S-Benzyl-L-cysteine Inhibits Growth and Photosynthesis, and Triggers Oxidative Stress in Ipomoea grandifolia
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Preparation and Growth Conditions
2.2. Biometric Parameters
2.3. Photosynthetic Parameters
2.4. Oxidative Stress Parameters
2.5. Enzyme Parameters
2.6. Statistical Analysis
3. Results
3.1. The Impact of SBC on Plant Growth
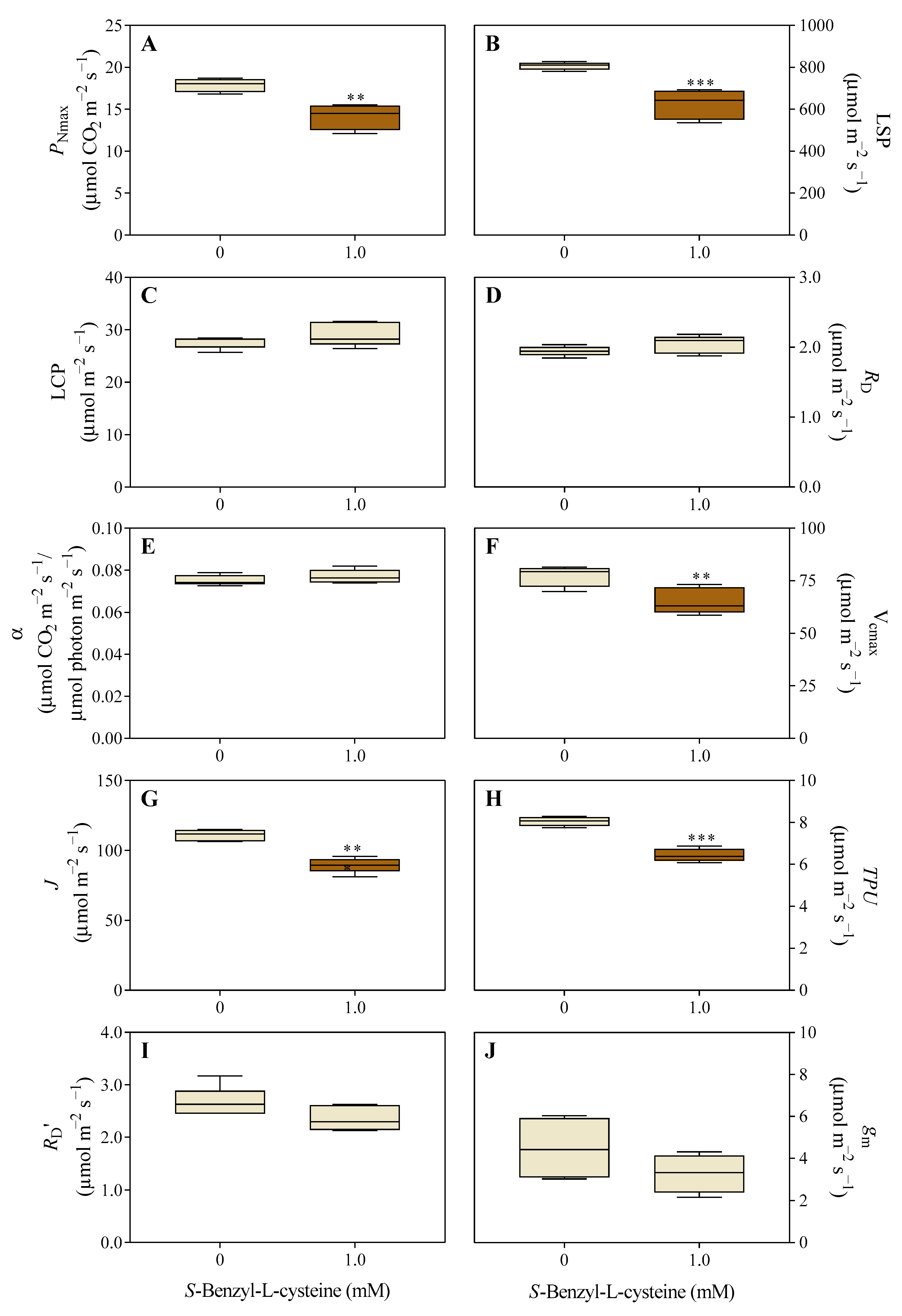
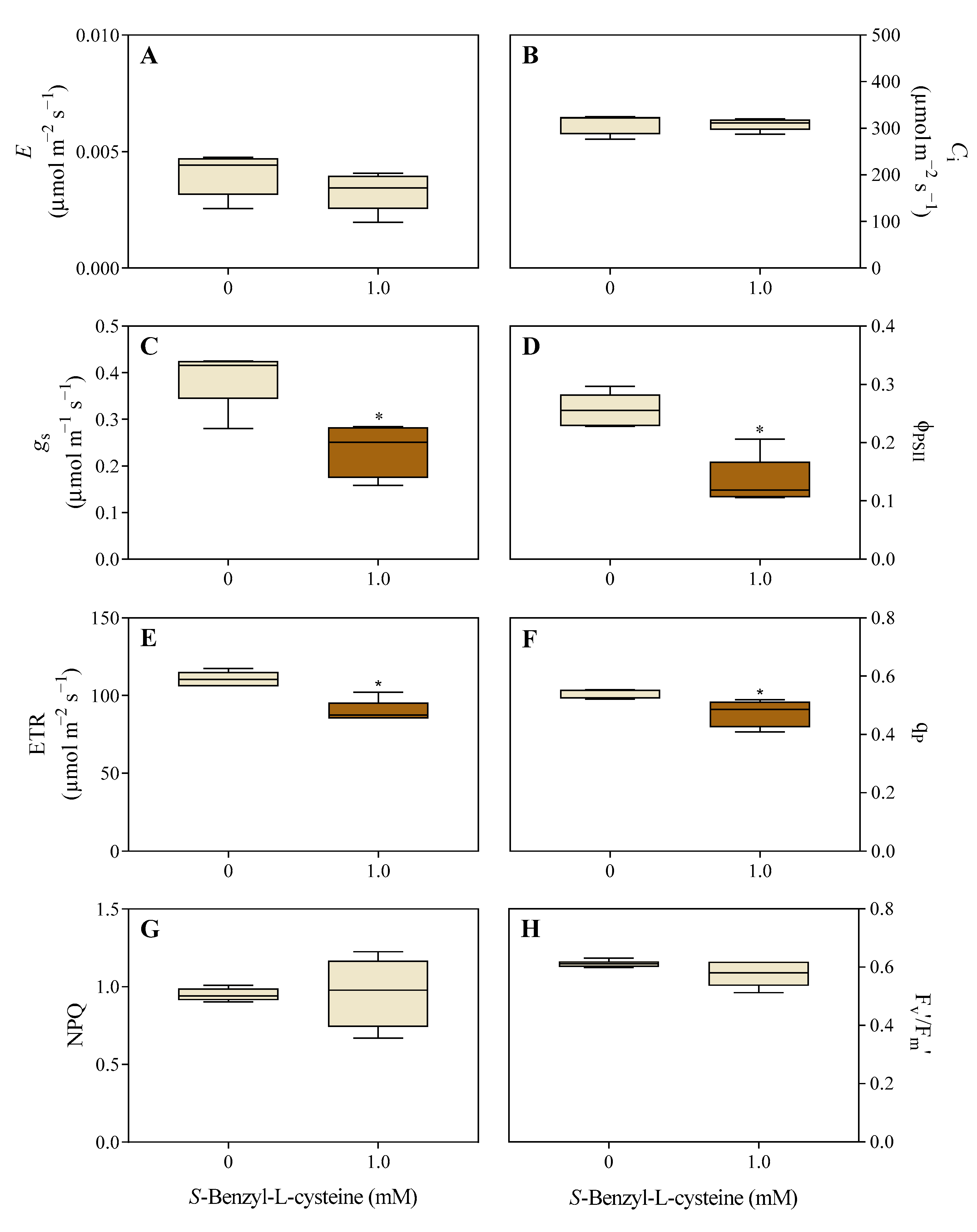
3.2. The Impact of SBC on Photosynthesis
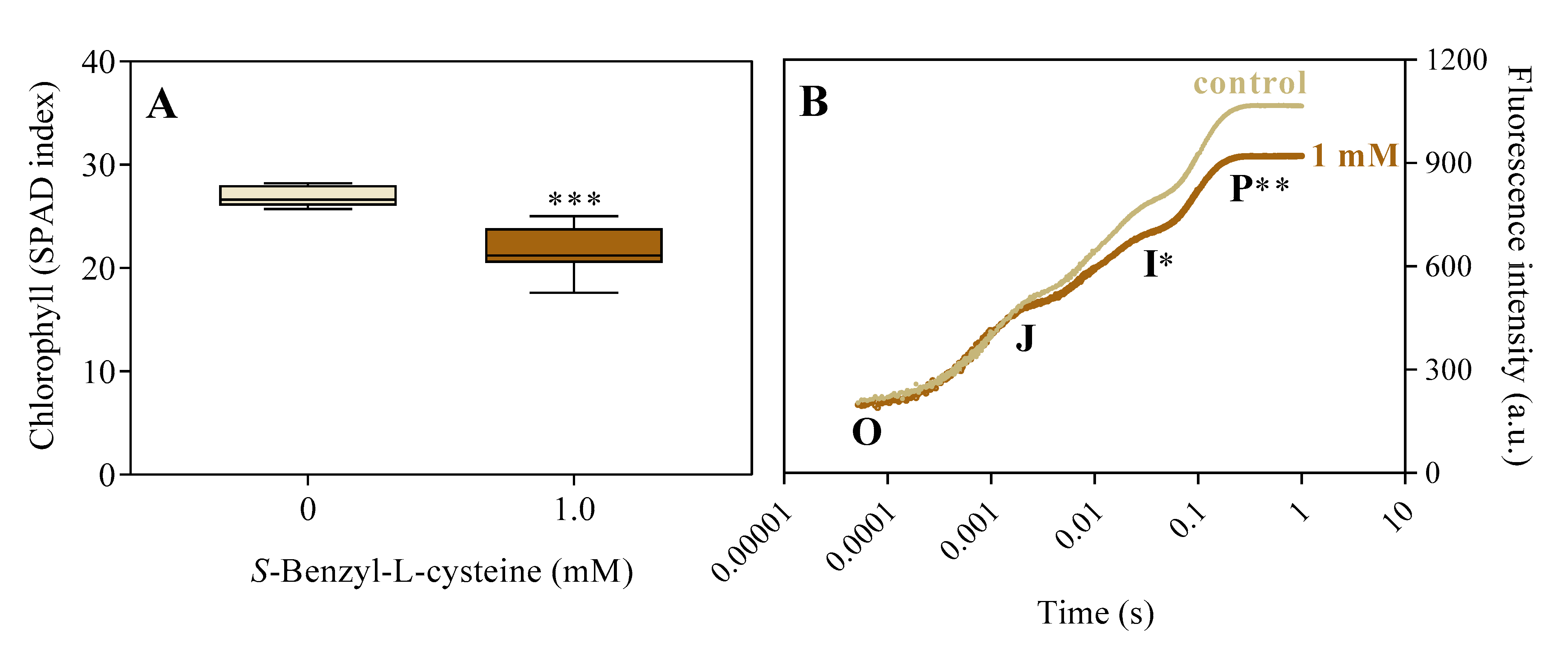
3.3. The Impact of SBC and Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | photosynthetic quantum yield |
| CAT | catalase |
| Ci | intercellular CO2 concentration |
| E | transpiration rate |
| ETR | electron transport rate through PSII |
| F0 | minimal fluorescence yield of the dark-adapted state |
| Fm | maximum fluorescence yield of the dark-adapted state |
| Fv | variable fluorescence |
| Fv/Fm | maximum quantum yield of PSII photochemistry |
| Fv′/Fm′ | effective photochemical quantum efficiency |
| gm | mesophilic conductance |
| gs | stomatal conductance |
| J | photosynthetic electron transport rate |
| LCP | light-compensation point |
| LSP | light-saturation point |
| MDA | malondialdehyde |
| NPQ | nonphotochemical quenching |
| OAS-TL | O-acetylserine(thiol) lyase |
| OJIP | chlorophyll a fluorescence transient |
| PNmax | light-saturated net photosynthetic rate |
| POD | peroxidase |
| QA | primary quinone acceptor |
| qP | photochemical quenching coefficient |
| RD | day respiration rate |
| RD′ | dark respiration rate |
| SBC | S-benzyl-L-cysteine |
| SOD | superoxide dismutase |
| TPU | triose phosphate utilization |
| Vcmax | maximum carboxylation rate of Rubisco |
| ϕPSII | quantum yield of photosystem II photochemistry |
References
- Gazziero, D.L.P.; Brighenti, A.M.; Lollato, R.P.; Pitelli, R.A.; Voll, E.; Oliveira, E.; Moriyama, R.T. Manual de Identificação de Plantas Daninhas da Cultura da Soja, 2nd ed.; Embrapa Soja: Londrina, Brazil, 2015; ISSN 2176-2937. [Google Scholar]
- Barroso, A.A.M.; Ferreira, P.S.H.; Martins, D. Growth and Development of Ipomoea Weed. Planta Daninha 2019, 37, e019186421. [Google Scholar] [CrossRef]
- Heap, I. International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org/Home.aspx (accessed on 1 January 2024).
- Pazuch, D.; Trezzi, M.M.; Guimarães, A.C.D.; Barancelli, M.V.J.; Pasini, R.; Vidal, R.A. Evolução Da Tolerância Ao Glyphosate Em Populações de Corda-de-Viola. Planta Daninha 2017, 35, e017159430. [Google Scholar] [CrossRef][Green Version]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123849052. [Google Scholar]
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Li, M.; Luo, R.; Zhao, F.J.; Salt, D.E. Epigenetic Regulation of Sulfur Homeostasis in Plants. J. Exp. Bot. 2019, 70, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Koprivova, A.; Kopriva, S. Sulfation Pathways in Plants. Chem. Biol. Interact. 2016, 259, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur Nutrition: Impacts on Plant Development, Metabolism, and Stress Responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M. Structural Biology of Plant Sulfur Metabolism: From Sulfate to Glutathione. J. Exp. Bot. 2019, 70, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Dey, S. The Cysteine Regulatory Complex from Plants and Microbes: What Was Old Is New Again. Curr. Opin. Struct. Biol. 2013, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.S.; Hawkesford, M.J. Modulation of Cyanoalanine Synthase and O-Acetylserine (Thiol) Lyases A and B Activity by β-Substituted Alanyl and Anion Inhibitors. J. Exp. Bot. 2002, 53, 439–445. [Google Scholar] [CrossRef][Green Version]
- Foletto-Felipe, M.D.P. O-Acetilserina(Tiol) Liase: Estudos in Silico, In Vitro e In Vivo; Universidade Estadual de Maringá: Maringá, Brazil, 2021. [Google Scholar]
- Chan, K.X.; Wirtz, M.; Phua, S.Y.; Estavillo, G.M.; Pogson, B.J. Balancing Metabolites in Drought: The Sulfur Assimilation Conundrum. Trends Plant Sci. 2013, 18, 18–29. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Lobo, F.D.; de Barros, M.P.; Dalmagro, H.J.; Dalmolin, Â.C.; Pereira, W.E.; de Souza, É.C.; Vourlitis, G.L.; Rodríguez Ortíz, C.E. Fitting Net Photosynthetic Light-Response Curves with Microsoft Excel—A Critical Look at the Models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- Ye, Z.P. A New Model for Relationship between Irradiance and the Rate of Photosynthesis in Oryza Sativa. Photosynthetica 2007, 45, 637–640. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting Photosynthetic Carbon Dioxide Response Curves for C3 Leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Loriaux, S.D.; Avenson, T.J.; Welles, J.M.; Mcdermitt, D.K.; Eckles, R.D.; Riensche, B.; Genty, B. Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant Cell Environ. 2013, 36, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Chlorophyll a Fluorescence Induction: Can Just a One-Second Measurement Be Used to Quantify Abiotic Stress Responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and Detection of Reactive Oxygen Species (ROS), Lipid Peroxidation, and Electrolyte Leakage in Plants. In Plant Stress Tolerance: Methods in Molecular Biology; Sunkar, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–297. ISBN 978-1-60761-701-3. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplast. I. Kinetics and Stoichiometry of Fatty Acids Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Biomembranes—Part C: Biological Oxidations. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of Antioxidant Enzymes to Transfer from Elevated Carbon Dioxide to Air and Ozone Fumigation, in the Leaves and Roots of Wild-Type and a Catalase-Deficient Mutant of Barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Tománková, K.; Luhová, L.; Petřivalský, M.; Peč, P.; Lebeda, A. Biochemical Aspects of Reactive Oxygen Species Formation in the Interaction between Lycopersicon Spp. and Oidium neolycopersici. Physiol. Mol. Plant Pathol. 2006, 68, 22–32. [Google Scholar] [CrossRef]
- Dos Santos, W.D.; Ferrarese, M.L.L.; Nakamura, C.V.; Mourão, K.S.M.; Mangolin, C.A.; Ferrarese-Filho, O. Soybean (Glycine max) Root Lignification Induced by Ferulic Acid. The Possible Mode of Action. J. Chem. Ecol. 2008, 34, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Foletto-Felipe, M.; Abrahão, J.; de Cássia Siqueira-Soares, R.; de Carvalho Contesoto, I.; Grizza, L.H.E.; de Almeida, G.H.G.; Constantin, R.P.; Philippsen, G.S.; Seixas, F.A.V.; Bueno, P.S.A.; et al. Inhibition of O-Acetylserine (Thiol) Lyase as a Promising New Mechanism of Action for Herbicides. Plant Physiol. Biochem. 2023, 204, 108127. [Google Scholar] [CrossRef] [PubMed]
- Lunde, C.; Zygadlo, A.; Simonsen, H.T.; Nielsen, P.L.; Blennow, A.; Haldrup, A. Sulfur Starvation in Rice: The Effect on Photosynthesis, Carbohydrate Metabolism, and Oxidative Stress Protective Pathways. Physiol. Plant. 2008, 134, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Bucher, S.F.; Bernhardt–Römermann, M.; Römermann, C. Chlorophyll Fluorescence and Gas Exchange Measurements in Field Research: An Ecological Case Study. Photosynthetica 2018, 56, 1161–1170. [Google Scholar] [CrossRef]
- Dabrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of Chlorophyll a Fluorescence and Plant Gas Exchange Parameters as Indicators of Drought Tolerance in Perennial Ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef]
- Parizotto, A.V.; Marchiosi, R.; Bubna, G.A.; Bevilaqua, J.M.; Ferro, A.P.; Ferrarese, M.L.L.; Ferrarese-Filho, O. Benzoxazolin-2-(3H)-One Reduces Photosynthetic Activity and Chlorophyll Fluorescence in Soybean. Photosynthetica 2017, 55, 386–390. [Google Scholar] [CrossRef]
- Marchiosi, R.; de Souza Bido, G.; Böhm, P.A.F.; Soares, A.R.; da Silva, H.A.; Ferro, A.P.; de Lourdes Lucio Ferrarese, M.; Ferrarese-Filho, O. Photosynthetic Response of Soybean to L-DOPA and Aqueous Extracts of Velvet Bean. Plant Growth Regul. 2016, 80, 171–182. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yu, J.Q. Allelochemicals and Photosynthesis. In Allelopathy: A Physiological Process with Ecological Implications; 2006; pp. 127–139. ISBN 9781402042805. [Google Scholar]
- Bortolo, T.D.S.C.; Marchiosi, R.; Viganó, J.; de Cássia Siqueira-Soares, R.; Ferro, A.P.; Barreto, G.E.; de Souza Bido, G.; Abrahão, J.; Dos Santos, W.D.; Ferrarese-Filho, O. Trans-Aconitic Acid Inhibits the Growth and Photosynthesis of Glycine max. Plant Physiol. Biochem. 2018, 132, 490–496. [Google Scholar] [CrossRef]
- Everard, J.D.; Cucci, R.; Kann, S.C.; Flore, J.A.; Loescher, W.H. Gas Exchange and Carbon Partitioning in the Leaves of Celery (Apium graveolens L.) at Various Levels of Root Zone Salinity. Plant Physiol. 1994, 106, 281–292. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Reigosa, M.J. Allelochemical Stress Inhibits Growth, Leaf Water Relations, PSII Photochemistry, Non-Photochemical Fluorescence Quenching, and Heat Energy Dissipation in Three C 3 Perennial Species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef] [PubMed]
- Lazár, D. The Polyphasic Chlorophyll a Fluorescence Rise Measured under High Intensity of Exciting Light. Funct. Plant Biol. 2006, 33, 9–30. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Photosynthesis in Intact Leaves of C3 Plants: Physics, Physiology and Rat Limitation. Bot. Rev. 1985, 5, 53–105. [Google Scholar] [CrossRef]
- Sharkey, T.D. What Gas Exchange Data Can Tell Us about Photosynthesis. Plant Cell Environ. 2016, 39, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Bernacchi, C.J. Gas Exchange Measurements, What Can They Tell Us about the Underlying Limitations to Photosynthesis? Procedures and Sources of Error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Octobre, G.; Delprat, N.; Doumèche, B.; Leca-Bouvier, B. Herbicide Detection: A Review of Enzyme- and Cell-Based Biosensors. Environ. Res. 2024, 249, 118330. [Google Scholar] [CrossRef]
- Fuerst, E.P.; Norman, M.A. Interactions of Herbicides with Photosynthetic Electron Transport. Weed Sci. 1991, 39, 458–464. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet Oxygen Production in Photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Mudassir Maqbool, M.; Iqbal, J.; Nadeem, M.; Faiz, S.; Noor, H.; Hamid, Y.; Yuan, X.; Pingyi, G. The Growth, Physiological and Biochemical Response of Foxtail Millet to Atrazine Herbicide. Saudi J. Biol. Sci. 2021, 28, 6471–6479. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H. Prometryne-Induced Oxidative Stress and Impact on Antioxidant Enzymes in Wheat. Ecotoxicol. Environ. Saf. 2009, 72, 1687–1693. [Google Scholar] [CrossRef]


| SBC (mM) | Root | Stem | Leaf | |
|---|---|---|---|---|
| Length (cm) | 0 | 21.22 ± 0.97 | 6.02 ± 0.67 | 5.60 ± 0.16 |
| 1.0 | 14.87 ± 1.10 * | 3.42 ± 0.21 * | 4.02 ± 0.19 * | |
| Fresh mass (g) | 0 | 0.36 ± 0.02 | 0.30 ± 0.02 | 0.79 ± 0.05 |
| 1.0 | 0.16 ± 0.02 * | 0.12 ± 0.01 * | 0.34 ± 0.03 * | |
| Dry mass (g) | 0 | 0.02 ± 0.001 | 0.02 ± 0.002 | 0.10 ± 0.007 |
| 1.0 | 0.01 ± 0.001 * | 0.01 ± 0.001 * | 0.05 ± 0.004 * |
| SBC (mM) | Root | Leaf | |
|---|---|---|---|
| Reactive oxygen species | 0 | 0.40 ± 0.068 | 4.40 ± 0.543 |
| (fluorescence μg−1) | 1.0 | 0.62 ± 0.035 * | 6.61 ± 0.623 * |
| Conjugated dienes | 0 | 1.79 ± 0.042 | 5.24 ± 0.414 |
| (μmol g−1) | 1.0 | 2.89 ± 0.163 * | 9.27 ± 0.639 * |
| Malondialdehyde | 0 | 1.90 ± 0.067 | 19.7 ± 2.041 |
| (nmol g−1) | 1.0 | 2.48 ± 0.055 * | 21.9 ± 1.707 |
| Superoxide dismutase | 0 | 0.01 ± 0.001 | 0.06 ± 0.004 |
| (U mg−1) | 1.0 | 0.01 ± 0.002 | 0.05 ± 0.003 |
| Catalase | 0 | 0.02 ± 0.004 | 0.13 ± 0.006 |
| (μmol min−1 mg−1) | 1.0 | 0.03 ± 0.004 | 0.14 ± 0.006 |
| Peroxidase | 0 | 0.10 ± 0.011 | 0.12 ± 0.018 |
| (μmol min−1 g−1) | 1.0 | 0.13 ± 0.003 * | 0.16 ± 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martarello, D.C.I.; Grizza, L.H.E.; Foletto-Felipe, M.d.P.; Mendonça, A.P.d.S.; Constantin, R.P.; Ferro, A.P.; dos Santos, W.D.; Constantin, R.P.; Marchiosi, R.; Ferrarese-Filho, O. S-Benzyl-L-cysteine Inhibits Growth and Photosynthesis, and Triggers Oxidative Stress in Ipomoea grandifolia. Agronomy 2024, 14, 1633. https://doi.org/10.3390/agronomy14081633
Martarello DCI, Grizza LHE, Foletto-Felipe MdP, Mendonça APdS, Constantin RP, Ferro AP, dos Santos WD, Constantin RP, Marchiosi R, Ferrarese-Filho O. S-Benzyl-L-cysteine Inhibits Growth and Photosynthesis, and Triggers Oxidative Stress in Ipomoea grandifolia. Agronomy. 2024; 14(8):1633. https://doi.org/10.3390/agronomy14081633
Chicago/Turabian StyleMartarello, Danielly Caroline Inacio, Luiz Henryque Escher Grizza, Marcela de Paiva Foletto-Felipe, Ana Paula da Silva Mendonça, Renato Polimeni Constantin, Ana Paula Ferro, Wanderley Dantas dos Santos, Rodrigo Polimeni Constantin, Rogerio Marchiosi, and Osvaldo Ferrarese-Filho. 2024. "S-Benzyl-L-cysteine Inhibits Growth and Photosynthesis, and Triggers Oxidative Stress in Ipomoea grandifolia" Agronomy 14, no. 8: 1633. https://doi.org/10.3390/agronomy14081633
APA StyleMartarello, D. C. I., Grizza, L. H. E., Foletto-Felipe, M. d. P., Mendonça, A. P. d. S., Constantin, R. P., Ferro, A. P., dos Santos, W. D., Constantin, R. P., Marchiosi, R., & Ferrarese-Filho, O. (2024). S-Benzyl-L-cysteine Inhibits Growth and Photosynthesis, and Triggers Oxidative Stress in Ipomoea grandifolia. Agronomy, 14(8), 1633. https://doi.org/10.3390/agronomy14081633







