The Impact of Continuous Cropping on Phenolic Acids in Muskmelon Soil and the Colonization of Trichoderma viride
Abstract
1. Introduction
2. Materials and Methods
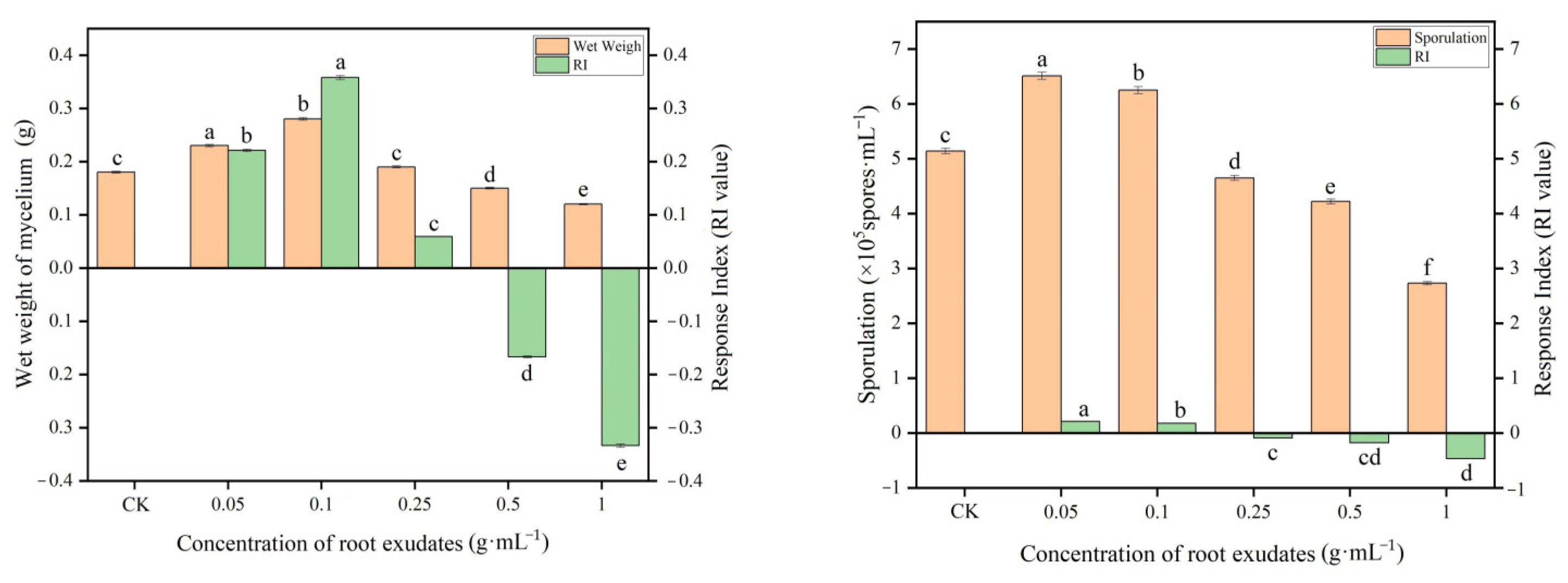
2.1. Detection of the Effect of Muskmelon Root Exudates on the Growth of T. viride T23
2.2. Detection of the Effect of Muskmelon Root Exudates on Enzymatic Activity of T. viride T23
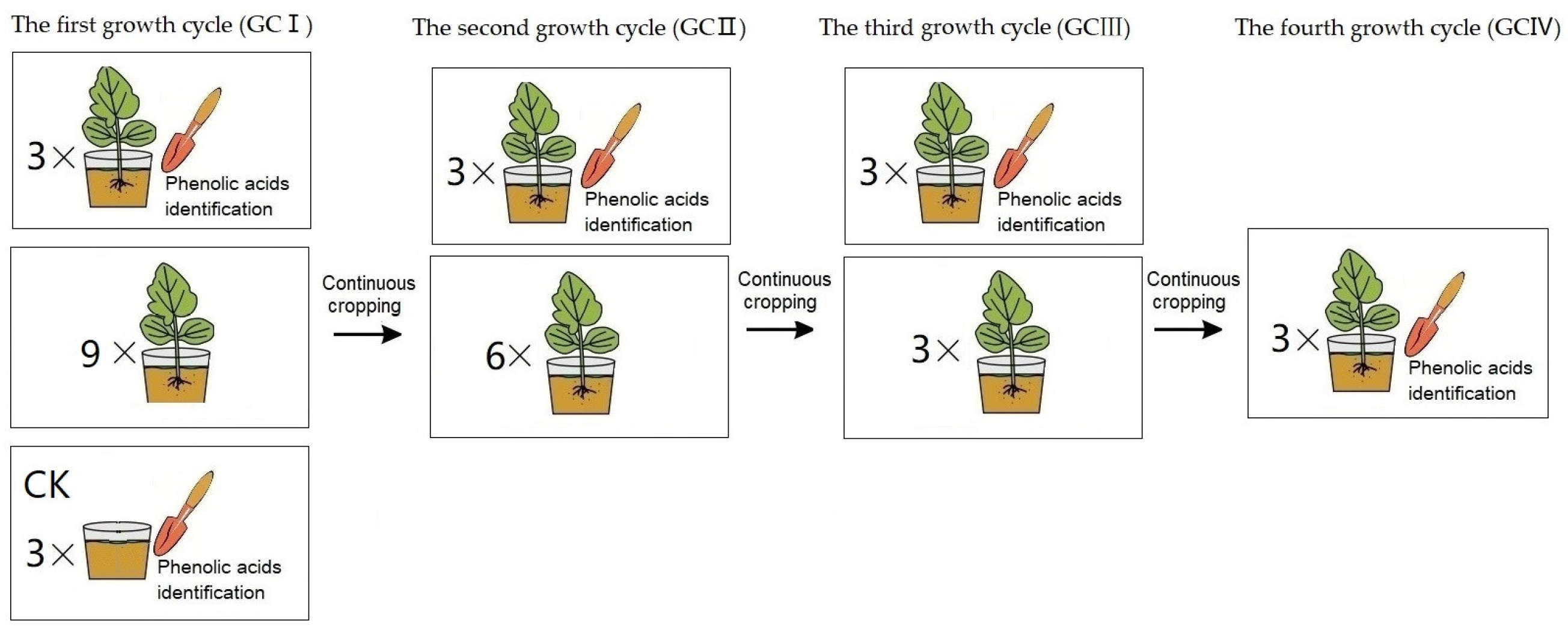
2.3. Effect of Muskmelon Continuous Cropping on Phenolic Acid Content in Soil
2.4. Inoculation Assay
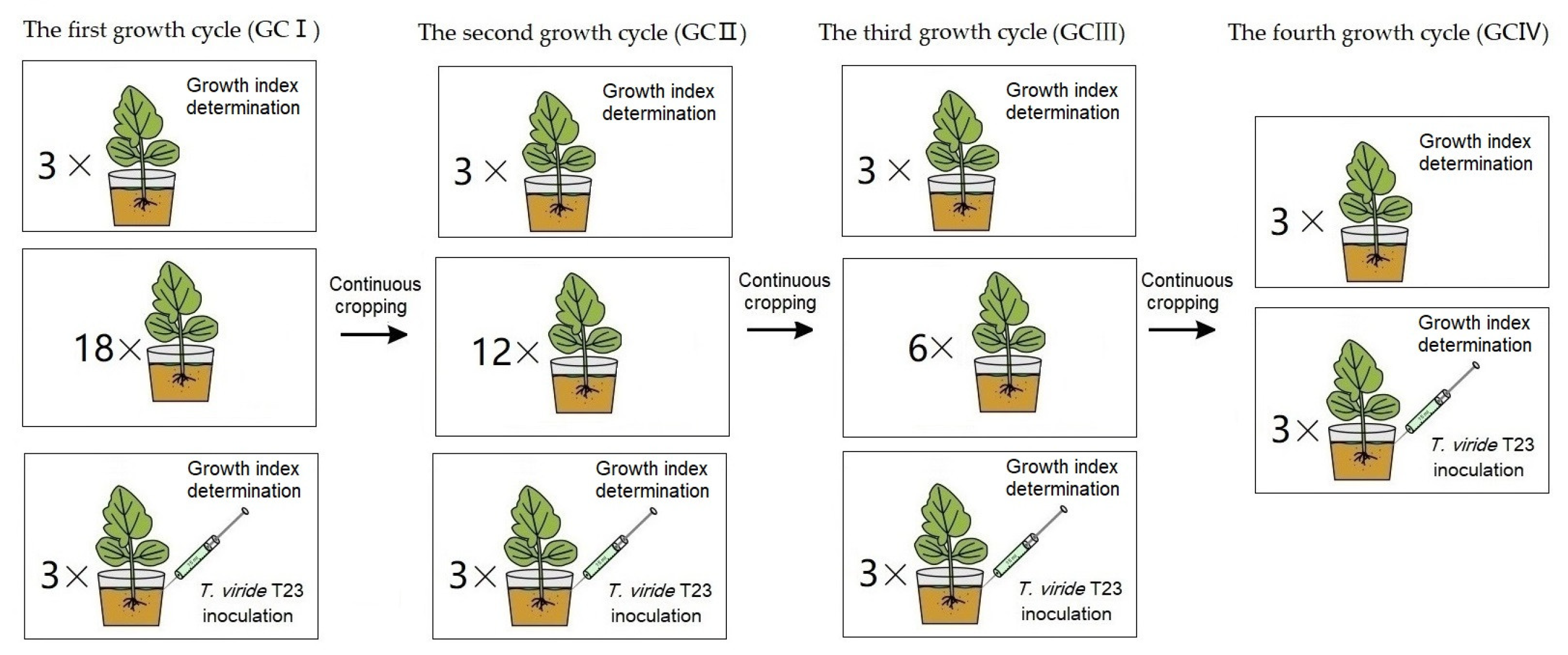
2.5. Effect of T. viride T23 on Continuous Cropping Muskmelon
2.6. Data Analysis
3. Results
3.1. Effect of Muskmelon Root Exudates on the Growth of T. viride T23
3.2. Effect of Muskmelon Root Exudates on Enzymatic Activity of T. viride T23
3.3. Changes of the Phenolic Acid Concentration of the Replanted Muskmelon Soil
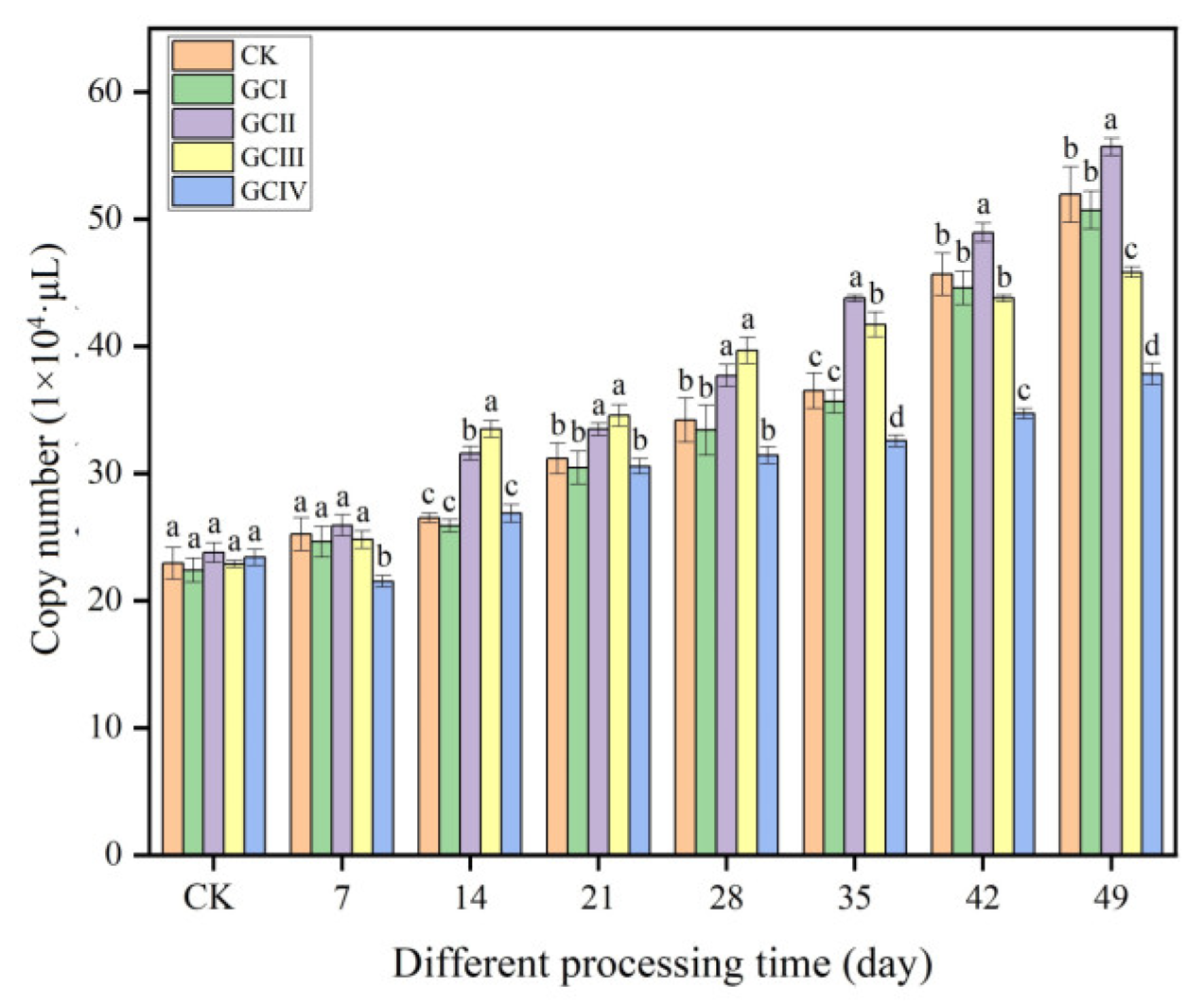
3.4. Effect of Continuous Cropping on the Colonization of T. viride T23
3.5. Effect of Continuous Cropping on Promotional Role of T. viride T23
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wonglom, P.; Sunpapao, A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo). J. Phytopath. 2020, 168, 204–210. [Google Scholar] [CrossRef]
- Gava, C.A.T.; Pinto, J.M. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol. Control 2016, 97, 13–20. [Google Scholar]
- Yang, R.X.; Gao, Z.G.; Liu, X.; Yao, Y.; Cheng, Y. Root exudates from muskmelon (Cucumis melon. L) induce autotoxicity and promote growth of Fusarium oxysporum f. sp. melonis. Allelopath. J. 2014, 33, 175–188. [Google Scholar]
- Wipornpan, N.; Worawoot, A.; Jaturong, K.; Saisamorn, L.; Nakarin, S. Evaluation of a newly identified endophytic fungus, Trichoderma phayaoense for plant growth promotion and biological control of gummy stem blight and wilt of muskmelon. Front. Microbiol. 2021, 21, 634772. [Google Scholar]
- Gauri, S.S.; Mandal, S.M.; Dey, S.; Pati, B.R. Biotransformation of p-coumaric acid and 2,4-dichlorophenoxy acetic acid by Azotobacter sp. strain SSB81. Bioresour. Technol. 2012, 126, 350–353. [Google Scholar] [CrossRef]
- Wu, H.; Lin, W. A commentary and development perspective on the consecutive monoculture problems of medicinal plants. Chin. J. Eco-Agric. 2020, 28, 775–793. [Google Scholar]
- Siles, J.A.; Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient inalpine forest soils: What are the driving factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, J.; Huang, W.; Wu, H.; Chen, J.; Yang, Y. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci. Rep. 2015, 5, 15871. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef]
- Wang, S.N.; Yang, R.X.; Liu, X.; Yao, Y.; Gao, Z.G. Overcoming the autotoxicity of muskmelon by Trichoderma spp. Allelopath. J. 2016, 39, 29–42. [Google Scholar]
- Ioanna, K.; Alexandros, T.; Antonios, M.; Angeliki, K.; Stella, K.; Charikleia, Z.; Varvara, K.; Artemis, K.; Antigolena, F.; Aristidis, K. Effect of colonization of Trichoderma harzianum on growth development and CBD content of hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, W.W.; Xue, M.; Liu, Z.; Zhang, Q.M.; Hou, J.M.; Xing, M.Y.; Wang, R.; Liu, T. The combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control fusarium wilt in cowpea. J. Fungi 2021, 7, 685. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, O.A.; Abdel-lateif, K.S.; Bakr, R.A. Genetic diversity and biocontrol efficacy of indigenous Trichoderma isolates against Fusarium wilt of pepper. J. Basic Microb. 2019, 60, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Metwally, R.A.; Al-Amri, S.M. Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett. Appl. Microbiol. 2020, 70, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bunbury-Blanchette, A.L.; Walker, A.K. Trichoderma species show biocontrol potential in dual culture and greenhouse bioassays against Fusarium basal rot of onion. Biol. Control 2019, 130, 127–135. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Tang, B.; Dong, Y.; He, M.; Liu, J.; Wu, K.; Guan, H. Effects of different planting years of healthy Panax notoginseng on the rhizosphere microbial community in Wenshan of Yunnan province. J. Microbiol. 2020, 9, 2857–2866. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef]
- Bastakoti, S.; Belbase, S.; Manandhar, S.; Arjyal, C. Trichoderma species as biocontrol agent against soil borne fungal pathogen. Nepal J. Biotechnol. 2017, 5, 39–45. [Google Scholar] [CrossRef]
- Yariv, B.; Udi, L.; Alvaro, C.I.; Tohge, T.; Alisdair, R.F.; Chet, A.V.; Lothar, W. Trichoderma-Plant Root Colonization: Escaping EarlyPlant Defense Responses and Activation of the Antioxidant Machinery for Saline Stress Tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar]
- Montaser, F.A. Improvement of biocontrol of damping-off and root rot/wilt of faba bean by salicylic acid and hydrogen peroxide. Mycobiology 2013, 41, 47–55. [Google Scholar]
- Asad, S.A.; Ali, N.; Hameed, A.; Khan, S.A.; Ahmad, R.; Bilal, M.; Shahzad, M.; Tabassum, A. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Pol. J. Microbiol. 2014, 63, 95–103. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd-Allahm, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Tang, L.; Gao, Z.G.; Zhuagn, J.A.; Zhao, H. Effect of microelements on genetic variation of biocontrol agent Trichoderma T23. J. Shenyang Agric. Univ. 2008, 39, 423–426. (In Chinese) [Google Scholar]
- Willamson, G.B.; Richardson, D. Bioassays for Allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef]
- Saleh, R.M.; Kabli1, S.A.; Al-Garni1, S.M.; Al-Ghamdi, M.A.; Abdel-Aty, A.M.; Mohamed, S.A. Solid-state fermentation by Trichoderma viride for enhancing phenolic content, antioxidant and antimicrobialactivities in ginger. Microbiology 2018, 67, 161–167. [Google Scholar]
- Abu-Tahon, M.A.; Isaac, G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020, 66, 32–40. [Google Scholar] [CrossRef]
- Faria, M.L.; Kolling, D.; Camassola, M. Comparison of Pennicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Biores. Technol. 2008, 99, 1417–1424. [Google Scholar]
- Wang, Y.Y.; Du, J.; Gao, K.X. Screening and identification of biocontrol Trichoderma to wilt of bitter gourd and detection of its colonization by qPCR. Shandong Agric. Sci. 2018, 50, 110–115. (In Chinese) [Google Scholar]
- Cai, H.G.; Mi, G.H.; Chen, F.J. Genotypic variation of leaf SPAD value, nitrogen and nitrate content in maize. Plant Nutr. Fertil. Sci. 2010, 16, 866–873. [Google Scholar]
- Jin, X.; Wu, F.; Zhou, X. Different toxic effects of ferulic and p-hydroxybenzoic acids on cucumber seedling growth were related to their different influences on rhizosphere microbial composition. Biol. Fertil. Soils 2020, 56, 125–136. [Google Scholar] [CrossRef]
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of Volatiles from the Endophytic Fungus Trichoderma asperelloides PSU-P1 in Biocontrol Potential and in Promoting the Plant Growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Trichoderma-Not just for biocontrol anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Troian, R.F.; Steindorff, A.S.; Ramada, M.H.; Arruda, W.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: Evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol. Lett. 2014, 36, 2095–2101. [Google Scholar] [CrossRef]
- Chen, S.L.; Zhou, B.L.; Lin, S.S.; Li, X.; Xi, H.J.; Yin, Y.L.; Ye, X.L. Allelopathic effects of cinnamic acid and vanillin on soil microbes, soil enzymes activities and growth of grafted eggplants. Allelopath. J. 2011, 28, 29–40. [Google Scholar]
- Martínez-Medina, A.; Del Mar Alguacil, M.; Pascual, J.A.; Van Wees, S.C. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]
- Yin, C.M.; Wang, G.S.; Li, Y.Y.; Che, J.S.; Shen, X.; Chen, X.S. A new method for analysis of phenolic acids in the soil-soil from replanted apple orchards was investigated. China Agric. Sci. 2013, 46, 4612–4619. (In Chinese) [Google Scholar]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Miki, T.; Doi, H. Leaf phenological shifts and plant-microbe-soil interactions can determine forest productivity and nutrient cycling under climate change in an ecosystem model. Ecol. Res. 2016, 31, 263–274. [Google Scholar] [CrossRef]
- Luo, L.; Yang, L.; Yan, Z.; Jiang, B.; Li, S.; Huang, H. Ginsenosides in root exudates of Panax notoginseng drive the change of soil microbiota through carbon source different utilization. Plant Soil. 2020, 455, 139–153. [Google Scholar] [CrossRef]
- Benizri, E.; Baudoin, E.; Guckert, A. Root Colonization by Inoculated Plant Growth-Promoting Rhizobacteria. Biocontrol Sci. Technol. 2001, 11, 557–574. [Google Scholar] [CrossRef]
- Ruangwong, O.U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol mechanisms of Trichoderma koningiopsis PSU3-2 against postharvest anthracnose of chili pepper. J. Fungi 2021, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Ru, Z.; Di, W. Trichoderma spp. from rhizosphere soil and their antagonism against Fusarium sambucinum. Afr. J. Biotechnol. 2012, 11, 4180–4186. [Google Scholar]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma asperellum T76-14 released volatile organic compounds against postharvest fruit rot in muskmelons (Cucumis melo) caused by Fusarium incarnatum. J. Fungi 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Bai, Y.X.; Wang, G.; Cheng, Y.D.; Shi, P.Y.; Yang, C.C.; Yang, H.W.; Xu, Z.L. Soil acidification in continuous lycropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef] [PubMed]
- Blum, U.; Gerig, T.M. Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: Nutrient culture studies. J. Chem. Ecol. 2005, 31, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]
- Wu, J.J.; Zhu, J.H.; Zhang, D.Q.; Cheng, H.Y.; Hao, B.Q.; Cao, A.C.; Yan, D.D.; Wang, Q.X.; Li, Y. Beneficial effect on the soil microenvironment of Trichoderma applied after fumigation for cucumber production. PLoS ONE 2022, 17, e0266347. [Google Scholar] [CrossRef]
- James, T.T.; Harting, R.; Samer, S.; Charles, M.K.; Gerhard, H.B.; Benjamin, A.H. Adhesion as a Focus in Trichoderma–Root Interactions. J. Fungi 2022, 8, 372. [Google Scholar] [CrossRef]



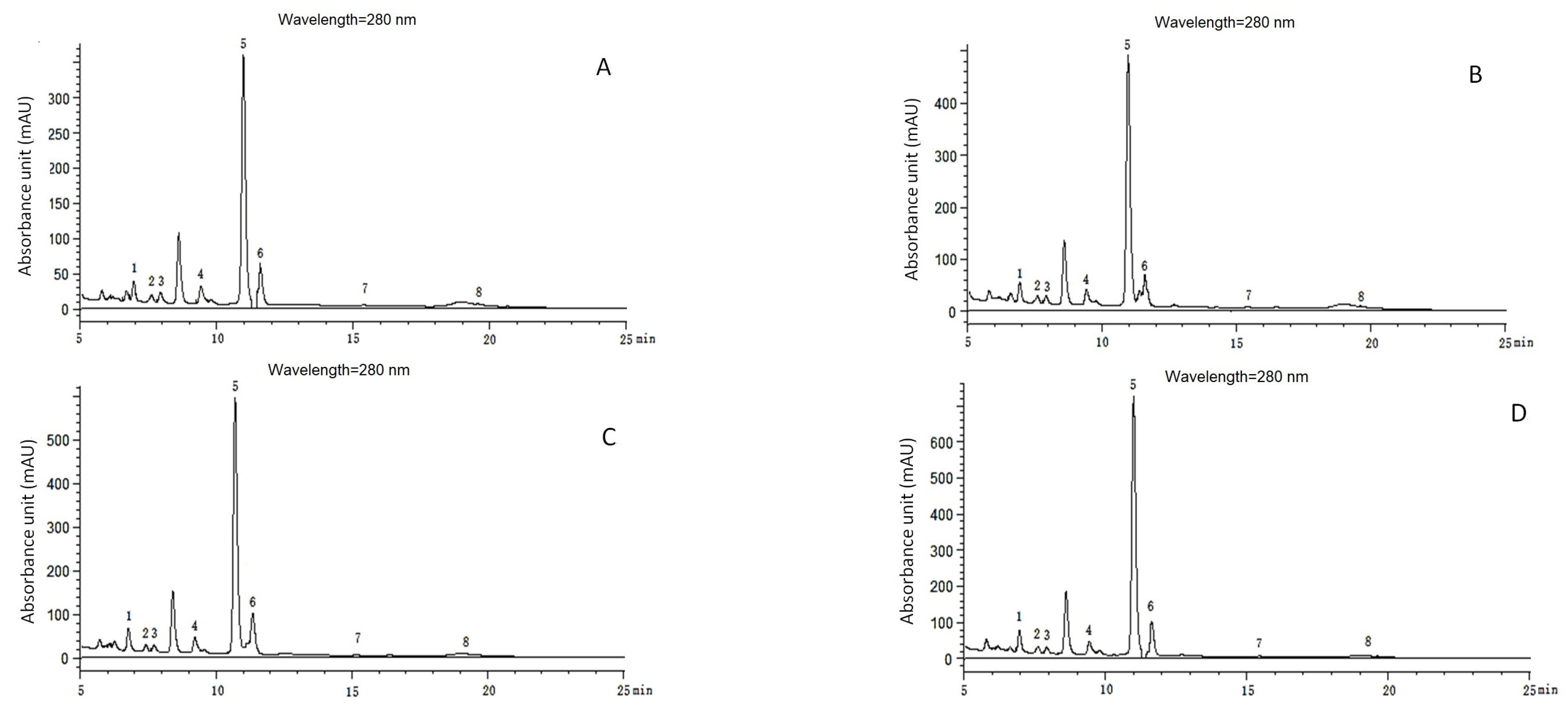
| Phenolic Acids | Linear Range (mg·L−1) | Regression Equation | R-Square Value | LOD (μg·kg−1) | LOQ (μg·kg−1) |
|---|---|---|---|---|---|
| ρ-hydroxybenzoic acid | 0.10–30.0 | y = 23.80x − 12.88 | 0.9997 | 7 | 23 |
| Vanillic acid | 0.10–30.0 | y = 36.24x + 9.53 | 0.9986 | 11 | 35 |
| Syringic acid | 0.10–30.0 | y = 56.96x − 53.37 | 0.9991 | 8 | 26 |
| Vanillin | 0.10–30.0 | y = 74.21x + 0.66 | 0.9985 | 9 | 29 |
| Coumaric acid | 0.10–30.0 | y = 87.93x − 62.99 | 0.9993 | 10 | 31 |
| Ferulic acid | 0.10–30.0 | y = 49.18x − 36.54 | 0.9971 | 7 | 22 |
| Benzoic acid | 0.10–30.0 | y = 6.60x − 4.90 | 0.9972 | 10 | 35 |
| Cinnamic acid | 0.10–30.0 | y = 164.9x − 132.04 | 0.9964 | 8 | 26 |
| Treatments | The Control CK | Growth Cycle I | Growth Cycle II | Growth Cycle III | Growth Cycle IV |
|---|---|---|---|---|---|
| ρ-hydroxybenzoic acid | 1.14 ± 0.09 d | 25.51 ± 0.73 c | 33.86 ± 0.67 bc | 44.10 ± 0.85 ab | 49.85 ± 0.96 a |
| Vanillic acid | 0.86 ± 0.03 d | 9.36 ± 0.28 c | 12.28 ± 0.25 bc | 13.80 ± 0.28 ab | 16.21 ± 0.32 a |
| Syringic acid | 0.48 ± 0.07 d | 7.21 ± 0.21 c | 8.95 ± 0.18 bc | 10.11 ± 0.21 ab | 10.50 ± 0.22 a |
| Vanillin | 0.58 ± 0.03 d | 6.53 ± 0.19 c | 8.28 ± 0.16 bc | 8.60 ± 0.17 ab | 9.92 ± 0.19 a |
| Coumaric acid | 0.89 ± 0.02 d | 43.60 ± 1.21 c | 55.71 ± 1.07 bc | 73.01 ± 1.41 ab | 92.76 ± 1.79 a |
| Ferulic acid | - | 16.06 ± 0.46 c | 22.18 ± 0.43 b | 26.70 ± 0.52 ab | 29.84 ± 0.57 a |
| Benzoic acid | - | 19.21 ± 0.55 c | 28.75 ± 0.56 bc | 35.30 ± 0.68 ab | 43.43 ± 0.83 a |
| Cinnamic acid | - | 1.14 ± 0.03 a | 1.83 ± 0.04 a | 1.62 ± 0.03 a | 1.46 ± 0.02 a |
| Treatments | Stem Diameter | Leaf Area | Fresh Weight | Dry Weight | SPAD |
|---|---|---|---|---|---|
| (mm) | (cm2) | (g·Plant−1) | (g·Plant−1) | ||
| GCⅠ | 9.84 ± 0.43 ab | 210.09 ± 8.21 b | 234.48 ± 9.71 bc | 28.05 ± 2.19 ab | 44.26 ± 3.22 abc |
| GCⅠ + T23 | 10.20 ± 0.24 a | 227.17 ± 6.34 a | 257.21 ± 5.67 a | 31.45 ± 2.03 a | 48.32 ± 1.79 a |
| GCⅡ | 9.45 ± 0.29 b | 206.14 ± 4.26 bc | 221.07 ± 7.72 cd | 26.24 ± 2.01 bc | 43.36 ± 2.56 bcd |
| GCⅡ + T23 | 9.76 ± 0.21 ab | 219.44 ± 5.81 ab | 238.46 ± 6.98 b | 28.89 ± 1.49 ab | 46.48 ± 1.38 ab |
| GCⅢ | 8.12 ± 0.22 cd | 184.46 ± 6.59 d | 206.31 ± 8.48 d | 20.68 ± 1.25 d | 39.44 ± 2.11 de |
| GCⅢ + T23 | 8.68 ± 0.48 c | 195.05 ± 8.35 cd | 211.37 ± 4.58 d | 22.21 ± 1.55 cd | 40.51 ± 2.62 cd |
| GCⅣ | 7.31 ± 0.32 e | 166.23 ± 8.71 e | 153.22 ± 7.09 e | 18.38 ± 1.88 d | 34.26 ± 1.33 f |
| GCⅣ + T23 | 7.57 ± 0.51 de | 158.68 ± 6.88 e | 160.41 ± 7.29 e | 20.77 ± 1.59 d | 35.18 ± 1.63 ef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Liu, B.; Teng, A.; Zhang, L.; Wang, H.; Yang, Z.; Li, J.; Xia, Y.; Wang, J. The Impact of Continuous Cropping on Phenolic Acids in Muskmelon Soil and the Colonization of Trichoderma viride. Agronomy 2024, 14, 1344. https://doi.org/10.3390/agronomy14071344
Yang R, Liu B, Teng A, Zhang L, Wang H, Yang Z, Li J, Xia Y, Wang J. The Impact of Continuous Cropping on Phenolic Acids in Muskmelon Soil and the Colonization of Trichoderma viride. Agronomy. 2024; 14(7):1344. https://doi.org/10.3390/agronomy14071344
Chicago/Turabian StyleYang, Ruixiu, Bo Liu, Ao Teng, Lu Zhang, Hongling Wang, Zhijuan Yang, Jinshi Li, Yingjun Xia, and Jiaqing Wang. 2024. "The Impact of Continuous Cropping on Phenolic Acids in Muskmelon Soil and the Colonization of Trichoderma viride" Agronomy 14, no. 7: 1344. https://doi.org/10.3390/agronomy14071344
APA StyleYang, R., Liu, B., Teng, A., Zhang, L., Wang, H., Yang, Z., Li, J., Xia, Y., & Wang, J. (2024). The Impact of Continuous Cropping on Phenolic Acids in Muskmelon Soil and the Colonization of Trichoderma viride. Agronomy, 14(7), 1344. https://doi.org/10.3390/agronomy14071344





