Abstract
The cultivation of Panax ginseng C.A. Meyer, a valuable medicinal plant, presents a number of challenges due to its physiology and life cycle. The composition of the soil and the microbiome living in it are important for plant growth and root quality. Modern analytical methods were used to identify differences in the rhizosphere soils of plants in the forest and in the plots. Microbiological and molecular genetic methods were used to isolate and identify bacterial isolates from these soils, allowing for the establishment of a working collection of potentially useful bacterial strains. Increases in soil pH in the plots and changes in the amount of macronutrients partially explained the changes in the activity of the forest and plot isolates and the composition of the cultivated strains. The cultivated strains belonged to the rhizosphere-dominant phyla Pseudomonadota, Bacillota, and Actinomycetota of the main functional groups of soil potassium, phosphorus, and nitrogen transformations. The ratio of bacteria functional groups was comparable in the forest and in the plots. The most common phylum of cultured microorganisms was Bacillota, while the main differences were observed in the functional group of potassium-solubilizing bacteria belonging to the phyla Pseudomonadota.
1. Introduction
Panax ginseng C.A. Meyer (Araliaceae) is a unique perennial plant that has been known in the Asia–Pacific region for over a thousand years. The wide range of uses of ginseng (medicine, cosmetics, food industry) makes it one of the most cultivated medicinal plants. The global ginseng market, including ginseng root and processed products, is estimated to be worth $2084 million [1]. According to various sources, the Asian ginseng market, of which Korea alone accounts for $1140 million, will grow at an annual rate of 9.80–10.4% from 2024 to 2031, reaching $17.7 billion, according to Cognitive Market Research [2,3]. The value of ginseng roots to humans depends on the amount and diversity of the major secondary metabolites (ginsenosides) they produce. Throughout the growing area, wild roots have attracted attention for centuries for their value and high productivity. The wild roots of the Ussuri taiga are particularly valued, despite the ban on its collection and export [4]. At present, Primorsky Krai in the Russian Far East is the only place in the world where the forest areas of ginseng are represented by three wild-growing populations: the Sikhote-Alin, the Blue Mountains, and the Khasan (Russia) populations [5]. The Khasan population also includes the area of Jilin and Heilongjiang provinces (China). The Far Eastern region is the northernmost border of the species’ range. In these areas, the natural growing conditions for the species are more severe than in other regions. Despite this, the area under ginseng cultivation in the Russian Far East is expanding and farmers are facing problems typical of ginseng cultivation in other regions. In addition, the structure of ginseng roots and their lack of mechanical tissues, which provide elasticity and rigidity to the plant’s organs, make dense cultivation of ginseng roots particularly susceptible to infection. When phytopathogenic microorganisms invade, the lack of mechanical barriers quickly destroys the ginseng roots and the infection spreads rapidly between plants through the soil [6,7,8,9].
Various microorganisms in the soil rhizosphere have been shown to suppress pathogen invasion, promote plant growth and development, and assist in the uptake of nutrients from the soil, thus ensuring the rational use of soil elements [10,11]. Currently, several strategies have been proposed to manage the plant rhizosphere microbiome in order to enhance immunity and increase plant biomass and growth for different agronomic cultivars. This helps to reduce the use of fertilizers and pesticides [6,9,12,13,14,15].
Based on bioinformatic analysis of a large set of metagenomic data, it has been shown that the major phyla belong to the copiotrophic phyla Bacteroidota and Pseudomonadota. Due to the abundance of plant organic matter in the rhizosphere soil, some of the bacteria of these phyla (Bacteroidota and Pseudomonadota) are active in carbon and nitrogen transformations [16]. In the rhizosphere soil of ginseng, the most abundant genera belonging to Verrucomicrobiota, Acidobacteriota, and Pseudomonadota were identified. Gemmatimonadetes, Planctomycetes, Firmicutes, Bacteroidetes, Actinobacteria, and Gemmatimonadales were found in lesser numbers. The uniqueness of ginseng rhizosphere soils and the decrease in microbiome diversity with increasing root age were noted [17]. Pseudomonadota, Bacillota, Actinomycetota, and Bacteroidota were also the dominant phyla of endophytic bacterial microorganisms in cultivated ginseng [18,19,20]. Chowdhury et al. [21] isolated, identified, and cultured thirty-five genera of endophytes in mountain ginseng. Most of them belonged to the genera Pseudomonas, Bacillus, and Paenibacillus, while the species Lysinibacillus, Micrococcus, Microbacterium, Arthrobacter, Serratia, Staphylococcus, Pantoea, Chryseobacterium, Erwinia, Stenotrophomonas and Sphingobium, Psychrobacillus, Burkholderia, Sphingomonas, Methylobacterium, Acinetobacter, and Raoultella occurred in relatively small numbers. Strains producing siderophores, solubilizing phosphates, IAA-like indole derivatives, and HCN derivatives with ß-glucosidase activity have been cultured [21,22]. Some of these microorganisms not only promote the growth and development of ginseng plants but also directly participate in their metabolism and ginsenoside formation [23,24]. Among the bacteria studied in the undergrowth of wild, cultivated, and simulated ginseng, phyla such as Pseudomonadota, Acidobacteriota, Actinomycetota, and Chloroflexota were predominant [25]. The bacterial community of wild ginseng grown in China was dominated by Pseudomonadota, Actinomycetota, and Acidobacteriota [22,26,27].
Cultivation time, plant age, and growing region have been shown to significantly influence the microbiome of ginseng rhizosphere and endosphere communities [11,21,28,29]. All of this diversity is closely related to basic soil physical properties such as soil pH level, complex underline rock composition, and chemical element availability [16,30]. However, for the ginseng-growing area on the east coast of the Pacific Ocean (Primorsky Region of the Russian Far East), no comprehensive studies have been conducted on this issue.
The main objective of this work is to compare the composition of the cultivated bacterial strains in the rhizosphere of Panax ginseng C.A. Meyer depending on soil properties in different habitats, in the forest, and on the plots. To this end, the difference in soil characteristics between forest and experimental plots was identified and rhizosphere isolates from forest and experimental plots were characterized via direct culturing. The established collection of cultivated bacterial strains could be used to create a biofertilizer for successful ginseng cultivation.
2. Materials and Methods
2.1. Soil Sampling and Plant Materials
Soil Sampling
Eleven soil samples were selected for the analysis of macro- and micro-elemental composition. Two samples were collected from the wild population of Blue Mounting from broadleaved forest with understory of Pinus koraiensis Sieb. et Zucc. The territory is located in the southern part of the Sikhote-Alin mountain. The forest soils are classified as Dystric Cambisols according to the World Reference Base for Soil Resources [31]. These are the soils that cover the largest part of Korean pine-broadleaved forests in the Primorsky region.
The key species in the studied forest area are Quercus mongolica Fisch. ex Ledeb, Acer mono Maxim., Tilia amurensis Rupr., Fraxinus mandshurica Rupr, and Populus tremula L. The shrub layer is sparse, dominated by Philadelphus tenuifolius Rupr. et Maxim and Eleutherococcus senticoccus (Rupr. et Maxim.) Maxim. The herbage layer is mosaic. Two types of sinusia in the grass cover are distinguished—sedge and forb with a predominance of Carex campylorhina V. Krecz. The total projective cover of species is 70%, including 18 species. And small-herb synusia, the projective cover of species, does not exceed 5%. It is represented by a small number of species—Carex pallida C.A. Mey, Galium davuricum Turcz. ex Ledeb, Convallaria keiskei Miq., Anemone udensis Trautv., and C. A. Mey.
Previously, ginseng plants from natural habitats were transplanted and distributed to artificial plots in the experimental nursery in the forest (Spassky District, Primorsky Region, 44°44′59.9′′ N, 133°6′45.7′′ E) (Table 1). The population affiliation of the plants was analyzed and confirmed using different types of genetic markers [5]. Ginseng plants and their offspring from the various wild populations according to their origin grow on the separate plots for one to twenty years [5]. Soil samples were collected from five replicate points randomly distributed over the plots. In total, nine soil samples were taken from nine artificial plots with plants from three wild populations (Khasan, Sikhote-Alin, and Blue Mountain) and the plants were brought from Korea and China. Minimal agronomic practices were applied to these plots in the middle of the growing season. The plots had not been treated with chemical fertilizers and pesticides for the last four years since 2020.

Table 1.
Soil sampling scheme used for macro-and micro-analysis and plants ages.
For bacteria isolate, ginseng plants from two wild habitats of the Blue Mounting population and wild plants of the Blue Mounting population transplanted to plots in 2008 were selected. There were no visible signs of infection on any of the plants used for this work. Eight soil samples from roots with the same area were evenly mixed and considered as one replicate. After sampling, the probes were immediately taken to the laboratory to keep them fresh at 4 °C. The samples of rhizosphere soil were seeded on agar nutrient medium (AGM) to obtain pure cultures of microorganisms. Bacterial strains were isolated from all samples. Rhizosphere soil samples from five healthy plants were collected from five replicate plots distributed throughout the root system by digging to a depth of 20 cm.
2.2. Soil Properties and Elemental Analysis
Soil pH was measured electrometrically with the pH meter Mettler Toledo S220-Kit in a 1:2.5 soil/deionized water ratio after shaking the suspensions for 1 h based on Pansu and Gautheyrou [32].
The analysis of total carbon (TC) and nitrogen (TN) contents was performed using a Flash2000 elemental analyzer (Thermo Scientific, Cambridge, UK) with a CHNS configuration. Elemental data were analyzed using the Eager Xperience software 1.2. The samples were analyzed in a quartz reactor at 900 °C with a column temperature of 65 °C. Helium and oxygen flow rates into the reactor were 140 and 100 mL min−1, respectively. The time cycle was 380 s and the oxygen injection time was 15 s. The organic analytical standard for cystine OAS (Thermo FS, Cambridge, UK) and the K factor calibration method were used for quality control. Soil organic matter (SOM) was determined via the wet combustion method of Tyurin, as previously described by Timofeeva et al. [33]. This method is included into the register of methods approved for the determination of organic matter in soils of the Russian Federation GOST 26213-91 [34]. The content of total carbon, total nitrogen, and soil organic matter was determined on dried (105 °C) soil samples.
The total macro and trace element contents in soil samples were determined via energy-dispersive X-ray fluorescence spectroscopy (EDX), according to M-02-0604-2007 [35]. The determination of element content was carried out on an EDX 800HS-P analyzer (Shimadzu, Japan) equipped with a rhodium cathode in the format of quantitative analysis and in a vacuum environment using state standard reference samples: GSO 901-76, GSO 902-76, GSO 903-76, GSO 2498-83, GSO 2499-83, GSO 2500-83, GSO 2507-83, GSO 2509-83 (Institute of Applied Physics of Irkutsk State University, Irkutsk, Russia). Measurement parameters: voltage—50 kV., current—100 mA., determination time—300 s., “dead” time—20%., and collimator size—10 mm. The accuracy of the energy-dispersive X-ray fluorescence spectroscopy was controlled via analysis of the standard soil sample that contained the corresponding element. One standard soil sample was included for every five unknown samples. Acceptable recoveries for the standards were 5–8% of the certified values.
The content of available-to-plants potassium (AK) and phosphorus (AP) forms was determined photometrically (extraction with 0.2 M HCl) according to the GOST R 54650-2011 [36] guidelines. The content of available-to-plants nitrogen (AN) forms was determined using the method of Tyurin and Kononova (extraction with 0.5 N H2SO4) as described by Spirina and Solov’eva [37].
The average content of elements calculated from the values obtained for 3 analytical replicates of the measurement of each experimental soil sample is given in Table 2.

Table 2.
Main parameters and content of macro elements in soil samples (g/kg) (x + s, n = 3).
2.3. For Microbiological Investigation
Soil samples were collected using sterile tools and packed into sterile material according to Barillot et al. [38]. The samples were packed into cooled bags and transported to the laboratory for immediate investigation.
2.3.1. Determination of Target Groups of Microorganisms
The determination of the quantitative composition of soil microorganisms of different functional groups was carried out by sowing dilutions of soil suspension on solid elective media. Three replicates were performed for each experimental group; for each replicate, 10 g of soil was dispensed into flasks with saline (100 mL). The flask was shaken for 20 min at room temperature and at 150 rpm on an Elmi S-3.02L.A20 shaker (Elmi, Riga, Latvia) to separate microbial cells from soil particles. After the completion of the process, the suspension from the flask was plated onto agar media: fishmeal hydrolysate agar (FMHA), starch-ammonia agar (SAA), Ashby agar, Pikovsky agar, and Aleksandrov medium intended for the isolation of nitrogen mineralization (NM), nitrogen immobilization (NIm/AOB), nitrogen fixers (NF), phosphorus-solubilizing (PSB) and potassium-solubilizing bacteria (KSB), respectively. The dishes were then incubated at 24 °C for 3–5 days until colonies of microorganisms appeared. The number of microorganisms was expressed in colony-forming units (CFUs) per 1 g of soil.
FMHA used ready-made media produced in Obolensk, Russia. Pikovsky agar was used as ready-made media produced in HiMedia, Laboratories Pvt. Ltd., Maharashtra, India. SAA, Ashby agar, and Aleksandrov medium were prepared independently according to the preparation instructions. SAA had the following composition in g: starch—10.0, (NH4)2 SO4—2.0, K2HPO4—1.0, MgSO4—1.0, CaCO3—3.0, agar-agar—20.0, and distilled water—1000 mL. Ashby’s agar had the following composition in g: sucrose—20.0, K2HPO4—0.2, MgSO4 ·7H2O—0.2, NaCI—0.2, FeSO4—0.1, CaCO3—5.0, agar-agar—20.0, and distilled water—1000 mL. Aleksandrov medium had the following composition in g: glucose—5, MgSO4·7H2O—0.005158, FeCl3—0.1, CaCO3—2.0, potassium feldspar powder—3.0, calcium phosphate—2.0, and agar-agar—20 [39].
2.3.2. Molecular Genetic Identification of Bacteria and Phylogenetic Analysis
Genomic DNA was isolated from pure bacterial cultures grown on the agar medium for the isolation of corresponding groups of bacteria using a commercial kit “NK-sorbent Base” (Litekh, Moscow, Russia) according to the manufacturer’s protocol. The analysis of the 16S rRNA gene fragment was carried out using a set of reagents “BioMaster HS-Taq PCR-Color (2×)” (Biolabmix, Novosibirsk, Russia) and universal bacterial primers 27F (5′–AGAGTTTGATCATGGCTCAG–3′) and 1350R (5′–GACGGGCGGTGTGTACAAG–3′) [40]. Amplification was carried out on a T100 Thermal Cycler device (BioRad, Hercules, CA, USA) with the following mode: 94 °C—4 min (1 cycle); 94 °C—60 s, 48 °C—60 s, and 72 °C—90 s (5 cycles); 92 °C—60 s, 50 °C—110 s, and 72 °C—90 s (10 cycles); 92 °C—60 s, 52 °C—60 s, and 72 °C—60 s (10 cycles); 92 °C—60 s, 54 °C—60 s, and 72 °C—110 s (10 cycles); and 72 °C—10 min (1 cycle) [41]. The resulting PCR products were separated in an electrophoresis chamber (at a field strength of about 2 V/cm) in a 1% agarose gel with the addition of ethidium bromide. The results were taken into account on a transilluminator under ultraviolet radiation. To purify amplification products from reaction mixture residues, the ExoSAP-IT Express kit (Thermo FS, Waltham, MA, USA) was used.
PCR products were sequenced using the Sanger method with the Big Dye Terminator v.3.1 Cycle Sequencing Kit reagents (Thermo FS, Waltham, MA, USA) to prepare nucleotide sequences for reading on a Nanofor 05 genetic analyzer (Synthol, Moscow, Russia). Phylogenetic analysis was carried out by searching for homologous sequences in the international data bank (GenBank) using the BLAST program (ver. 2.16.0) [42] (http://www.ncbi.nlm.nih.gov/blast (accessed on 30 November 2024)).
2.4. Statistical Analysis
For comparison among data sets the analysis of variance (ANOVA) followed by multiple comparison procedures was employed. The CFUs (log CFUs per 1 g of wet soil) were transformed logarithmically to normalize the distribution for statistical analysis. Statistically significant correlations between variables (p < 0.05) were determined in STATISTICA v. 13.1. (TIBCO Software Inc., Palo Alto, CA, USA) using Spearman’s rank correlation.
3. Results
3.1. Physicochemical Characteristics and Element Composition of P. ginseng Soils
First, two pH values of soil samples (pH H2O and pH KCl) were determined (Table 2). Compared to the other soil samples studied, the pH values of the forest soils were more acidic (No. 1 and 2). The pH indicators of the rhizosphere soils of the same populations from different plots have similar pH H2O values. Most of the samples (No. 3, 5, 7, 9, and 11) have a slightly acidic soil solution reaction. At the same time, samples from Korea with red fruits (No. 10) have values close to forest soils, in contrast to other samples. The acidity of samples No. 4 and 6 is characterized as neutral. According to the value of exchangeable acidity (pH KCl), soil samples No. 1, 2, 5, 9, and 10 are classified as acidic, samples No. 3, 4, 7, and 11 as moderately acidic, and samples No. 6 and 8 as weakly acidic (Table 2).
Our data indicate that the rhizosphere soils of the same populations from the different plots have similar pH values regardless of plant age. At the same time, cluster analysis (UPGMA) based on soil acidity grouped the samples into clusters according to their populations, with the exception of Samples 6 and 8.
All investigated rhizosphere soil properties of the eleven samples are presented in Table 2. Cluster analysis (UPGMA) based on all edaphic parameters divided the samples into two large groups, regardless of their origin or age, while forest soil samples were separated as a distinct cluster (Figure 1). The forest soil samples were always assigned to a separate group by each parameter.
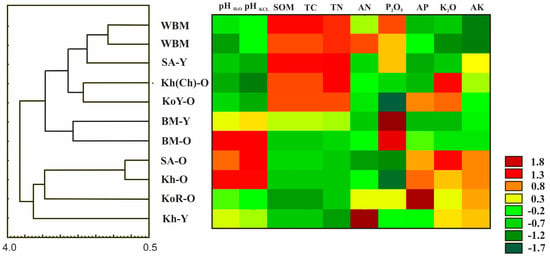
Figure 1.
UPGMA dendrogram and heat map of the eleven soil samples.
At all sites, the content of macro elements in the soil samples corresponds to the level of element content in the upper part of the Dystric Cambisol profiles developed on rhyolite in the natural landscapes of the regions studied (Table 2). The contents of Na2O, MgO, Al2O3, P2O5, K2O, TiO2, MnO, and Fe2O3 in the rhizosphere were lower in the forest samples than in the cultivated plots (Table 2 and Supplementary Table S1). The contents of SiO2 and CaO in the forest soil samples were similar or slightly higher than those in the cultivated plots. However, the AP and AK contents of the forest soils were significantly lower than those of the plot soils (p < 0.05). Furthermore, a difference in AK was found between old soil samples from plots and plots with young plants. Compared to the forest soil samples, ANOVA showed that the AN level was compatible with other samples, with the exception of Kh-Y.
At the same time, the amount of carbon in the forest soil samples remained at a high level and was comparable to the amount of carbon in cultivated plots with older plants. The content of SOM was variable among the studied soil samples, and only two samples from old plots and one wild site had a high amount of SOM (WBM, Ch-O, KoY-O). The maximum value was found in the rhizosphere sample under young plants (SA-Y), with the biggest difference in comparison to the old rhizosphere sample (Table 2). The amount of TN is practically the same between samples taken from the forest and plots and repeated the distribution pattern of SOM and TC. The amount of available nitrogen in the new plot with 4-year-old plants of the Khasan population (Kh-Y) was highest and differed four-fold compared to samples from the plants of northern origin (BM-Y, BM-O, SA-Y, SA-O). Total phosphorus showed an inverse distribution compared to available phosphorus. Samples showing an increase in total phosphorus had the lowest available phosphorus values, especially in the rhizosphere of forest soils. The values of total and available potassium were comparable in each sample, although the minimum values were observed in forest soils. At the same time, the amount of available forms of potassium was almost three times lower in the forest soils than in the plots. Correlation analysis confirmed the relationship between the main soil parameters such as TN, TC, AN, SOM, etc. (Supplementary Table S2).
The soils sampled from the forest plots were found to have a trace element composition typical of soils formed in the natural landscapes of the study region (Supplementary Table S3). The contents of V, Ni, Co, Zr, and Mo in the plot soils were found to be similar to those in the upper horizons of the forest soils. The main difference in trace element composition between the soil samples studied was the increase in Cu content in the plot soils. The results showed that the total Cu content in the plot soils was 2.07–4.48 times higher than in the forest soils. Furthermore, this result suggests that the accumulation of Cu in the plot soils poses an ecological risk to the soil environment. The contents of Zn, Rb, and Pb in most of the samples of plot soils were found to be higher than those in forest soils.
3.2. Bacterial Cultured Strains of P. ginseng Rhizosphere Soil from Forest and Plots
A total of 61 strains of bacteria were isolated and stored. Data based on morphological analysis and sequenced 16S DNA sequences showed that the strains belonged to three dominant phyla: Bacillota, Pseudomonadota, and Actinomycetota. At the same time, the ratio of phyla containing isolated cultured strains in rhizosphere soil from the forest and rhizosphere samples from the plots was compatible, 53.6%, 28.6%, and 17.8% and 60%, 15%, and 25%, respectively.
Differences in the ratio of the three phyla in the microbiomes are mainly due to the two phyla, Pseudomonadota and Actinomycetota. Phylum Bacillota, despite its high abundance in both samples (53.6% and 60%), was represented by only four genera (Peribacillus, Bacillus, Lysinibacillus, and Brevibacillus) with several strains of bacteria close to each other. Some strains were very similar in their nucleotide sequences, such as the species B. cereus, but differed in their functional properties (Figure 2, Supplementary Table S4). The remaining strains were identified as Bacillus mycoides, Bacillus subtilis, Bacillus pumilus, Peribacillus simplex, Lysinibacillus xylanilyticus, L. parviboronicapiens, and Brevibacillus laterosporus.
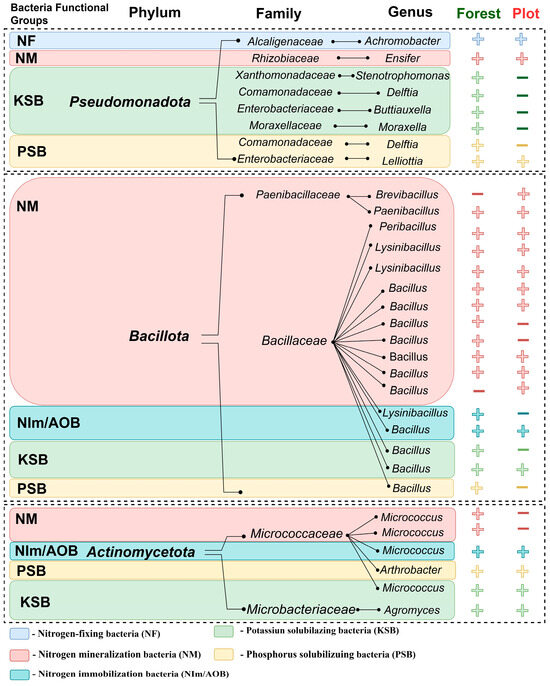
Figure 2.
Functional groups of bacteria from plot and forest rhizosphere soils. “+ or −” indicates strain presence or absence in the rhizosphere soil samples, respectively.
The greatest number of the phylum Pseudomonadota families in the forest significantly exceeded that of other phylum isolated from the plots rhizosphere and was represented by six genera: Alcaligenaceae, Xanthomonadaceae, Comamonadaceae, Moraxellaceae, Enterobacteriaceae, and Rhizobiaceae. The smallest numbers of cultivated strains were found in the phylum Actinomycetota, which was represented by isolates of four genera: Micrococcus, Arthrobacter, and Agromyces. Three isolates of the genus Micrococcus were close to Micrococcus yunnanensis (Supplementary Table S4) and differed in strain characteristics.
The composition of bacterial communities is very closely related to the microbial function that influences soil composition. In our case, cultivated strains of bacteria responsible for nitrogen mineralization (NM) were detected in all bacterial phyla. However, the majority of these cultivated strains belonged to the Bacillaceae family. The main differences were observed in the group of potassium-solubilizing bacteria (KSB). Most of the cultivated strains of this group were isolated from forest soils and belonged to different families of the phylum Pseudomonadota. They were not observed among the strains isolated from the plots (Figure 2). Although the number of strains decreased, the relative number of strains with NM and NF properties increased in the plots. At the same time, the strains of Micrococcus cultivated in the plots lost their nitrogen-mineralizing properties. Minor differences were observed only in the ratio of phosphorus-solubilizing bacteria (PSB) to nitrogen immobilizing (NIm/AOB). Nitrogen-fixing bacteria (NF) were represented by two strains belonging to the genus Achromobacter (Pseudomonadota).
Therefore, the ratio of the main functional groups of microorganisms did not change much (Figure 3A), despite the large difference in the quantitative ratio of microbial activities (Figure 3B). The phosphate of the solubilizing group of bacteria was quantitatively slightly higher in the soils of the plots than in the soils of the forest, but its percentage was lower in the plots than in the forest. The opposite trend was observed for the groups involved in nitrogen turnover; the microbial abundance of the nitrogen-mineralizing (NM), nitrogen-immobilizing (NIm/AOB), and nitrogen-fixing (NF) groups was dramatically lower in the plots. Quantitatively cultured nitrogen-mineralizing microorganisms are 25 times less represented in the plot soils than in the forest soils, while the proportion of this group in the microbiome of plot soils is 10% higher (Figure 3B). Thus, strains isolated from the rhizosphere of forest soils were better adapted to environmental conditions and cultivation.
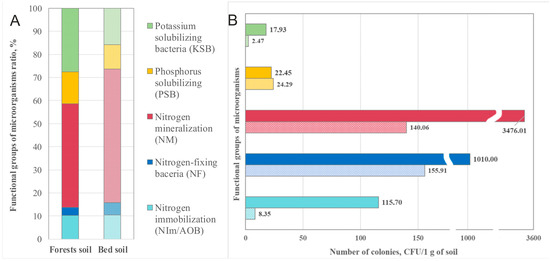
Figure 3.
Functional groups of cultivated forms of bacteria of ginseng rhizosphere soils in forests and plots. (A) The ratio of functional groups of cultivated strains and % (B) average number of cultivated strains by functional groups; CFUs/1 g soil × 105.
4. Discussion
Currently, the study of plant–soil interactions through a small region surrounding roots and inhabited by numerous organisms (rhizosphere) is one of the hottest topics in modern ecology and agricultural engineering [6,8,13]. Microorganisms in the rhizosphere have been shown to promote plant growth and development, protect plants from external pathogens, and convert soil nutrients into a form available to plants [9,11,16,21,43]. In turn, the plant shapes the environment by releasing various chemical compounds through its roots [44,45]. All this leads to a harmonious ecological interaction between plant and soil that is stable under conditions of rapid environmental change. To maintain this balance, there is a shift towards a safer and more environmentally friendly type of fertilizer based on soil microbial strains that can benefit agricultural species and do not pollute the environment [14,25,46,47]. Microbial-based products available for agriculture depend heavily on cultivable strains that can be produced in bioreactors. Culturing allows us to assess the technological properties of a strain, for example, its interaction with different plant species, its growth rate, its survival rate after lyophilization, and its potential uses and viability. The availability of different representatives of the plant microbiota in culture collections is crucial for the successful use of microorganisms and/or microbiome engineering approaches in this field [48,49].
Ginseng is one of the most important species cultivated in the Asia–Pacific region. Its cultivation presents many challenges, including the spread of infection, the duration of cultivation, and the depletion of soil resources. To increase root productivity and reduce the risk of infectious diseases, transplanting ginseng at different ages is widely used. After these manipulations, soil restoration is difficult and leads to soil resource limitation [6,7,50]. Forest planting methods for ginseng, called wild modeling, are also used to reduce infectious diseases and improve root quality [22,25,27]. Therefore, soil parameters are one of the fundamental factors influencing the growth and health of ginseng plants as well as the surrounding microbiome.
Ginseng is thought to require a slightly acidic and nutrient-rich soil. In most studies by Chinese scientists, the pH of the rhizosphere and soils with young and annual plants is closer to neutral and oxidizes with age [25,28,29]. At the same time, the observed diversity of microbiome species in all plant rhizosphere soils studied decreased with plant age [28,29]. When rhizosphere soils of infected plants were examined, pH and physicochemical parameters such as available nitrogen, total and available phosphorus (TP and AP), and available potassium (AK) decreased [8,29], except for an increase in available potassium (AK) as shown in [29]. During ginseng cultivation in Korean fields, soil pH also decreased over time [51]. In our case, the opposite situation was observed with pH level. Although the growing season of the plants in the old plots and in the forest was almost the same, the pH level increased significantly during cultivation in the plots (Table 2). In most of the plots, the soil pH was neutral, although there were no visible signs of infection. Wang with coauthors [6], when studying the development of infection in five different fields in the Changbai Mountains, found that soil mound candles, total iron (Fe) concentration, and total manganese (Mn) concentration were significantly higher in the group of infected plants than in the group of healthy plants. Our results showed that not only in the plots but also in the forest soils in the study area that there was an excess of iron compared to the average level in Primorsky Krai [52]. Our results showed that the total Cu content in the plot soil was 2.07–4.48 times higher than in the forest soil. This result is consistent with the results of earlier systematic treatment of P. ginseng from plots with copper sulfate to eliminate fungal infections. In addition, this result indicates that the accumulation of Cu in plot soil carries an ecological risk to the soil environment. Zn, Rb, and Pb contents were higher in most of the plot soil samples than in forest soils. These results suggest that external sources of these elements have contributed to the soil composition, possibly through chemical fertilizers. Four years is not enough for these chemical compounds to be consumed, or there are not enough bacteria in the soil to help convert the chemicals into forms that plants can utilize.
Previously, Wang’s data [4] showed that SOM accumulation in soils with old ginseng plants was associated with a lower soil pH level. They suggested that this inhibited the transformation of SOM. In our study, SOM was not significantly different between forest and plots and was independent of soil pH (Table 2). It is likely that high SOM levels in old plant plots are related to the establishment of a balance of available carbon forms.
Edaphic environmental factors such as soil pH, carbon, nitrogen, and other elemental contents play a crucial role in the formation of the rhizosphere microbiome and, consequently, in plant adaptation, growth, and development. Rhizosphere soils are reservoirs of endophytic bacteria. These bacteria can move within the plant from the rhizosphere to the aerial parts of the plant [53,54,55]. At the same time, studies on Arabidopsis endophytes have shown that although endophytic bacteria can move between niches in plants starting from the rhizosphere, there is evidence of microbiota specialization in their respective niche [56,57]. Over such a long period of cultivation in the rhizosphere of plants, it is likely that a balance has been established to maintain a specific microbiological composition and stable rhizoassociations have formed despite high pH levels. At the same time, the ratio of the main functional groups of microorganisms involved in soil element turnover did not change significantly (Figure 3A). The reduction in some macronutrients also did not affect the visible manifestations of infection in plants, despite the fact that plants with limited mineral resources are often affected by pathogens.
Interestingly, cluster analysis identified two large groups based on soil parameters. A single cluster was formed by young plants from different Far Eastern populations and wild and cultivated plants from the Blue Mountain population. Surprisingly, this group also included long-growing plants from Korea with yellow seeds (Figure 1). This may be due to the fact that young plants have higher nutrient requirements for growth and development than mature ginseng plants. In addition, in the forest, nutrient uptake is very active due to the close proximity of woody vegetation and litter. The second group combined plants brought from China and Korea with plants from Far Eastern populations that had been growing in neighboring plots for a long time.
Some authors suggest that despite specific plant nutrient requirements, such as disease control mechanisms and edaphic habitat conditions, the rhizosphere environment provides generally similar conditions for the microbiome. Because rhizosphere soils perform similar functions for plants, the bacterial diversity of the rhizosphere is reduced to four major phyla, Bacillota, Pseudomonadota, Actinomycetota, and Bacteroidota, compared to bulk soils [16]. We were only able to grow strains belonging to three of these: Bacillota, Pseudomonadota, and Actinomycetota.
In the works of authors studying the metagenome of the rhizosphere and endosphere microbial communities of ginseng, the phylum composition is consistent with the strains cultivated by us, except for the genera presented in the works of Chinese authors [29]. Most of the bacterial endophytes isolated from P. ginseng cultivated in the mountains of Korea are capable of producing siderophores and belong to the phylum Pseudomonadota [21]. In a study of ginseng cultivated in the mountains, Pseudomonadota was the most common order and Bacillota was the least common order [21]. In the Chaibangshan Mountains, the dominant groups were also Pseudomonadota, Actinomicetota, and Acidobacteriota in samples from different years and did not vary much [29]. The same dominant species were also identified for rhizosphere soils of 7- and 9-year-old wild-simulated ginseng plants [22].
In a more detailed study of the composition of functional groups, the greatest diversity of cultivated bacterial forms was observed in forest soils. Differences were found at the level of genera within each phylum. As mentioned above, despite the large difference in the quantitative ratio of microorganisms, the ratio of different functional groups of microorganisms involved in the main processes of soil formation did not change (Figure 3). The main difference was observed in strains belonging to the phylum Pseudomonadota, at the expense of the K-solubilizing group. At the same time, it was not possible to isolate cultivated strains of genera of this phylum belonging to this group from plot soils. The strains of potassium-solubilizing bacteria (KSB) at the level of Bacillota were few and did not differ in diversity. The abundance of microorganisms of the potassium-solubilizing group also decreased in the plots (Figure 3B). Meanwhile, both total and available K were deficient in the forest soils compared to the plots (Table 2). This may be due to the active consumption of this element by the forest vegetation.
The cultivated bacterial strains capable of nitrogen mineralization belonged mainly to two phyla, Actinomycetota and Bacillota. It was observed that the number of nitrogen-mineralizing bacteria in the forest soil was 25 times higher than in the litter soil (Figure 3B). This may be due to the fact that the bacterial strains isolated from forest soil have a wider range of adaptability and a high rate of nitrogen conversion from unavailable to available form. A decrease in the ratio of these parameters in the plots was practically observed for all functional groups, except for the phosphorus-solubilizing group. Despite the fact that there were more bacterial strains isolated in pure culture capable of solubilizing phosphorus in forest soils, their activity was lower compared to the activity of strains isolated from rhizosphere soils of the plots (Figure 3B). The amount of phosphorus available in the forest was very low compared to the amount in the plots. This may be due to its rapid uptake due to the presence of a large number of roots of associated woody species surrounding ginseng plants. All phosphate-solubilizing bacterial strains were assigned to two phyla, Bacillota and Pseudomonadota. It is known from the literature that bacteria belonging to several genera, such as Pseudomonas, Agrobacterium, and Bacillus, can be used as plant biostimulants to increase P availability and thus improve the efficiency of phosphorus use for agronomic purposes [58,59,60,61].
The level of total nitrogen was practically the same in the forest and in the plots, while the amount of available nitrogen was significantly reduced in the plots compared to the forest. At the same time, we observed that the growth activity of plot strains was significantly reduced in the nitrogen-mineralizing group. The lack of bacterial numbers in this group probably results in an inability to convert total nitrogen into a form available to plants. The changes in the phylum Bacillota, whose strains predominated in this functional group despite their high representation in both samples, concerned the genus Bacillus Many Bacillus species can reduce the incidence of soil-borne diseases by producing chitinases and acting as biocontrol agents [62,63,64].
From the rhizosphere soil of the plots, we could not identify strains of the genus Ensifer (Pseudomonadota) and strains of Micrococcus (Actinomycetota), which perform the function of nitrogen mineralization. Of greatest interest among the cultivated strains are the rare strains isolated from forest soils that belong to the main functional groups. Such strains make it possible to manage and, in many cases, restore the forest and litter microbiome.
5. Conclusions
The investigation of cultured bacterial strains from the rhizosphere of ginseng growing in different habitats, in the forest, and on experimental plots, revealed differences in their composition and activity. The data obtained showed that cultured strains of Pseudomonadota, Bacillota, and Actinomycetota were the most abundant in the rhizosphere of ginseng. At the same time, the main functional groups of potassium and phosphate solubilization and nitrogen cycling, to which the isolated strains belonged, maintained their ratio in different variants, forests and plots, indicating the preservation of the importance of these processes for plant growth and development. At the same time, the abundance of isolates of the main functional groups N and K isolated from the forest was 6–25 times higher than the values for the plot isolates. Understanding the difference in activity of cultivated strains is important for the strategy of effective use of these strains for agronomic purposes. Ginseng-associated cultured isolates from natural habitats can be used as resources for the production of bacterial biofertilizers that could bring ginseng cultivation conditions closer to natural, thus improving conventional and wild cultivation (for the purpose of artificial wild cultivation).
Supplementary Materials
The following supporting information can be downloaded at the following website: https://www.mdpi.com/article/10.3390/agronomy14123019/s1, Table S1: Spearman’s correlation analyses of the soil properties; Table S2: Content of macroelements in soil samples taken from under ginseng plants; Table S3. Content of microelements in soil samples taken from under ginseng plants (ppm = 1⋅10−6); Table S4: Cultivated bacterial strains and NCBI GenBank accession numbers.
Author Contributions
Conceptualization, T.Y.G. and Y.N.Z.; methodology, A.V.K., M.L.S. and Y.O.T.; software, A.V.K. and V.M.K.; validation, A.V.K. and V.M.K.; formal analysis, T.Y.G., A.V.K., and V.M.K.; investigation, D.A.R., A.V.K., Y.V.V. and Y.O.T.; resources, T.Y.G. and Y.N.Z.; writing—original draft preparation, T.Y.G., M.L.S. and Y.O.T.; writing—review and editing, T.Y.G. and Y.N.Z.; visualization, V.M.K. and P.A.P.; supervision, Y.N.Z.; project administration, T.Y.G.; funding acquisition, T.Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out with financial support from the Russian Science Foundation grant no. 23-26-00213.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We used the equipment from the Center for Collective Use “Biotechnology and Genetic Engineering” of the Federal Scientific Center for Biodiversity of Terrestrial Biota of East Asia (Far East Branch, Russian Academy of Sciences).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baeg, I.-H.; So, S.-H. The World Ginseng Market and the Ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-W.; Xu, X.-T.; Cheng, H.; Sang, Z.; Shi, Y.-H. Standardization of Panax ginseng: Current Status of Global Trade, Demands, and Development. Am. J. Chin. Med. 2023, 51, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tang, M.; Wei, H.; Feng, Z.; Yu, N. Global Ginseng Trade Networks: Structural Characteristics and Influencing Factors. Front. Pharmacol. 2023, 14, 1119183. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, Y.; Tomskikh, A.; Gorpenchenko, T. The History and the Future Prospects of Ginseng Study in the Russian Far East. Vestn. FEB RAS 2022, 4, 101–116. [Google Scholar] [CrossRef]
- Zhuravlev, Y.N.; Koren, O.G.; Reunova, G.D.; Muzarok, T.I.; Gorpenchenko, T.Y.; Kats, I.L.; Khrolenko, Y.A. Panax ginseng Natural Populations: Their Past, Current State and Perspectives. Acta Pharmacol. Sin. 2008, 29, 1127–1136. [Google Scholar] [CrossRef]
- Wang, Q.X.; Xu, C.L.; Sun, H.; Ma, L.; Li, L.; Zhang, D.D.; Zhang, Y.Y. Analysis of the Relationship between Rusty Root Incidences and Soil Properties in Panax ginseng. IOP Conf. Ser.Earth Environ. Sci. 2016, 41, 012001. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Xu, C.; Ma, L.; Li, M.; Shao, C.; Guan, Y.; Liu, N.; Liu, Z.; Zhang, S.; et al. Analysis of Rhizosphere Bacterial and Fungal Communities Associated with Rusty Root Disease of Panax ginseng. Appl. Soil. Ecol. 2019, 138, 245–252. [Google Scholar] [CrossRef]
- Bian, X.; Xiao, S.; Zhao, Y.; Xu, Y.; Yang, H.; Zhang, L. Comparative Analysis of Rhizosphere Soil Physiochemical Characteristics and Microbial Communities between Rusty and Healthy Ginseng Root. Sci. Rep. 2020, 10, 15756. [Google Scholar] [CrossRef]
- Benaissa, A. Rhizosphere: Role of Bacteria to Manage Plant Diseases and Sustainable Agriculture—A Review. J. Basic Microbiol. 2024, 64, e2300361. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Brunel, C.; Pouteau, R.; Dawson, W.; Pester, M.; Ramirez, K.S.; van Kleunen, M. Towards Unraveling Macroecological Patterns in Rhizosphere Microbiomes. Trends Plant Sci. 2020, 25, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Berezhnaya, V.V.; Klykov, A.G.; Sidorenko, M.L.; Sleptsova, N.A.; Timofeeva, Y.O. Use of Microbial Strains to Increase Yields of Spring Soft Wheat (Triticum aestivum L.). Russ. Agricult. Sci. 2021, 47, 1–5. [Google Scholar] [CrossRef]
- Sidorenko, M.L.; Sleptsova, N.A.; Bykovskaya, A.N.; Berezhnaya, V.V.; Klykov, A.G. Effects of nitrogen-fixing and phos-phate-solubilizing microorganisms from the far east agricultural soils on the cereal seed germination. Sel’skokhozyaistvennaya Biol. [Agric. Biol.] 2021, 56, 146–157. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Dou, X.; Liao, D.; Li, K.; An, C.; Li, G.; Dong, Z. Microbial Fertilizers Improve Soil Quality and Crop Yield in Coastal Saline Soils by Regulating Soil Bacterial and Fungal Community Structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, Y.; Xu, M.; Ma, L.; Adams, J.; Shi, Y. Insights into plant–microbe interactions in the rhizosphere to pro-motesustainable agriculture in the new crops era. New Crops 2024, 1, 100004. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere Bacteriome Structure and Functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Li, Y.; Ying, Y.; Ding, W. Dynamics of Panax ginseng Rhizospheric Soil Microbial Community and Their Metabolic Function. Evid.-Based Complement. Altern. Med. 2014, 2014, 160373. [Google Scholar] [CrossRef]
- Hong, C.E.; Jo, S.; Jo, I.-H.; Park, J.M. Diversity and Antifungal Activity of Endophytic Bacteria Associated with Panax ginseng Seedlings. Plant Biotechnol. Rep. 2018, 12, 409–418. [Google Scholar] [CrossRef]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Bang, K.H.; Jo, I.H. Metagenomic Analysis of Bacterial Endophyte Community Structure and Functions in Panax ginseng at Different Ages. 3 Biotech. 2019, 9, 300. [Google Scholar] [CrossRef]
- Goodwin, P.H. The Endosphere Microbiome of Ginseng. Plants 2022, 11, 415. [Google Scholar] [CrossRef]
- Chowdhury, M.E.K.; Jeon, J.; Rim, S.O.; Park, Y.-H.; Lee, S.K.; Bae, H. Composition, Diversity and Bioactivity of Culturable Bacterial Endophytes in Mountain-Cultivated Ginseng in Korea. Sci. Rep. 2017, 7, 10098. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Y.; Gao, Q.; Li, X.; Zeng, Y.; Guo, Y.; Zhang, H.; Qin, Z. Metagenomic Exploration of the Rhizosphere Soil Microbial Community and Their Significance in Facilitating the Development of Wild-Simulated Ginseng. Appl. Environ. Microbiol. 2024, 90, e02335-23. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiong, K.; Wen, W.; Li, L.; Xu, D. Functional Endophytes Regulating Plant Secondary Metabolism: Current Status, Prospects and Applications. Int. J. Mol. Sci. 2023, 24, 1153. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Gao, Y.; Zang, P.; Zheng, T. Effects of a Co-Bacterial Agent on the Growth, Disease Control, and Quality of Ginseng Based on Rhizosphere Microbial Diversity. BMC Plant Biol. 2024, 24, 647. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Zhao, L.; Wang, M.; Sun, M. Diversity and Structure of the Rhizosphere Microbial Communities of Wild and Cultivated Ginseng. BMC Microbiol. 2022, 22, 2. [Google Scholar] [CrossRef]
- He, C.; Wang, R.; Ding, W.; Li, Y. Effects of Cultivation Soils and Ages on Microbiome in the Rhizosphere Soil of Panax ginseng. Appl. Soil Ecol. 2022, 174, 104397. [Google Scholar] [CrossRef]
- Yun, Y.-B.; Kim, K.; Huh, J.-H.; Um, Y. Relationships between Rhizosphere Environments and Growth of 10-Year-Old Wild-Simulated Ginseng. Agronomy 2023, 13, 1313. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, L.; Zhang, L.; Liu, C.; Han, M. Effects of Cultivation Ages and Modes on Microbial Diversity in the Rhizosphere Soil of Panax ginseng. J. Ginseng Res. 2016, 40, 28–37. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Li, M.; Xu, C.; Zhang, Y. Different Age-Induced Changes in Rhizosphere Microbial Composition and Function of Panax ginseng in Transplantation Mode. Front. Plant Sci. 2020, 11, 563240. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports; FAO: Rome, Italy, 2014; Volume 106. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis Mineralogical, Organic and Inorganic Methods; Springer: Berlin, Germany, 2006. [Google Scholar]
- Timofeeva, Y.; Purtova, L.; Emelyanov, A.; Burdukovskii, M.; Kiseleva, I.; Sidorenko, M. Contents, distribution, and fractionation of soil organic carbon and trace elements in soils under a green manure application. Soil Water Res. 2021, 16, 50–58. [Google Scholar] [CrossRef]
- GOST 26213-91; Soils. Methods for Determination of Organic Matter. Standartinform: Moscow, Russia, 1992. (In Russian)
- Shimadzu Europa GmbH. Available online: https://www.shimadzu.eu/products/elemental-analysis/edx-fs/edx-700080008100/applications.html#03 (accessed on 7 November 2024).
- GOST R 54650 2011 Soils. Determination of Mobile Phosphorus and Potassium Compounds by Kirsanov Method Modified by CINAO; Standartinform: Moscow, Russia, 2011. (In Russian) [Google Scholar]
- Spirina, V.; Solov’eva, T. Agrochemical Methods for Studying Soils, Plants and Fertilizers: Textbook; Tomsk State University: Tomsk, Russia, 2014. [Google Scholar]
- Barillot, C.D.C.; Sarde, C.-O.; Bert, V.; Tarnaud, E.; Cochet, N. A Standardized Method for the Sampling of Rhizosphere and Rhizoplan Soil Bacteria Associated to a Herbaceous Root System. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium Solubilizing Rhizobacteria (KSR): Isolation, Identification, and K-Release Dynamics from Waste Mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid Determination of 16S Ribosomal RNA Sequences for Phylogenetic Analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef]
- Denisova, L.I.; Bel’kova, N.L.; Tulokhonov, I.I.; Zaĭchikov, E.F. Diversity of bacteria at various depths in the southern part of lake Baikal as detected by 16S rRNA sequencing. Mikrobiologiia 1999, 68, 547–556. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Liang, W.; Liu, S. Rhizosphere Microbiome: The Emerging Barrier in Plant-Pathogen Interactions. Front. Microbiol. 2021, 12, 772420. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. The Rhizosphere Microbial Complex in Plant Health: A Review of Interaction Dynamics. J. Integr. Agric. 2022, 21, 2168–2182. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Newberger, D.; Vivanco, J.M. The Rhizosphere Microbiome: Plant-Microbial Interactions for Resource Acquisition. J. Appl. Microbiol. 2022, 133, 2864–2876. [Google Scholar] [CrossRef]
- Zhang, R.; Vivanco, J.M.; Shen, Q. The Unseen Rhizosphere Root–Soil–Microbe Interactions for Crop Production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef]
- Solanki, M.K.; Joshi, N.C.; Singh, P.K.; Singh, S.K.; Santoyo, G.; Basilio de Azevedo, L.C.; Kumar, A. From Concept to Reality: Transforming Agriculture through Innovative Rhizosphere Engineering for Plant Health and Productivity. Microbiol. Res. 2024, 279, 127553. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, F.; Mengoni, A.; Riva, V.; Borin, S. Bacterial Culturing Is Crucial to Boost Sustainable Agriculture. Trends Microbiol. 2023, 31, 1–4. [Google Scholar] [CrossRef]
- Youseif, S.H.; Abd El-Megeed, F.H.; Humm, E.A.; Maymon, M.; Mohamed, A.H.; Saleh, S.A.; Hirsch, A.M. Comparative Analysis of the Cultured and Total Bacterial Community in the Wheat Rhizosphere Microbiome Using Culture-Dependent and Culture-Independent Approaches. Microbiol. Spectr. 2021, 9, e00678-21. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, J.; Li, Y.; Fang, H.; Niu, W.; Li, X.; Zhang, Y.; Ding, W.; Chen, S. Manipulation of Microbial Community in the Rhizosphere Alleviates the Replanting Issues in Panax ginseng. Soil. Biol. Biochem. 2018, 125, 64–74. [Google Scholar] [CrossRef]
- Nguyen, N.-L.; Kim, Y.-J.; Hoang, V.-A.; Subramaniyam, S.; Kang, J.-P.; Kang, C.H.; Yang, D.-C. Bacterial Diversity and Community Structure in Korean Ginseng Field Soil Are Shifted by Cultivation Time. PLoS ONE 2016, 11, e0155055. [Google Scholar] [CrossRef]
- Kostenkov, N.M. Oxidation-Reduction Regimes in Periodically Moistened Soils; Nauka: Moscow, Russia, 1987. (In Russian) [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of Bacterial Endophytes and Their Proposed Role in Plant Growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner Plant Values: Diversity, Colonization and Benefits from Endophytic Bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.-G.; Wang, J.-T.; Singh, B.; Han, L.-L.; Shen, J.-P.; Li, P.-P.; Wang, G.-B.; Wu, C.-F.; Ge, A.-H.; et al. Host Selection Shapes Crop Microbiome Assembly and Network Complexity. New Phytol. 2021, 229, 1091–1104. [Google Scholar] [CrossRef]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional Overlap of the Arabidopsis Leaf and Root Microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef]
- Chu, L.L.; Bae, H. Bacterial Endophytes from Ginseng and Their Biotechnological Application. J. Ginseng Res. 2022, 46, 1–10. [Google Scholar] [CrossRef]
- Comeau, D.; Balthazar, C.; Novinscak, A.; Bouhamdani, N.; Joly, D.L.; Filion, M. Interactions Between Bacillus spp., Pseudomonas spp. and Cannabis sativa Promote Plant Growth. Front. Microbiol. 2021, 12, 715758. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Potential Use of Bacillus Spp. as an Effective Biostimulant against Abiotic Stresses in Crops—A Review. Curr. Res. Biotechnol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Wang, T.; Li, Z.; Liang, H.; Jiang, C.; Tang, H.; Gao, J.; Jiang, Y.; Chen, C. The Novel Pseudomonas Thivervalensis Strain JI6 Promotes Growth and Controls Rusty Root Rot Disease in Panax ginseng. Biol. Control 2024, 193, 105514. [Google Scholar] [CrossRef]
- Chowdhury, M.D.E.K.; Bae, H. Bacterial Endophytes Isolated from Mountain-Cultivated Ginseng (Panax Ginseng Mayer) Have Biocontrol Potential against Ginseng pathogens. Biol. Control 2018, 126, 97–108. [Google Scholar] [CrossRef]
- Anckaert, A.; Arguelles Arias, A.; Hoff, G.; Calonne-Salmon, M.; Declerck, S.; Ongena, M. The Use of Bacillus spp. as Bacterial Biocontrol Agents to Control Plant Diseases; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Chu, L.L.; Huy, N.Q.; Tung, N.H. Microorganisms for Ginsenosides Biosynthesis: Recent Progress, Challenges, and Perspectives. Molecules 2023, 28, 1437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).