Dynamic Changes in Physicochemical Properties and Microbial Community in Three Types of Recycled Manure Solids for Dairy Heifers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals
2.2. Preparation of Bedding Materials
2.3. Bedding Material Sampling and Physicochemical Analysis
2.4. Gas Concentration Measurement
2.5. DNA Extraction and Illumina MiSeq Sequencing of 16S rRNA and ITS1 Amplicons
2.6. Bioinformatic Analysis
2.7. Statistical Analysis
3. Results and Discussion
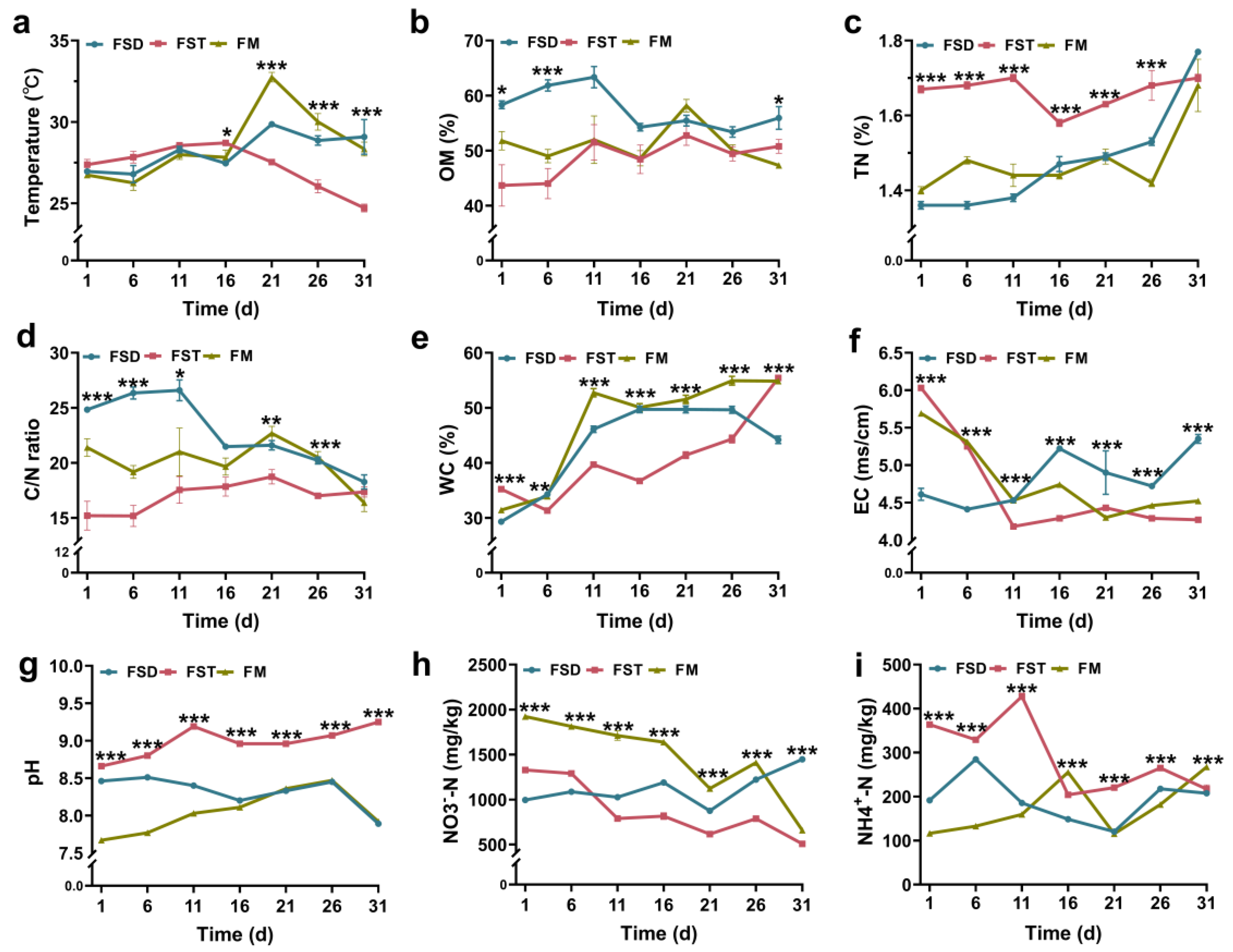
3.1. Changes in the Physicochemical Properties of the Bedding Material
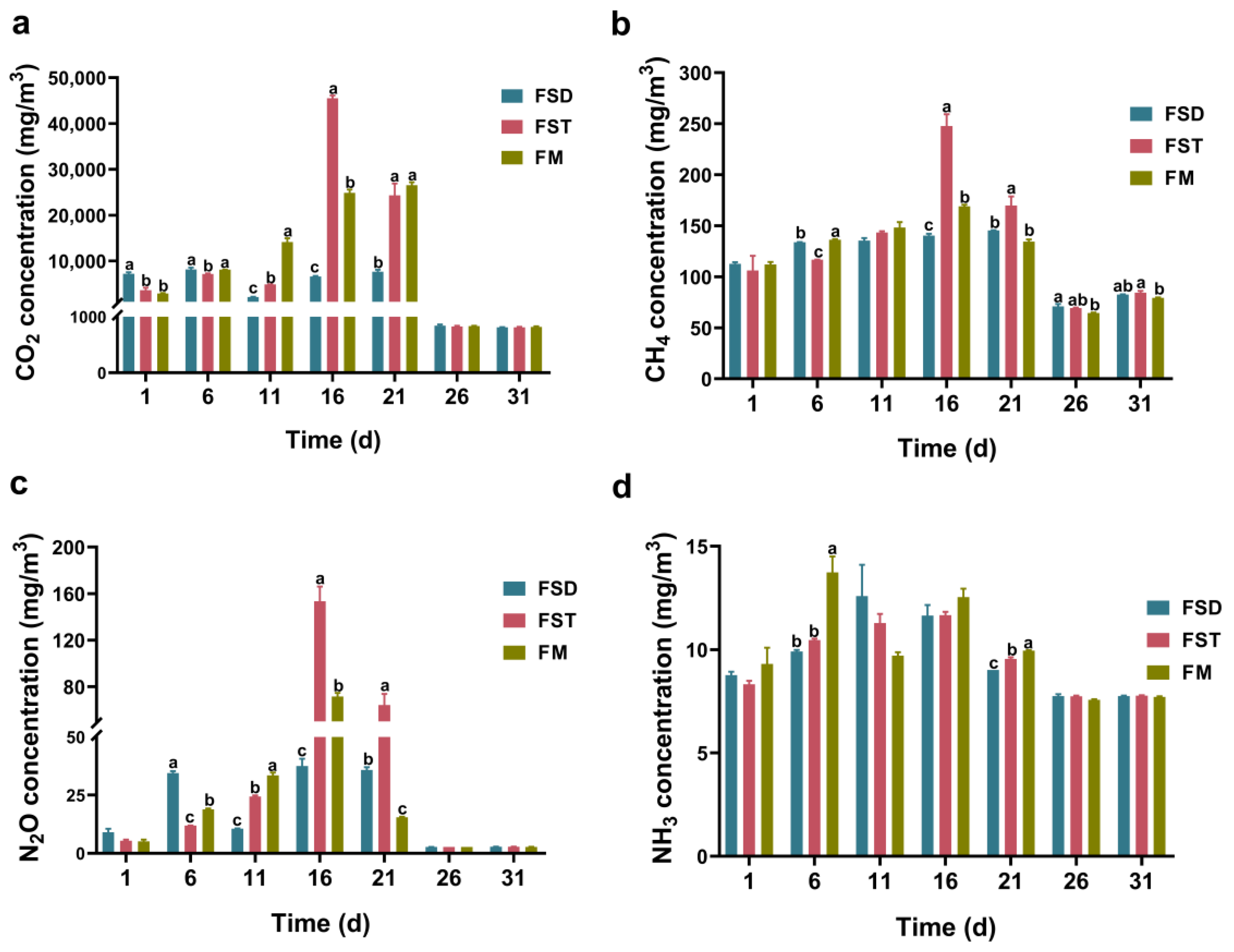
3.2. Emissions of CO2, N2O, NH3 and CH4 in Bedding Materials
3.3. Changes in Microbial Diversity and Abundance
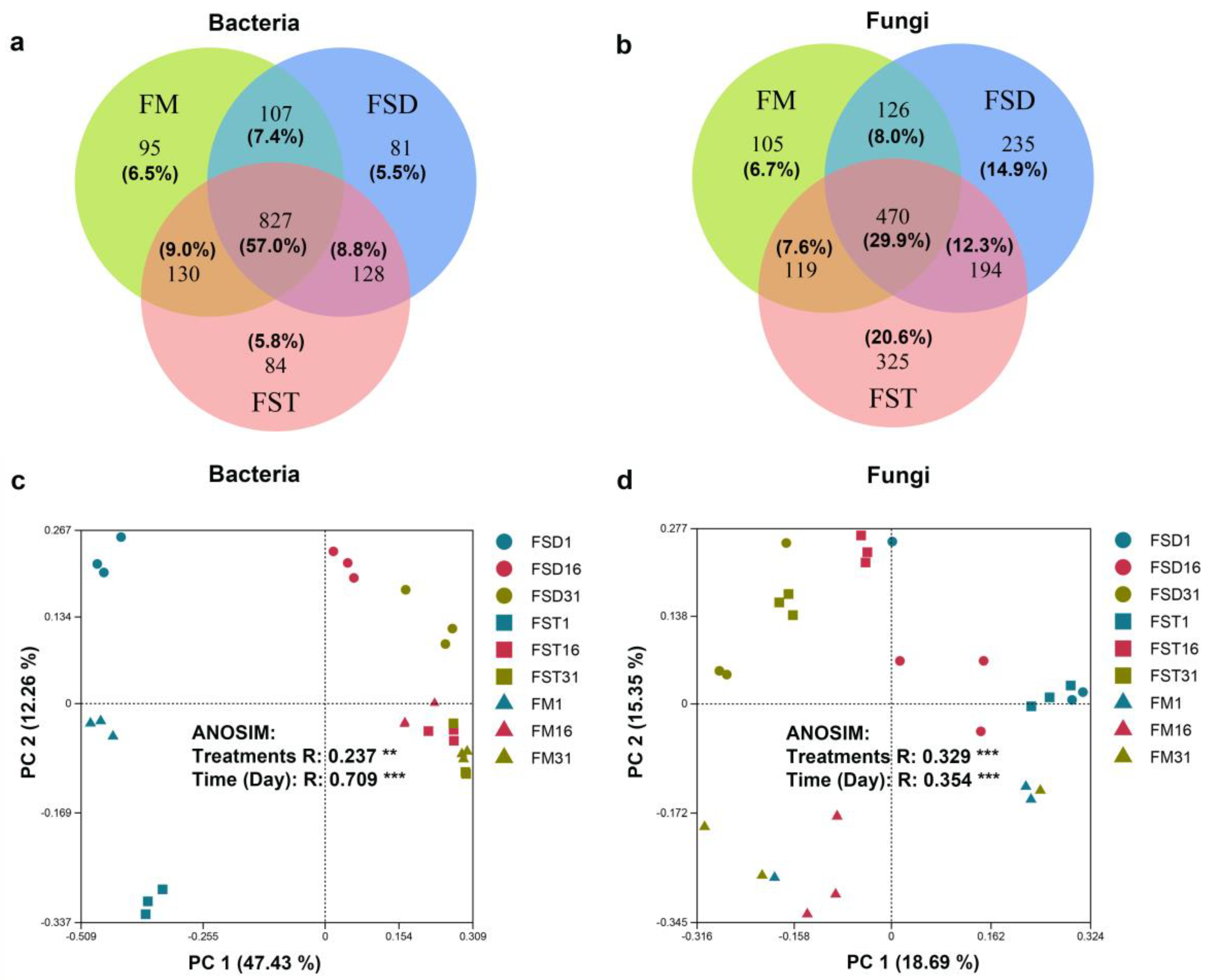
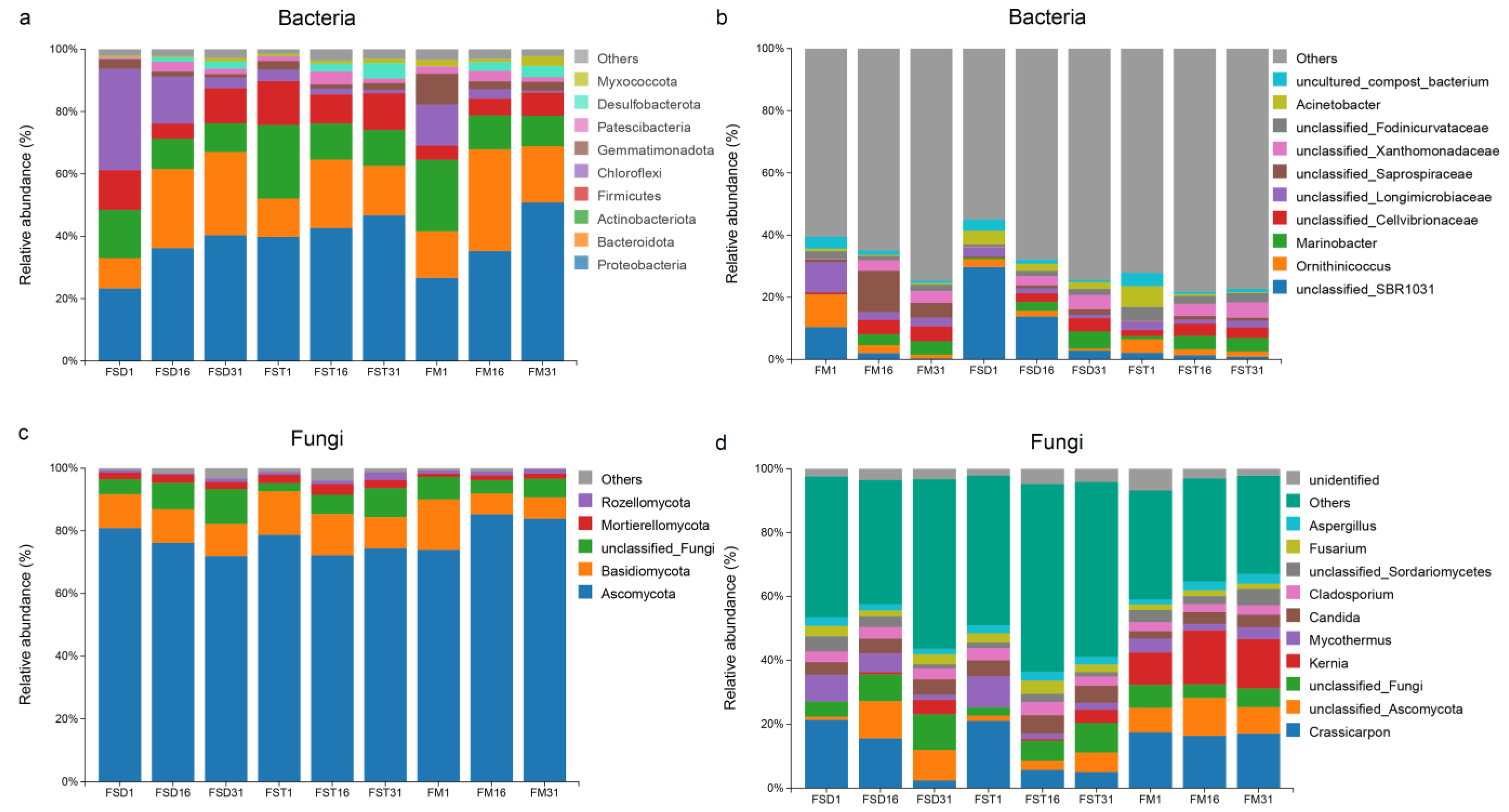
3.4. Evolution of Microbial Community
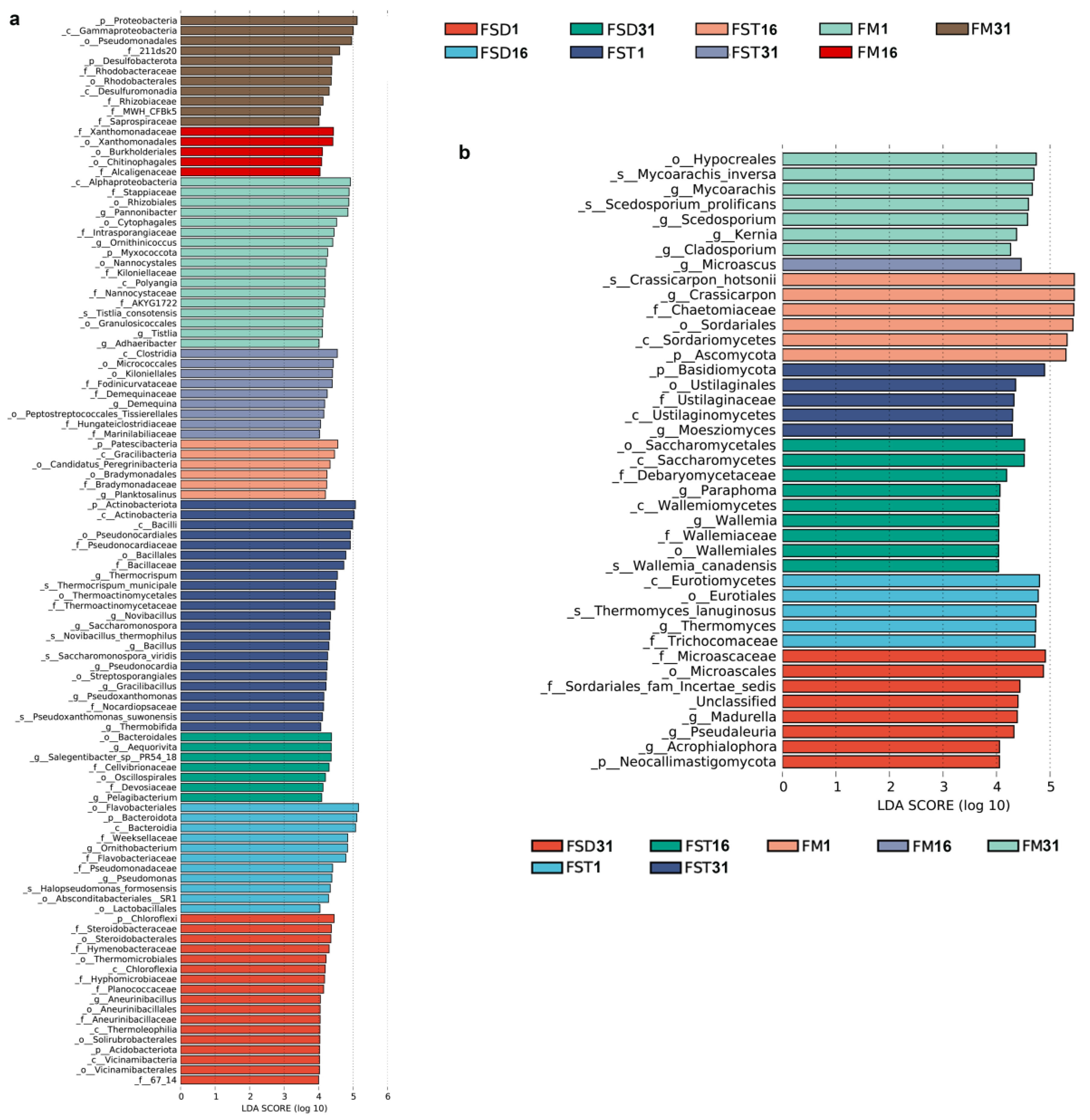
3.5. Influence of Environmental Factors on the Microbial Community


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, M.; Zhou, H.; Shen, Y.; Meng, H.; Li, R.; Zhou, J.; Cheng, H.; Zhang, X.; Ding, J.; Wang, J.; et al. Effect of aeration rates on enzymatic activity and bacterial community succession during cattle manure composting. Bioresour. Technol. 2020, 304, 122928. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhou, H.; Meng, H.; Ding, J.; Shen, Y.; Cheng, H.; Zhang, X.; Li, R.; Fan, S. Amino acid profile characterization dur-ing the co-composting of a livestock manure and maize straw mixture. J. Clean. Prod. 2021, 278, 123494. [Google Scholar] [CrossRef]
- Škrbić, B.; Marinković, V.; Spaić, S.; Milanko, V.; Branovački, S. Profiles of polycyclic aromatic hydrocarbons in smoke from combustion and thermal decomposition of poplar wood pellets and sawdust. Microchem. J. 2018, 139, 9–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Qiao, Y.; Li, S.; Chen, Y.; Hu, C. Mitigation of carbon and nitrogen losses during pig manure composting: A meta-analysis. Sci. Total Environ. 2021, 783, 147103. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.L.; Cheong, D.Y. Chapter 26—Agricultural waste management in food processing. In Handbook of Farm, Dairy and Food Machinery Engineering, 3rd ed.; Kutz, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 673–716. ISBN 978-0-12-814803-7. [Google Scholar]
- Gagnon, M.; Hamelin, L.; Fréchette, A.; Dufour, S.; Roy, D. Effect of recycled manure solids as bedding on bulk tank milk and implications for cheese microbiological quality. J. Dairy Sci. 2020, 103, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, J.; Fréchette, A.; Thériault, W.; Dufour, S.; Fravalo, P.; Thibodeau, A. Comparison of microbiota of recycled manure solids and straw bedding used in dairy farms in eastern Canada. J. Dairy Sci. 2022, 105, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.O.; Sommer, S.G.; Béline, F.; Burton, C.; Dach, J.; Dourmad, J.Y.; Leip, A.; Misselbrook, T.; Nicholson, F.; Poulsen, H.D.; et al. Recycling of livestock manure in a whole-farm perspective. Livest. Sci. 2007, 112, 180–191. [Google Scholar] [CrossRef]
- Fournel, S.; Godbout, S.; Ruel, P.; Fortin, A.; Duquette-Lozeau, K.; Létourneau, V.; Généreux, M.; Lemieux, J.; Potvin, D.; Côté, C.; et al. Production of recycled manure solids for use as bedding in Canadian dairy farms: II. Composting methods. J. Dairy Sci. 2019, 102, 1847–1865. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity as-sessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Huhe; Jiang, C.; Wu, Y.; Cheng, Y. Bacterial and fungal communities and contribution of physicochemical factors during cattle farm waste composting. MicrobiologyOpen 2017, 6, e00518. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.; Qiu, X.; Zhang, J. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour. Technol. 2019, 272, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Wang, B.; Zhao, Y.; Yang, W.; Ogundeji, A.; Deng, L.; Egbeagu, U.U.; Yu, S.; Zhao, L.; Li, D.; et al. Composted biochar affects structural dynamics, function and co-occurrence network patterns of fungi community. Sci. Total Environ. 2021, 775, 145672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Convers. Biorefinery 2023, 13, 5445–5468. [Google Scholar] [CrossRef]
- Huang, G.F.; Wong, J.W.C.; Wu, Q.T.; Nagar, B.B. Effect of C/N on composting of pig manure with sawdust. Waste Manag. 2004, 24, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Q.; Wang, J.; Ma, Y.; Cao, B. Responses of heavy metals mobility and resistant bacteria to adding time of activated carbon during chicken manure composting. Environ. Pollut. 2021, 290, 118070. [Google Scholar] [CrossRef] [PubMed]
- Robles, I.; Kelton, D.F.; Barkema, H.W.; Keefe, G.P.; Roy, J.P.; von Keyserlingk, M.A.G.; DeVries, T.J. Bacterial concen-trations in bedding and their association with dairy cow hygiene and milk quality. Animal 2020, 14, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Llonch, L.; Castillejos, L.; Mainau, E.; Manteca, X.; Ferret, A. Effect of forest biomass as bedding material on compost-bedded pack performance, microbial content, and behavior of nonlactating dairy cows. J. Dairy Sci. 2020, 103, 10676–10688. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cai, A.; Descovich, K.; Fu, T.; Lian, H.; Gao, T.; Phillips, C.J.C. A comparison of rice husks and peanut shells as bed-ding materials on dairy cows’ preferences, behaviour, and health. Animals 2021, 11, 1887. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Dong, L.; Hu, H.; Meng, L.; Liu, H.; Zheng, N.; Wang, J. Microbial characteristics and safety of dairy ma-nure composting for reuse as dairy bedding. Biology 2020, 10, 13. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, X.; Chen, C.; Yang, L. Effects of fermented manure bedding thickness on bulls’ growth, behavior, and wel-fare as well as barn gases concentration in the barn. Animals 2022, 12, 925. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Huang, H.; Sun, E.; Butterly, C.; Xu, Y.; He, H.; Zhang, J.; Chang, Z. Spectroscopic evidence for hyper-thermophilic pretreatment intensifying humification during pig manure and rice straw composting. Bioresour. Technol. 2019, 294, 122131. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Y.; Han, Y.; Qian, W.; Li, G.; Luo, W. Performance of mature compost to control gaseous emissions in kitchen waste composting. Sci. Total Environ. 2019, 657, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, H.; Zhang, H.; Xun, L.; Chen, G.; Wang, L. Thermomyces lanuginosus is the dominant fungus in maize straw composts. Bioresour. Technol. 2015, 197, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Shamrikova, E.V.; Kondratenok, B.M.; Tumanova, E.A.; Vanchikova, E.V.; Lapteva, E.M.; Zonova, T.V.; Lu-Lyan-Min, E.I.; Davydova, A.P.; Libohova, Z.; Suvannang, N. Transferability between soil organic matter measurement methods for database harmonization. Geoderma 2022, 412, 115547. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, X.; Wan, Y.; Deng, R.; Zhu, H.; Wang, X.; Wang, S.; Wang, X. Effect of different livestock manure ratios on the decomposition process of aerobic composting of wheat straw. Agronomy 2023, 13, 2916. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A guide for selecting the most appropriate method for ammonium determination in water analysis. TrAC Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Ferraz, P.F.P.; Ferraz, G.A.E.S.; Leso, L.; Klopcic, M.; Barbari, M.; Rossi, G. Properties of conventional and alternative bedding materials for dairy cattle. J. Dairy Sci. 2020, 103, 8661–8674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Wang, F.; Wang, A.; Yan, P. Evaluation of gaseous concentrations, bacterial diversity and microbial quantity in different layers of deep litter system. Asian-Australas. J. Anim. Sci. 2017, 30, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Y.; Shan, G.; He, X.-S.; Huang, J.; Li, Q. Inoculation with compost-born thermophilic complex microbial consortium induced organic matters degradation while reduced nitrogen loss during co-composting of dairy manure and sugarcane leaves. Waste Biomass Valorization 2019, 10, 2467–2477. [Google Scholar] [CrossRef]
- Guo, H.; Gu, J.; Wang, X.; Yu, J.; Nasir, M.; Zhang, K.; Sun, W. Microbial driven reduction of N2O and NH3 emissions during composting: Effects of bamboo charcoal and bamboo vinegar. J. Hazard. Mater. 2020, 390, 121292. [Google Scholar] [CrossRef]
- Bohacz, J. Changes in mineral forms of nitrogen and sulfur and enzymatic activities during composting of lignocellulosic waste and chicken feathers. Environ. Sci. Pollut. Res. 2019, 26, 10333–10342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, H.; Wang, B.; Chen, C.; Zou, X.; Cheng, T.; Li, J. Aerobic co-composting of mature compost with cattle manure: Organic matter conversion and microbial community characterization. Bioresour. Technol. 2023, 382, 129187. [Google Scholar] [CrossRef] [PubMed]
- Smolders, G.; Ouweltjes, W. On Farm Development of Bedded Pack Dairy Barns in the Netherrlands; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2014. [Google Scholar]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Leso, L.; Barbari, M.; Lopes, M.A.; Damasceno, F.A.; Galama, P.; Taraba, J.L.; Kuipers, A. Invited review: Compost-bedded pack barns for dairy cows. J. Dairy Sci. 2020, 103, 1072–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lü, F.; Shao, L.; He, P. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Zhou, S.; Guo, H.; Geng, B.; Peng, X.; Zhao, G.; Xie, Y. Insights into functional microbial succession during nitrogen transformation in an ectopic fermentation system. Bioresour. Technol. 2019, 284, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, H.; Tang, S.; Wei, K.; Peng, H.; Lu, G.; Dang, Z. Effects of benzo [a] pyrene (BaP) on the composting and microbial community of sewage sludge. Chemosphere 2019, 222, 517–526. [Google Scholar] [CrossRef]
- Bargougui, L.; Guergueb, Z.; Chaieb, M.; Mekki, A. Co-composting of olive industry wastes with poultry manure and evaluation of the obtained compost maturity. Waste Biomass Valorization 2020, 11, 6235–6247. [Google Scholar] [CrossRef]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Sun, X.; Lu, P.; Jiang, T.; Schuchardt, F.; Li, G. Influence of bulking agents on CH4, N2O, and NH3 emissions during rapid composting of pig manure from the Chinese Ganqinfen system. J. Zhejiang Univ. Sci. B 2014, 15, 353–364. [Google Scholar] [CrossRef] [PubMed]
- El Kader, N.A.; Robin, P.; Paillat, J.-M.; Leterme, P. Turning, compacting and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresour. Technol. 2007, 98, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Rashad, F.M.; Saleh, W.D.; Moselhy, M.A. Bioconversion of rice straw and certain agro-industrial wastes to amendments for organic farming systems: 1. Composting, quality, stability and maturity indices. Bioresour. Technol. 2010, 101, 5952–5960. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, X.; Cheng, W. Biochar combined with ferrous sulfate reduces nitrogen and carbon losses during agricultural waste composting and enhances microbial diversity. Process Saf. Environ. Prot. 2022, 162, 531–542. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Zhang, Z.; Wang, Q.; Shen, F.; Li, R.; Li, D.; Ren, X.; Wang, M.; Chen, H.; Zhao, J. New insight with the effects of biochar amendment on bacterial diversity as indicators of biomarkers support the thermophilic phase during sewage sludge composting. Bioresour. Technol. 2017, 238, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-Z.; Ma, S.-C.; Wang, S.-P.; Wang, T.-T.; Sun, Z.-Y.; Tang, Y.-Q.; Deng, Y.; Kida, K. A comparative study of composting the solid fraction of dairy manure with or without bulking material: Performance and microbial community dynamics. Bioresour. Technol. 2018, 247, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Vejmelkova, D.; Lücker, S.; Streshinskaya, G.M.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Kleerbezem, R.; van Loosdrecht, M.; Muyzer, G.; Daims, H. Nitrolancea hollandica gen. nov., sp. nov., a chemolithoautotrophic nitrite-oxidizing bacterium isolated from a bioreactor belonging to the phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2014, 64, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Khan, S.; Li, Y.; Zheng, N.; Yao, H. Influence of biochars on the accessibility of organochlorine pesticides and microbial community in contaminated soils. Sci. Total Environ. 2019, 647, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, Y.; Liu, H.; Xie, S.; Abbas, F. Impact of different nitrogen source on the compost quality and greenhouse gas emissions during composting of garden waste. Process Saf. Environ. Prot. 2019, 124, 326–335. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, L.; Liu, Y.; Xiong, J.; Su, Y.; Lan, Z.; Han, L.; Huang, G. Effect of micro-aerobic conditions based on semipermeable membrane-covered on greenhouse gas emissions and bacterial community during dairy manure storage at industrial scale. Environ. Pollut. 2022, 299, 118879. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Gao, D. The threshold of influent ammonium concentration for nitrate over-accumulation in a one-stage deammonification system with granular sludge without aeration. Sci. Total Environ. 2018, 634, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Gu, J.; Wang, X.; Hu, T.; Wang, J.; Yu, J.; Dai, X.; Lei, L. Effects of shrimp shell powder on antibiotic resistance genes and the bacterial community during swine manure composting. Sci. Total Environ. 2021, 752, 142162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pan, Y.; Yang, X.; Liu, J.; Miao, H.; Ren, Y.; Zhang, C.; Yan, G.; Lv, J.; Li, Y. Beneficial influences of pelelith and dicyandiamide on gaseous emissions and the fungal community during sewage sludge composting. Environ. Sci. Pollut. Res. 2019, 26, 8928–8938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, G.; Chen, Y.; Yu, M.; Yu, Z.; Li, H.; Yu, Y.; Huang, H. Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Bioresour. Technol. 2011, 102, 2950–2956. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.C.; Zhang, H.H.; Shi, H.L.; Hu, T.; Ngo, H.H. Characteristics of nitrogen transformation and microbial community in an aerobic composting reactor under two typical temperatures. Bioresour. Technol. 2013, 137, 270–277. [Google Scholar] [CrossRef]
| Items | Water Content (%) | Organic Matter (%) | Total N (%) | C/N ratio | Bulk Density (kg/m3) | Porosity (%) | |
|---|---|---|---|---|---|---|---|
| Materials | |||||||
| Cow manure | 81.43 | 69.12 | 1.97 | 20.38 | — | — | |
| Sawdust | 13.94 | 79.43 | 0.26 | 176.52 | — | — | |
| Wheat straw | 8.56 | 73.33 | 0.48 | 89.84 | — | — | |
| FSD 1 | 67.89 | 76.11 | 1.01 | 43.87 | 267.10 | 61.85 | |
| FST 1 | 67.3 | 71.91 | 1.16 | 36.03 | 294.47 | 63.58 | |
| FM 1 | 67.02 | 67.25 | 1.05 | 37.10 | 334.27 | 61.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wu, B.; Hao, W.; Li, G.; Yan, P.; Yang, X.; Mao, S.; Wei, S. Dynamic Changes in Physicochemical Properties and Microbial Community in Three Types of Recycled Manure Solids for Dairy Heifers. Agronomy 2024, 14, 1132. https://doi.org/10.3390/agronomy14061132
Zhao C, Wu B, Hao W, Li G, Yan P, Yang X, Mao S, Wei S. Dynamic Changes in Physicochemical Properties and Microbial Community in Three Types of Recycled Manure Solids for Dairy Heifers. Agronomy. 2024; 14(6):1132. https://doi.org/10.3390/agronomy14061132
Chicago/Turabian StyleZhao, Chongchong, Bin Wu, Weiguang Hao, Guowen Li, Peishi Yan, Xingming Yang, Shengyong Mao, and Shengjuan Wei. 2024. "Dynamic Changes in Physicochemical Properties and Microbial Community in Three Types of Recycled Manure Solids for Dairy Heifers" Agronomy 14, no. 6: 1132. https://doi.org/10.3390/agronomy14061132
APA StyleZhao, C., Wu, B., Hao, W., Li, G., Yan, P., Yang, X., Mao, S., & Wei, S. (2024). Dynamic Changes in Physicochemical Properties and Microbial Community in Three Types of Recycled Manure Solids for Dairy Heifers. Agronomy, 14(6), 1132. https://doi.org/10.3390/agronomy14061132






