MicroRNA164 Regulates Perennial Ryegrass (Lolium perenne L.) Adaptation to Changing Light Intensity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Experiment Treatments
2.2. Sampling and Measurements
2.2.1. Morphological Comparison of Transgenic Plants Grown under Different
Light Intensity
2.2.2. Plant Leaf Electron Microscopy
2.2.3. Measurement of Physiological Parameters
2.3. Experimental Design and Statistical Analysis
3. Results and Discussion
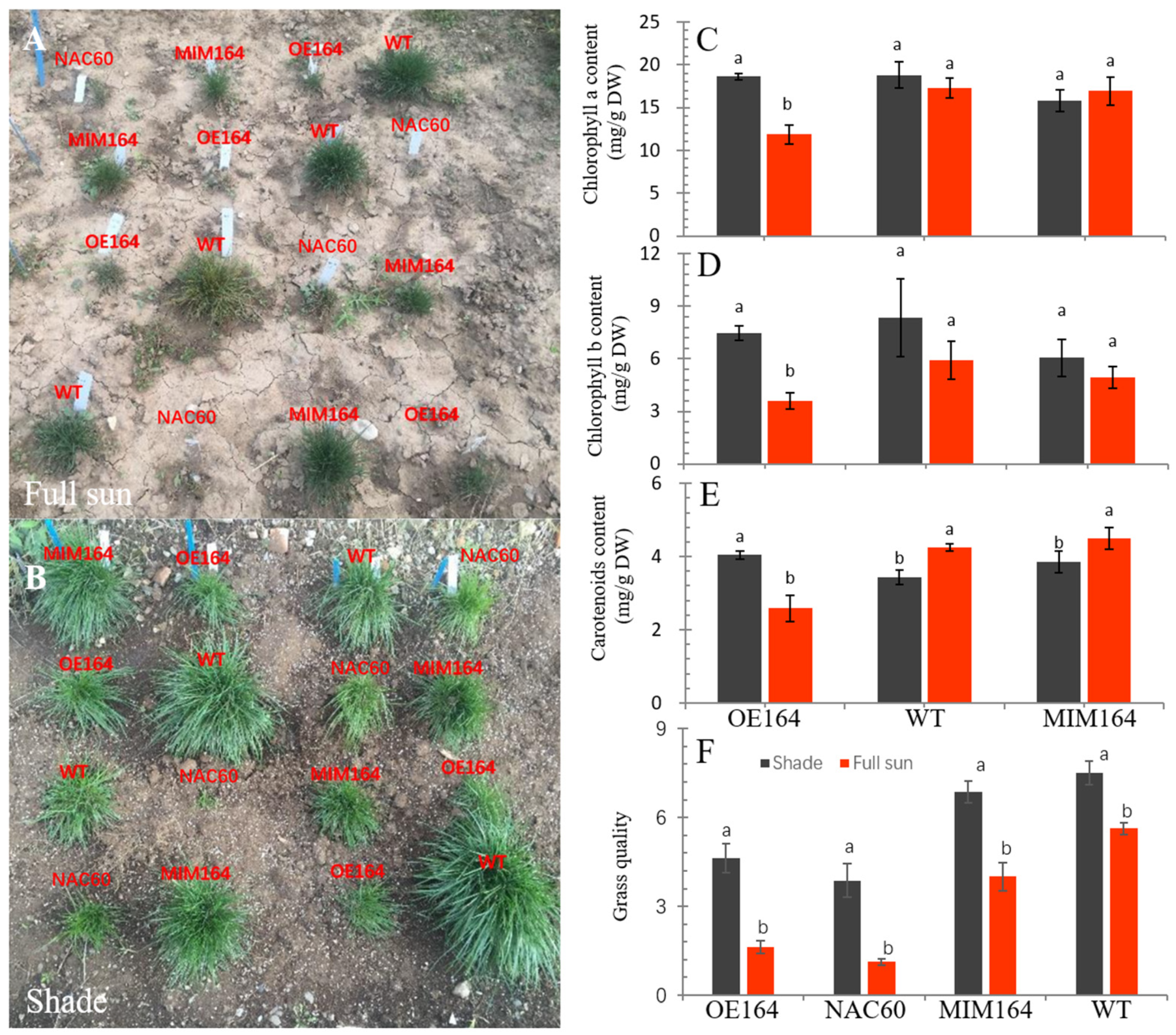
3.1. OE164, NAC60, MIM164, and WT Plants Performed Differently under Shade or Full Sun Field Conditions
3.1.1. Grass Quality and Photosynthetic Pigments
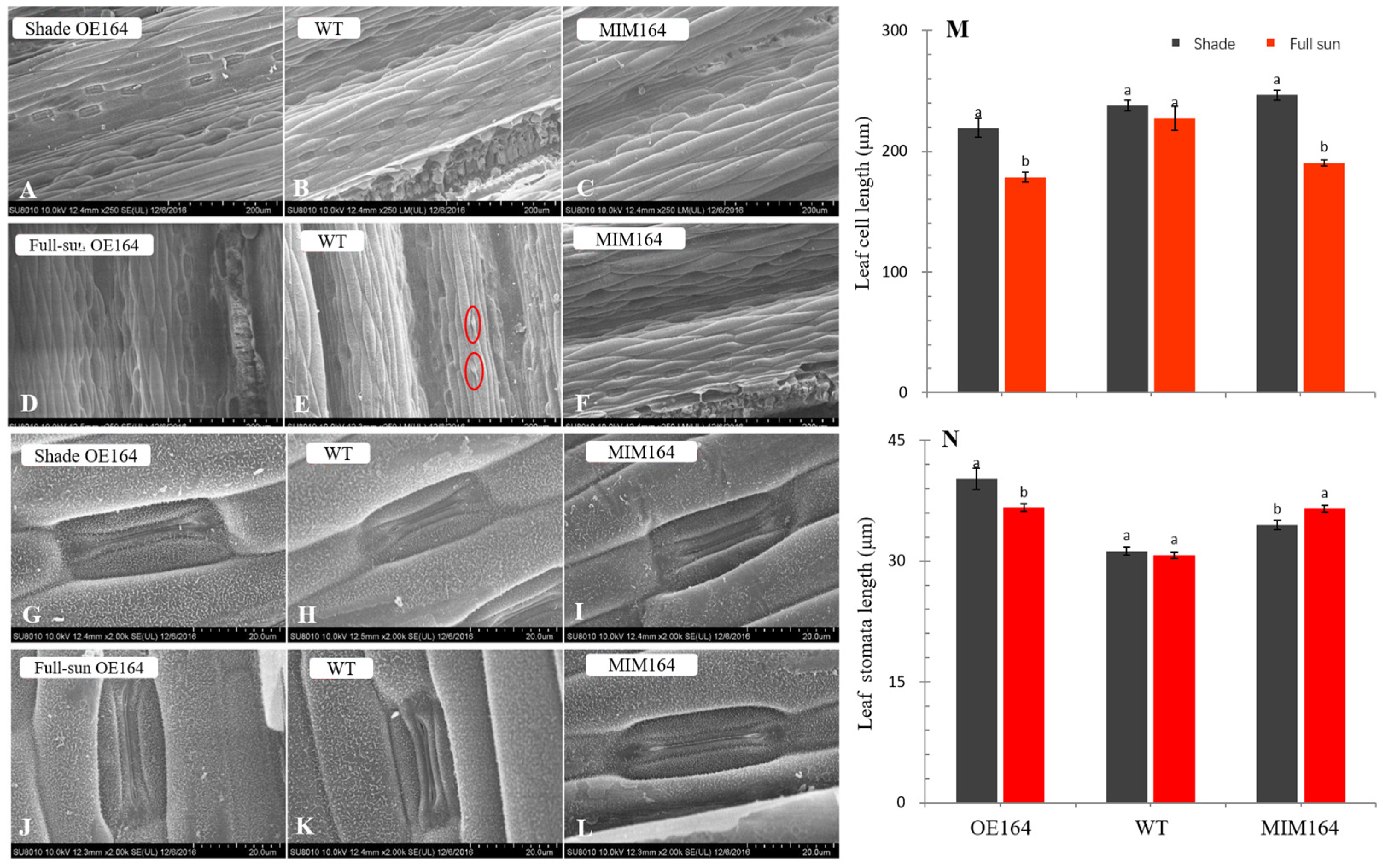
3.1.2. Leaf SEM and TEM Analysis
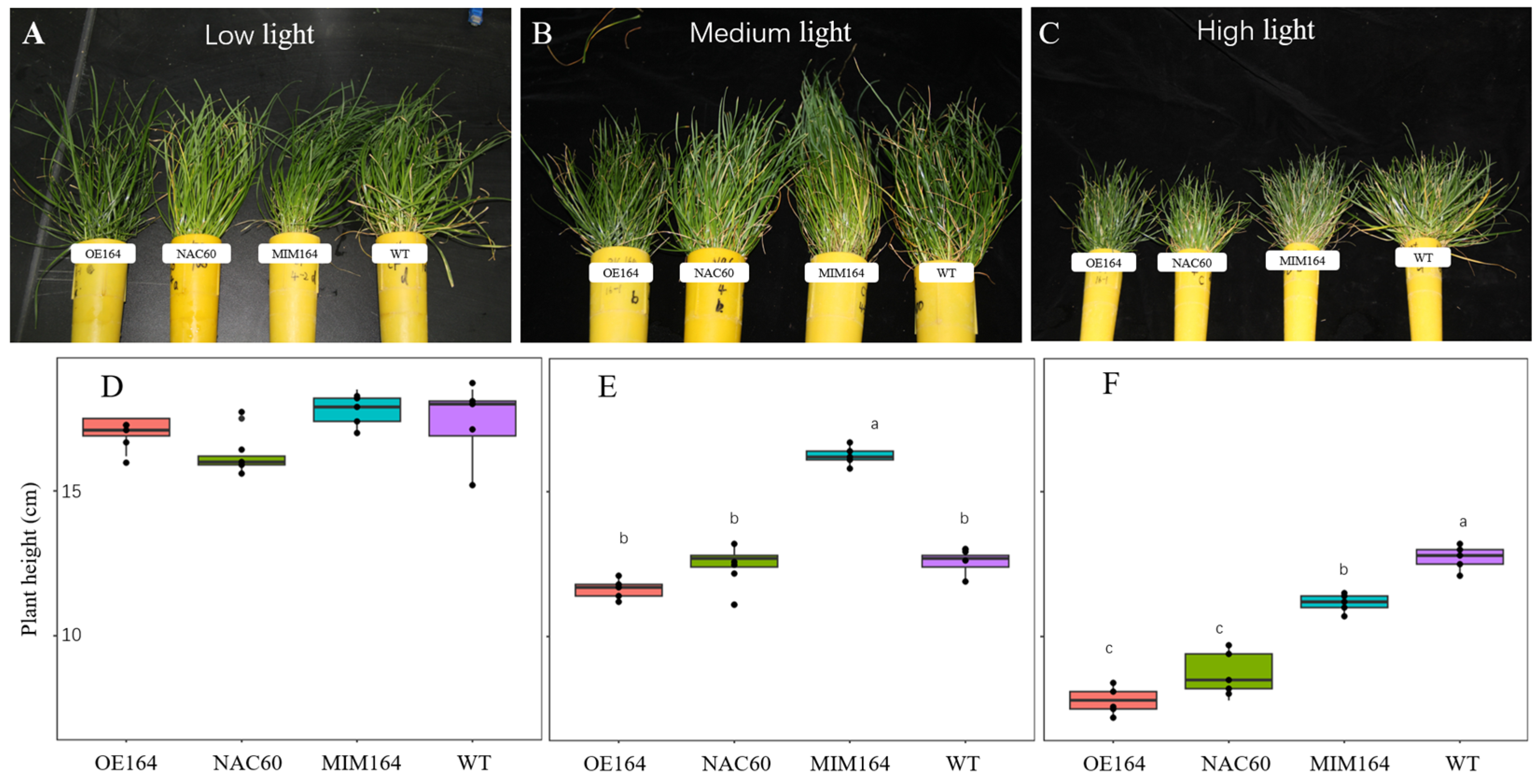
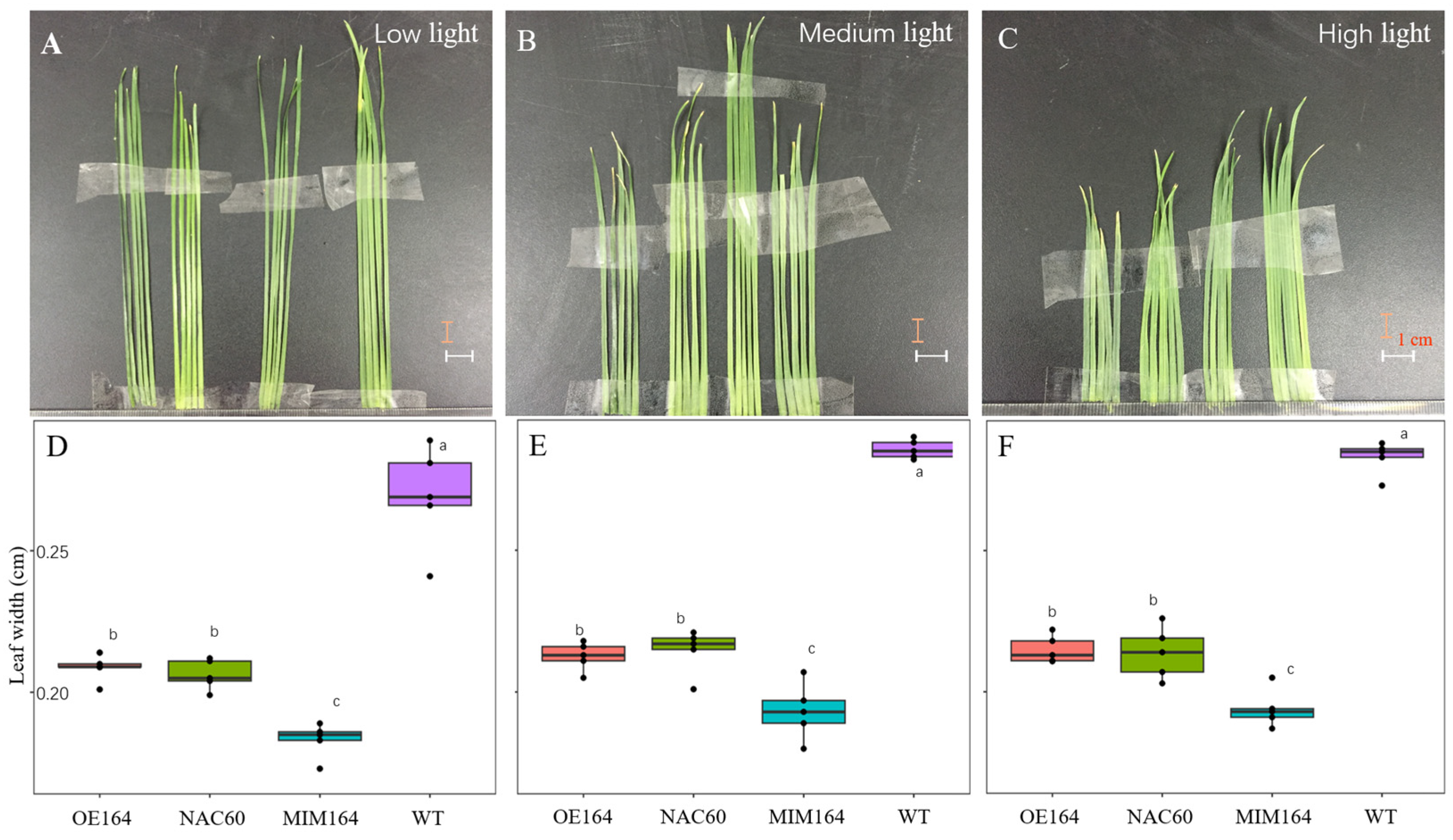
3.2. OE164, NAC60, MIM164, and WT Plants Responded Similarly to Various Light Intensities in Growth Chambers but to Different Extents
3.2.1. The Morphology of OE164, NAC60, MIM164, and WT Plants
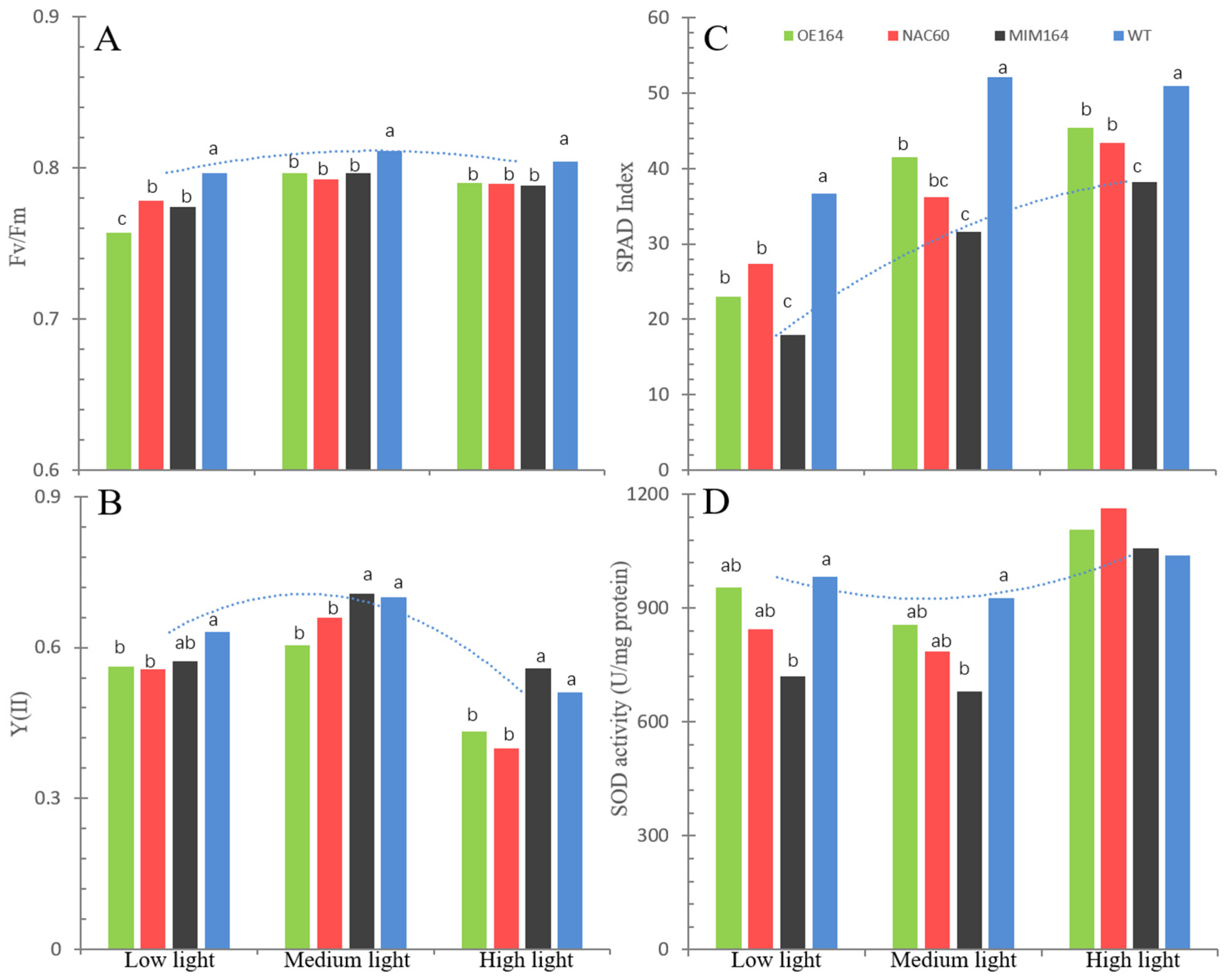
3.2.2. The Physiology of OE164, NAC60, MIM164, and WT Plants
3.3. MiR164 Plays a Role in Regulating Plant Adaptation to Changing Light Intensity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does it Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kuroda, H.; Ozawa, S.I.; Saito, K.; Dogra, V.; Scholz, M.; Zhang, G.X.; de Vitry, C.; Ishikita, H.; Kim, C.; et al. Characterization of tryptophan oxidation affecting D1 degradation by FtsH in the photosystem II quality control of chloroplasts. eLife 2023, 12, RP88822. [Google Scholar] [CrossRef] [PubMed]
- Schulze, E.D.; Beck, E.; Muller-Hohenstein, K. Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- de Wit, M.; Galvão, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Hernando, C.E.; Garcia, C.; Mateos, J.L. Casting Away the Shadows: Elucidating the Role of Light-mediated Posttranscriptional Control in Plants. Photochem. Photobiol. 2017, 93, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.T.; Zhou, C.Z.; Chen, L.; Zaripov, T.; Zhan, D.M.; Weng, J.J.; Lin, Y.L.; Lai, Z.X.; Guo, Y.Q. Integrated Transcriptome, microRNA, and Phytochemical Analyses Reveal Roles of Phytohormone Signal Transduction and ABC Transporters in Flavor Formation of Oolong Tea (Camellia sinensis) during Solar Withering. J. Agric. Food Chem. 2020, 68, 12749–12767. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.M.; Wang, X.; Ijaz, M.; Rahman, M.U.; Oranab, S.; Ali, M.A.; Fiaz, S. Molecular aspects of microRNAs and phytohormonal signaling in response to drought stress: A review. Curr. Issues Mol. Biol. 2022, 44, 3695–3710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Song, Y.; Xue, X.; Xue, R.; Jiang, H.; Zhou, Y.; Qi, X.; Wang, Y. Differential Transcription Profiling Reveals the MicroRNAs Involved in Alleviating Damage to Photosynthesis under Drought Stress during the Grain Filling Stage in Wheat. Int. J. Mol. Sci. 2024, 25, 5518. [Google Scholar] [CrossRef]
- Song, X.W.; Li, Y.; Cao, X.F.; Qi, Y.J. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.R.; Liu, Y.; Wang, H.; Ma, X.J.; Wang, B.B.; Wu, G.X.; Wang, H.Y. Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nat. Commun. 2017, 8, 348. [Google Scholar] [CrossRef]
- Sunkar, R.; Zhou, X.; Zheng, Y.; Zhang, W.; Zhu, J.K. Identification of novel and candidate miRNAs in rice by high throughput sequencing. BMC Plant Biol. 2008, 8, 25. [Google Scholar] [CrossRef]
- Chi, Q.; Du, L.Y.; Ma, W.; Niu, R.Y.; Wu, B.W.; Guo, L.J.; Ma, M.; Liu, X.L.; Zhao, H.X. The miR164-TaNAC14 module regulates root development and abiotic-stress tolerance in wheat seedlings. J. Integr. Agric. 2023, 22, 981–998. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, K.; Xiong, L. Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. J. Exp. Bot. 2014, 65, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Jeon, H.S.; Kim, H.G.; Park, O.K. An Arabidopsis NAC transcription factor NAC4 promotes pathogen-induced cell death under negative regulation by microRNA164. New Phytol. 2017, 214, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, S.Q.; Wang, L.P.; Wu, D.; Cheng, H.L.; Du, X.; Mao, D.D.; Zhang, C.L.; Jiang, X.C. miR164c and miR168a regulate seed vigor in rice. J. Integr. Plant Biol. 2020, 62, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.R.; Joshi, G.; Pandey, R.; Kukreja, B.; Goel, S.; Jagannath, A.; Kumar, A.; Katiyar-Agarwal, S.; Agarwal, M. A Genome-Wide Perspective of miRNAome in Response to High Temperature, Salinity and Drought Stresses in Brassica juncea (Czern) L. PLoS ONE 2014, 9, e92456. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhao, H.S.; Sun, H.Y.; Dong, L.L.; Lou, Y.F.; Gao, Z.M. Expression Analysis of miR164b and Its Target Gene PeNAC1 in Phyllostachys edulis under Stress. For. Res. 2015, 28, 605–611. [Google Scholar]
- Zhang, M.; Liang, S.J.; Hang, X.M.; Xiang, Y.X.; Cheng, Z.L.; Li, W.J.; Shi, J.M.; Huang, L.; Sun, Y.Q. Identification of heavy-ion radiation-induced microRNAs in rice. Adv. Space Res. 2011, 47, 1054–1061. [Google Scholar] [CrossRef]
- Peng, X.; Feng, C.; Wang, Y.T.; Zhang, X.; Wang, Y.Y.; Sun, Y.T.; Xiao, Y.Q.; Zhai, Z.F.; Zhou, X.; Du, B.Y.; et al. MiR164g-MsNAC022 acts as a novel module mediating drought response by transcriptional regulation of reactive oxygen species scavenging systems in apple. Hortic. Res. 2022, 9, uhac192. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, L.; Terzaghi, W.; Guo, Y.; Li, J.G. Molecular mechanisms underlying coordinated responses of plants to shade and environmental stresses. Plant J. 2024, 117, 1893–1913. [Google Scholar] [CrossRef]
- Bell, G.E.; Danneberger, T.K.; McMahon, M.J. Spectral Irradiance Available for Turfgrass Growth in Sun and Shade. Crop Sci. 2000, 40, 189–195. [Google Scholar] [CrossRef]
- Gardner, D.S.; Goss, R.M. Management of Turfgrass in Shade. Turfgrass Biol. Use Manag. 2013, 56, 219–247. [Google Scholar]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Ye, W.; Liu, Y.; Li, D.; Wang, K.; Zhang, W. Overexpression of a Chimeric Gene, OsDST-SRDX, Improved Salt Tolerance of Perennial Ryegrass. Sci. Rep. 2016, 6, 27320. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; Valli, A.; Todesco, M.; Mateos, I.; Puga, M.I.; Rubio-Somoza, I.; Leyva, A.; Weigel, D.; Garcia, J.A.; Paz-Ares, J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 2007, 39, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Ma, N. Genetic Transformation of miR164 and Target Mimicry miR164 Gene on Perennial Ryegrass. Master’s Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- Wang, Z.Y.; Xia, Y.Q.; Lin, S.Y.; Wang, Y.R.; Guo, B.H.; Song, X.N.; Ding, S.C.; Zheng, L.Y.; Feng, R.Y.; Chen, S.L.; et al. Osa-miR164a targets OsNAC60 and negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. Plant J. 2018, 95, 584–597. [Google Scholar] [CrossRef]
- Xu, C.; Huang, X.; Ma, N.; Liu, Y.; Xu, A.; Zhang, X.; Li, D.; Li, Y.; Zhang, W.; Wang, K. MicroRNA164 Affects Plant Responses to UV Radiation in Perennial Ryegrass. Plants 2024, 13, 1242. [Google Scholar] [CrossRef]
- Fowke, L.C. Transmission and Scanning Electron Microscopy for Plant Protoplasts, Cultured Cells and Tissues. In Plant Cell, Tissue and Organ Culture: Fundamental Methods; Gamborg, O.L., Phillips, G.C., Eds.; Springer: Berlin Heidelberg, Germany, 1995; pp. 229–238. [Google Scholar]
- Hang, N.; Shi, T.R.; Liu, Y.R.; Ye, W.X.; Taier, G.; Sun, Y.; Wang, K.H.; Zhang, W.J. Overexpression of Os-microRNA408 enhances drought tolerance in perennial ryegrass. Physiol. Plant. 2020, 172, 733–747. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Wang, K.H.; Zhang, X.Z.; Ervin, E. Antioxidative responses in roots and shoots of creeping bentgrass under high temperature: Effects of nitrogen and cytokinin. J. Plant Physiol. 2012, 169, 492–500. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, J.; Dayananda, B.; Li, J. Effect of light intensities on the photosynthesis, growth and physiological performances of two maple species. Front. Plant Sci. 2022, 13, 999026. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.F.; Tang, J.M.; Mallik, A.; Shi, Y.C.; Zou, R.; Li, J.T.; Wei, X. Eco-physiological basis of shade adaptation of Camellia nitidissima, a rare and endangered forest understory plant of Southeast Asia. BMC Ecol. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.J.; Ranney, T.G.; Pharr, D.M. Photosynthesis, Chlorophyll Fluorescence, and Carbohydrate Content of Illicium Taxa Grown under Varied Irradiance. J. Am. Soc. Hortic. Sci. 2004, 129, 46–53. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hong, S.H.; Kim, Y.W.; Lee, I.H.; Jun, J.H.; Phee, B.K.; Rupak, T.; Jeong, H.; Lee, Y.; Hong, B.S.; et al. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 4023–4036. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai, T.; Song, H.; Xu, X. MiR164 is involved in delaying senescence of strawberry (Fragaria ananassa) fruit by negatively regulating NAC transcription factor genes under low temperature. Russ. J. Plant Physiol. 2017, 64, 251–259. [Google Scholar] [CrossRef]
- Li, Z.H.; Peng, J.Y.; Wen, X.; Guo, H.W. ETHYLENE-INSENSITIVE3 Is a Senescence-Associated Gene That Accelerates Age-Dependent Leaf Senescence by Directly Repressing miR164 Transcription in Arabidopsis. Plant Cell 2013, 25, 3311–3328. [Google Scholar] [CrossRef] [PubMed]
- Moschen, S.; Luoni, S.B.; Paniego, N.B.; Hopp, H.E.; Dosio, G.A.A.; Fernandez, P.; Heinz, R.A. Identification of Candidate Genes Associated with Leaf Senescence in Cultivated Sunflower (Helianthus annuus L.). PLoS ONE 2014, 9, e104379. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.E.; Le, T.Y.T.; Stromberg, C.A.E. Light Environment and Epidermal Cell Morphology in Grasses. Int. J. Plant Sci. 2015, 176, 832–847. [Google Scholar] [CrossRef]
- Barillot, R.; De Swaef, T.; Combes, D.; Durand, J.L.; Escobar-Gutiérrez, A.J.; Martre, P.; Perrot, C.; Roy, E.; Frak, E. Leaf elongation response to blue light is mediated by stomatal-induced variations in transpiration in Festuca arundinacea. J. Exp. Bot. 2020, 72, 2642–2656. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Chen, C.; Jin, Z.X.; Yang, Z.N.; Li, Y.L. Leaf anatomy, photosynthesis, and chloroplast ultrastructure of Heptacodium miconioides seedlings reveal adaptation to light environment. Environ. Exp. Bot. 2022, 195, 104780. [Google Scholar] [CrossRef]
- Roelfsema, M.R.; Hedrich, R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef]
- Pastenes, C.; Pimentel, P.; Lillo, J. Leaf movements and photoinhibition in relation to water stress in field-grown beans. J. Exp. Bot. 2005, 56, 425–433. [Google Scholar] [CrossRef]
- Phookaew, P.; Netrphan, S.; Sojikul, P.; Narangajavana, J. Involvement of miR164-and miR167-mediated target gene expressions in responses to water deficit in cassava. Biol. Plant. 2014, 58, 469–478. [Google Scholar] [CrossRef]
- Lin, D.B.; Zhu, X.E.; Qi, B.L.; Gao, Z.; Tian, P.; Li, Z.W.; Lin, Z.T.; Zhang, Y.X.; Huang, T.B. SlMIR164A regulates fruit ripening and quality by controlling SlNAM2 and SlNAM3 in tomato. Plant Biotechnol. J. 2022, 20, 1456–1469. [Google Scholar] [CrossRef]
- Chapa-Dubocq, X.; Makarov, V.; Javadov, S. Simple kinetic model of mitochondrial swelling in cardiac cells. J. Cell. Physiol. 2018, 233, 5310–5321. [Google Scholar] [CrossRef] [PubMed]
- Rurek, M. Plant mitochondria under a variety of temperature stress conditions. Mitochondrion 2014, 19, 289–294. [Google Scholar] [CrossRef]
- Scott, I.; Logan, D.C. Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytol. 2008, 177, 90–101. [Google Scholar] [CrossRef]
- Fina, J.; Casadevall, R.; AbdElgawad, H.; Prinsen, E.; Markakis, M.N.; Beemster, G.T.S.; Casati, P. UV-B Inhibits Leaf Growth through Changes in Growth Regulating Factors and Gibberellin Levels. Plant Physiol. 2017, 174, 1110–1126. [Google Scholar] [CrossRef]
- IPCC. Electronic Book; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Sharma, S.; Chatterjee, S.; Kataria, S.; Joshi, J.; Datta, S.; Vairale, M.G.; Veer, V. A Review on Responses of Plants to UV-B Radiation Related Stress. UV-B Radiat. 2017, 75–97. [Google Scholar] [CrossRef]
- Huang, K.Y.; Liu, Y.R.; Shi, Y.L.; Tian, J.L.; Shi, T.R.; Peng, H.R.; Tian, X.J.; Zhang, W.J.; Wang, K.H. Overexpression of TaMBF1c improves thermo-tolerance of perennial ryegrass. Sci. Hortic. 2022, 295, 110812. [Google Scholar] [CrossRef]
- Reyes, T.H.; Pompeiano, A.; Ranieri, A.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Photosynthetic performance of five cool-season turfgrasses under UV-B exposure. Plant Physiol. Biochem. 2020, 151, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Guo, H.; Baskin, C.C.; Xiong, W.D.; Yang, C.; Li, Z.Y.; Song, H.; Wang, T.R.; Yin, J.N.; Wu, X.L.; et al. Effect of Light Intensity on Morphology, Photosynthesis and Carbon Metabolism of Alfalfa (Medicago sativa) Seedlings. Plants 2022, 11, 1688. [Google Scholar] [CrossRef]
- Wimalasekera, R. Effect of Light Intensity on Photosynthesis. In Photosynthesis, Productivity and Environmental Stress; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 65–73. [Google Scholar]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.J.; Hu, H.; Zhang, S.B. Seasonal variations in photosystem I compared with photosystem II of three alpine evergreen broad-leaf tree species. J. Photochem. Photobiol. B Biol. 2016, 165, 71–79. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Zhu, H.F.; Li, X.F.; Zhai, W.; Liu, Y.; Gao, Q.Q.; Liu, J.P.; Ren, L.; Chen, H.Y.; Zhu, Y.Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef]
- Lu, X.; Dun, H.; Lian, C.; Zhang, X.; Yin, W.; Xia, X. The role of peu-miR164 and its target PeNAC genes in response to abiotic stress in Populus euphratica. Plant Physiol. Biochem. 2017, 115, 418–438. [Google Scholar] [CrossRef]
- Tsai, W.A.; Sung, P.H.; Kuo, Y.W.; Chen, M.C.; Jeng, S.T.; Lin, J.S. Involvement of microRNA164 in responses to heat stress in Arabidopsis. Plant Sci. 2023, 329, 111598. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.; Brown, L.K.; George, T.S.; Pakeman, R.J.; Palmer, S.; Ramsay, L.; Schoeb, C.; Schurch, N.; Wilkinson, M.J. Active and adaptive plasticity in a changing climate. Trends Plant Sci. 2022, 27, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, S.; Milla, R. Differential plasticity to water and nutrients between crops and their wild progenitors. Environ. Exp. Bot. 2018, 145, 54–63. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Huang, X.; Liu, Y.; Ma, N.; Li, D.; Hu, Q.; Zhang, W.; Wang, K. MicroRNA164 Regulates Perennial Ryegrass (Lolium perenne L.) Adaptation to Changing Light Intensity. Agronomy 2024, 14, 1142. https://doi.org/10.3390/agronomy14061142
Zhang L, Huang X, Liu Y, Ma N, Li D, Hu Q, Zhang W, Wang K. MicroRNA164 Regulates Perennial Ryegrass (Lolium perenne L.) Adaptation to Changing Light Intensity. Agronomy. 2024; 14(6):1142. https://doi.org/10.3390/agronomy14061142
Chicago/Turabian StyleZhang, Liyun, Xin Huang, Yanrong Liu, Ning Ma, Dayong Li, Qiannan Hu, Wanjun Zhang, and Kehua Wang. 2024. "MicroRNA164 Regulates Perennial Ryegrass (Lolium perenne L.) Adaptation to Changing Light Intensity" Agronomy 14, no. 6: 1142. https://doi.org/10.3390/agronomy14061142
APA StyleZhang, L., Huang, X., Liu, Y., Ma, N., Li, D., Hu, Q., Zhang, W., & Wang, K. (2024). MicroRNA164 Regulates Perennial Ryegrass (Lolium perenne L.) Adaptation to Changing Light Intensity. Agronomy, 14(6), 1142. https://doi.org/10.3390/agronomy14061142






