the main stem. The method of McDonald et al. [36] was followed to determine the total phenols in the sage leaves. Briefly, powdered samples (1 g) were stirred with 50 mL of methanol (80%) at room temperature for two days. After that, the methanol was removed, and the obtained extract was kept at 4 °C. Then, a sample of 0.5 mL of diluted extract (1:10 g mL−1) or a standard phenolic compound (gallic acid, GA) was added to 5 mL of diluted (1:10) Folin–Ciocalteu reagent and 4 mL of sodium carbonate (1 M). Lastly, the optical density was monitored at 765 nm, and the total phenols were measured and are expressed as mg GAE g−1 DW.
2.8. Antioxidant Capacity
Three samples from each treatment were collected at the beginning of the flowering stage from the third leaf of the main stem to determine the antioxidant capacity. To investigate the antioxidant capacity in the sage leaves, the Ferric Reducing Antioxidant Power (FRAP) assay was calorimetrically performed, as reported by Benzie and Strain [37]. Firstly, the FRAP reagent was prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tri-2-pyridyl-1,3,5-triazin), and 20 mM ferric chloride (10:1:1, v/v/v). Next, a volume of 0.1 mL of leaf methanolic extract was added to 3.0 mL of FRAP reagent and kept at 37 °C for 8 min. FRAP was then measured by monitoring the optical density at 593 nm using a UV–VIS spectrophotometer, where the blank sample was ascorbic acid and the FRAP values were calculated and are presented as mg ascorbic acid equivalent (AAE) per 100 g FW (mg AAE 100 g−1 FW).
2.9. Determination of Phytohormone Contents
Three samples from each treatment were collected at the beginning of the flowering stage from the third leaf of the main stem for phytohormone determination. The GC-MS method was used to assess the contents of indole-3-acetic acid (IAA), gibberellic acid (GA3), and cytokinin (CK) in the sage leaves, as reported by [38], with minor modifications. Fresh leaf samples (0.1 g) were extracted in ice-cold extraction solvent [2 mL; methanol/water/HCl (6N); 80/19.9/0.1; v/v/v]. After that, the extract was centrifuged at 4 °C for 5 min at 25,000× g. The obtained supernatants were concentrated to 50 µL under N stream and then stored at −80 °C until investigation. For the IAA assessment, 50 µL of the supernatant was derivatized in 40 µL of methyl chloroformate and was then concentrated under the N stream to 20 µL. Then, 0.5 mg of Na2SO4 was added to dry the organic phase. For CKs and GA3, a sample of 50 µL from the dried supernatant was derivatized in 100 µL of N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) by heating for 45 min at 85 °C. To perform the GC-MS investigation, 1 µL was injected into the GC-MS running in the selective ion mode. All phytohormone standards and samples were analyzed using a Varian GC (CP-3800, Varian, Inc. 2700 Mitchell Drive, Walnut Creek, CA, USA) fitted with a CP-5MS column (30 m × 0.25 mm × 0.25 µm film thickness; Saturn 2200, Varian, Inc. 2700 Mitchell Drive, Walnut Creek, CA, USA). Helium was used as a carrier gas with a flow rate of 1 mL min−1. The temperature program for IAA was performed as follows: the column was held at 50 ºC for 3 min, then increased to 200 °C at a rate of 4 °C min−1, and held for 5 min. The program for CK and GA3 was performed as follows: the column was held at 60 °C for 2 min, then increased to 160 °C at 20 °C min−1, and finally to 290 °C at 5 °C min−1. The injector and detector temperatures were 250 and 260 °C, respectively. IAA, CK, and GA3 were identified by comparing their retention times, linear retention indices, and the selected ions with those of the standards.
2.10. Determination of Nutrient Elements
Each sage leaf sample (0.5 g) was oven-dried at 70 °C for 48 h. Then, all samples were milled by a micro hammer mill to obtain a fine powder suitable for mineral analysis. Perchloric acid (HClO4) and sulfuric acid (H2SO4) (1:5) methodology was used to digest the dried samples for determination of the nutrient content, as previously reported [39]. The method of micro-Kjeldhl was used to determine the nitrogen content [40]. The phosphorus content was colorimetrically assessed by a spectrophotometer (Pharmacia, LKB-Novaspec II, Cambridge, UK) using the stannous chloride phosphomolybdic–sulfuric acid system, and the optical density was monitored at 660 nm [41]. The potassium content was determined using the flame photometer (Flame Photometer 410 Sherwood Scientific Ltd., Cambridge, UK) according to Prasad et al. [42]. The contents of Mg, Zn, and Fe were spectrophotometrically measured, as previously reported [39].
2.11. Statistical Analysis
For each season, an analysis of variance (ANOVA) was conducted using the SPSS (13.3 version) program (IBM, New York, NY, USA). Tukey–Kramer’s multiple range test at p ≤ 0.05 was utilized to separate the means. Data are presented as means ± SD (n = 3). A principal component analysis (PCA) was performed using JMP Data Analysis Software Version 9 (SAS Institute Inc., 270 Cary, NC, USA). The PCA analysis was performed using the average data of both seasons.
3. Results
3.1. Growth Attributes
The foliar application of BH or GE significantly enhanced the plant height and branch number of the sage plants compared with the controls in both experimental seasons, and the effect was more pronounced with GE (Table 3). Both characters were increased with increasing BH or GE levels. The elevation in the plant height was 31.53 and 41.62% in the first season and 31.13 and 44.41% in the second one when BH10 or GE10 was applied, respectively, relative to their controls. Similarly, the branch number increased by 83.30 and 123.96% in the first season and by 66.06 and 108.94% in the second season under both treatment applications (BH10 or GE10).

Table 3.
Impact of foliar spray with bee honey (BH) or ginger extract (GE) on plant height and branch number of sage plants.
3.2. Herb Yield
The data in Table 4 reveal that sage plants that were exogenously sprayed with various BH or GE levels produced significantly greater fresh and dry herb yields per plant compared with the controls in both seasons, more so with GE. The maximum herb yield was obtained by applying the GE10 treatment, and no further increase in herb yield was obtained with an increasing GE level in both seasons. The fresh and dry herb yields per plant were increased by 58.13 and 78.87% in the first season and by 61.03 and 84.21% in the second one in response to the GE10 treatment relative to the untreated plants. A comparable percentage increase for the fresh and dry herb yield was found with BH15.

Table 4.
Impact of foliar spray with bee honey (BH) or ginger extract (GE) on fresh and dry herb weights of sage plants.
3.3. Essential Oil Content
All applied levels of BH or GE significantly increased the essential oil percentage and essential oil yield per plant and per feddan in comparison with the untreated controls in both experimental seasons (Table 5). The percentage and yield of essential oil were increased with increasing BH levels, whereas GE10 applicated resulted in the highest essential oil content in the sage leaves in both seasons. Relative to the untreated plants, the essential oil yield per hectare was increased by 98.16 and 106.25% in response to BH15 treatment, but it was 127.91 and 138.89% after GE10 application in both seasons (Table 5).

Table 5.
Impact of foliar spray with bee honey (BH) or ginger extract (GE) on essential oil percentage and yield of sage plants.
3.4. Essential Oil Composition
The main constituents detected in the essential oil were 1.8-Cineole, α-Thujone, β-Thujone, camphor, borneol, and viridiflorol, which were elevated in response to the foliar application of BH or GE compared with the untreated plants (Table 6). Though this impact increased with increasing BH or GE levels, the main components were higher in the GE-treated plants than in the BH-treated ones. The highest percentage of the identified compounds (97.54%) was obtained by GE10 treatment, followed by BH15 treatment (96.49%), relative to their controls.

Table 6.
GC-MS analysis of sage essential oil in response to foliar spray with bee honey (BH) or ginger extract (GE).
3.5. Total Chlorophyll Content
The BH- and GE-treated plants exhibited significant chlorophyll content relative to the controls in both experimental seasons, more so with the GE foliar spray (Table 7). Among the various treatments, GE10 and GE15 showed the greatest chlorophyll contents in both seasons, without significant differences between either treatment. Compared with the controls, plants sprayed with GE10 had 20.54 and 20.21% higher chlorophyll levels, while the chlorophyll levels in the BH15-treated plants were 15.67 and 15.96% higher in both seasons, respectively (Table 7).

Table 7.
Impact of foliar spray with bee honey (BH) or ginger extract (GE) on total chlorophyll content, total phenolics, and ferric reducing antioxidant power (FRAP) of sage plants.
3.6. Total Phenolics
The total phenolics were considerably enhanced in response to BH or GE application compared with the controls, an increase was observed with increasing biostimulant levels, and GE was more effective than BH (Table 7). The total phenolics increased by 33.63 and 35.17% after the application of BH15 and 53.97 and 54.88% after GE15 application compared with the untreated controls in both seasons, respectively.
3.7. Antioxidant Capacity
The antioxidant capacity of the sage leaves measured by the Ferric Reducing Antioxidant Power (FRAP) showed that FRAP was significantly improved after applying BH or GE compared with the controls in both seasons (Table 7). FRAP was gradually increased with increasing the BH or GE level. The FRAP values in the GE-treated plants were higher than those in the BH-treated ones in both seasons. On a percentage basis, the increase in the antioxidant capacity was 38.65 and 38.37% after BH15and 65.03 and 69.81% after GE15 application compared with the untreated controls in both seasons, respectively.
3.8. Phytohormone Content
The endogenous phytohormones of sage leaves (IAA, GA3, and CK) showed significant elevation with BH or GE treatment, more so with GE application (Table 8). Generally, the highest IAA, GA3, and CK contents in both seasons were observed with the highest concentrations of both GE and BH (10 and 15 mg L−1), with no significant differences between them.

Table 8.
Impact of foliar spray with bee honey (BH) or ginger extract (GE) on endogenous phytohormones of sage plants.
3.9. Nutrient Content
The data in Table 9 indicate that all levels of BH or GE significantly enhanced the contents of the nutrients N, P, K, Fe, Zn, and Mg in the sage leaves compared with their controls in both experimental seasons. The contents of the previous nutrients increased with an increase in the BH or GE level. On a percentage basis, the impact was more pronounced with GE in both seasons.

Table 9.
Impact of foliar spray with bee honey (BH) or ginger extract (GE) on nutrient contents of sage plants.
3.10. Principal Component Analysis (PCA)
The PCA biplot results reveal the loading of the variables on the first two principal (PC1 and PC2) components, and both principal axes explain the variance (Figure 1). The arrows indicate the strength and direction of each parameter. The long arrows show that all variables are strongly implied in the plot. The biplot provides valuable information about the correlations among variables. The components of PC1 and PC2 successfully separate the effect of the BH and GE treatments. The effect of both biostimulants appear to group together, however, the untreated control is markedly varied. All variables show a positive correlation with PC1 and positively correlate with each other.
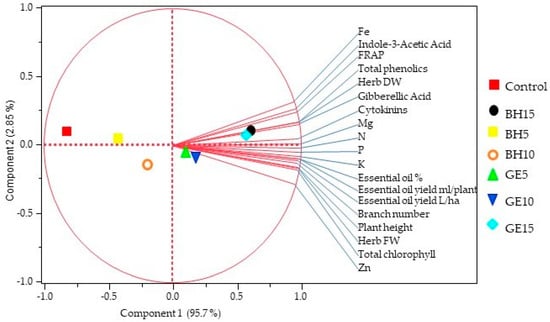
Figure 1.
Biplot of principal component analysis (PCA) for sage plants sprayed with bee honey (BH) or ginger extract (GE). BH5, BH10, and BH15 mean bee honey at 5, 10, and 15 g L−1, while GE5, GE10, and GE15 mean ginger extract at 5, 10, and 15 mg L−1, respectively. The circle around vectors represents perfect correlation. The arrows represent the variables, and the colored shapes represent various treatments. PCA analysis was performed using the average data of both seasons.
4. Discussion
Enhancing the growth and yield of medicinal and aromatic plants, including sage, via eco-friendly applications has attracted great interest in sustainable and organic production. Our study proved that BH and GE were effective biostimulants that improved sage growth, yield, and essential oil content. Both biostimulants have been reported to contain several bioactive molecules that considerably and efficiently participate in stimulating the growth and productivity of sage [20,25,31]. These molecules include flavonoids, phenolics, proteins, lipids, carbohydrates, carotenoids, citric acid, ascorbic acid, organic acids, and nutrient elements such as phosphorus, calcium, potassium, iron, zinc, copper, magnesium, and manganese. We assume that the bioactive ingredients included in GE or BH were most likely integrated into the sage metabolism and had a stimulatory impact on the primary and secondary metabolites that were used to build substances of various sage cells, and tissues, hence inducing growth and yield. Also, the elevation in phytohormone levels might have effectively regulated pathways related to improved growth and essential oil production. Altogether, this resulted in a promotional effect on sage growth and productivity. In support of our proposal, a previous publication showed that moringa leaf extract containing such components detected in GE markedly enhanced geranium growth and productivity [22]. Also, applying soluble sugars, one constituent found in BH, exhibited a dominant regulatory function and considerably enhanced the growth and yield of different plant species [28,43,44,45]. Accordingly, both GE and BH can be accepted as eco-friendly biostimulants because of their contents of several bioactive compounds. It is obvious that the current results support our hypothesis considering both biostimulants as promising effective plant growth promoters. A positive correlation was noticed in the PCA between growth characteristics such as the plant height, branch number, and herb yield of the sage plants following the application of BH or GE (Figure 1). This confirms the efficiency of both BH and GE as natural safe biostimulants for enhancing the crucial sage productivity.
The enhanced growth and yield attributes of the sage are most likely ascribed to the increased endogenous phytohormones, essential nutrients, chlorophyll, and antioxidant capacity triggered by BH and GE application. As various nutrients markedly affect the growth and development processes in plants [46] and receiving the proper levels of nutrient elements enhances the growth characteristics in several aromatic plants [16,47,48,49], stimulating essential nutrient elements with BH or GE treatment is proposed to contribute largely to the enhanced sage growth. In agreement with the current results, improved growth and yield have been observed in Syngonium in response to BH treatment [26] and damask rose in response GE [31]. Further, Taiz and Zeiger [50] reported that auxins enhance cell elongation and stem growth, GA3 enhances cell division and elongation, and cytokinins promote cell division and modify apical dominance. We, therefore, speculate that BH- or GE-induced phytohormones markedly participated in enhancing the fresh and dry herb yield, as well as the plant height, branch number, and, consequently, biomass production of sage. Moreover, chlorophylls play a fundamental role in photosynthesis, and changes in their levels are related to the efficiency of the photosynthetic machinery and, hence, plant growth and yield [51]. Thus, we assume that the elevated chlorophyll content due to GE or BH most probably contributed to the stimulated growth and yield of the sage, which is supported by a strong positive correlation observed in the PCA between the herb yield and chlorophyll content (Figure 1).
BH or GE foliar application not only enhanced the essential oil percentage and yield, but also improved the essential oil composition of the sage. Our finding is the first to show the positive impact of BH and GE on essential oil accumulation in sage plants. Therefore, we recommend BH and GE as promising biostimulants that may positively impact the physiological and biochemical processes that directly or indirectly affect the terpenoid pathway, hence improving the oil content of sage. BH and GB the improved essential oil content and its composition, which may be attributed to the detected important constituents in BH and GB that can promote the accumulation of the secondary metabolite in sage leaves. Our proposal is supported by the results of other researchers [22,25,45], who illustrated that the foliar application of soluble sugars or other biostimulants containing similar bioactive compounds found in BH markedly enhanced the essential oil accumulation in different aromatic species [22,45]. Consistently, the application of GE resulted in quantitative and qualitative modifications of the essential oil content of Origanum majorana [32] and Damask rose [31] as well. In this study, the BH- and GE-treated plants showed higher chlorophylls, total phenols, and antioxidant capacity. An improved chlorophyll content via another biostimulant (moringa leaf extract) was also shown in geranium, resulting in increased biomass production [22]. Increasing the total chlorophyll content in sage leaves by BH or GB could be interpreted by the presence of several active compounds induced by these biostimulants in the leaves [22,45], which might enhance chlorophyll biosynthesis and accumulation. In support, ref. [45] demonstrated that chlorophyll content was increased in response to polysaccharide application to chamomile. Furthermore, the Mg element, as a crucial chlorophyll constituent, as well as other nutrients, was promoted by BH and GB spray and most probably also enhanced chlorophyll biosynthesis, resulting in escalating the photosynthetic efficiency and, thus, the plant’s growth and productivity [20,28,31]. This result is consistent with the PCA that showed positive correlations between the Mg and chlorophyll content in the sage leaves. Consistently, ref. [31] also reported that the existence of Mg in ginger extract elevated chlorophyll biosynthesis and accumulation in damask rose. Our finding that either BH or GE increased the total phenolics of the sage leaves may be explained by the contention that phenolics and flavonoids are enriched in both biostimulants and, in turn, elevate the phenolics level in sage leaves [28,31]. The total phenolic content was similarly improved by the foliar application of BH to faba bean [20] and GE to Damask rose [31]. The current work also showed that sage leaves saw a significant improvement in their antioxidant capacity after applying BH or GE, which may be interpreted by the increased content of phenolic compounds in response to BH or GE treatment. Similar results have been previously observed in Damask Rose after GE [32] and in faba bean after BH [20,28] application. These studies illustrate marked enhancements of non-enzymatic, enzymatic antioxidants, and antioxidant capacity, which might be the case in our study. Similarly, the antioxidant capacity was increased by other biostimulant applications in several medicinal and aromatic species [22,45,52]. It is noteworthy that the antioxidant capacity is a very important factor in evaluating medicinal and aromatic plants [53], and based on that, phenolics have gained extensive attention due to their antioxidant activities and, thus, health-promoting properties [54].
The foliar application of BH or GE considerably increased the phytohormone levels (IAA, GA3, and CK) in the sage leaves, which is a novel result. This finding might be explained by the biostimulant induction of mineral nutrients, which are required for both protoplasm and phytohormone formation [27]. In agreement with our results, other biostimulants (propolis and maize grain extracts) have been reported to increase the endogenous hormone levels in common bean [55]. Therefore, we propose that the enhanced endogenous hormones efficiently promoted sage growth and herb productivity, which, in turn, increased the essential oil content observed in this research. Our proposal is supported by the finding that growth regulators showed a promotional effect on both the essential oil percentage and oil constituents via regulating the terpenoid pathway in several aromatic species [56,57]. In addition, Rady et al.’s [20] work on Vicia faba L. and Belal et al.’s [28] work on Phaseolus vulgaris L. reported BH improvements in the hormone levels that contributed to enhanced growth and productivity. Based on the above, we believe that elevated phytohormone levels in response to BH or GE application largely contributed to the observed improvement in the sage essential oil content. In support, the PCA indicated a strong positive correlation among the phenolic content, phytohormones, and essential oil content (Figure 1). Therefore, it appears that these positive findings triggered by both biostimulants can be ascribed to their marked improvements in phytohormone levels, antioxidant defense systems, osmoregulatory compounds, and improved chlorophyll content, all of which minimize ROS-catalyzed oxidative stress. This led to remarkable enhancements in sage integrity, photosynthetic efficiency, nutrient contents, and hormonal homeostasis, which eventually resulted in noticeable stimulation in sage growth, productivity, and production quality.
The BH- and GE-induced N, P, K, Fe, Zn, and Mg contents of the sage leaves could be interpreted by the fact that both biostimulants are rich in these nutrients [20,22], and, thus, their foliar spray enriched these nutrients in the sage leaves. Comparable findings are that foliar application of the biostimulant moringa leaf extract increased the nutrient elements in other aromatic species [21,31]. Also, a substantial increase in essential nutrients was reported in geranium leaves by applying moringa leaf extract as a biostimulant [22]. Increased essential nutrients in response to BH or GE treatment most likely contributed to the sage’s enhanced growth and productivity observed in the current study. In support, the foliar application of BH enlarged the root system size, hence enhancing the development of the absorbing root surface [28], which induced ion hemostasis and escalated the mineral content in the leaves [20]. The PCA similarly showed a positive correlation among the essential elements and the growth, yield, and oil content of the sage plants sprayed with BH or GE (Figure 1). Taken together and as proposed above, GE and BH induced phytohormones, nutrients, antioxidant capacity, and chlorophylls in the sage leaves, which seemed to play a pivotal role in improving the sage growth criteria and, hence, productivity.
5. Conclusions
Our study is the first to report that the foliar application of BH and GE enhances sage growth, herb production, and essential oil content, and GE is more effective. The positive beneficial impact of BH and GE is ascribed to the stimulation of phytohormone levels, nutritional status, chlorophyll content, and leaf antioxidant capacity, resulting in improved growth and branching, and, in turn, enhancing the herb and essential oil yield and its constituents. These positive marked improvements most probably result in minimizing ROS-catalyzed oxidative stress, which leads to remarkable enhancement in sage integrity, efficient photosynthesis, and hormonal homeostasis. This eventually leads to noticeable stimulation in growth, productivity, and production quality. We, therefore, strongly recommend BH and GE as innovative eco-friendly biostimulants for commercial application of sage production, as well as other aromatic species. However, further studies are required to explore more aromatic plants to support our findings.
Author Contributions
Conceptualization, F.A.S.H. and M.M.M.; methodology, M.M.M.; software, M.E.-S.; validation, M.E.-S. and M.M.F.M.; formal analysis, M.M.M.; investigation, M.E.-S.; resources, M.M.M.; data curation, M.E.-S.; writing—original draft preparation, F.A.S.H.; writing—review and editing, M.M.F.M.; visualization, M.M.M.; supervision, F.A.S.H.; project administration, M.E.-S.; funding acquisition, M.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The paper was funded by Taif University by Project No. (TU-DSPP-2024-134), Taif University, Taif, Saudi Arabia.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schippmann, U.; Leaman, D.; Cunningham, A.B. A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. In Medicinal and Aromatic Plants; Bogers, R.J., Ed.; UR Frontis Series; Springer: Dordrecht, The Netherlands, 2006; Volume 17, pp. 75–95. [Google Scholar]
- Said-Al Ahl, H.; Hussein, M.S.; Gendy, A.S.H.; Tkachenko, K.G. Quality of Sage (Salvia officinalis L.) Essential Oil Grown in Egypt. Int. J. Plant Res. 2015, 1, 119–123. Available online: http://www.aiscience.org/journal/ijpr (accessed on 5 June 2024).
- Bruneton, J. Pharmacognosy, Phytochemistry Medicinal Plants; Lavoisier Intercept: London, UK, 1999. [Google Scholar]
- El-Feky, A.M.; Aboulthana, W.M. Phytochemical and biochemical studies of sage (Salvia officinalis L.). UK J. Pharm. Biosci. 2016, 4, 56. [Google Scholar]
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) is not monophyletic: Implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef]
- Tisserand, R.; Balacs, T. Essential Oil Safety; Churchill Livingstone: New York, NY, USA, 1995. [Google Scholar]
- Demirci, B.; Hüsnü, K.; Baser, C.; Tümen, G. Composition of the essential oil of Salvia aramiensis Rech. fil. growing in Turkey. Flavour Frag. J. 2005, 17, 23–25. [Google Scholar] [CrossRef]
- Delamare Longaray, A.P.L.; Ivete, T.M.P.; Artico, L.; Atti-Serafini, L.; Echeverrrigary, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in south Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Özcan, B.; Birgul, E.M.; Coleri, A.; Yolcu, H.; Caliskan, M. In vitro antimicrobial and antioxidant activities of various extracts of Salvia microstegia (Boiss.) et. Bal. from Antakya, Turkey. Fresenius Environ. Bull. 2009, 18, 658–662. [Google Scholar]
- Kolak, U.; Kabouche, A.; Ozturk, M.; Kabouche, Z.; Topçu, G.; Ulubelen, A. Antioxidant diterpenoids from the roots of Salvia barrelieri. Phytochem. Anal. 2009, 20, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jung, S.N.; Son, K.H.; Kim, S.R.; Ha, T.Y.; Park, M.G.; Jo, I.G.; Park, J.G.; Choe, W.; Kim, S.; et al. Antidiabetes and antiobesity effect of cryptotanshinone via activation of AMP-activated protein ki-nase. Mol. Pharmacol. 2007, 72, 62–72. [Google Scholar] [CrossRef]
- Cardile, V.; Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Piozzi, F. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J. Ethnopharmacol. 2009, 126, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of Bioactive Properties and Phenolic Compounds in Different Extracts Prepared from Salvia officinalis L. Food Chem. 2014, 170, 378–385. [Google Scholar] [CrossRef]
- Pandey, V.; Patra, D.D. Crop productivity, aroma profile and antioxidant activity in Pelargonium graveolens L’Her. under integrated supply of various organic and chemical fertilizers. Ind. Crop. Prod. 2015, 67, 257–263. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Kahil, A.A.; Ali, E.F.; Hassan, F. Influence of bio-fertilizers on growth, yield and anthocyanin content of Hibiscus sabdariffa L. plant under Taif region conditions. Annu. Res. Rev. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hort. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.F.; Mostafa, N.Y.; Mazrou, R. Shelf-life extension of sweet basil leaves by edible coating with thyme volatile oil encapsulated chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 177, 517–525. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Al-Yasi, H.; Ali, E.F.; Alamer, K.; Hessini, K.; Attia, H.; El-Shazly, S. Mitigation of salt stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 2021, 101, 157–165. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Arena, M.E.; Postemsky, P.D.; Curvetto, N.R. Changes in the phenolic compounds and antioxidant capacity of Berberis microphylla G. Forst. Berries in relation to light intensity and fertilization. Sci. Hortic. 2017, 218, 63–71. [Google Scholar] [CrossRef]
- Yakhin, O.L.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Inés, M.; Craig, A.; Ordoñez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomón, V.; Maldonado, L. LWT—Food Science and Technology Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT Food Sci. Technol. 2011, 44, 1922–1930. [Google Scholar]
- El-Hanafy, S.H. A pilot study exploring the effects of bee honey as a bio-fertilizer on the morphological features and chemical constituents of Syngonium podophyllum plants. J. Product. Dev. 2007, 12, 299–314. [Google Scholar] [CrossRef][Green Version]
- Semida, W.M.; Abd El-Mageed, T.A.; Hemida, K.; Rady, M.M. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defence system. J. Hortic. Sci. Biotechnol. 2019, 94, 632–642. [Google Scholar] [CrossRef]
- Belal, H.E.E.; Abdelpary, M.A.M.; Desoky, E.-S.M.; Ali, E.F.; Al Kashgry, N.A.T.; Rady, M.M.; Semida, W.M.; Mahmoud, A.E.M.; Sayed, A.A.S. Effect of eco-friendly application of bee honey solution on yield, physio-chemical, antioxidants, and enzyme gene expressions in excessive nitrogen-stressed common bean (Phaseolus vulgaris L.) plants. Plants 2023, 12, 3435. [Google Scholar] [CrossRef]
- Yeh, H.; Chuang, C.; Chen, H.; Wan, C.; Chen, T.; Lin, L. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT—Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Chen, C.; Kuo, M.; Wu, C.; Ho, C. Pungent compounds of ginger (Zingiber officinale (L) Rosc) extracted by liquid carbon dioxide. J. Agric. Food Chem. 1986, 34, 477–480. [Google Scholar] [CrossRef]
- Ali, E.F.; Al-Yasi, H.M.; Issa, A.A.; Hessini, K.; Hassan, F.A.S. Ginger extract and fulvic acid foliar applications as novel practical approaches to improve the growth and productivity of damask rose. Plants 2022, 11, 412. [Google Scholar] [CrossRef]
- Shabana, M.H.; Balbaa, L.K.; Talaat, I.M. Effect of foliar applications of Zingiber officinale extracts on Origanum majorana. J. Herbs Spices Med. Plants 2017, 23, 89–97. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Metzner, H.; Rau, H.; Senger, H. Unter suchungen zur synchronisier barteit einzelner pigmentan angel mutanten von chlorela. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids Pigments of Photosynthetic Biomembranes. In Methods Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Phytohormone profiling of the sweet orange (Citrus sinensis (L.) Osbeck) leaves and roots using GC-MS-based method. J. Plant Physiol. 2016, 199, 12–17. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists International. Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Jackson, W.A. Nitrate acquisition and assimilation by higher plants: Processes in the root system. In Nitrogen in the Environment, Vol 2 Soil-Plant-Nitrogen Relationships; Nielsen, D.R., MacDonald, J.G., Eds.; Academic Press: New York, NY, USA, 1978; pp. 45–88. [Google Scholar]
- Prasad, R.; Shivay, Y.S.; Kumar, D.; Sharma, S.N. Learning by Doing Exercise in Soil Fertility—Practical Manual for Soil Fertility; Indian Agricultural Research Institute: New Delhi, India, 2006. [Google Scholar]
- Zahid, M.; Iqbal, N.; Muhammad, S.; Faisal, S.; Mahboob, W.; Hussain, M. Efficacy of exogenous applications of glucose in improving wheat crop (Triticum aestivum L.) performance under drought stress. Pak. J. Agric. Sci. 2018, 31, 264–273. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, L.; Duan, X.; Chai, X.; Huang, R.; Kang, Y.; Yang, X. Effects of exogenous sucrose and selenium on plant growth, quality, and sugar metabolism of pea sprouts. J. Sci. Food Agric. 2022, 102, 2855–2863. [Google Scholar] [CrossRef]
- Mazrou, R.; Ali, E.F.; Hassan, S.; Hassan, F.A.S. A pivotal role of chitosan nanoparticles in enhancing the essential oil productivity and antioxidant capacity in Matricaria chamomilla L. Horticulturae 2021, 7, 574. [Google Scholar] [CrossRef]
- Ahmed, M.; Hasanuzzaman, M.; Raza, M.A.; Malik, A.; Ahmad, S. Plant nutrients for crop growth, development and stress tolerance. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 43–92. Available online: https://link.springer.com/chapter/10.1007/978-3-030-45669-6_3 (accessed on 17 July 2024).
- Mekdad, A.A.A.; Abou El-Enin, M.M.; Rady, M.M.; Hassan, F.A.S.; Ali, E.F.; Shaaban, A. Impact of level of nitrogen fertilization and critical period for weed control in peanut (Arachis hypogaea L.). Agronomy 2021, 11, 909. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Rady, M.M.; Ali, E.F.; Hassan, F.A.S. Early sowing combined with adequate potassium and sulfur fertilization: Promoting Beta vulgaris (L.) yield, yield quality, and K- and S-use efficiency in a dry saline environment. Agronomy 2021, 11, 806. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.F.; Mahfouz, S. Comparison between different fertilization sources, irrigation frequency and their combinations on the growth and yield of coriander plant. Aust. J. Appl. Basic Sci. 2012, 6, 600–615. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the photosynthetic apparatus functions by chlorophyll fluorescence and P700 absorbance in C3 and C4 plants under physiological conditions and under salt stress. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar] [CrossRef] [PubMed]
- El-Serafy, R.S.; Dahab, A.A.; Ghanem, K.Z.; Elhakem, A.; Bahgat, A.; Venkatesh, J.; El-Sheshtawy, A.; Badawy, A. As a natural antioxidant: Sesbania Grandiflora leaf extract enhanced growth and yield performance, active ingredients and tolerance of Hibiscus Sabdariffa L. under salt-affected Soil. J. Soil Sci. Plant Nutr. 2024, 24, 3406–3420. [Google Scholar] [CrossRef]
- Ali, E.F.; Issa, A.A.; Al-Yasi, H.M.; Hessini, K.; Hassan, F.A.S. The efficacies of 1-methylcyclopropene and chitosan nanoparticles in preserving the postharvest quality of Damask rose and their underlying biochemical and physiological mechanisms. Biology 2022, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Behdad, A.; Mohsenzadeh, S.; Azizi, M.; Moshtaghi, N. Salinity effects on physiological and phytochemical characteristics and gene expression of two Glycyrrhiza glabra L. populations. Phytochemistry 2020, 171, 112236. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Rady, M.M. Presoaking application of propolis and maize grain extracts alleviates salinity stress in common bean (Phaseolus vulgaris L.). Sci. Hortic. 2014, 168, 210–217. [Google Scholar] [CrossRef]
- Bano, U.; Khan, A.F.; Mujeeb, F.; Maurya, N.; Tabassum, H.; Siddiqui, M.H.; Haneef, M.; Osama, K.; Farooqui, A. Effect of plant growth regulators on essential oil yield in aromatic plants. J. Chem. Pharm. Res. 2016, 8, 733–739. [Google Scholar]
- Khan, A.F.; Mujeeb, F.; Aha, F.; Farooqui, A. Effect of plant growth regulators on growth and essential oil content of palmarosa (Cymbopogon martinii). Asian J. Pharm. Clin. Res. 2015, 8, 373–376. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).