Identification and Transfer of a New Powdery Mildew Resistance Gene PmCAHM from Landrace Changanhongmai into Common Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Evaluation of Powdery Mildew Resistance
2.3. Molecular Marker and Nulli-Tetrasomic Analysis
2.4. Genetic Mapping and Data Analysis
3. Results
3.1. Genetic Analysis of Powdery Mildew Resistance Gene in Changanhongmai
3.2. Linkage Analysis of SSR Polymorphic Markers
3.3. Chromosomal Mapping and Genetic Linkage Map Construction
3.4. Breeding with PmCAHM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Curtis, T.; Halford, N.G. Food security: The challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Zhou, Y.; Xin, W.L.; Wei, Y.Q.; Zhang, J.L.; Guo, L.L. Wheat breeding in northern China: Achievements and technical advances. Crop J. 2019, 7, 718–729. [Google Scholar] [CrossRef]
- Bapela, T.; Shimelis, H.; Terefe, T.; Bourras, S.; Sánchez-Martín, J.; Douchkov, D.; Desiderio, F.; Tsilo, T.J. Breeding Wheat for Powdery Mildew Resistance: Genetic Resources and Methodologies—A Review. Agronomy 2023, 13, 1173. [Google Scholar] [CrossRef]
- Wicker, T.; Oberhaensli, S.; Parlange, F.; Buchmann, J.P.; Shatalina, M.; Roffler, S.; Ben-David, R.; Dolezel, J.; Simkova, H.; Schulze-Lefert, P.; et al. The wheat powdery mildew genome shows the unique evolution of an obligate biotroph. Nat. Genet. 2013, 45, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, E.; Shamanin, V.; Shoeva, O.; Kukoeva, T.; Morgounov, A.; Khlestkina, E. The Strategy for Marker-Assisted Breeding of Anthocyanin-Rich Spring Bread Wheat (Triticum aestivum L.) Cultivars in Western Siberia. Agronomy 2020, 10, 1603. [Google Scholar] [CrossRef]
- Vikas, V.K.; Kumar, S.; Archak, S.; Tyagi, R.K.; Kumar, J.; Jacob, S.; Sivasamy, M.; Jayaprakash, P.; Saharan, M.S.; Basandrai, A.K.; et al. Screening of 19,460 genotypes of wheat species for resistance to powdery mildew and identification of potential candidates using focused identification of germplasm strategy (FIGS). Crop Sci. 2020, 60, 2857–2866. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.-Z.; Sela, H.; Govta, L.; Klymiuk, V.; Roychowdhury, R.; Chawla, H.S.; Ens, J.; Wiebe, K.; Bocharova, V.; et al. Long-read genome sequencing accelerated the cloning of Pm69 by resolving the complexity of a rapidly evolving resistance gene cluster in wheat. bioRxiv 2022. [Google Scholar] [CrossRef]
- Aravindh, R.; Sivasamy, M.; Ganesamurthy, K.; Jayaprakash, P.; Gopalakrishnan, C.; Geetha, M.; Nisha, R.; Shajitha, P.; Peter, J.; Sindhu, P.A. Marker assisted stacking/pyramiding of stem rust, leaf rust and powdery mildew disease resistance genes (Sr2/Lr27/Yr30, Sr24/Lr24 and Sr36/Pm6) for durable resistance in wheat (Triticum aestivum L.). Elect. J. Plant Breed. 2020, 11, 907–915. [Google Scholar]
- Cao, A.; Xing, L.; Wang, X.; Yang, X.; Wang, W.; Sun, Y.; Qian, C.; Ni, J.; Chen, Y.; Liu, D.; et al. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 7727–7732. [Google Scholar] [CrossRef]
- Hu, Z.; Luo, J.; Wan, L.; Luo, J.; Li, Y.; Fu, S.; Liu, D.; Hao, M.; Tang, Z. Chromosomes polymorphisms of Sichuan wheat cultivars displayed by ND-FISH landmarks. Cereal Res. Commun. 2022, 50, 253–262. [Google Scholar] [CrossRef]
- Huang, X.Q.; Hsam, S.L.K.; Zeller, F.J. Identification of powdery mildew resistance genes in common wheat (Triticum aestivum L. em Thell.). IX. Cultivars, land races and breeding lines grown in China. Plant Breed. 2010, 116, 233–238. [Google Scholar] [CrossRef]
- Wu, N.; Lei, Y.; Pei, D.; Wu, H.; Liu, X.; Fang, J.; Guo, J.; Wang, C.; Guo, J.; Zhang, J.; et al. Predominant wheat-alien chromosome translocations in newly developed wheat of China. Mol. Breed. 2021, 41, 30. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, R.; Ma, P.; Du, H.; Zhang, H.; Wu, Q.; Yang, L.; Gong, S.; Liu, T.; Huo, N.; et al. Characterization of Pm68, a new powdery mildew resistance gene on chromosome 2BS of Greek durum wheat TRI 1796. Theor. Appl. Genet. 2021, 134, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, Z.; Ma, C.; Xia, Q.; Tian, X.; Sehgal, S.; Koo, D.H.; Friebe, B.; Ma, P.; Liu, W. A spontaneous wheat-Aegilops longissima translocation carrying Pm66 confers resistance to powdery mildew. Theor. Appl. Genet. 2020, 133, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, J.; Song, W.; Qiu, D.; Cui, L.; Wu, P.; Zhang, H.; Liu, H.; Yang, L.; Qu, Y.; et al. Pm61: A recessive gene for resistance to powdery mildew in wheat landrace Xuxusanyuehuang identified by comparative genomics analysis. Theor. Appl. Genet. 2018, 131, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Han, D.J.; Chen, X.M.; Gou, H.L.; Guo, S.J.; Rong, L.; Wang, Q.L.; Huang, L.L.; Kang, Z.S. Characterization and molecular mapping of stripe rust resistance gene Yr61 in winter wheat cultivar Pindong 34. Theor. Appl. Genet. 2014, 127, 2349–2358. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, X.; Zhong, X.; Tan, W.; Ma, C.; Wang, Y.; Tian, R.; Yang, S.; Li, X.; Xia, C.; et al. Transfer of the high-temperature adult-plant stripe rust resistance gene Yr62 in four Chinese wheat cultivars. Mol. Breed. 2023, 43, 44. [Google Scholar] [CrossRef]
- Gao, H.; Zhu, F.; Jiang, Y.; Wu, J.; Yan, W.; Zhang, Q.; Jacobi, A.; Cai, S. Genetic analysis and molecular mapping of a new powdery mildew resistant gene Pm46 in common wheat. Theor. Appl. Genet. 2012, 125, 967–973. [Google Scholar] [CrossRef]
- Huang, X.Q.; Wang, L.X.; Xu, M.X.; Roder, M.S. Microsatellite mapping of the powdery mildew resistance gene Pm5e in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2003, 106, 858–865. [Google Scholar] [CrossRef]
- Xue, F.; Wang, C.; Li, C.; Duan, X.; Zhou, Y.; Zhao, N.; Wang, Y.; Ji, W. Molecular mapping of a powdery mildew resistance gene in common wheat landrace Baihulu and its allelism with Pm24. Theor. Appl. Genet. 2012, 125, 1425–1432. [Google Scholar] [CrossRef]
- Ma, P.T.; Zhang, H.X.; Xu, H.X.; Xu, Y.F.; Cao, Y.W.; Zhang, X.T.; An, D.G. The gene confers broad-spectrum powdery mildew resistance in the multi-allelic chromosome region of the Chinese wheat cultivar YingBo 700. Mol. Breed. 2015, 35, 124. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, H.; Song, W.; Lu, M.; Huang, J.; Wu, L.; Wang, X.; Li, H. Genetic analysis and detection of the gene MlLX99 on chromosome 2BL conferring resistance to powdery mildew in the wheat cultivar Liangxing 99. Theor. Appl. Genet. 2013, 126, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zeller, F.; Hsam, S.; Wenzel, G.; Mohler, V. Chromosomal location of AFLP markers in common wheat utilizing nulli-tetrasomic stocks. Genome 2000, 43, 98–305. [Google Scholar] [CrossRef]
- Abe, A.; Kosugi, S.; Yoshida, K.; Natsume, S.; Takagi, H.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Mitsuoka, C.; Tamiru, M.; et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.; Van, d.M.J.; van den Heuvel, L.P.W.J.; Ooijen, J.; van ’t Verlaat, J.W. JoinMap® 4.0: Software for the Calculation of Genetic Linkage Maps in Experimental Populations. 2006. Available online: https://www.scienceopen.com/book?vid=b159fadd-708e-4c3e-9c91-bd395f88b847 (accessed on 21 March 2024).
- Xiao, M.; Song, F.; Jiao, J.; Wang, X.; Xu, H.; Li, H. Identification of the gene Pm47 on chromosome 7BS conferring resistance to powdery mildew in the Chinese wheat landrace Hongyanglazi. Theor. Appl. Genet. 2013, 126, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Li, G.; Cowger, C.; Carver, B.F.; Xu, X. Characterization of Pm59, a novel powdery mildew resistance gene in Afghanistan wheat landrace PI 181356. Theor Appl Genet. 2018, 131, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.K.; Li, Y.H.; Yang, B.J.; Yuan, H.B.; Jin, C.; Zhou, L.X.; Pei, H.C.; Zhao, L.F.; Li, Y.W.; Zhou, Y.L.; et al. Powdery mildew disease resistance and marker-assisted screening at the locus in wild diploid wheat. Crop J. 2020, 8, 252–259. [Google Scholar] [CrossRef]
- Petersen, S.; Lyerly, J.H.; Worthington, M.L.; Parks, W.R.; Cowger, C.; Marshall, D.S.; Brown-Guedira, G.; Murphy, J.P. Mapping of powdery mildew resistance gene Pm53 introgressed from Aegilops speltoides into soft red winter wheat. Theor. Appl. Genet. 2015, 128, 303–312. [Google Scholar] [CrossRef]
- Liu, W.; Koo, D.H.; Xia, Q.; Li, C.; Bai, F.; Song, Y.; Friebe, B.; Gill, B.S. Homoeologous recombination-based transfer and molecular cytogenetic mapping of powdery mildew-resistant gene Pm57 from Aegilops searsii into wheat. Theor. Appl. Genet. 2017, 130, 841–848. [Google Scholar] [CrossRef]
- Tan, C.C.; Li, G.Q.; Cowger, C.; Carver, B.F.; Xu, X.Y. Characterization of Pm63, a powdery mildew resistance gene in Iranian landrace PI 628024. Theor Appl Genet. 2019, 132, 1137–1144. [Google Scholar] [CrossRef]
- Lu, N.; Lu, M.X.; Liu, P.; Xu, H.X.; Qiu, X.L.; Hu, S.S.; Wu, Y.N.; Bai, S.L.; Wu, J.Z.; Xue, S.L. Fine mapping a broad-spectrum powdery mildew resistance gene in Chinese landrace Datoumai, PmDTM, and its relationship with Pm24. Plant Dis. 2020, 104, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Guo, L.; Wang, Z.Z.; Li, B.B.; Li, J.; Li, Y.H.; Qiu, D.; Shi, W.Q.; Yang, L.J.; Wang, N. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 2020, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Tosa, Y.; Tokunaga, H.; Ogura, H. Identification of a gene for resistance to wheatgrass powdery mildew fungus in the common wheat cultivar Chinese Spring. Genome 1988, 30, 612–614. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Huang, X.Q.; Zeller, F.J. Chromosomal location of genes for resistance to powderymildew in common wheat (Triticum aestivum L. em. Thell.). 6. Alleles at the Pm5 locus. Theor. Appl. Genet. 2002, 102, 127–133. [Google Scholar] [CrossRef]
- Xie, J.; Guo, G.; Wang, Y.; Hu, T.; Wang, L.; Li, J.; Qiu, D.; Li, Y.; Wu, Q.; Lu, P. A rare single nucleotide variant in Pm5e confers powdery mildew resistance in common wheat. New Phytol. 2020, 228, 1011–1026. [Google Scholar] [CrossRef]
- Xu, H.; Yi, Y.; Ma, P.; Qie, Y.; Fu, X.; Xu, Y.; Zhang, X.; An, D. Molecular tagging of a new broad-spectrum powdery mildew resistance allele Pm2c in Chinese wheat landrace Niaomai. Theor. Appl. Genet. 2015, 128, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Lillemo, M.; Asalf, B.; Singh, R.P.; Huerta-Espino, J.; Chen, X.M.; He, Z.H.; Bjornstad, A. The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor. Appl. Genet. 2008, 116, 1155–1166. [Google Scholar] [CrossRef]
- Peusha, H.; Enno, T.; Priilinn, O. Chromosomal location of powdery mildew resistance genes and cytogeneticanalysis of meiosis in common wheat cultivar Meri. Hereditas 2000, 132, 29–34. [Google Scholar] [CrossRef]
- Visioli, G.; Giannelli, G.; Agrimonti, C.; Spina, A.; Pasini, G. Traceability of Sicilian Durum Wheat Landraces and Historical Varieties by High Molecular Weight Glutenins Footprint. Agronomy 2021, 11, 143. [Google Scholar] [CrossRef]
- Pais, I.P.; Moreira, R.; Coelho, A.R.; Semedo, J.N.; Reboredo, F.H.; Coutinho, J.; Lidon, F.C.; Maçãs, B.; Scotti-Campos, P. Unveiling the Impact of Growth Traits on the Yield of Bread Wheat Germplasm Subjected to Waterlogging. Agriculture 2024, 14, 241. [Google Scholar] [CrossRef]
- Sirangelo, T.M. NLR- and mlo-Based Resistance Mechanisms against Powdery Mildew in Cannabis sativa. Plants 2024, 13, 105. [Google Scholar] [CrossRef]
- Luo, K.; He, D.; Guo, J.; Li, G.; Li, B.; Chen, X. Molecular Advances in Breeding for Durable Resistance against Pests and Diseases in Wheat: Opportunities and Challenges. Agronomy 2023, 13, 628. [Google Scholar] [CrossRef]
- Gharib, M.A.A.H.; Qabil, N.; Salem, A.H.; Ali, M.M.A.; Awaad, H.A.; Mansour, E. Characterization of wheat landraces and commercial cultivars based on morpho-phenological and agronomic traits. Cereal Res. Commun. 2021, 49, 149–159. [Google Scholar] [CrossRef]
- Cheng, P.; Guo, M.; Hao, X.; Guo, X.; Yao, Q.; Guo, Q.; Li, Q.; Wang, B. Evaluation of powdery mildew resistance and molecular detection of resistance genes in an international wheat collection. Crop Prot. 2022, 160, 106033. [Google Scholar] [CrossRef]
- Xue, S.L.; Wang, H.; Ma, Y.Y.; Sun, T.P.; Wang, Y.X.; Meng, F.; Wang, X.T.; Yang, Z.H.; Zhang, J.L.; Du, J.X.; et al. Fine mapping of powdery mildew resistance gene PmXNM in a Chinese wheat landrace Xiaonanmai. Theor. Appl. Genet. 2024, 137, 35. [Google Scholar] [CrossRef]

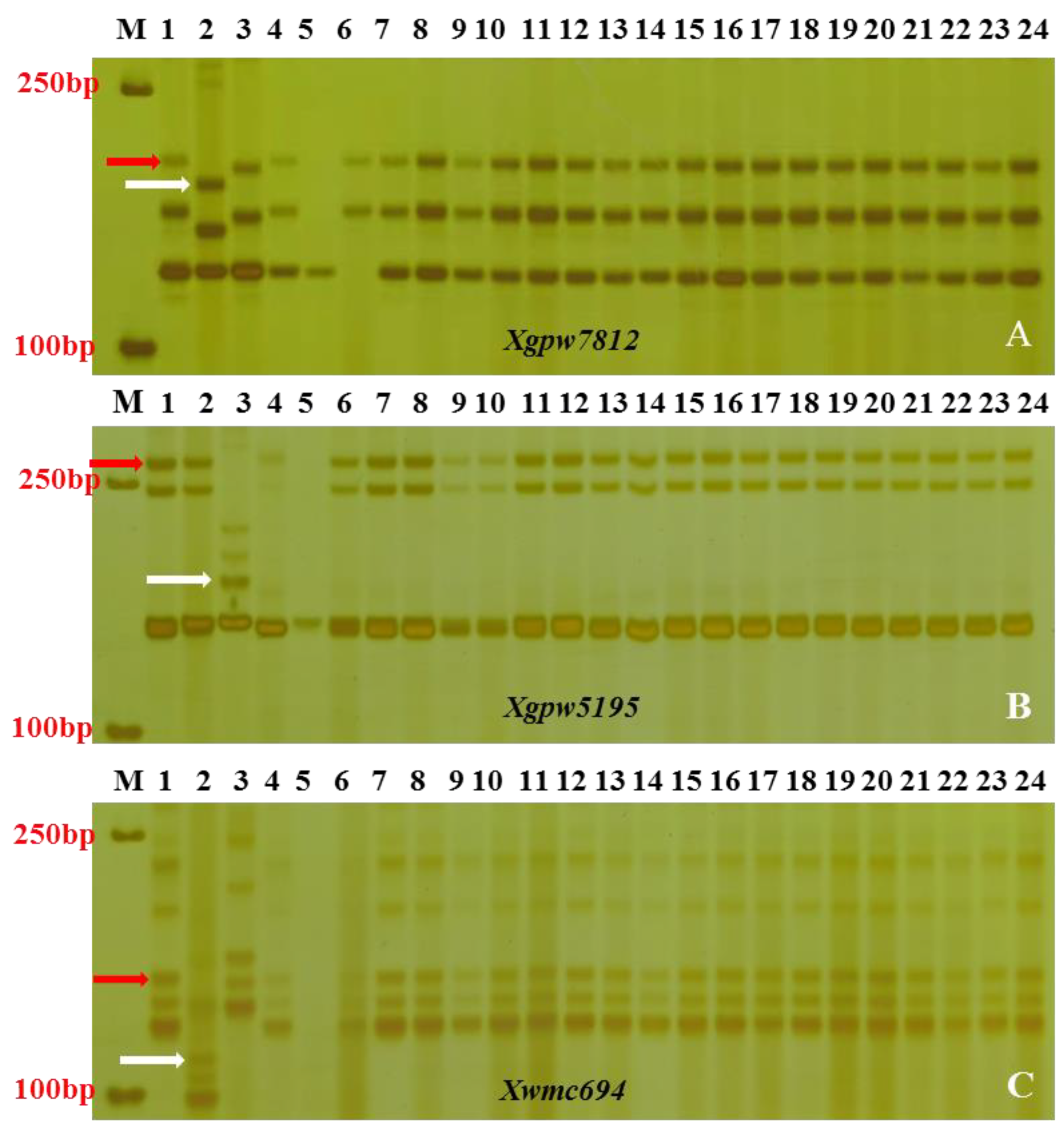
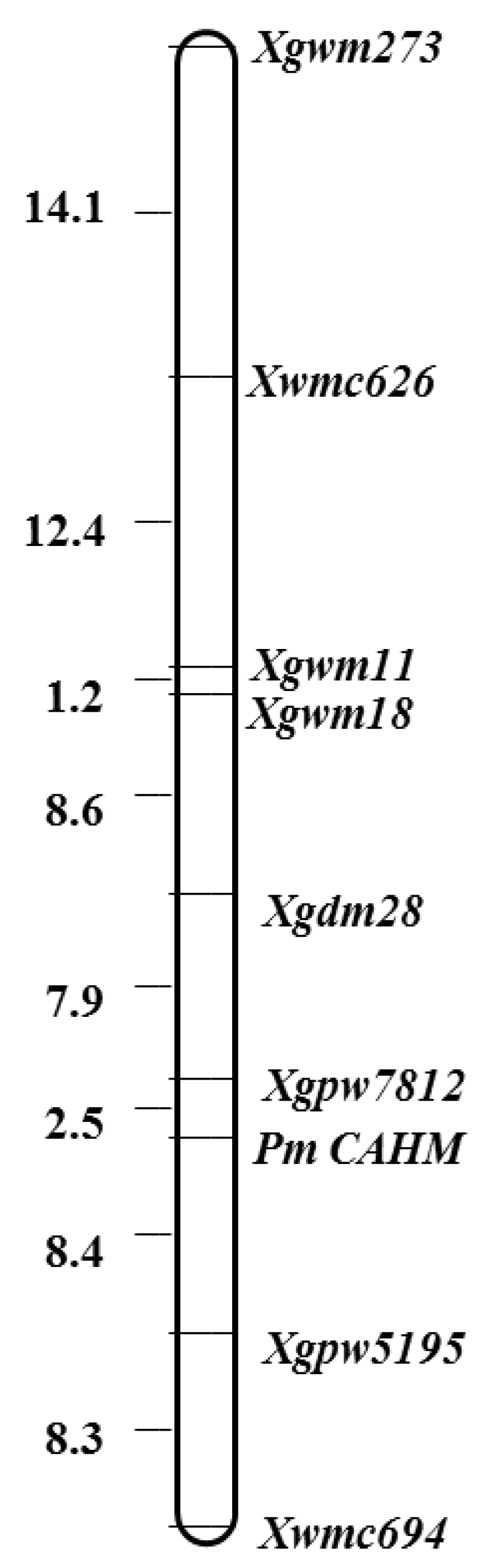

| Materials | Observed Plant Number | Expected Ratio | χ2 Value | p-Value | ||
|---|---|---|---|---|---|---|
| Resistant | Segregating | Susceptible | ||||
| CAHM | 10 | |||||
| SY225 | 10 | |||||
| F1 | 10 | |||||
| F2 | 122 | 54 | 3:1 | 2.73 | 0.90 | |
| F2:3 | 47 | 75 | 54 | 1:2:1 | 4.4 | 0.96 |
| Marker | Forward-Primer (5′-3′) | Reverse-Primer (5′-3′) | Tm °C/t (h) |
|---|---|---|---|
| Xgpw7812 | ACTGTCCTCGATTGTGTGTCC | CTTTATCAGGCATGGAACTGC | 60 |
| Xgwm273 | ATTGGACGGACAGATGCTTT | AGCAGTGAGGAAGGGGATC | 60 |
| Xwmc626 | AGCCCATAAACATCCAACACGG | AGGTGGGCTTGGTTACGCTCTC | 60 |
| Xgwm11 | GGATAGTCAGACAATTCTTGTG | GTGAATTGTGTCTTGTATGCTTCC | 62 |
| Xgwm1818 | TGGCGCCATGATTGCATTATCTTC | GGTTGCTGAAGAACCTTATTTAGG | 62 |
| Xgdm2828 | ATCTGACTTCATGGTTTATAT | TCAAGAATGAAGACATAGTT | 62 |
| Xwmc694 | ATTTGCCCTTGTGAGCCGTT | GACCTGGGTGGGACCCATTA | 58 |
| Xgpw5195 | CGACTCTCGCTTCAGCTTG | GGTTCTTCACGCCATTGATT | 60 |
| Materials | TGW (g) | Plant Height (cm) | Spike Length (cm) | Spike Grain Number | Infection Type |
|---|---|---|---|---|---|
| Changanhongmai | 45.0 | 120 | 8.5 | 42 | 0 |
| FY1718 | 44.5 | 80 | 9.0 | 46 | 4 |
| NW1748 | 45.9 | 95 | 9.2 | 46 | 0–1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, Y.; Han, G.; Fan, J.; Tan, Q.; Liu, G.; Zhang, H.; Wang, Y. Identification and Transfer of a New Powdery Mildew Resistance Gene PmCAHM from Landrace Changanhongmai into Common Wheat. Agronomy 2024, 14, 667. https://doi.org/10.3390/agronomy14040667
Chen X, Wang Y, Han G, Fan J, Tan Q, Liu G, Zhang H, Wang Y. Identification and Transfer of a New Powdery Mildew Resistance Gene PmCAHM from Landrace Changanhongmai into Common Wheat. Agronomy. 2024; 14(4):667. https://doi.org/10.3390/agronomy14040667
Chicago/Turabian StyleChen, Xueyan, Yongfu Wang, Guohao Han, Jianzhong Fan, Qingqing Tan, Guoxia Liu, Hong Zhang, and Yajuan Wang. 2024. "Identification and Transfer of a New Powdery Mildew Resistance Gene PmCAHM from Landrace Changanhongmai into Common Wheat" Agronomy 14, no. 4: 667. https://doi.org/10.3390/agronomy14040667
APA StyleChen, X., Wang, Y., Han, G., Fan, J., Tan, Q., Liu, G., Zhang, H., & Wang, Y. (2024). Identification and Transfer of a New Powdery Mildew Resistance Gene PmCAHM from Landrace Changanhongmai into Common Wheat. Agronomy, 14(4), 667. https://doi.org/10.3390/agronomy14040667






