Exogenous Melatonin Affects the Morphometric Characteristics and Glucosinolates during the Initial Growth Stages of Broccoli
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Materials
2.3. Glucosinolates Extraction
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. Statistical Analysis
3. Results
3.1. Morphometric Traits
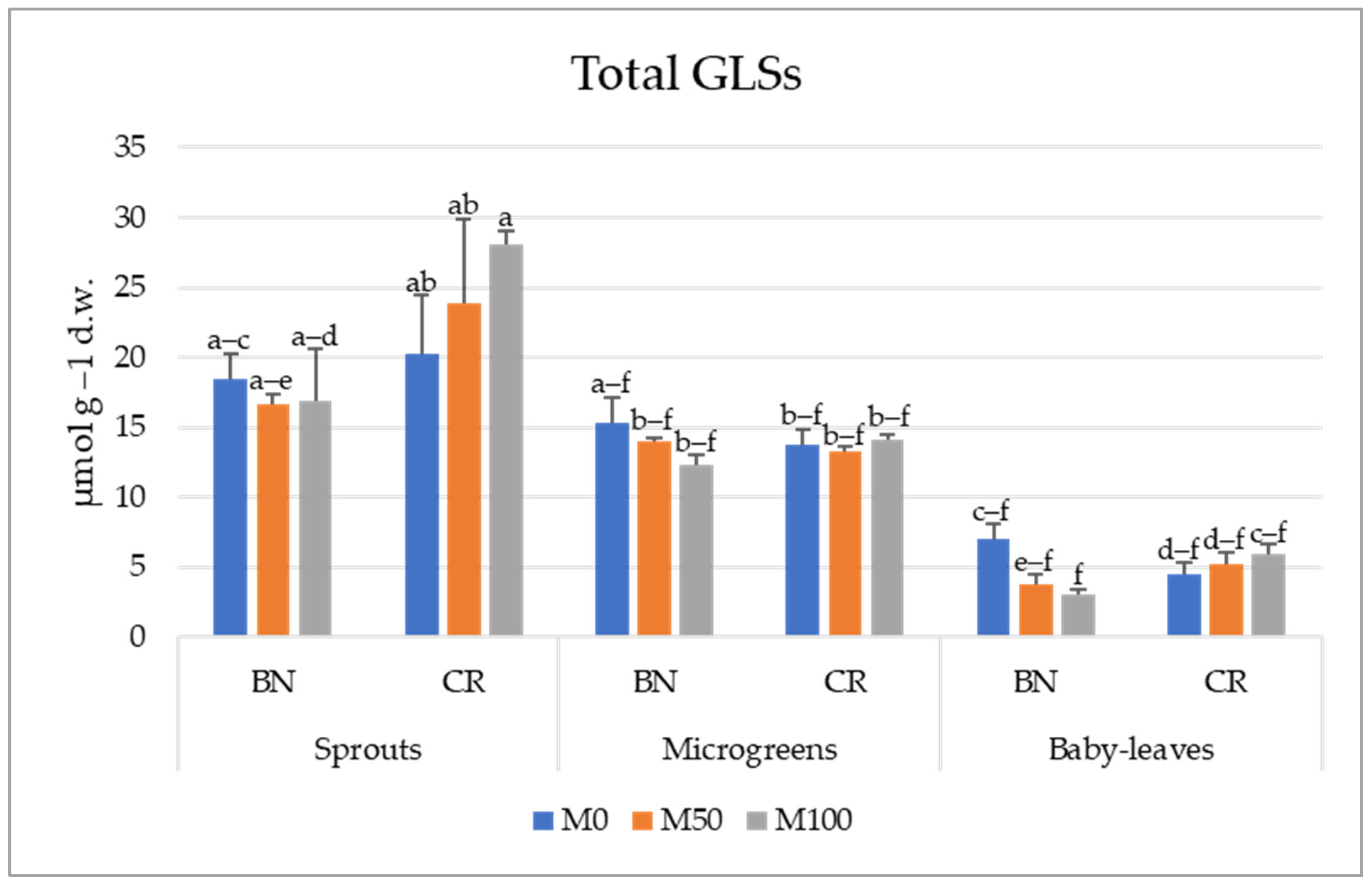
3.2. Glucosinolates Profile
3.3. The Variation of Individual Glucosinolates
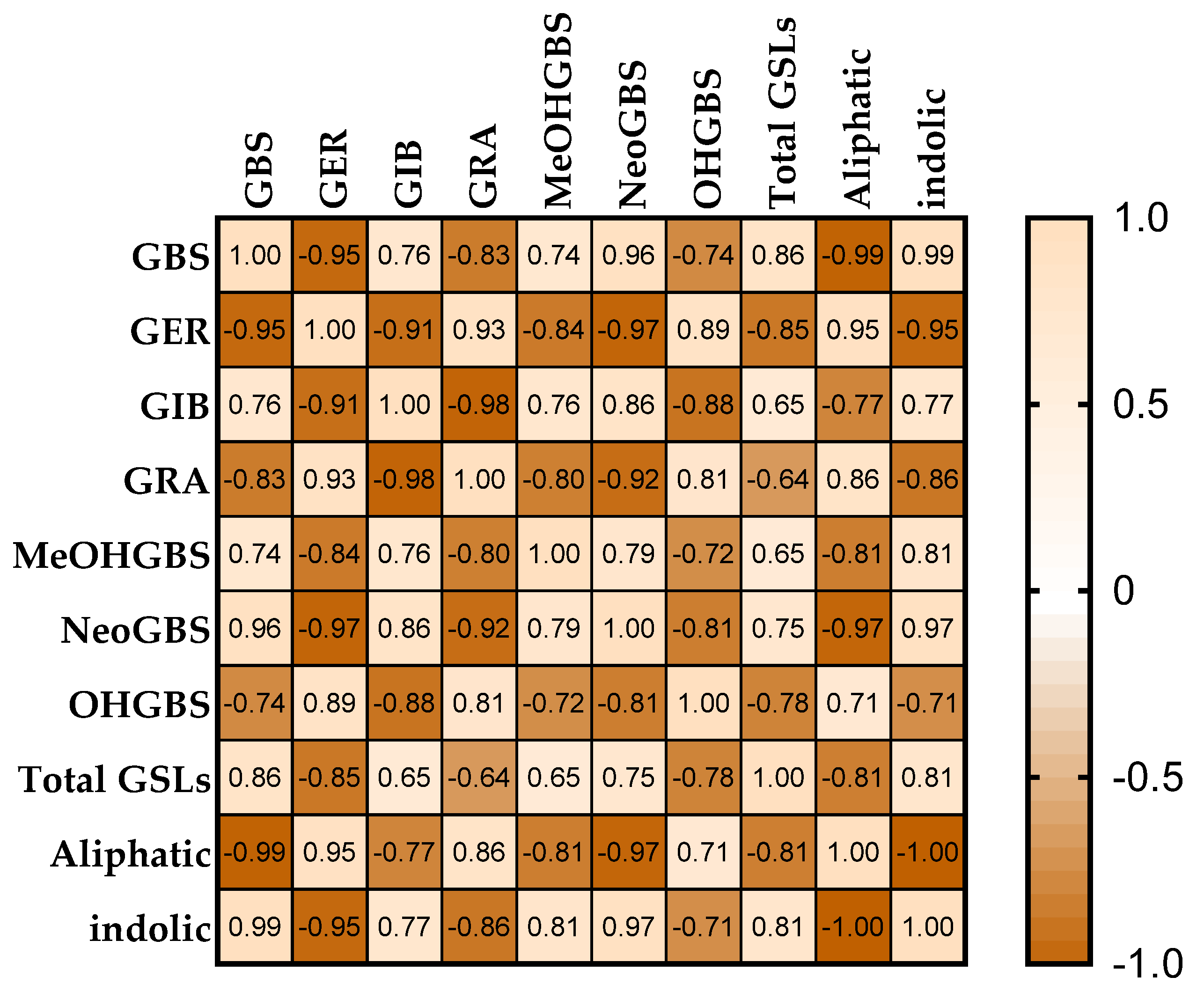
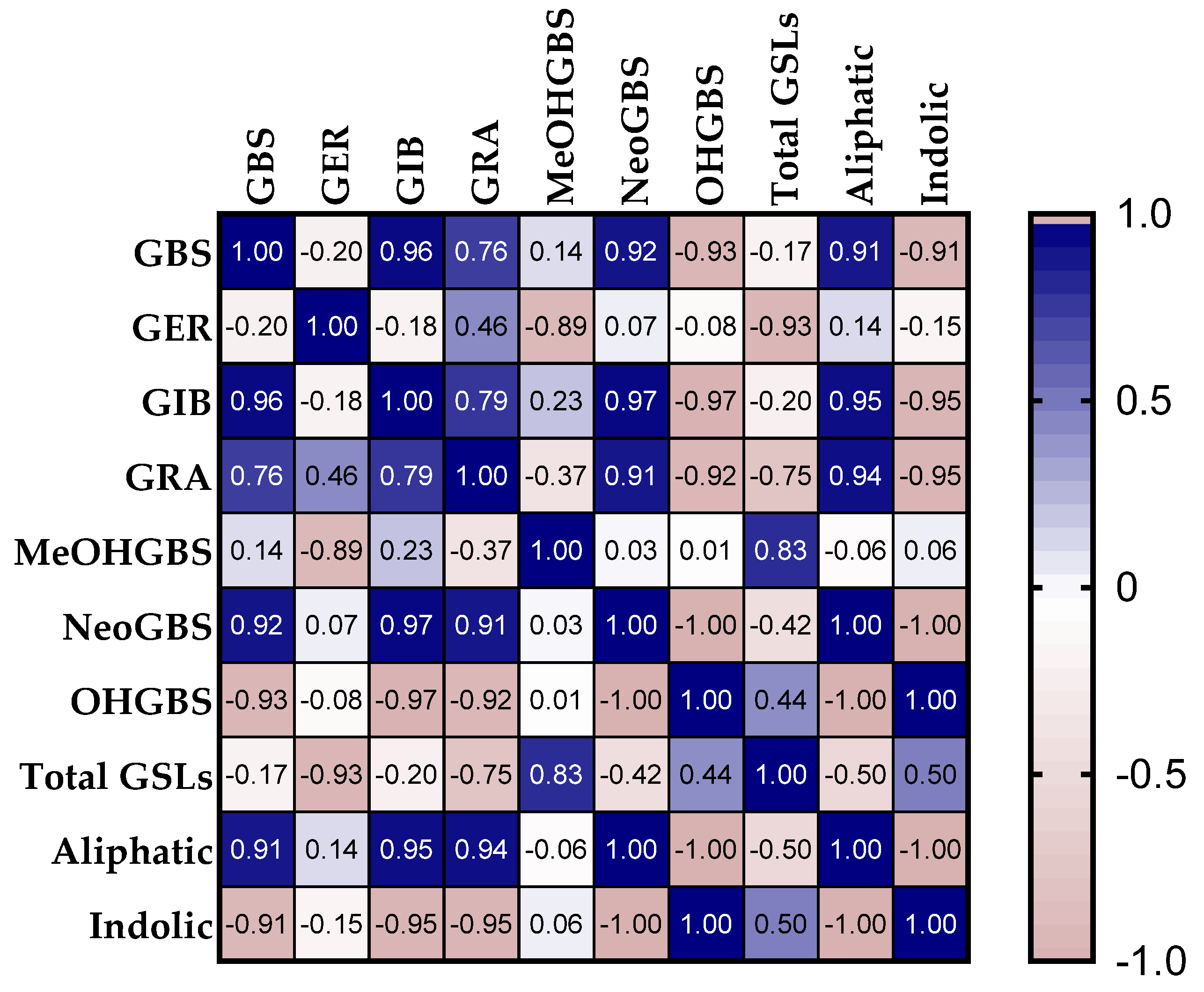
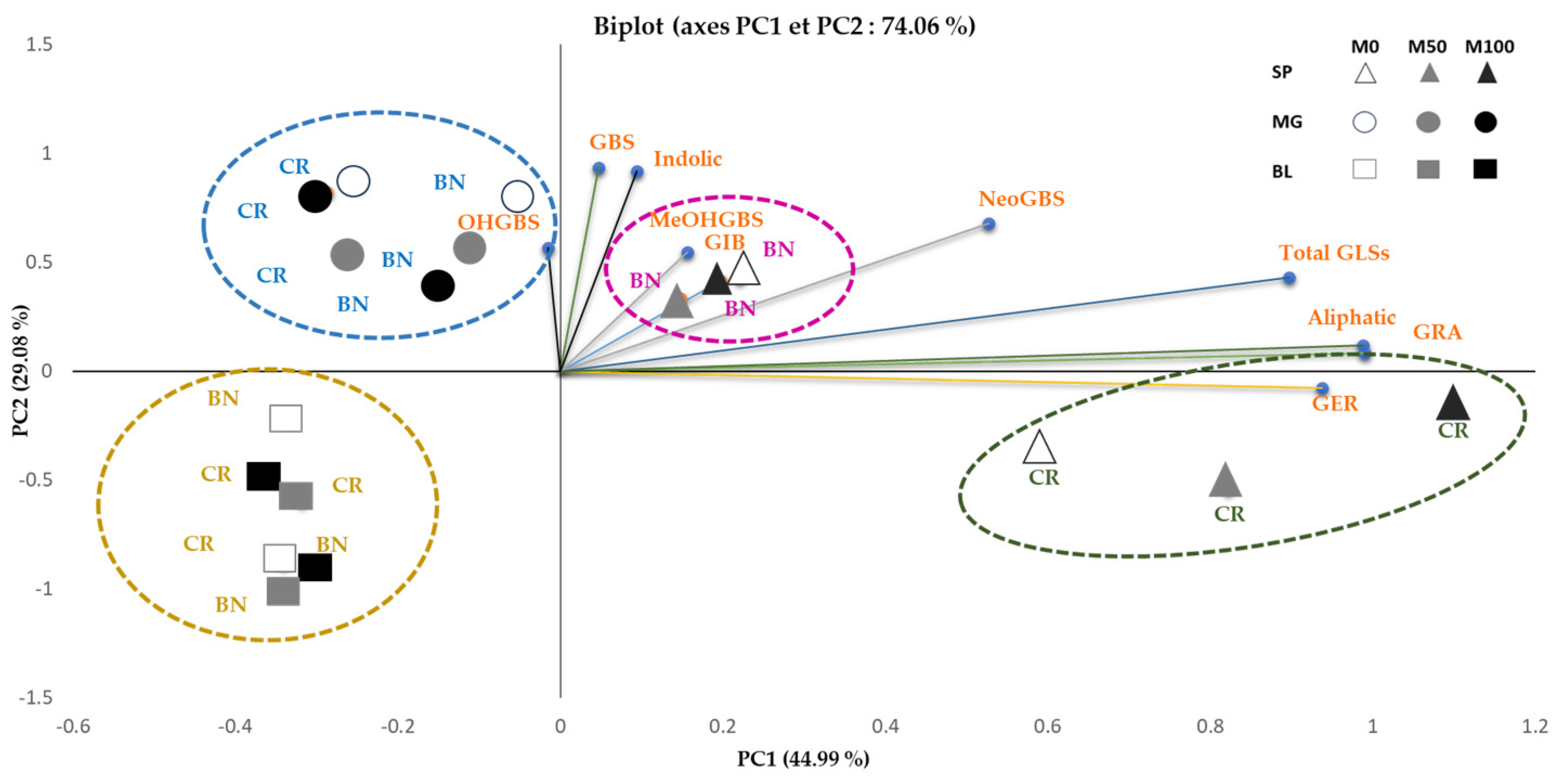
3.4. Correlation and Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raza, A.; Hafeez, M.B.; Zahra, N.; Shaukat, K.; Umbreen, S.; Tabassum, J.; Charagh, S.; Ahmad Khan, R.S.; Hasanuzzaman, M. The plant family Brassicaceae: Introduction, Biology, and Importance. In The Plant Family Brassicaceae; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 1–43. [Google Scholar] [CrossRef]
- Rakow, G. Species Origin and Economic Importance of Brassica. In Brassica; Pua, E.C., Douglas, C.J., Eds.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 54. [Google Scholar] [CrossRef]
- Gómez-Campo, C. (Ed.) Biology of Brassica coenospecies; Elsevier: Amsterdam, The Netherlands, 1999; ISBN 9780444502780. [Google Scholar]
- Al-Shehbaz, I.A. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 2012, 61, 931–954. [Google Scholar] [CrossRef]
- Maggioni, L.; von Bothmer, R.; Poulsen, G.; Branca, F.; Bagger Jørgensen, R. Genetic diversity and population structure of leafy kale and Brassica rupestris Raf. in south Italy. Hereditas 2014, 151, 145–158. [Google Scholar] [CrossRef]
- Arena, D.; Treccarichi, S.; Di Bella, M.C.; Achkar, N.; Ben Ammar, H.; Picchi, V.; Lo Scalzo, R.; Amari, M.; Branca, F. Evaluation of Brassica oleracea L. crops and wild relatives for bio-morphometric and biochemical characteristics. Acta Hortic. 2022, 1355, 71–80. [Google Scholar] [CrossRef]
- Ben Ammar, H.; Picchi, V.; Arena, D.; Treccarichi, S.; Bianchi, G.; Lo Scalzo, R.; Marghali, S.; Branca, F. Variation of Bio-Morphometric Traits and Antioxidant Compounds of Brassica oleracea L. Accessions in Relation to Drought Stress. Agronomy 2022, 12, 2016. [Google Scholar] [CrossRef]
- Bianchi, G.; Picchi, V.; Tava, A.; Doria, F.; Walley, P.G.; Dever, L.; Di Bella, M.C.; Arena, D.; Ben Ammar, H.; Lo Scalzo, R.; et al. Insights into the phytochemical composition of selected genotypes of organic kale (Brassica oleracea L. var. acephala). J. Food Compost Anal. 2024, 125, 105721. [Google Scholar] [CrossRef]
- Branca, F.; Arena, D.; Argento, S.; Frustaci, F.; Ciccarello, L.; Melilli, M.G. Recovery of healthy compounds in waste bracts of globe artichoke heads. Acta Hortic. 2021, 1354, 361–366. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Kobira, K.; Keck, A.S.; Juvik, J.A.; Jeffery, E.H. Correlation analyses of phytochemical composition, chemical, and cellular measures of antioxidant activity of broccoli (Brassica oleracea L. Var. italica). J. Agric. Food Chem. 2005, 53, 7421–7431. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Wu, X.; Huang, H.; Childs, H.; Wu, Y.; Yu, L.; Pehrsson, P.R. Glucosinolates in Brassica vegetables: Characterization and factors that influence distribution, content, and intake. Annu. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J.; VanEtten, C.H. Glucosinolates and their breakdown products in food and food plants. Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar] [CrossRef]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef] [PubMed]
- Jongen, W. Glucosinolates in Brassica: Occurrence and significance as cancer-modulating agents. Proc. Nutr. Soc. 1996, 55, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 501–508. [Google Scholar] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea—A dose-dependent effect. Protoplasma 2020, 257, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin Mediates Enhancement of Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The Physiological Function of Melatonin in Plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in plants–diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, J.; Harmens, H.; Zheng, X.; Zhang, C. Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiol. Biochem. 2020, 149, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Nawaz, G.; Cao, Q.; Xu, T. Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci. Hortic. 2022, 291, 110560. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Zheng, H.; Zheng, L. Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 125562. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, W.; Yu, Y.; Hong, S.B.; Zhu, Z.; Zang, Y. Melatonin regulated glucosinolate profile via modulation of genes related with sulfur and nitrogen metabolism in Brassica rapa ssp. pekinensis. Ind. Crops Prod. 2022, 177, 114538. [Google Scholar] [CrossRef]
- Karumannil, S.; Khan, T.A.; Kappachery, S.; Gururani, M.A. Impact of Exogenous Melatonin Application on Photosynthetic Machinery under Abiotic Stress Conditions. Plants 2023, 12, 2948. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R., Eds.; Food Engineering Series; Springer: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and Visible Spectrum LED Lighting as Abiotic Elicitors of Bioactive Compounds in Sprouts, Microgreens, and Baby Leaves—A Comprehensive Review including Their Mode of Action. Foods 2022, 11, 265. [Google Scholar] [CrossRef]
- Ragusa, L.; Picchi, V.; Tribulato, A.; Cavallaro, C.; Lo Scalzo, R.; Branca, F. The effect of the germination temperature on the phytochemical content of broccoli and rocket sprouts. Int. J. Food Sci. Nutr. 2017, 68, 411–420. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Cattaneo, C.; Pagliarini, E.; Artés-Hernández, F. How does the phytochemical composition of sprouts and microgreens from Brassica vegetables affect the sensory profile and consumer acceptability? Postharvest Biol. Technol. 2023, 203, 112411. [Google Scholar] [CrossRef]
- Di Gioia, F.; De Bellis, P.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini (Brassica rapa L.) microgreens. J. Sci. Food Agric. 2017, 97, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic Control of Natural Variation in Arabidopsis Glucosinolate Accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W. Sprouts and microgreens—Novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth-stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef]
- Wang, Q.; An, B.; Shi, H.; Luo, H.; He, C. High concentration of melatonin regulates leaf development by suppressing cell proliferation and endoreduplication in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 991. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Chen, F.; Liao, M.; Tang, Y.; Liang, D.; Xia, H.; Lai, Y.; Wang, X.; Chen, C.; et al. Effects of melatonin on the growth and cadmium characteristics of Cyphomandra betacea seedlings. Environ. Monit. Assess 2018, 190, 119. [Google Scholar] [CrossRef]
- Sardar, H.; Ramzan, M.A.; Naz, S.; Ali, S.; Ejaz, S.; Ahmad, R.; Altaf, M.A. Exogenous Application of Melatonin Improves the Growth and Productivity of Two Broccoli (Brassica oleracea L.) Cultivars Under Salt Stress. J. Plant Growth Regul. 2023, 42, 5152–5166. [Google Scholar] [CrossRef]
- Xiong, F.; Zhuo, F.; Reiter, R.J.; Wang, L.; Wei, Z.; Deng, K.; Song, Y.; Qanmber, G.; Feng, L.; Yang, Z.; et al. Hypocotyl elongation inhibition of melatonin is involved in repressing brassinosteroid biosynthesis in Arabidopsis. Front. Plant Sci. 2019, 10, 1082. [Google Scholar] [CrossRef]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef]
- Di Bella, M.C.; Treccarichi, S.; Arena, D.; Nicotra, R.; Mazzaglia, A.; Melilli, M.G.; Bartoszek, A.; Kusznierewicz, B.; Parchem, K.; Branca, F. Evaluation of Sicilian landraces of broccoli (B. oleracea var. italica Plenck) for quality traits. Acta Hortic. 2022, 1354, 343–350. [Google Scholar] [CrossRef]
- Jambor, T.; Knizatova, N.; Valkova, V.; Tirpak, F.; Greifova, H.; Kovacik, A.; Lukac, N. Microgreens as a functional component of the human diet: A review. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5870. [Google Scholar] [CrossRef]
- Perez-Balibrea, S.; Moreno, D.A.; Garcia-Viguera, C. Glucosinolates in broccoli sprouts (Brassica oleracea var. italica) as conditioned by sulphate supply during germination. J. Food Sci. 2010, 75, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, R.; Guo, Q.; Gu, Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J. Funct. Foods 2014, 9, 70–77. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive compounds and bioactivities of Brassica oleracea L. var. italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Omidbakhshfard, M.A.; Sokolowska, E.M.; Di Vittori, V.; de Souza, L.P.; Kuhalskaya, A.; Brotman, Y.; Alseekh, S.; Fernie, A.R.; Skirycz, A. Multi-omics analysis of early leaf development in Arabidopsis thaliana. Patterns 2021, 2, 100235. [Google Scholar] [CrossRef] [PubMed]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Lee, J.G. Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm. Molecules 2020, 25, 1860. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Erland, L.A.E. A Systematic Review of Melatonin in Plants: An Example of Evolution of Literature. Front. Plant Sci. 2021, 12, 683047. [Google Scholar] [CrossRef]
- Kim, M.J.; Chiu, Y.C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.M. Cultivar-specific changes in primary and secondary metabolites in pak choi (Brassica rapa, Chinensis group) by methyl jasmonate. Int. J. Mol. Sci. 2017, 18, 4. [Google Scholar] [CrossRef]
- Ishihara, A.; Matsuda, F.; Miyagawa, H.; Wakasa, K. Metabolomics for metabolically manipulated plants: Effects of tryptophan overproduction. Metabolomics 2007, 3, 319–334. [Google Scholar] [CrossRef][Green Version]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A Biostimulant Based on Protein Hydrolysates Promotes the Growth of Young Olive Trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]


| M0 | M50 | M100 | Mean | ||||||||
| BN | CR | BN | CR | BN | CR | BN | CR | ||||
| W (g) | 0.52 ± 0.12 d | 0.81 ± 0.17 a | 0.66 ± 0.20 b | 0.71 ± 0.25 b | 0.64 ± 0.12 bc | 0.68 ± 0.05 b | 0.61 ± 0.02 c | 0.85 ± 0.06 a | 0.73 ± 0.17 a | 0.61 ± 0.09 b | 0.76 ± 0.11 a |
| HL (mm) | 21.08 ± 0.09 b | 21.35 ± 0.10 b | 21.22 ± 0.02 b | 20.18 ± 0.02 b | 28.23 ± 0.13 a | 24.21 ± 0.57 a | 19.58 ± 0.11 b | 31.68 ± 0.11 a | 25.63 ± 0.86 a | 20.28 ± 0.08 b | 27.09 ± 0.53 a |
| CL (mm) | 9.45 ± 0.06 | 10.77 ± 0.05 | 10.11 ± 0.09 | 10.12 ± 0.05 | 10.13 ± 0.04 | 10.12 ± 0.01 | 10.13 ± 0.04 | 10.76 ± 0.05 | 10.45 ± 0.04 | 9.90 ± 0.04 b | 10.55 ± 0.08 a |
| CW (mm) | 11.19 ± 0.03 ab | 10.21 ± 0.03 b | 10.70 ± 0.07 | 12.63 ± 0.02 a | 10.14 ± 0.02 b | 11.38 ± 0.17 | 10.92 ± 0.07 b | 10.33 ± 0.07 b | 10.63 ± 0.08 | 11.58 ± 0.09 a | 10.22 ± 0.01 b |
| Significance of the differences via the ANOVA Newman–Keuls method | |||||||||||
| M | GE | M × GE | |||||||||
| W (g) | n.s. | * | n.s. | ||||||||
| HL (mm) | *** | *** | *** | ||||||||
| CL (mm) | n.s. | ** | n.s. | ||||||||
| CW (mm) | n.s. | *** | * | ||||||||
| M0 | M50 | M100 | Mean | ||||||||
| BN | CR | BN | CR | BN | CR | BN | CR | ||||
| W (g) | 1.15 ± 0.16 d | 1.96 ± 0.22 ab | 1.56 ± 0.58 b | 1.32 ± 0.11 c | 2.01 ± 0.14 a | 1.66 ± 0.48 a | 1.09 ± 0.16 d | 1.86 ± 0.26 b | 1.47 ± 0.55 c | 1.18 ± 0.12 b | 1.94 ± 0.07 a |
| HL (mm) | 22.29 ± 0.23 c | 27.20 ± 0.16 b | 24.75 ± 3.47 | 20.84 ± 0.09 c | 32.26 ± 0.03 a | 26.55 ± 0.81 | 20.38 ± 0.08 c | 32.62 ± 0.06 a | 26.50 ± 0.87 | 21.17 ± 1.00 b | 30.69 ± 0.30 a |
| CL (mm) | 9.55 ± 0.25 | 10.86 ± 0.16 | 10.21 ± 0.92 | 10.92 ± 0.17 | 10.21 ± 0.29 | 10.57 ± 0.50 | 10.36 ± 0.22 | 11.80 ± 0.02 | 11.08 ± 1.02 | 10.28 ± 0.69 | 10.96 ± 0.80 |
| CW(mm) | 9.94 ± 0.42 | 9.56 ± 0.24 | 9.75 ± 0.27 | 10.29 ± 0.06 | 10.14 ± 0.31 | 10.22 ± 0.11 | 10.24 ± 0.07 | 10.13 ± 0.11 | 10.19 ± 0.08 | 10.16 ± 0.19 | 9.94 ± 0.33 |
| LL (mm) | 11.06 ± 0.04 | 13.32 ± 0.13 | 12.19 ± 1.59 | 11.71 ± 0.07 | 13.22 ± 0.13 | 12.46 ± 1.07 | 11.14 ± 0.05 | 12.68 ± 0.01 | 11.91 ± 1.09 | 11.31 ± 0.35 b | 13.07 ± 0.34 a |
| LW (mm) | 7.96 ± 0.03 | 9.90 ± 0.11 | 8.93 ± 1.37 | 8.28 ± 0.02 | 10.11 ± 0.07 | 9.19 ± 1.30 | 7.64 ± 0.05 | 9.57 ± 0.06 | 8.61 ± 11.37 | 7.96 ± 0.32 b | 9.86 ± 0.27 a |
| Significance of the differences via the ANOVA Newman–Keuls method | |||||||||||
| M | GE | M × GE | |||||||||
| W (g) | *** | *** | * | ||||||||
| HL (mm) | n.s. | *** | *** | ||||||||
| CL (mm) | n.s. | *** | n.s. | ||||||||
| CW (mm) | n.s. | n.s. | n.s. | ||||||||
| LL (mm) | n.s. | *** | n.s. | ||||||||
| LW (mm) | n.s. | *** | n.s. | ||||||||
| M0 | M50 | M100 | Mean | ||||||||
| BN | CR | BN | CR | BN | CR | BN | CR | ||||
| W (g) | 6.01 ± 0.47 | 6.88 ± 0.64 | 6.44 ± 0.62 | 5.85 ± 0.71 | 7.27 ± 1.53 | 6.56 ± 1.00 | 6.02 ± 1.12 | 7.75 ± 2.20 | 6.89 ± 1.22 | 5.96 ± 0.09 b | 7.31 ± 0.44 a |
| SL (mm) | 49.61 ± 0.10 ab | 57.79 ± 0.27 a | 53.70 ± 0.58 | 52.28 ± 0.23 ab | 48.03 ± 0.16 b | 50.16 ± 0.30 | 50.21 ± 0.18 ab | 54.83 ± 0.35 ab | 52.52 ± 0.33 | 50.70 ± 0.14 | 53.55 ± 0.50 |
| N (n) | 3.23 ± 0.29 | 3.10 ± 0.10 | 3.17 ± 0.09 | 3.00 ± 0.27 | 3.10 ± 0.22 | 3.05 ± 0.07 | 3.17 ± 0.11 | 3.10 ± 0.22 | 3.13 ± 0.05 | 3.13 ± 0.12 | 3.10 ± 0.45 |
| LL (mm) | 28.71 ± 0.07 | 29.08 ± 0.11 | 28.90 ± 0.03 | 29.72 ± 0.09 | 29.47 ± 0.07 | 29.60 ± 0.02 | 28.78 ± 0.21 | 31.36 ± 0.17 | 30.07 ± 0.18 | 29.07 ± 0.06 | 29.97 ± 0.12 |
| LW (mm) | 19.53 ± 0.03 b | 21.27 ± 0.11 ab | 20.40 ± 0.12 b | 21.39 ± 0.09 a | 21.21 ± 0.02 ab | 21.30 ± 0.01 ab | 20.40 ± 0.06 b | 23.43 ± 0.19 a | 21.91 ± 0.21 a | 20.44 ± 0.09 b | 21.97 ± 0.13 a |
| Significance of the differences via the ANOVA Newman–Keuls method | |||||||||||
| M | GE | M × GE | |||||||||
| W (g) | n.s. | *** | n.s. | ||||||||
| SL (mm) | n.s. | n.s. | ** | ||||||||
| N (n) | n.s. | n.s. | n.s. | ||||||||
| LL (mm) | n.s. | n.s. | n.s. | ||||||||
| LW (mm) | * | ** | * | ||||||||
| Total GLSs | GRA | GER | GIB | GBS | MeOHGBS | NeoGBS | OHGBS | Aliphatic | Indolic | |
| M | ||||||||||
| M0 | 13.22 ± 2.25 | 4.42 ± 1.71 | 0.48 ± 0.27 | 0.43 ± 0.25 | 3.47 ± 0.64 | 1.01 ± 0.01 | 0.68 ± 0.15 | 2.27 ± 0.41 | 5.78 ± 2.21 | 7.44 ± 1.02 |
| M50 | 12.81 ± 3.04 | 4.52 ± 2.08 | 0.51 ± 0.32 | 0.68 ± 0.42 | 3.11 ± 0.68 | 1.04 ± 0.02 | 0.59 ± 0.14 | 2.02 ± 0.46 | 6.08 ± 2.69 | 6.72 ± 1.08 |
| M100 | 13.38 ± 3.62 | 4.58 ± 2.31 | 0.66 ± 0.49 | 0.79 ± 0.51 | 2.99 ± 0.69 | 1.01 ± 0.01 | 0.62 ± 0.15 | 2.24 ± 0.55 | 6.52 ± 3.15 | 6.86 ± 1.17 |
| GS | ||||||||||
| Sprouts | 20.68 ± 1.84 a | 10.24 ± 1.50 a | 1.32 ± 0.48 a | 1.31 ± 0.53 a | 3.05 ± 0.17 b | 1.03 ± 0.02 a | 0.91 ± 0.09 a | 1.89 ± 0.23 b | 13.84 ± 1.75 a | 6.88 ± 0.26 b |
| Microgreens | 13.81 ± 0.40 b | 2.83 ± 0.24 b | 0.29 ± 0.02 b | 0.56 ± 0.26 b | 5.02 ± 0.15 a | 1.01 ± 0.01 ab | 0.73 ± 0.08 b | 3.08 ± 0.55 a | 3.96 ± 0.45 b | 9.85 ± 0.47 a |
| Baby leaves | 4.92 ± 0.60 c | 0.51 ± 0.07 c | 0.02 ± 0.08 b | 0.04 ± 0.01 c | 1.52 ± 0.26 c | 0.99 ± 0.01 b | 0.25 ± 0.04 c | 1.56 ± 0.28 b | 0.62 ± 0.09 c | 4.31 ± 0.51 c |
| GE | ||||||||||
| BN | 11.93 ± 1.95 | 3.58 ± 1.0 b | 0.22 ± 0.0 b | 1.19 ± 0.3 c | 3.37 ± 0.5 a | 1.02 ± 0.01 a | 0.75 ± 0.14 a | 1.52 ± 0.17 b | 5.27 ± 1.43 | 6.66 ± 0.79 b |
| CR | 14.34 ± 2.80 | 5.43 ± 2.0 a | 0.88 ± 0.3 a | 0.08 ± 0.0 b | 3.02 ± 0.5 b | 0.11 ± 0.01 b | 0.51 ± 0.07 b | 2.84 ± 0.39 a | 6.99 ± 2.64 | 7.35 ± 0.92 a |
| Significance of the differences via ANOVA Student–Newman–Keuls | ||||||||||
| M | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| GS | *** | *** | *** | *** | *** | * | *** | *** | *** | *** |
| GE | * | * | *** | *** | n.s. | * | *** | *** | n.s. | n.s. |
| GS × M | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| GS × GE | * | ** | *** | *** | n.s. | n.s. | *** | *** | ** | n.s. |
| M × GE | n.s. | n.s. | n.s. | n.s. | ** | n.s. | n.s. | n.s. | n.s. | n.s. |
| GS × M × GE | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, D.; Ben Ammar, H.; Rodriguez, V.M.; Velasco, P.; Garcia, G.; Calì, R.; Branca, F. Exogenous Melatonin Affects the Morphometric Characteristics and Glucosinolates during the Initial Growth Stages of Broccoli. Agronomy 2024, 14, 286. https://doi.org/10.3390/agronomy14020286
Arena D, Ben Ammar H, Rodriguez VM, Velasco P, Garcia G, Calì R, Branca F. Exogenous Melatonin Affects the Morphometric Characteristics and Glucosinolates during the Initial Growth Stages of Broccoli. Agronomy. 2024; 14(2):286. https://doi.org/10.3390/agronomy14020286
Chicago/Turabian StyleArena, Donata, Hajer Ben Ammar, Victor Manuel Rodriguez, Pablo Velasco, Gresheen Garcia, Riccardo Calì, and Ferdinando Branca. 2024. "Exogenous Melatonin Affects the Morphometric Characteristics and Glucosinolates during the Initial Growth Stages of Broccoli" Agronomy 14, no. 2: 286. https://doi.org/10.3390/agronomy14020286
APA StyleArena, D., Ben Ammar, H., Rodriguez, V. M., Velasco, P., Garcia, G., Calì, R., & Branca, F. (2024). Exogenous Melatonin Affects the Morphometric Characteristics and Glucosinolates during the Initial Growth Stages of Broccoli. Agronomy, 14(2), 286. https://doi.org/10.3390/agronomy14020286






