Assessment of Soil Spatial Variability in Agricultural Ecosystems Using Multivariate Analysis, Soil Quality Index (SQI), and Geostatistical Approach: A Case Study of the Mnasra Region, Gharb Plain, Morocco
Abstract
1. Introduction
2. Materials and Methods
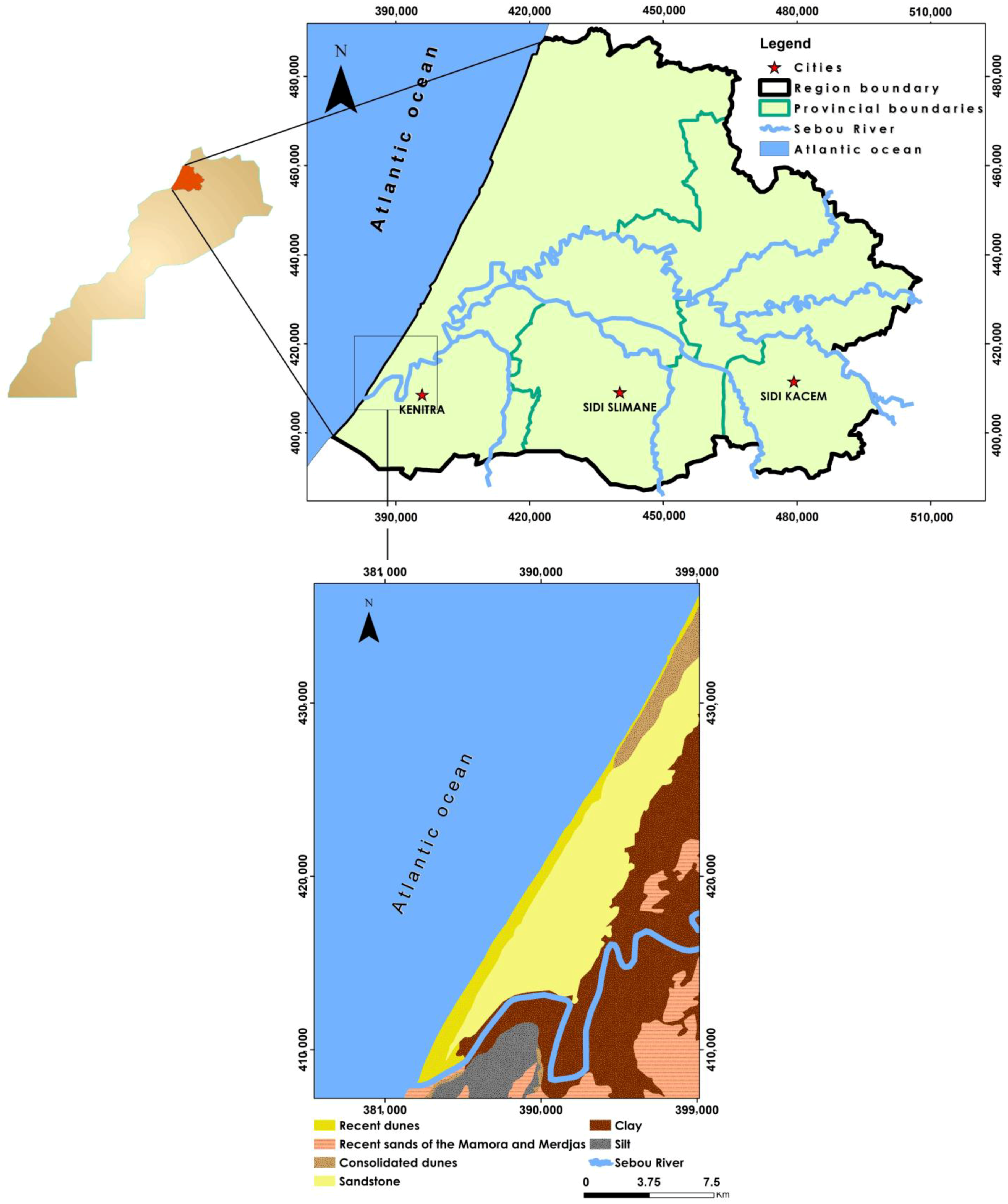
2.1. Description of the Study Area
2.2. Soil Sampling Collection and Analysis
2.2.1. Soil Physical Properties
2.2.2. Soil Chemical Properties
2.3. Statistical Analysis
2.4. Soil Quality Index (SQI)
2.5. Geostatistics Analysis
3. Results and Discussion
3.1. Soil Physical Properties
3.1.1. Particle Size Distribution and Soil Texture
3.1.2. Soil Moisture, Bulk Density, Particle Density, and Soil Porosity
3.2. Soil Chemical Properties
3.2.1. Soil pH, Electrical Conductivity, Soil Organic Matter, CaCO3 Content, and Cation Exchange Capacity
3.2.2. Soil Macronutrients
3.2.3. Soil Micronutrients
3.3. Relationships between Soil Properties
3.3.1. The Correlation Studies between Various Physicochemical Parameters
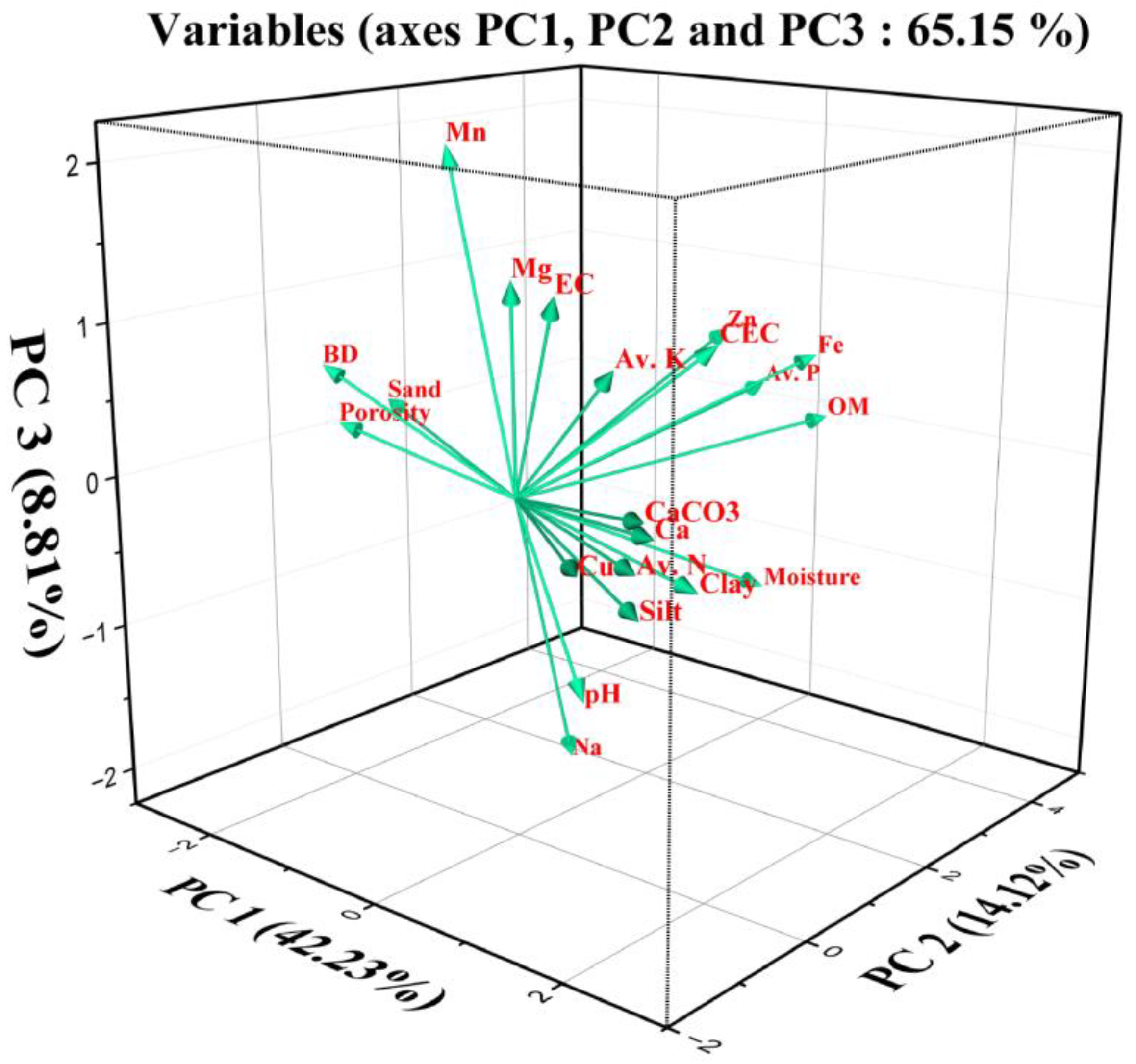
3.3.2. Principal Component Analysis
3.4. Assessment of SQI According to PCA
3.5. Geostatistical Analysis and Spatial Distribution of Soil Properties
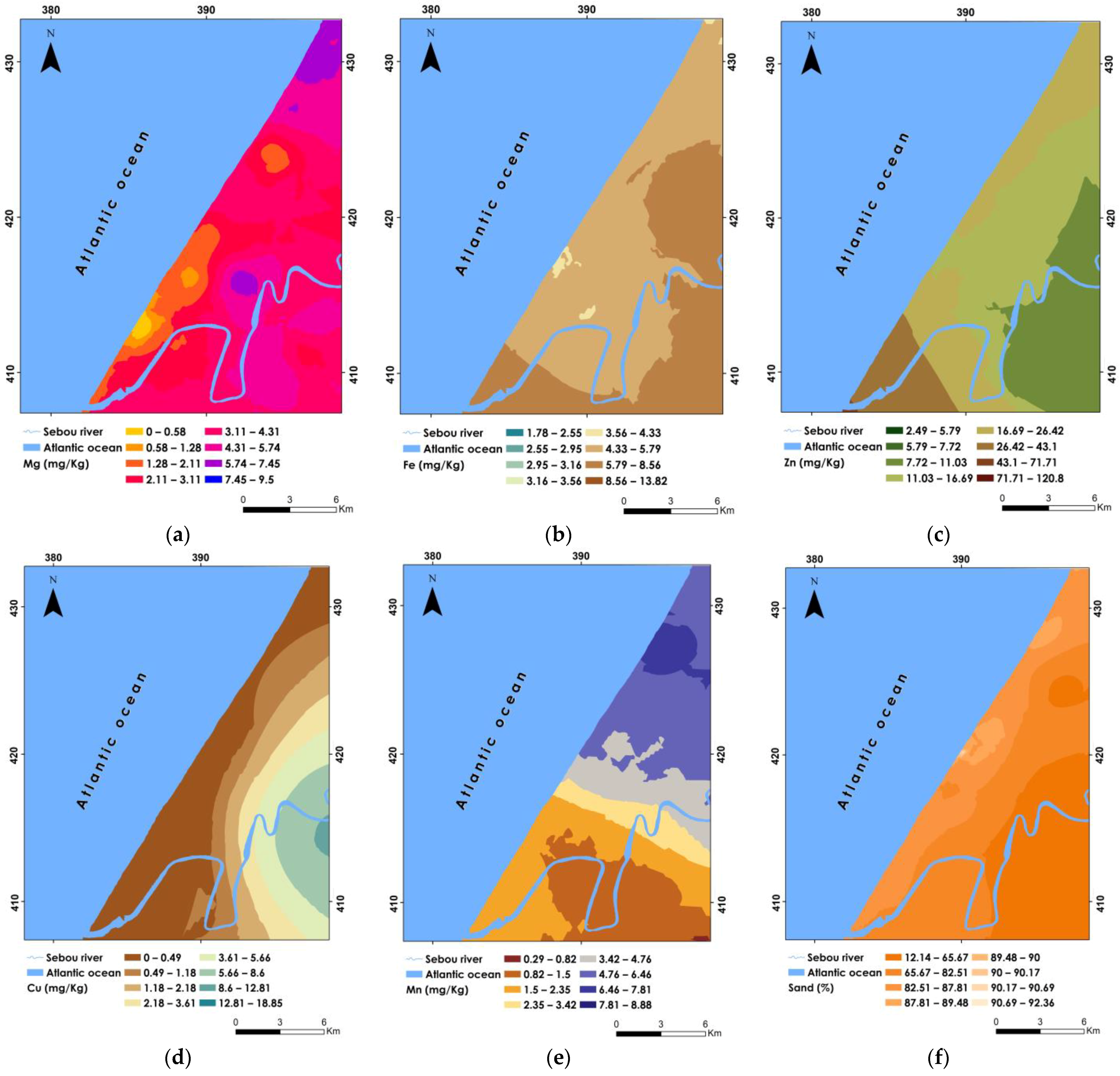
3.5.1. Spatial Variability of Soil Properties
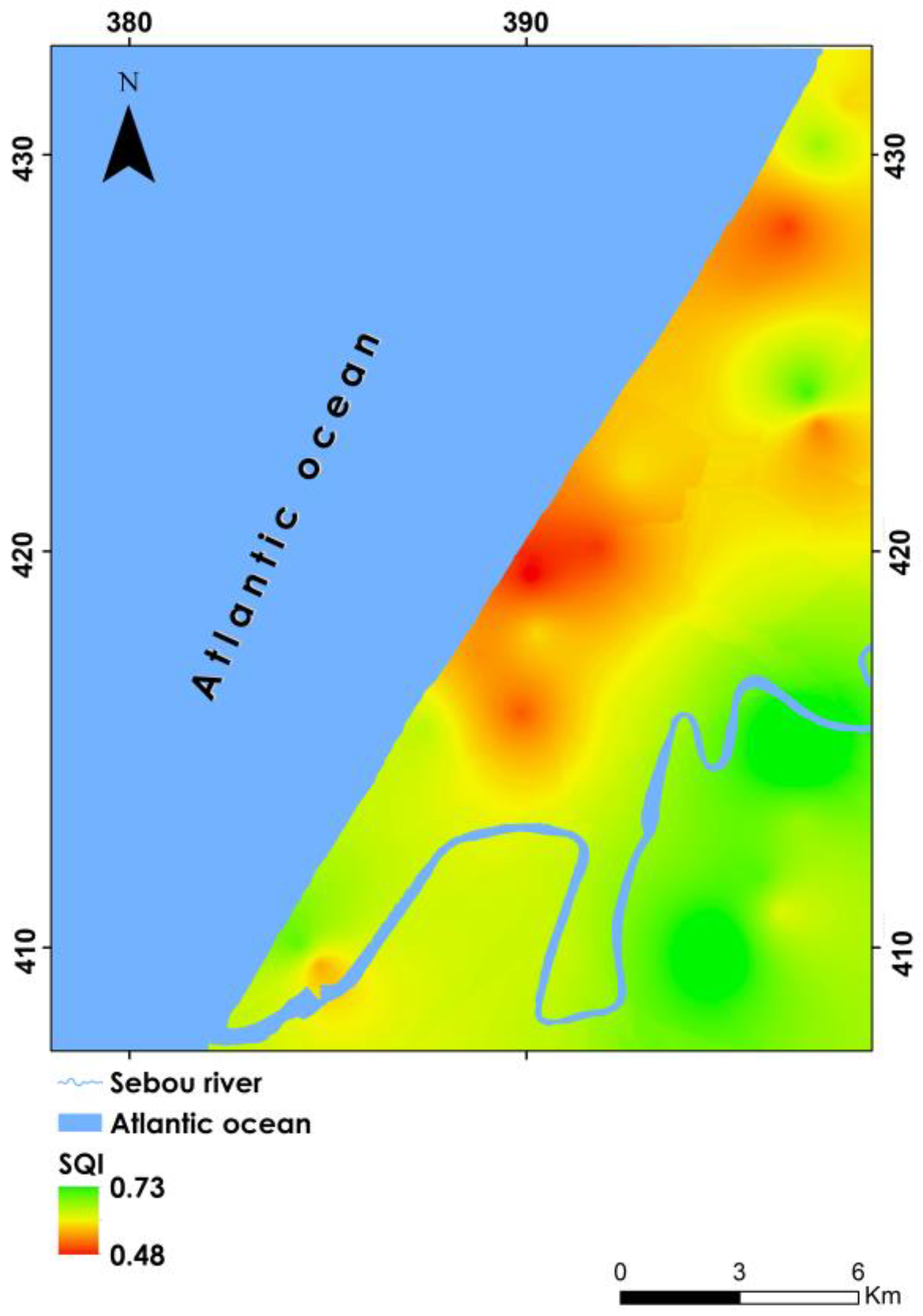
3.5.2. Spatial Analysis of SQI
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasir, M.J.; Haider, M.F.; Ali, Z.; Akhtar, W.; Alam, S. Evaluation of Soil Quality through Simple Additive Soil Quality Index (SQI) of Tehsil Charsadda, Khyber Pakhtunkhwa, Pakistan. J. Saudi Soc. Agric. Sci. 2024, 23, 42–54. [Google Scholar] [CrossRef]
- Kebebew, S.; Bedadi, B.; Erkossa, T.; Yimer, F.; Wogi, L. Effect of Different Land-Use Types on Soil Properties in Cheha District, South-Central Ethiopia. Sustainability 2022, 14, 1323. [Google Scholar] [CrossRef]
- Ismaili, M.; Krimissa, S.; Namous, M.; Abdelrahman, K.; Boudhar, A.; Edahbi, M.; Lebrini, Y.; Htitiou, A.; Maimouni, S.; Benabdelouhab, T. Mapping Soil Suitability Using Phenological Information Derived from MODIS Time Series Data in a Semi-Arid Region: A Case Study of Khouribga, Morocco. Heliyon 2024, 10, e24101. [Google Scholar] [CrossRef] [PubMed]
- Lago-Olveira, S.; Ouhemi, H.; Idrissi, O.; Moreira, M.T.; González-García, S. Promoting More Sustainable Agriculture in the Moroccan Drylands by Shifting from Conventional Wheat Monoculture to a Rotation with Chickpea and Lentils. Clean. Environ. Syst. 2024, 12, 100169. [Google Scholar] [CrossRef]
- Silatsa, F.B.T.; Kebede, F. A Quarter Century Experience in Soil Salinity Mapping and Its Contribution to Sustainable Soil Management and Food Security in Morocco. Geoderma Reg. 2023, 34, e00695. [Google Scholar] [CrossRef]
- Cárceles Rodríguez, B.; Durán-Zuazo, V.H.; Soriano Rodríguez, M.; García-Tejero, I.F.; Gálvez Ruiz, B.; Cuadros Tavira, S. Conservation Agriculture as a Sustainable System for Soil Health: A Review. Soil Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- Aggag, A.M.; Alharbi, A. Spatial Analysis of Soil Properties and Site-Specific Management Zone Delineation for the South Hail Region, Saudi Arabia. Sustainability 2022, 14, 16209. [Google Scholar] [CrossRef]
- Liu, R.; Pan, Y.; Bao, H.; Liang, S.; Jiang, Y.; Tu, H.; Nong, J.; Huang, W. Variations in Soil Physico-Chemical Properties along Slope Position Gradient in Secondary Vegetation of the Hilly Region, Guilin, Southwest China. Sustainability 2020, 12, 1303. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, S.; Wen, C.; Xu, X.; Xiao, X.; Zhou, J.; Yang, X.; Li, R.; Zhang, J.; Fang, X. Anthropogenic Effects on Soils in the Eastern Tibetan Plateau Revealed by Geochemical Elemental Characteristics. Environ. Res. 2024, 252, 118794. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Tong, Y.; Sun, H.; Zhao, Y.; Zhang, P. Regional Spatial Variability of Soil Organic Carbon in 0–5 m Depth and Its Dominant Factors. Catena 2023, 231, 107326. [Google Scholar] [CrossRef]
- Enjavinezhad, S.M.; Baghernejad, M.; Abtahi, S.A.; Ghasemi-Fasaei, R.; Zarei, M. Effects of Topography, Climate, Mineralogy and Physicochemical Properties on Potassium Forms in Various Soils of Fars Province, Southern Iran. Phys. Chem. Earth Parts A/B/C 2024, 133, 103539. [Google Scholar] [CrossRef]
- Dakak, H.; Dekkaki, H.C.; Zouahri, A.; Moussadek, R.; Iaaich, H.; Yachou, H.; Ghanimi, A.; Douaik, A. Soil Salinity Prediction and Mapping Using Electromagnetic Induction and Spatial Interpolation. Environ. Sci. Proc. 2023, 16, 76. [Google Scholar] [CrossRef]
- Acharki, S.; Qorchi, F.E.; Arjdal, Y.; Amharref, M.; Bernoussi, A.S.; Aissa, H.B. Soil Erosion Assessment in Northwestern Morocco. Remote Sens. Appl. Soc. Environ. 2022, 25, 100663. [Google Scholar] [CrossRef]
- Reilly, K.; Cavigelli, M.; Szlavecz, K. Agricultural Management Practices Impact Soil Properties More than Soil Microarthropods. Eur. J. Soil Biol. 2023, 117, 103516. [Google Scholar] [CrossRef]
- Marion, L.F.; Schneider, R.; Cherubin, M.R.; Colares, G.S.; Wiesel, P.G.; Costa, A.B.d.; Lobo, E.A. Development of a Soil Quality Index to Evaluate Agricultural Cropping Systems in Southern Brazil. Soil Tillage Res. 2022, 218, 105293. [Google Scholar] [CrossRef]
- Jalhoum, M.E.M.; Abdellatif, M.A.; Mohamed, E.S.; Kucher, D.E.; Shokr, M. Multivariate Analysis and GIS Approaches for Modeling and Mapping Soil Quality and Land Suitability in Arid Zones. Heliyon 2024, 10, e27577. [Google Scholar] [CrossRef]
- Karlen, D.L.; Veum, K.S.; Sudduth, K.A.; Obrycki, J.F.; Nunes, M.R. Soil Health Assessment: Past Accomplishments, Current Activities, and Future Opportunities. Soil Tillage Res. 2019, 195, 104365. [Google Scholar] [CrossRef]
- Aranguren, R.; Cañón, J. Assessing Differential Land Use Impacts on Soil Quality: A Method Based on Log-Response Ratios and Polygonal Projections. J. Environ. Manag. 2023, 348, 119442. [Google Scholar] [CrossRef]
- Baroudy, A.A.E. Mapping and Evaluating Land Suitability Using a GIS-Based Model. Catena 2016, 140, 96–104. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Shalaby, A.; Mohamed, E.S. Comparison of Two Soil Quality Indices Using Two Methods Based on Geographic Information System. Egypt. J. Remote Sens. Space Sci. 2019, 22, 127–136. [Google Scholar] [CrossRef]
- Moore, F.; Sheykhi, V.; Salari, M.; Bagheri, A. Soil Quality Assessment Using GIS-Based Chemometric Approach and Pollution Indices: Nakhlak Mining District, Central Iran. Environ. Monit. Assess. 2016, 188, 214. [Google Scholar] [CrossRef]
- Raiesi, F. A Minimum Data Set and Soil Quality Index to Quantify the Effect of Land Use Conversion on Soil Quality and Degradation in Native Rangelands of Upland Arid and Semiarid Regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Ray, S.K.; Duraisami, V.P.; Tiwary, P.; Chandran, P.; Nimkar, A.M.; Anantwar, S.G. Soil Quality Index (SQI) as a Tool to Evaluate Crop Productivity in Semi-Arid Deccan Plateau, India. Geoderma 2016, 282, 70–79. [Google Scholar] [CrossRef]
- Feng, S.; Wen, H.; Ni, S.; Wang, J.; Cai, C. Degradation Characteristics of Soil-Quality-Related Physical and Chemical Properties Affected by Collapsing Gully: The Case of Subtropical Hilly Region, China. Sustainability 2019, 11, 3369. [Google Scholar] [CrossRef]
- Leul, Y.; Assen, M.; Damene, S.; Legass, A. Effects of Land Use Types on Soil Quality Dynamics in a Tropical Sub-Humid Ecosystem, Western Ethiopia. Ecol. Indic. 2023, 147, 110024. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Comparison of Soil Quality Index Using Three Methods. PLoS ONE 2014, 9, e105981. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.M.; Schott, L.R.; Piaskowski, J.; Chapagain, A.; Yost, J.L.; Brooks, E.; Kahl, K.; Johnson-Maynard, J. Evaluating Intra-Field Spatial Variability for Nutrient Management Zone Delineation through Geospatial Techniques and Multivariate Analysis. Sustainability 2024, 16, 645. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.K.; Mohamed, E.S.; Wagdi, E.M.; Shahin, S.A.; Aldosari, A.A.; Lasaponara, R.; Alnaimy, M.A. Quantitative Evaluation of Soil Quality Using Principal Component Analysis: The Case Study of El-Fayoum Depression Egypt. Sustainability 2021, 13, 1824. [Google Scholar] [CrossRef]
- El Azhari, H.; Cherif, E.; El Halimi, R.; El Mustapha, A.; Ou Larbi, Y.; Coren, F.; Salmoun, F. Predicting the Production and Depletion of Rare Earth Elements and Their Influence on Energy Sector Sustainability through the Utilization of Multilevel Linear Prediction Mixed-Effects Models with R Software. Sustainability 2024, 16, 1951. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Romano, G.; Sasso, P.D.; Liuzzi, G.T.; Gentile, F. Multi-Criteria Decision Analysis for Land Suitability Mapping in a Rural Area of Southern Italy. Land Use Policy 2015, 48, 131–143. [Google Scholar] [CrossRef]
- Sanad, H.; Mouhir, L.; Zouahri, A.; Moussadek, R.; El Azhari, H.; Yachou, H.; Ghanimi, A.; Oueld Lhaj, M.; Dakak, H. Assessment of Groundwater Quality Using the Pollution Index of Groundwater (PIG), Nitrate Pollution Index (NPI), Water Quality Index (WQI), Multivariate Statistical Analysis (MSA), and GIS Approaches: A Case Study of the Mnasra Region, Gharb Plain, Morocco. Water 2024, 16, 1263. [Google Scholar] [CrossRef]
- Kazemi, H.; Sadeghi, S.; Akinci, H. Developing a Land Evaluation Model for Faba Bean Cultivation Using Geographic Information System and Multi-Criteria Analysis (A Case Study: Gonbad-Kavous Region, Iran). Ecol. Indic. 2016, 63, 37–47. [Google Scholar] [CrossRef]
- Gupta, A.; Kamble, T.; Machiwal, D. Comparison of Ordinary and Bayesian Kriging Techniques in Depicting Rainfall Variability in Arid and Semi-Arid Regions of North-West India. Environ. Earth Sci. 2017, 76, 512. [Google Scholar] [CrossRef]
- Behera, S.K.; Mathur, R.K.; Shukla, A.K.; Suresh, K.; Prakash, C. Spatial Variability of Soil Properties and Delineation of Soil Management Zones of Oil Palm Plantations Grown in a Hot and Humid Tropical Region of Southern India. Catena 2018, 165, 251–259. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.K. A GIS-Based Approach to Identify the Spatial Variability of Salt Affected Soil Properties and Delineation of Site-Specific Management Zones: A Case Study from Egypt. Soil Sci. Annu. 2020, 71, 76–85. [Google Scholar] [CrossRef]
- Aabad, M.; Bouaziz, A.; Falisse, A.; Martin, J.F. Improving Yield and Water Use Efficiency of Sugarcane under Drip. Rev. Marocaine Des Sci. Agron. Et Vétérinaires 2016, 32–40. [Google Scholar]
- Aziane, N.; Khaddari, A.; IbenTouhami, M.; Zouahri, A.; Nassali, H.; Elyoubi, M.S. Evaluation of Groundwater Suitability for Irrigation in the Coastal Aquifer of Mnasra (Gharb, Morocco). Mediterr. J. Chem. 2020, 10, 197–212. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1989. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1986; pp. 363–375. ISBN 978-0-89118-864-3. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Pearson Prentice Hall: London, UK, 2008; ISBN 978-0-13-513387-3. [Google Scholar]
- Rowell, D.L. Soil Science: Methods & Applications; Routledge: London, UK, 2014; ISBN 978-1-315-84485-5. [Google Scholar]
- Page, A.L. (Ed.) Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; International Science Publishers Inc.: New York, NY, USA, 1947. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis; Technical Paper; International Soil Reference and Information Centre: Wageningen, The Netherlands, 1986. [Google Scholar]
- Baruah, T.C.; Barthakur, H.P. A Textbook of Soil Analysis; Vikas Publishing House PVT Ltd.: New Delhi, India, 1997. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall Inc.: London, UK, 1958. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A.; United States Department of Agriculture. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954.
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.; Hastie, T.; Iodice D’Enza, A.; Markos, A.; Tuzhilina, E. Principal Component Analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Cude, C.G. Oregon Water Quality Index a Tool for Evaluating Water Quality Management Effectiveness 1. JAWRA J. Am. Water Resour. Assoc. 2001, 37, 125–137. [Google Scholar] [CrossRef]
- Walck, C. Hand-Book on Statistical Distributions for Experimentalists; Stockholms Universitet: Stockholm, Sweden, 1996. [Google Scholar]
- Liu, Y.; Liu, W.; Wu, L.; Liu, C.; Wang, L.; Chen, F.; Li, Z. Soil Aggregate-Associated Organic Carbon Dynamics Subjected to Different Types of Land Use: Evidence from 13C Natural Abundance. Ecol. Eng. 2018, 122, 295–302. [Google Scholar] [CrossRef]
- Cafarelli, B.; Castrignanò, A.; De Benedetto, D.; Palumbo, A.D.; Buttafuoco, G. A Linear Mixed Effect (LME) Model for Soil Water Content Estimation Based on Geophysical Sensing: A Comparison of an LME Model and Kriging with External Drift. Environ. Earth Sci. 2015, 73, 1951–1960. [Google Scholar] [CrossRef]
- Burgess, T.M.; Webster, R. Optimal Interpolation and Isarithmic Mapping of Soil Properties. J. Soil Sci. 1980, 31, 315–331. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Shao, M.A. Spatial Variability of Soil Physical Properties in a Region of the Loess Plateau of PR China Subject to Wind and Water Erosion. Land Degrad. Dev. 2013, 24, 296–304. [Google Scholar] [CrossRef]
- Lark, R.M. Estimating Variograms of Soil Properties by the Method-of-Moments and Maximum Likelihood. Eur. J. Soil Sci. 2000, 51, 717–728. [Google Scholar] [CrossRef]
- Gundogdu, K.S.; Guney, I. Spatial Analyses of Groundwater Levels Using Universal Kriging. J. Earth Syst. Sci. 2007, 116, 49–55. [Google Scholar] [CrossRef]
- Toru, T.; Kibret, K. Carbon Stock under Major Land Use/Land Cover Types of Hades Sub-Watershed, Eastern Ethiopia. Carbon Balance Manag. 2019, 14, 7. [Google Scholar] [CrossRef]
- Kaur, T.; Sehgal, S.K.; Singh, S.; Sharma, S.; Dhaliwal, S.S.; Sharma, V. Assessment of Seasonal Variability in Soil Nutrients and Its Impact on Soil Quality under Different Land Use Systems of Lower Shiwalik Foothills of Himalaya, India. Sustainability 2021, 13, 1398. [Google Scholar] [CrossRef]
- Alharbi, A.B.; Aggag, A.M. Land Evaluation for Alternative Crops of Alfalfa Using GIS in South Hail, Saudi Arabia. Alex. Sci. Exch. J. 2020, 41, 419–433. [Google Scholar] [CrossRef]
- Sahu, C.; Basti, S.; Pradhan, R.; Kumar, S. Physicochemical Properties of Soil under Different Land Use Practices Located near Bhawanipatna Town in Odisha, India. Int. J. Environ. Sci. 2016, 6, 941–953. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Kharal, S.; Khanal, B.; Panday, D. Assessment of Soil Fertility under Different Land-Use Systems in Dhading District of Nepal. Soil Syst. 2018, 2, 57. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Tiwary, P.; Chandran, P.; Ray, S.K.; Duraisami, V.P. Pedogenic Processes and Soil–Landform Relationships for Identification of Yield-Limiting Soil Properties. Soil Res. 2017, 55, 273–284. [Google Scholar] [CrossRef]
- Gowthamchand; Dhaliwal, S.S.; Sharma, V.; Verma, G.; Singh, J.; Kaur, M. Variation of Physico-Chemical Properties among Different Soil Orders under Different Land Use Systems of the Majha Region in North-Western India. Sustainability 2023, 15, 4779. [Google Scholar] [CrossRef]
- Hazelton, P.A.; Murphy, B.W.; Hazelton, P.A.; Murphy, B.W. What Do All the Numbers Mean?—A Guide to the Interpretation of Soil Test Results; CSIRO Publishing: Melbourne, Australia, 2007; ISBN 978 0 64309 223 9. [Google Scholar]
- Reddy, D.T.P. Critical Levels of Micro and Secondary Nutrients in Soils and Crops for Optimum Plant Nutrition. Int. J. Sci. Res. 2021, 6, 594–595. [Google Scholar]
- Verma, R.R.; Manjunath, B.L.; Singh, N.P.; Kumar, A.; Asolkar, T.; Chavan, V.; Srivastava, T.K.; Singh, P. Soil Mapping and Delineation of Management Zones in the Western Ghats of Coastal India. Land Degrad. Dev. 2018, 29, 4313–4322. [Google Scholar] [CrossRef]
- Howladar, M.F.; Deb, P.K.; Muzemder, A.T.M.S.H.; Ahmed, M. Evaluation of Water Resources around Barapukuria Coal Mine Industrial Area, Dinajpur, Bangladesh. Appl. Water Sci. 2014, 4, 203–222. [Google Scholar] [CrossRef]
- Bahir, M.; Ouhamdouch, S.; Ouazar, D.; Moçayd, N.E. Climate Change Effect on Groundwater Characteristics within Semi-Arid Zones from Western Morocco. Groundw. Sustain. Dev. 2020, 11, 100380. [Google Scholar] [CrossRef]
- Selmy, S.A.H.; Abd Al-Aziz, S.H.; Ibrahim, A.G.; Jiménez-Ballesta, R. Impact of Short-Term Cultivation on Some Selected Properties of Sandy Soil in an Arid Environment. Soil Syst. 2022, 6, 82. [Google Scholar] [CrossRef]
- Ferreira, V.; Panagopoulos, T.; Andrade, R.; Guerrero, C.; Loures, L. Spatial Variability of Soil Properties and Soil Erodibility in the Alqueva Reservoir Watershed. Solid Earth 2015, 6, 383–392. [Google Scholar] [CrossRef]
- Aldabaa, A.; Yousif, I. Geostatistical Approach for Land Suitability Assessment of Some Desert Soils. Egypt. J. Soil Sci. 2020, 60, 195–209. [Google Scholar] [CrossRef]
- Rangel-Peraza, J.G.; Padilla-Gasca, E.; López-Corrales, R.; Medina, J.R.; Bustos-Terrones, Y.; Amabilis-Sosa, L.E.; Rodríguez-Mata, A.E.; Osuna-Enciso, T. Robust Soil Quality Index for Tropical Soils Influenced by Agricultural Activities. J. Agric. Chem. Environ. 2017, 06, 199–221. [Google Scholar] [CrossRef][Green Version]
- Martinez, M.; Gutiérrez-Romero, V.; Jannsens, M.; Ortega-Blu, R. Biological Soil Quality Indicators: A Review. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 319–328. [Google Scholar]
- Chivenge, P.P.; Murwira, H.K.; Giller, K.E.; Mapfumo, P.; Six, J. Long-Term Impact of Reduced Tillage and Residue Management on Soil Carbon Stabilization: Implications for Conservation Agriculture on Contrasting Soils. Soil Tillage Res. 2007, 94, 328–337. [Google Scholar] [CrossRef]
- Fisher, R.; Binkley, D. Ecology and Management of Forest Soils, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; Volume 489, ISBN 0-471-19426-3. [Google Scholar]
- Toohey, R.C.; Boll, J.; Brooks, E.S.; Jones, J.R. Effects of Land Use on Soil Properties and Hydrological Processes at the Point, Plot, and Catchment Scale in Volcanic Soils near Turrialba, Costa Rica. Geoderma 2018, 315, 138–148. [Google Scholar] [CrossRef]
- Ghorbani-Dashtaki, S.; Homaee, M.; Loiskandl, W. Towards Using Pedotransfer Functions for Estimating Infiltration Parameters. Hydrol. Sci. J. 2015, 61, 1477–1488. [Google Scholar] [CrossRef]
- Murphy, B.W. Impact of Soil Organic Matter on Soil Properties—A Review with Emphasis on Australian Soils. Soil Res. 2015, 53, 605–635. [Google Scholar] [CrossRef]
- Berns, A.E.; Knicker, H. Soil Organic Matter. In eMagRes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 43–54. ISBN 978-0-470-03459-0. [Google Scholar]



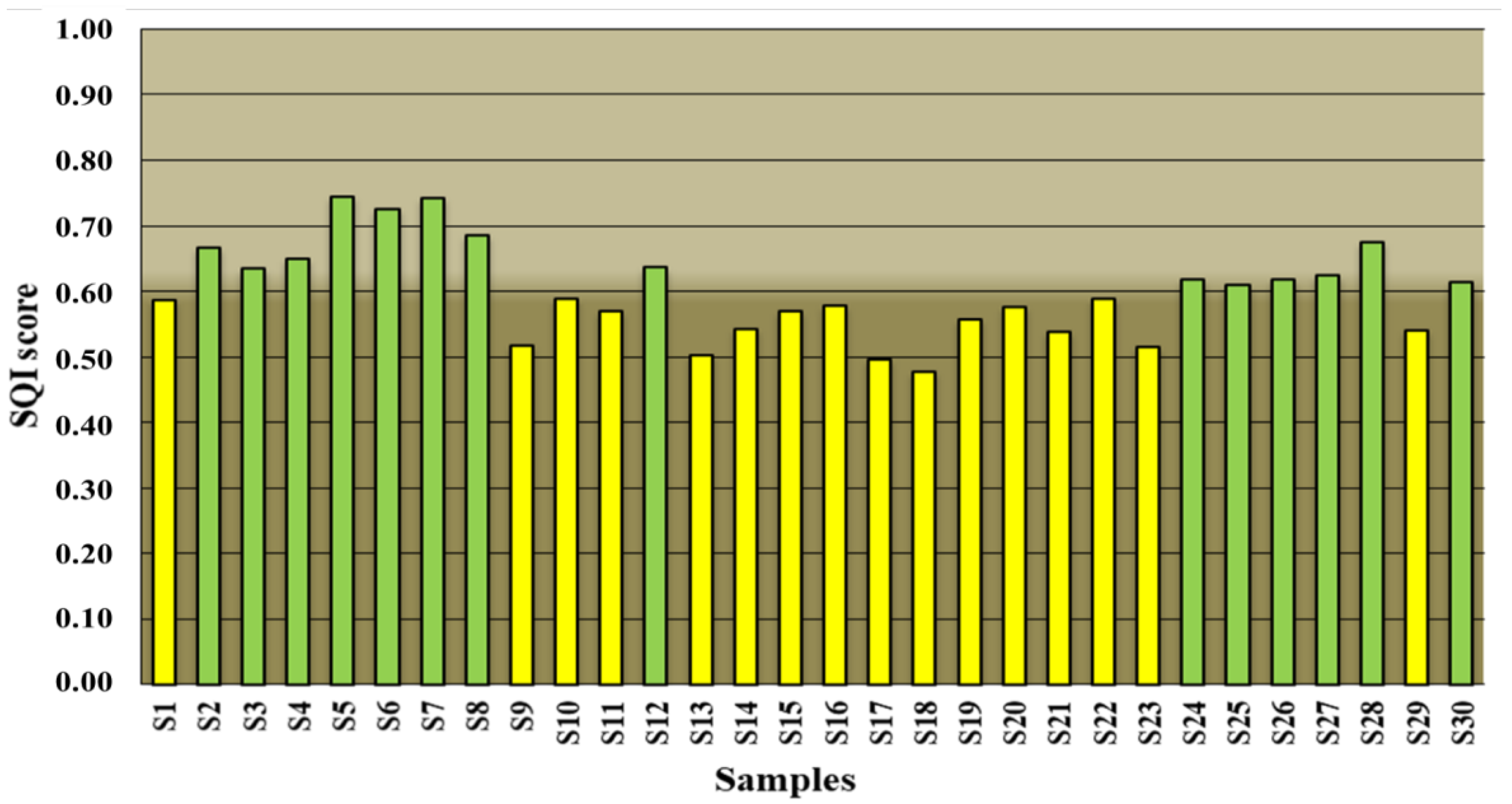



| SQI Values | SQI Interpretation |
|---|---|
| From 0.80 to 1 | Very good |
| From 0.60 to 0.79 | Good |
| From 0.35 to 0.59 | Fair |
| From 0.20 to 0.34 | Bad |
| From 0 to 0.19 | Very bad |
| Samples | Sand (%) | Silt (%) | Clay (%) | Textural Class | Moisture (%) | Bulk Density (g/cm3) | Particle Density (g/cm3) | Total Porosity (%) |
|---|---|---|---|---|---|---|---|---|
| S1 | 88.21 | 6.21 | 5.57 | Sand | 0.90 | 1.58 | 2.90 | 45.52 |
| S2 | 28.21 | 45.43 | 26.36 | Loam | 1.40 | 1.46 | 2.30 | 36.52 |
| S3 | 24.71 | 54.57 | 20.71 | Silty loam | 6.70 | 1.41 | 2.30 | 38.70 |
| S4 | 22.86 | 48.21 | 28.93 | Clay Loam | 3.10 | 1.44 | 2.30 | 37.39 |
| S5 | 19.64 | 47.86 | 32.50 | Silty clay loam | 3.70 | 1.45 | 2.20 | 34.09 |
| S6 | 12.14 | 55.79 | 32.07 | Silty clay loam | 4.00 | 1.40 | 2.20 | 36.36 |
| S7 | 20.29 | 37.71 | 42.00 | Clay | 7.50 | 1.38 | 2.10 | 34.29 |
| S8 | 21.00 | 57.71 | 21.29 | Silty loam | 11.40 | 1.39 | 2.30 | 39.57 |
| S9 | 90.21 | 6.86 | 2.93 | Sand | 1.80 | 1.58 | 2.80 | 43.57 |
| S10 | 87.86 | 7.57 | 4.57 | Sand | 2.50 | 1.55 | 2.60 | 40.38 |
| S11 | 89.79 | 2.79 | 7.43 | Sand | 1.50 | 1.56 | 2.60 | 40.00 |
| S12 | 88.29 | 5.14 | 6.57 | Sand | 3.30 | 1.55 | 2.60 | 40.38 |
| S13 | 90.21 | 2.86 | 6.93 | Sand | 1.30 | 1.57 | 2.80 | 43.93 |
| S14 | 90.07 | 7.93 | 2.00 | Sand | 3.20 | 1.57 | 2.80 | 43.93 |
| S15 | 88.07 | 0.43 | 11.50 | Loamy sand | 3.70 | 1.49 | 2.50 | 40.40 |
| S16 | 89.64 | 5.43 | 4.93 | Sand | 0.90 | 1.52 | 2.60 | 41.54 |
| S17 | 91.36 | 5.43 | 3.21 | Sand | 3.60 | 1.59 | 2.80 | 43.21 |
| S18 | 92.36 | 5.14 | 2.50 | Sand | 3.89 | 1.59 | 2.80 | 43.21 |
| S19 | 83.36 | 11.93 | 4.71 | Loamy sand | 5.25 | 1.54 | 2.60 | 40.77 |
| S20 | 81.00 | 9.14 | 9.86 | Loamy sand | 4.79 | 1.53 | 2.60 | 41.15 |
| S21 | 79.86 | 7.79 | 12.36 | Sand loam | 4.71 | 1.49 | 2.50 | 40.40 |
| S22 | 81.79 | 8.71 | 9.50 | Loamy sand | 3.96 | 1.53 | 2.60 | 41.15 |
| S23 | 90.07 | 5.86 | 4.07 | Sand | 5.26 | 1.58 | 2.80 | 43.57 |
| S24 | 88.14 | 7.14 | 4.71 | Sand | 2.69 | 1.55 | 2.60 | 40.38 |
| S25 | 86.00 | 5.64 | 8.36 | Loamy sand | 3.20 | 1.52 | 2.60 | 41.54 |
| S26 | 86.21 | 7.50 | 6.29 | Loamy sand | 2.28 | 1.55 | 2.60 | 40.38 |
| S27 | 87.50 | 10.21 | 2.29 | Sand | 8.14 | 1.56 | 2.70 | 42.22 |
| S28 | 81.14 | 4.71 | 14.14 | Sand loam | 5.21 | 1.46 | 2.30 | 36.52 |
| S29 | 81.64 | 5.21 | 13.14 | Sand loam | 4.97 | 1.48 | 2.45 | 39.59 |
| S30 | 83.43 | 12.43 | 4.14 | Loamy sand | 1.29 | 1.53 | 2.60 | 41.15 |
| Parameters | Minimum | Maximum | Mean | Variance | Std. Dev. | CV | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| pH | 6.61 | 8.33 | 7.46 | 0.14 | 0.38 | 0.05 | −0.08 | 0.06 |
| EC (dS/m) | 0.15 | 3.05 | 0.76 | 0.52 | 0.72 | 0.95 | 1.98 | 3.64 |
| OM (%) | 1.61 | 10.08 | 3.4 | 2.8 | 1.67 | 0.49 | 2.37 | 8.03 |
| CaCO3 (%) | 0 | 4.28 | 0.68 | 1.14 | 1.06 | 1.56 | 2.01 | 3.8 |
| CEC (cmol/kg) | 1.34 | 3.11 | 2.09 | 0.27 | 0.52 | 0.24 | 0.44 | −0.96 |
| Macronutrients of soils | ||||||||
| Av. N (mg/kg) | 65.56 | 193.53 | 107.72 | 1452.26 | 38.1 | 0.35 | 0.94 | −0.18 |
| Av. P (mg/kg) | 0.82 | 103.47 | 25.81 | 653.6 | 25.56 | 0.99 | 1.63 | 2.41 |
| Ex. K (mg/kg) | 65 | 362.5 | 177.5 | 9721.55 | 98.59 | 0.55 | 0.62 | −1.01 |
| Na (mg/kg) | 2.83 | 6.54 | 4.78 | 0.39 | 0.62 | 0.13 | −0.38 | 3.67 |
| Ca (mg/kg) | 3 | 34.5 | 14.9 | 104.16 | 10.2 | 0.68 | 0.75 | −0.91 |
| Mg (mg/kg) | 0 | 9.5 | 3.51 | 7.18 | 2.67 | 0.76 | 0.55 | −0.53 |
| Micronutrients of soils | ||||||||
| Fe (mg/kg) | 1.77 | 13.82 | 5.44 | 9.35 | 3.05 | 0.56 | 1.23 | 1 |
| Zn (mg/kg) | 2.49 | 120.8 | 17.75 | 487 | 22.06 | 1.24 | 3.88 | 17.22 |
| Cu (mg/kg) | 0 | 18.85 | 1.72 | 16.84 | 4.1 | 2.38 | 3.15 | 10.87 |
| Mn (mg/kg) | 0.28 | 8.87 | 3.47 | 6.94 | 2.63 | 0.75 | 0.56 | −0.96 |
| Variables | pH | EC | OM | CEC | Av. N | Av. P | Ex. K | Na | Ca | Mg | Fe | Zn | Cu | Mn | Sand | Silt | Clay | Moisture | BD | Porosity | CaCO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||||||||||||
| EC | 0.358 | 1 | |||||||||||||||||||
| OM | 0.248 | 0.166 | 1 | ||||||||||||||||||
| CEC | 0.318 | 0.434 | 0.421 | 1 | |||||||||||||||||
| Av. N | 0.297 | 0.290 | 0.670 | 0.571 | 1 | ||||||||||||||||
| Av. P | −0.148 | −0.062 | 0.509 | 0.187 | −0.028 | 1 | |||||||||||||||
| Ex. K | 0.267 | 0.282 | 0.449 | 0.528 | 0.394 | 0.019 | 1 | ||||||||||||||
| Na | 0.017 | −0.206 | 0.070 | −0.296 | −0.198 | 0.200 | −0.293 | 1 | |||||||||||||
| Ca | 0.487 | 0.413 | 0.263 | 0.585 | 0.728 | −0.142 | 0.529 | −0.303 | 1 | ||||||||||||
| Mg | 0.038 | 0.174 | 0.082 | 0.214 | 0.149 | 0.034 | 0.102 | −0.331 | 0.004 | 1 | |||||||||||
| Fe | −0.013 | 0.131 | 0.631 | 0.456 | 0.364 | 0.541 | 0.278 | −0.028 | 0.407 | −0.033 | 1 | ||||||||||
| Zn | −0.174 | 0.013 | 0.664 | −0.024 | −0.264 | 0.457 | −0.043 | 0.030 | −0.009 | −0.122 | 0.466 | 1 | |||||||||
| Cu | 0.303 | 0.319 | 0.059 | 0.441 | 0.619 | −0.222 | 0.291 | −0.003 | 0.666 | 0.232 | 0.223 | −0.196 | 1 | ||||||||
| Mn | −0.373 | 0.147 | −0.175 | 0.118 | −0.247 | −0.037 | −0.001 | −0.167 | −0.305 | 0.204 | 0.024 | −0.029 | −0.052 | 1 | |||||||
| Sand | −0.518 | −0.278 | −0.276 | −0.742 | −0.819 | 0.113 | −0.476 | 0.186 | −0.802 | −0.187 | −0.326 | 0.266 | −0.675 | 0.229 | 1 | ||||||
| Silt | 0.512 | 0.254 | 0.213 | 0.697 | 0.777 | −0.203 | 0.466 | −0.192 | 0.799 | 0.191 | 0.317 | −0.310 | 0.714 | −0.200 | −0.978 | 1 | |||||
| Clay | 0.472 | 0.290 | 0.357 | 0.739 | 0.804 | 0.059 | 0.442 | −0.155 | 0.718 | 0.159 | 0.306 | −0.160 | 0.531 | −0.255 | −0.929 | 0.830 | 1 | ||||
| Moisture | 0.266 | −0.070 | 0.472 | 0.307 | 0.240 | 0.447 | 0.153 | 0.275 | 0.211 | 0.069 | 0.595 | 0.051 | 0.294 | −0.101 | −0.399 | 0.414 | 0.328 | 1 | |||
| BD | −0.491 | −0.159 | −0.436 | −0.685 | −0.685 | −0.005 | −0.383 | 0.059 | −0.620 | −0.126 | −0.359 | 0.070 | −0.560 | 0.288 | 0.882 | −0.819 | −0.896 | −0.508 | 1 | ||
| Porosity | −0.457 | −0.260 | −0.406 | −0.622 | −0.594 | −0.095 | −0.355 | 0.064 | −0.558 | −0.153 | −0.213 | −0.065 | −0.402 | 0.223 | 0.774 | −0.672 | −0.868 | −0.273 | 0.834 | 1 | |
| CaCO3 | 0.466 | 0.439 | 0.205 | 0.665 | 0.787 | −0.051 | 0.498 | −0.229 | 0.815 | 0.155 | 0.299 | −0.173 | 0.608 | −0.118 | −0.873 | 0.829 | 0.855 | 0.209 | −0.668 | −0.757 | 1 |
 | |||||||||||||||||||||
| Variables | Components | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | ||
| pH | Factor loadings | 0.554 | −0.124 | −0.315 | −0.312 | 0.375 |
| EC | 0.402 | −0.116 | 0.441 | −0.344 | 0.148 | |
| OM | 0.403 | 0.779 | 0.051 | −0.198 | 0.222 | |
| CEC | 0.795 | 0.123 | 0.342 | 0.070 | 0.031 | |
| Av. N | 0.830 | −0.143 | −0.032 | 0.083 | −0.196 | |
| Av. P | 0.014 | 0.818 | 0.087 | 0.150 | 0.077 | |
| Ex. K | 0.555 | −0.037 | 0.303 | −0.133 | −0.227 | |
| Na | −0.203 | 0.288 | −0.651 | 0.212 | 0.013 | |
| Ca | 0.848 | −0.100 | 0.026 | −0.278 | −0.288 | |
| Mg | 0.199 | −0.121 | 0.458 | 0.385 | 0.594 | |
| Fe | 0.441 | 0.705 | 0.199 | 0.111 | −0.376 | |
| Zn | −0.111 | 0.763 | 0.206 | −0.424 | −0.008 | |
| Cu | 0.684 | −0.220 | −0.031 | 0.231 | −0.172 | |
| Mn | −0.224 | −0.065 | 0.680 | 0.409 | −0.055 | |
| Sand | −0.968 | 0.136 | 0.095 | −0.098 | 0.011 | |
| Silt | 0.927 | −0.193 | −0.094 | 0.144 | −0.074 | |
| Clay | 0.932 | −0.020 | −0.086 | 0.006 | 0.101 | |
| Moisture | 0.431 | 0.535 | −0.263 | 0.488 | −0.004 | |
| BD | −0.884 | −0.091 | 0.214 | −0.094 | −0.126 | |
| Porosity | −0.807 | −0.085 | 0.100 | 0.105 | −0.289 | |
| CaCO3 | 0.894 | −0.159 | 0.076 | −0.073 | −0.059 | |
| Eigenvalue | 8.869 | 2.965 | 1.849 | 1.284 | 1.038 | |
| Variability (%) | 42.235 | 14.117 | 8.805 | 6.115 | 4.940 | |
| Cumulative (%) | 42.235 | 56.352 | 65.157 | 71.272 | 76.213 | |
| Variables | Component | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | ||
| pH | Component Score Coefficient Matrix (CSC) | 0.186 | −0.072 | −0.232 | −0.275 | 0.368 |
| EC | 0.135 | −0.067 | 0.324 | −0.304 | 0.145 | |
| OM | 0.135 | 0.452 | 0.037 | −0.174 | 0.218 | |
| CEC | 0.267 | 0.072 | 0.251 | 0.062 | 0.030 | |
| Av. N | 0.279 | −0.083 | −0.023 | 0.073 | −0.192 | |
| Av. P | 0.005 | 0.475 | 0.064 | 0.133 | 0.076 | |
| Ex. K | 0.186 | −0.021 | 0.223 | −0.118 | −0.222 | |
| Na | −0.068 | 0.167 | −0.479 | 0.188 | 0.013 | |
| Ca | 0.285 | −0.058 | 0.019 | −0.245 | −0.283 | |
| Mg | 0.067 | −0.070 | 0.337 | 0.340 | 0.584 | |
| Fe | 0.148 | 0.409 | 0.147 | 0.098 | −0.370 | |
| Zn | −0.037 | 0.443 | 0.151 | −0.374 | −0.008 | |
| Cu | 0.230 | −0.128 | −0.023 | 0.204 | −0.169 | |
| Mn | −0.075 | −0.038 | 0.500 | 0.361 | −0.054 | |
| Sand | −0.325 | 0.079 | 0.070 | −0.086 | 0.011 | |
| Silt | 0.311 | −0.112 | −0.069 | 0.127 | −0.072 | |
| Clay | 0.313 | −0.012 | −0.063 | 0.005 | 0.099 | |
| Moisture | 0.145 | 0.311 | −0.194 | 0.430 | −0.004 | |
| BD | −0.297 | −0.053 | 0.157 | −0.083 | −0.124 | |
| Porosity | −0.271 | −0.050 | 0.074 | 0.092 | −0.283 | |
| CaCO3 | 0.300 | −0.092 | 0.056 | −0.064 | −0.058 | |
| Eigenvalue | 8.869 | 2.965 | 1.849 | 1.284 | 1.038 | |
| Variability (%) | 42.235 | 14.117 | 8.805 | 6.115 | 4.940 | |
| Cumulative (%) | 42.235 | 56.352 | 65.157 | 71.272 | 76.213 | |
| Samples | z-pH | z-EC | z-OM | z-CEC | z-Av. N | z-Av. P | z-Ex. K | z-Na | z-Ca | z-Mg | z-Fe | z-Zn | z-Cu | z-Mn | z-Sand | z-Silt | z-Clay | z-Moisture | z-BD | z-Porosity | z-CaCO3 | SQI-PC1 | SQI-PC2 | SQI-PC3 | SQI-PC4 | SQI-PC5 | CSQI-1 | CSQI-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.12 | 0.95 | 0.38 | 1.06 | 1.35 | 0.42 | 0.33 | 0.60 | 1.38 | 0.94 | 1.23 | 0.16 | 0.71 | 1.21 | 0.59 | 0.55 | 0.59 | 1.27 | 1.17 | 1.81 | 0.38 | 1.40 | 0.76 | 1.31 | 0.87 | −0.77 | 0.83 | 0.59 |
| S2 | 0.86 | 0.11 | 0.41 | 1.75 | 0.70 | 0.98 | 1.88 | 0.51 | 1.63 | 0.57 | 0.69 | 0.54 | 0.28 | 0.92 | 1.52 | 1.52 | 1.35 | 1.06 | 0.83 | 1.37 | 0.89 | 1.99 | 1.08 | 1.36 | 0.40 | −0.99 | 1.09 | 0.67 |
| S3 | 0.96 | 0.04 | 0.07 | 1.08 | 0.90 | 0.86 | 0.63 | 0.37 | 1.48 | 1.30 | 0.16 | 0.49 | 4.18 | 0.27 | 1.64 | 2.00 | 0.83 | 1.21 | 1.67 | 0.60 | 0.64 | 2.34 | 0.08 | 0.70 | 1.33 | −0.74 | 1.11 | 0.63 |
| S4 | 1.06 | 0.16 | 0.13 | 0.89 | 1.43 | 0.94 | 1.88 | 3.10 | 1.67 | 0.74 | 0.01 | 0.59 | 0.25 | 0.85 | 1.71 | 1.67 | 1.59 | 0.33 | 1.17 | 1.06 | 2.10 | 2.02 | 0.62 | 0.00 | 0.34 | −0.76 | 0.92 | 0.65 |
| S5 | 0.16 | 1.99 | 0.44 | 1.22 | 2.25 | 0.70 | 1.12 | 049 | 1.92 | 0.55 | 0.54 | 0.56 | 2.34 | 1.30 | 1.82 | 1.65 | 1.92 | 0.07 | 1.00 | 2.23 | 3.37 | 3.22 | −0.20 | 2.31 | 0.08 | −1.89 | 1.45 | 0.74 |
| S6 | 2.26 | 2.62 | 0.13 | 1.89 | 1.52 | 0.47 | 0.96 | 0.15 | 1.72 | 0.94 | 0.54 | 0.52 | 0.97 | 0.74 | 2.08 | 2.07 | 1.88 | 0.06 | 1.83 | 1.42 | 1.84 | 2.86 | −0.32 | 2.17 | −1.04 | −0.19 | 1.28 | 0.73 |
| S7 | 0.10 | 0.50 | 1.33 | 0.96 | 2.25 | 3.04 | 0.33 | 0.20 | 0.55 | 1.30 | 1.32 | 0.47 | 0.32 | 0.90 | 1.80 | 1.11 | 2.81 | 1.55 | 2.17 | 2.16 | 1.49 | 1.99 | 2.64 | 2.11 | 1.52 | −0.56 | 1.46 | 0.74 |
| S8 | 1.04 | 0.53 | 1.50 | 0.66 | 1.06 | 0.77 | 0.18 | 0.39 | 1.28 | 0.18 | 2.28 | 0.41 | 0.76 | 0.08 | 1.77 | 2.17 | 0.88 | 3.22 | 2.00 | 0.29 | 0.55 | 2.15 | 2.60 | 0.19 | 0.90 | −1.04 | 1.30 | 0.69 |
| S9 | 1.09 | 0.65 | 0.90 | 0.24 | 0.41 | 0.34 | 0.74 | 0.67 | 0.92 | 0.01 | 0.37 | 0.47 | 0.42 | 1.92 | 0.66 | 0.52 | 0.83 | 0.88 | 1.17 | 1.12 | 0.64 | 0.81 | 0.79 | 1.10 | 0.25 | −0.53 | 0.54 | 0.52 |
| S10 | 0.05 | 0.83 | 0.90 | 0.98 | 0.12 | 0.52 | 0.43 | 0.34 | 0.87 | 1.49 | 0.59 | 0.46 | 0.42 | 0.64 | 0.57 | 0.48 | 0.68 | 0.59 | 0.67 | 0.00 | 0.64 | 1.32 | 0.98 | 1.47 | 0.40 | 050 | 0.87 | 0.59 |
| S11 | 0.03 | 0.62 | 0.02 | 1.46 | 0.49 | 0.59 | 0.58 | 1.06 | 0.45 | 0.18 | 0.85 | 1.69 | 0.42 | 005 | 0.64 | 0.73 | 0.41 | 1.01 | 0.83 | 0.14 | 0.22 | 1.03 | 1.68 | 0.60 | 0.05 | −0.61 | 0.70 | 0.57 |
| S12 | 1.12 | 3.15 | 0.93 | 0.66 | 0.86 | 0.08 | 0.89 | 1.06 | 0.87 | 1.86 | 0.85 | 0.30 | 0.42 | 1.23 | 0.59 | 0.61 | 0.49 | 0.24 | 0.67 | 0.00 | 0.35 | 1.85 | 0.48 | 2.10 | −0.24 | 1.04 | 1.07 | 0.64 |
| S13 | 0.99 | 0.21 | 0.16 | 0.43 | 0.86 | 0.30 | 0.48 | 0.58 | 0.58 | 0.37 | 0.67 | 0.35 | 0.42 | 1.22 | 0.66 | 0.73 | 0.46 | 1.10 | 1.00 | 1.25 | 0.64 | 0.79 | 0.58 | 0.70 | 0.76 | −0.66 | 0.49 | 0.50 |
| S14 | 1.84 | 0.11 | 0.33 | 0.24 | 0.94 | 0.20 | 0.79 | 0.13 | 0.92 | 1.30 | 0.49 | 0.09 | 0.42 | 2.04 | 0.65 | 0.46 | 0.92 | 0.29 | 1.00 | 1.25 | 0.64 | 1.04 | −0.08 | 1.52 | 0.62 | 0.12 | 0.61 | 0.54 |
| S15 | 0.03 | 0.38 | 0.78 | 0.43 | 0.57 | 0.15 | 1.80 | 0.51 | 0.14 | 1.31 | 0.31 | 0.24 | 0.42 | 0.96 | 0.58 | 0.86 | 0.03 | 0.07 | 033 | 0.00 | 0.64 | 1.11 | 0.37 | 1.46 | 0.52 | 0.11 | 0.69 | 0.57 |
| S16 | 1.06 | 0.65 | 0.90 | 0.22 | 0.66 | 0.96 | 0.99 | 1.48 | 0.58 | 0.18 | 0.66 | 0.41 | 0.42 | 0.10 | 0.64 | 0.59 | 0.65 | 1.27 | 0.17 | 0.41 | 0.64 | 1.48 | 1.56 | −0.31 | 0.21 | −0.10 | 0.82 | 0.58 |
| S17 | 0.26 | 0.04 | 0.13 | 0.52 | 1.02 | 1.03 | 1.50 | 0.36 | 0.48 | 0.37 | 0.47 | 0.10 | 0.42 | 0.09 | 0.70 | 0.59 | 0.80 | 0.12 | 1.33 | 1.00 | 0.13 | 0.67 | 0.51 | 0.77 | 0.20 | −0.88 | 0.39 | 0.50 |
| S18 | 0.03 | 0.62 | 0.02 | 0.52 | 0.37 | 0.91 | 0.66 | 0.03 | 0.48 | 0.75 | 0.46 | 0.25 | 0.42 | 1.02 | 0.73 | 0.61 | 0.87 | 0.01 | 1.33 | 1.00 | 0.38 | 0.40 | 0.35 | 1.64 | 0.43 | −0.44 | 0.36 | 0.48 |
| S19 | 0.73 | 0.76 | 0.55 | 0.56 | 0.74 | 0.45 | 1.06 | 0.75 | 0.68 | 0.94 | 0.02 | 0.25 | 0.42 | 1.34 | 0.42 | 0.25 | 0.67 | 0.59 | 0.50 | 0.13 | 0.38 | 1.23 | 0.46 | 1.12 | 0.47 | 0.31 | 0.73 | 0.56 |
| S20 | 2.21 | 0.74 | 0.67 | 0.60 | 1.11 | 0.37 | 0.81 | 0.42 | 0.87 | 0.57 | 0.08 | 0.02 | 0.42 | 0.73 | 0.33 | 0.40 | 0.19 | 0.39 | 0.33 | 0.27 | 0.64 | 1.68 | 0.16 | 0.48 | −0.32 | 0.51 | 0.78 | 0.58 |
| S21 | 0.23 | 0.64 | 0.70 | 0.81 | 0.08 | 0.50 | 0.79 | 2.78 | 0.92 | 0.75 | 0.72 | 0.25 | 0.42 | 0.92 | 0.29 | 0.47 | 0.05 | 0.36 | 0.33 | 0.00 | 0.64 | 1.07 | 1.21 | 0.13 | 0.72 | −0.08 | 0.67 | 0.54 |
| S22 | 0.60 | 0.37 | 0.33 | 0.94 | 0.66 | 0.20 | 0.76 | 0.44 | 0.48 | 2.23 | 0.83 | 0.15 | 0.42 | 0.95 | 0.36 | 0.42 | 0.22 | 0.04 | 0.33 | 0.27 | 0.64 | 1.29 | 0.28 | 1.64 | 0.88 | 0.68 | 0.81 | 0.59 |
| S23 | 0.65 | 0.53 | 0.36 | 0.94 | 0.74 | 0.55 | 0.53 | 1.20 | 0.14 | 1.31 | 0.82 | 0.09 | 0.42 | 1.02 | 0.65 | 0.57 | 0.72 | 0.59 | 1.17 | 1.12 | 0.13 | 0.65 | 0.75 | 1.03 | 1.13 | 0.08 | 0.54 | 0.51 |
| S24 | 0.57 | 0.69 | 1.07 | 1.23 | 1.02 | 0.65 | 0.99 | 0.36 | 0.30 | 0.57 | 1.20 | 0.38 | 0.42 | 1.20 | 0.58 | 0.50 | 0.67 | 0.50 | 0.67 | 0.00 | 0.12 | 1.50 | 1.34 | 1.51 | 0.42 | −0.18 | 0.97 | 0.62 |
| S25 | 1.61 | 0.64 | 0.92 | 0,75 | 0.94 | 0.84 | 1.14 | 1.08 | 0.72 | 1.31 | 0.47 | 0.69 | 0.42 | 0.09 | 0.51 | 0.58 | 0.33 | 0.28 | 0.17 | 0.41 | 0.64 | 1.73 | 1.07 | 0.50 | −0.19 | 0.68 | 0.95 | 0.61 |
| S26 | 0.81 | 0.73 | 0.78 | 1.02 | 0.57 | 0.66 | 1.09 | 0.27 | 0.97 | 1.31 | 0.83 | 0.27 | 0.42 | 1.06 | 0.52 | 0.48 | 0.52 | 0.68 | 0.67 | 0.00 | 0.64 | 1.70 | 0.93 | 1.60 | 0.41 | 0.26 | 1.03 | 0.62 |
| S27 | 0.34 | 0.45 | 0.04 | 0.57 | 0.04 | 2.08 | 0.99 | 0.32 | 0.06 | 0.19 | 1.60 | 0.57 | 0.42 | 0.62 | 0.56 | 0.34 | 0.89 | 1.83 | 0.83 | 0.65 | 0.38 | 0.92 | 2.29 | 0.91 | 1.08 | −0.70 | 0.82 | 0.62 |
| S28 | 0.16 | 0.01 | 4.00 | 0.93 | 0.66 | 2.23 | 0.10 | 0.51 | 0.30 | 0.57 | 2.74 | 4.67 | 0.42 | 0.27 | 0.34 | 0.63 | 0.21 | 0.57 | 0.83 | 1.37 | 0.13 | 1.09 | 6.03 | 1.78 | −1.09 | −0.42 | 1.38 | 0.67 |
| S29 | 0.18 | 0.35 | 0.13 | 0.92 | 0.25 | 0.17 | 0.91 | 0.41 | 0.97 | 0.19 | 0.79 | 0.09 | 0.42 | 0.60 | 0.36 | 0.60 | 0.12 | 0.47 | 0.50 | 0.28 | 0.64 | 1.15 | 0.42 | 0.84 | 0.25 | −0.82 | 0.60 | 0.54 |
| S30 | 0.88 | 0.73 | 0.67 | 1.08 | 0.16 | 0.42 | 0.99 | 0.56 | 1.17 | 0.18 | 0.98 | 0.56 | 0.42 | 0.97 | 0.42 | 0.22 | 0.72 | 1.10 | 0.33 | 0.27 | 0.64 | 1.66 | 1.26 | 0.95 | 0.07 | −0.42 | 0.95 | 0.61 |
| Soil Properties | Best-Fitted Model | Nugget | Partial Sill | Sill | Nugget/Sill | SDC | Prediction Errors | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | RMSE | MSE | RMSSE | ASE | |||||||
| pH | Pentaspherical | 14.00 | 0.01 | 14.01 | 1.00 | Weak | 0.0002 | 0.4121 | 0.0001 | 1.0040 | 0.4124 |
| EC | Pentaspherical | 0.21 | 0.31 | 0.52 | 0.40 | Moderate | 0.0001 | 0.6436 | −0.0121 | 1.0279 | 0.6294 |
| OM | Exponential | 2.23 | 0.86 | 3.09 | 072 | Moderate | −0.0491 | 1.8557 | −0.0308 | 1.1149 | 1.6535 |
| CEC | Exponential | 0.20 | 0.20 | 0.40 | 0.50 | Moderate | 0.0064 | 0.4698 | 0.0061 | 0.9902 | 0.4774 |
| Av. N | Rational Quadratic | 134.36 | 1569.66 | 1704.02 | 0.08 | Strong | 0.0227 | 20.4236 | −0.0074 | 0.8568 | 25.7317 |
| Av. P | Rational Quadratic | 482.63 | 198.86 | 681.49 | 0.71 | Moderate | −0.3996 | 25.6871 | −0.0168 | 1.0083 | 25.4855 |
| Ex. K | Spherical | 3848.17 | 8340.20 | 12,188.37 | 0.32 | Moderate | 0.0218 | 96.2504 | −0.0131 | 1.0418 | 91.4128 |
| Na | Spherical | 0.00 | 0.45 | 0.45 | 0.00 | Strong | 0.0001 | 0.5846 | −0.0105 | 1.0390 | 0.5285 |
| Ca | K-Bessel | 52.22 | 85.12 | 137.34 | 0.38 | Moderate | 0.1509 | 7.6766 | 0.0114 | 0.9176 | 8.1934 |
| Mg | Circular | 2.63 | 5.11 | 7.74 | 0.34 | Moderate | −0.0325 | 2.8651 | 0.0044 | 1.0653 | 2.6544 |
| Fe | J-Bessel | 7.46 | 2.15 | 9.61 | 0.78 | Weak | −0.0835 | 3.2337 | −0.0272 | 1.0882 | 2.9493 |
| Zn | Gaussian | 293.68 | 498.12 | 791.80 | 0.37 | Moderate | −0.2277 | 23.5815 | −0.0053 | 1.2340 | 18.4944 |
| Cu | K-Bessel | 9.53 | 13.19 | 22.72 | 0.42 | Moderate | 0.0000 | 3.1222 | −0.0008 | 0.9045 | 3.5267 |
| Mn | Stable | 3.41 | 3.41 | 6.82 | 0.50 | Moderate | −0.0051 | 2.0940 | −0.0030 | 0.9645 | 2.1785 |
| Sand | Rational Quadratic | 313.16 | 580.08 | 893.24 | 0.35 | Moderate | −0.5418 | 21.7406 | −0.0104 | 0.8659 | 25.7928 |
| Silt | Pentaspherical | 141.53 | 294.18 | 435.71 | 0.32 | Moderate | 0.4637 | 14.6749 | 0.0210 | 0.9392 | 15.7000 |
| Clay | Rational Quadratic | 8.72 | 100.29 | 109.01 | 0.08 | Strong | 0.2189 | 9.4223 | 0.0117 | 1.0640 | 8.7242 |
| Porosity | J-Bessel | 5.47 | 2.81 | 8.28 | 0.66 | Moderate | 0.0182 | 2.7933 | 0.0037 | 1.0681 | 2.6031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanad, H.; Moussadek, R.; Mouhir, L.; Oueld Lhaj, M.; Dakak, H.; El Azhari, H.; Yachou, H.; Ghanimi, A.; Zouahri, A. Assessment of Soil Spatial Variability in Agricultural Ecosystems Using Multivariate Analysis, Soil Quality Index (SQI), and Geostatistical Approach: A Case Study of the Mnasra Region, Gharb Plain, Morocco. Agronomy 2024, 14, 1112. https://doi.org/10.3390/agronomy14061112
Sanad H, Moussadek R, Mouhir L, Oueld Lhaj M, Dakak H, El Azhari H, Yachou H, Ghanimi A, Zouahri A. Assessment of Soil Spatial Variability in Agricultural Ecosystems Using Multivariate Analysis, Soil Quality Index (SQI), and Geostatistical Approach: A Case Study of the Mnasra Region, Gharb Plain, Morocco. Agronomy. 2024; 14(6):1112. https://doi.org/10.3390/agronomy14061112
Chicago/Turabian StyleSanad, Hatim, Rachid Moussadek, Latifa Mouhir, Majda Oueld Lhaj, Houria Dakak, Hamza El Azhari, Hasna Yachou, Ahmed Ghanimi, and Abdelmjid Zouahri. 2024. "Assessment of Soil Spatial Variability in Agricultural Ecosystems Using Multivariate Analysis, Soil Quality Index (SQI), and Geostatistical Approach: A Case Study of the Mnasra Region, Gharb Plain, Morocco" Agronomy 14, no. 6: 1112. https://doi.org/10.3390/agronomy14061112
APA StyleSanad, H., Moussadek, R., Mouhir, L., Oueld Lhaj, M., Dakak, H., El Azhari, H., Yachou, H., Ghanimi, A., & Zouahri, A. (2024). Assessment of Soil Spatial Variability in Agricultural Ecosystems Using Multivariate Analysis, Soil Quality Index (SQI), and Geostatistical Approach: A Case Study of the Mnasra Region, Gharb Plain, Morocco. Agronomy, 14(6), 1112. https://doi.org/10.3390/agronomy14061112







