Rapid Conversion of the Popular Normal-Oleic Peanut Cultivar 308 to High-Oleic Variants via Floral Organ Injection of Pingyangmycin
Abstract
1. Introduction
2. Materials and Methods
2.1. Peanut Cultivar for Chemical Mutagen Injection and Its Cultivation
2.2. Floral Injection of Chemical Mutagen Solution and Harvesting of M1 Seeds
2.3. Planting of M1 Plants
2.4. Quality Analysis
2.5. FAD2A and FAD2B Genotyping
2.6. Productivity Evaluation of Mutant Lines
2.7. Statistical Analysis
3. Results
3.1. Identification of High-Oleic M1 Plants Using NIR Screening
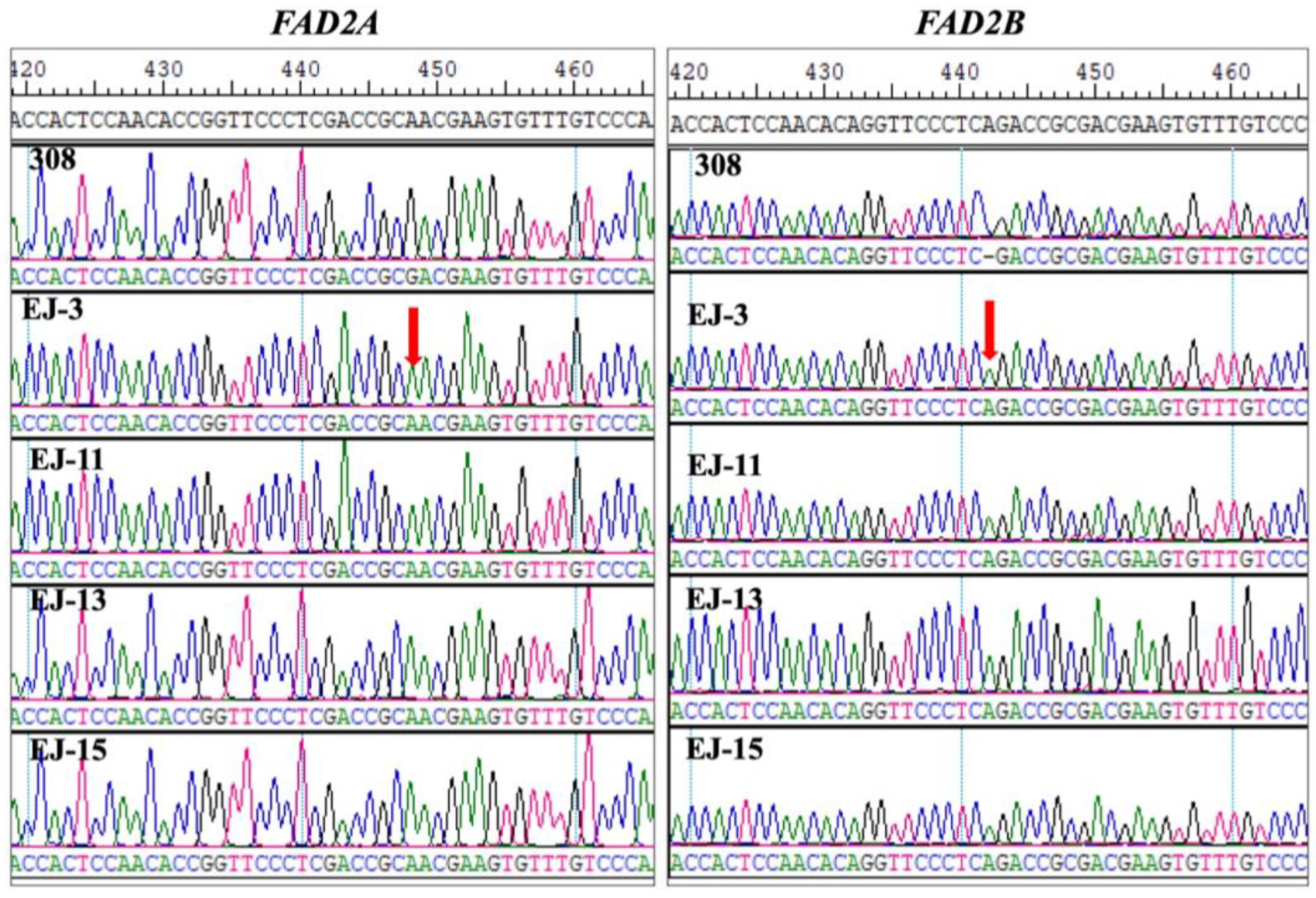
3.2. FAD2A/FAD2B Genotyping of High-Oleic Mutants
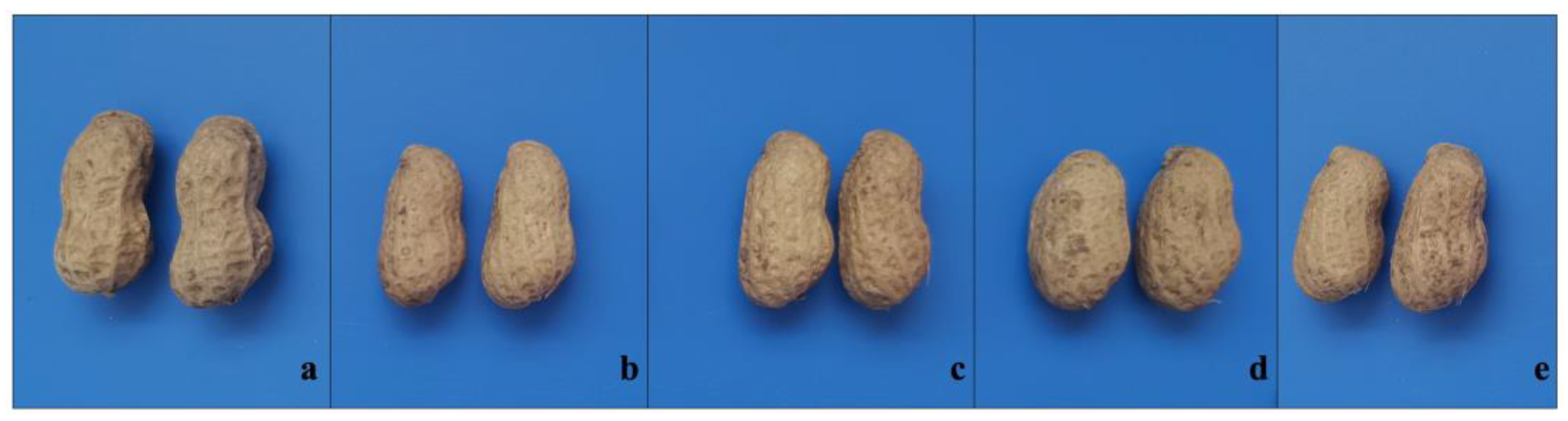
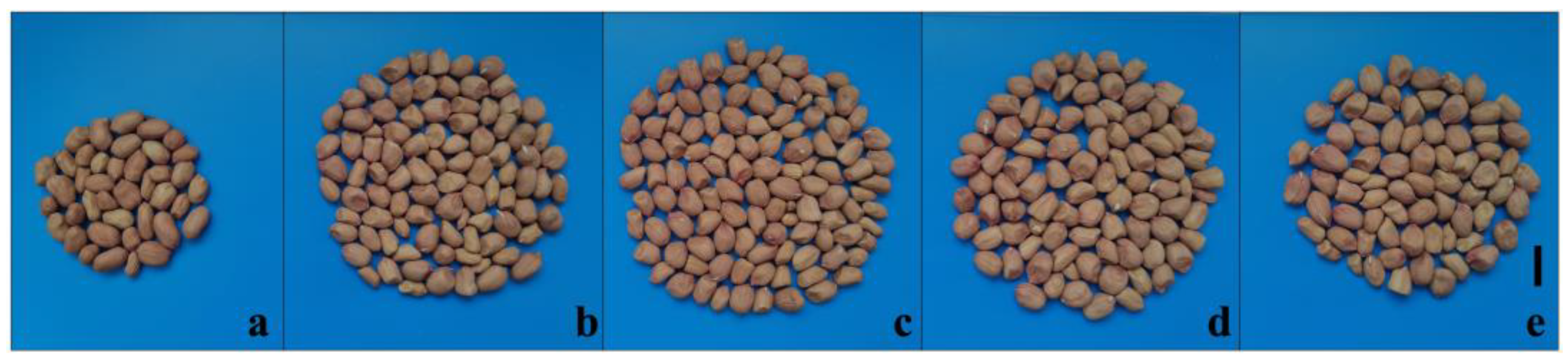
3.3. Pod Morphology and Preliminary Productivity Evaluation of M1 Plants
3.4. Quality Evaluation, Field Performance and Selection of M2 Plants
3.5. Quality and Yield Evaluation of the High-Oleic Mutant Lines
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reddy, P. Groundnut; Publications and Information Division, Indian Council of Agricultural Research, Krishi Anusandhan Bhavan, Pusa: New Delhi, India, 1988. [Google Scholar]
- Smartt, J. The Groundnut Crop: A Scientific Basis for Improvement; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Lusas, E.W.; Erickson, D.R.; Nip, W.-K. Food Uses of Whole Oil and Protein Seeds; Amer Oil Chemists Society: Champaign, IL, USA, 1989. [Google Scholar]
- Łozowicka, B.; Kaczyński, P.; Iwaniuk, P.; Rutkowska, E.; Socha, K.; Orywal, K.; Farhan, J.A.; Perkowski, M. Nutritional compounds and risk assessment of mycotoxins in ecological and conventional nuts. Food Chem. 2024, 458, 140222. [Google Scholar] [CrossRef] [PubMed]
- Nkuna, R.T.; Wang, C.T.; Wang, X.Z.; Tang, Y.Y.; Wang, Z.W.; Zhang, J.C. Sodium azide induced high-oleic peanut (Arachis hypogaea L.) mutant of Virginia type. Genet. Resour. Crop Evol. 2021, 68, 1759–1767. [Google Scholar] [CrossRef]
- Han, H.W.; Wang, Z.W.; Wang, X.Z.; Sun, X.S.; Fang, C.Q.; Wang, C.T. Identification of high-oleic peanut chemical mutants and functional analysis of mutated FAD2B gene. Plant Genet. Resour. 2022, 20, 15–21. [Google Scholar] [CrossRef]
- Wang, C.T.; Yu, S.L.; Zhu, L.G. High Oleic Acid Peanuts in China; Shanghai Science and Technology Press: Shanghai, China, 2021. [Google Scholar]
- Wang, C.T.; Wang, X.Z.; Tang, Y.Y.; Chen, D.X.; Zhang, J.C.; Cui, F.G.; Yu, S.L. High yielding mutants achieved by injecting EMS into peanut flower organs. J. Nucl. Agric. Sci. 2010, 24, 239–242. [Google Scholar]
- Wang, C.T.; Wang, X.Z.; Tang, Y.Y.; Zhang, J.C.; Chen, D.X.; Xu, J.Z.; Yang, X.D.; Song, G.S.; Cui, F.G. Huayu 40, a groundnut cultivar developed through EMS mutagenesis. J. SAT Agric. Res. 2011, 9, 1–2. [Google Scholar]
- Wang, C.T.; Tang, Y.Y.; Wang, X.Z.; Wu, Q.; Sun, Q.X.; Gong, Q.X.; Yang, Z.; Song, G.S.; Wang, Z.W. Breeding of Huayu 9610, a high-yielding export-type conventional large-podded peanut cultivar, through chemical mutagenesis combined with intersectional hybridization. Seeds 2016, 35, 100–101. [Google Scholar]
- International Rice Research Institute. Genetic Manipulation in Crops: Proceedings of the International Symposium on Genetic Manipulation in Crops: The 3rd International Symposium on Haploidy, the 1st International Symposium on Somatic Cell Genetics in Crops, Beijing, October 1984; Natural Resources and the Environment Series; Cassell Tycooly: London, UK, 1988. [Google Scholar]
- Zhang, R.; Li, W.; Pan, S.; Dai, L.; Liu, S. Application of chemical mutagenesis in improving germplasm resource. Mol. Plant Breed. 2017, 15, 5189–5196. [Google Scholar]
- Sui, J.; Wang, Y.; Wang, P.; Qiao, L.; Sun, S.; Hu, X.; Chen, J.; Wang, J. Generation of peanut drought tolerant plants by pingyangmycin-mediated in vitro mutagenesis and hydroxyproline-resistance screening. PLoS ONE 2015, 10, e0119240. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, L.; Liu, Y.; Zhao, M.; Wang, X.; Qiao, L.; Sui, J.; Li, G.; Zhu, H.; Yu, S. Development of peanut varieties with high oil content by in vitro mutagenesis and screening. J. Integr. Agric. 2020, 19, 2974–2982. [Google Scholar] [CrossRef]
- Wan, S.B. Peanut Cultivation Science in China; Shanghai Sci & Tech Press: Shanghai, China, 2003. [Google Scholar]
- Wang, C.T.; Zhang, J.C. Peanut Genetic Improvement; Shanghai Sci & Tech Press: Shanghai, China, 2013. [Google Scholar]
- Tang, Y.Y.; Wang, X.Z.; Liu, T.; Wu, Q.; Sun, Q.X.; Wang, Z.W.; Zhang, X.; Wang, C.T.; Shao, J.F. A near infrared spectroscopy model for predicting sucrose content of sun-dried peanut seeds. Shandong Agric. Sci. 2018, 50, 159–162. [Google Scholar]
- Liu, T.; Wang, C.T.; Tang, Y.Y.; Hu, D.Q.; Wang, X.Z.; Wu, Q.; Sun, Q.X.; Wang, Z.W.; Song, G.S.; Shi, C.R.; et al. A near infrared spectroscopy model for predicting vitamin E content in sun-dried peanut seeds. Shandong Agric. Sci. 2018, 50, 163–166. [Google Scholar]
- Yang, C.D.; Guan, S.Y.; Tang, Y.Y.; Wang, X.Z.; Wu, Q.; Gong, Q.X.; Wang, C.T. Rapid non-destructive determination of fatty acids in single groundnut seeds by gas chromatography. J. Peanut Sci. 2012, 41, 21–26. [Google Scholar]
- Patel, M.; Jung, S.; Moore, K.; Powell, G.; Ainsworth, C.; Abbott, A. High-oleate peanut mutants result from a MITE insertion into the FAD2 gene. Theor. Appl. Genet. 2004, 108, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.T.; Wang, C.T.; Yu, S.L.; Wang, X.Z.; Tang, Y.Y.; Chen, D.X.; Zhang, J.C. Simple method to prepare DNA templates from a slice of peanut cotyledonary tissue for Polymerase Chain Reaction. Electron. J. Biotechnol. 2010, 13, 9. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Zhang, C.-X. Data Processing System (DPS) Software with Experimental Design, Statistical Analysis and Data Mining Developed for Use in Entomological Research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Han, H.W.; Yu, S.T.; Wang, Z.W.; Yang, Z.; Jiang, C.J.; Wang, X.Z.; Sun, X.S.; Wang, C.T. In planta genetic transformation to produce CRISPRed high-oleic peanut. Plant Growth Regul. 2023, 101, 443–451. [Google Scholar] [CrossRef]
- Badigannavar, A.M.; Mondal, S. Advances in Mutation Breeding of Groundnut (Arachis hypogaea L.). In Mutation Breeding for Sustainable Food Production and Climate Resilience; Penna, S., Jain, S.M., Eds.; Springer Nature: Singapore, 2023; pp. 487–519. ISBN 9789811697203. [Google Scholar]
- Chen, T.; Huang, L.; Wang, M.; Huang, Y.; Zeng, R.; Wang, X.; Wang, L.; Wan, S.; Zhang, L. Ethyl methyl sulfonate-induced mutagenesis and its effects on peanut agronomic, yield and quality traits. Agronomy 2020, 10, 655. [Google Scholar] [CrossRef]


| Treatment No. | Pingyangmycin Concentration (mg/L) | Time of Injection | Injection Site |
|---|---|---|---|
| EJ-1 | 0.75 | The day of anthesis | Inside corolla (keel) |
| EJ-2 | 1.60 | The day of anthesis | Inside corolla (keel) |
| EJ-3 | 2.45 | The day of anthesis | Inside corolla (keel) |
| EJ-4 | 3.30 | The day of anthesis | Inside corolla (keel) |
| EJ-5 | 4.15 | The day of anthesis | Inside corolla (keel) |
| EJ-6 | 5.00 | The day of anthesis | Inside corolla (keel) |
| EJ-7 | 0.75 | The day of anthesis | Inside calyx tube |
| EJ-8 | 1.60 | The day of anthesis | Inside calyx tube |
| EJ-9 | 2.45 | The day of anthesis | Inside calyx tube |
| EJ-10 | 3.30 | The day of anthesis | Inside calyx tube |
| EJ-11 | 4.15 | The day of anthesis | Inside calyx tube |
| EJ-12 | 5.00 | The day of anthesis | Inside calyx tube |
| EJ-13 | 0.75 | A day before anthesis | Inside calyx tube |
| EJ-14 | 1.60 | A day before anthesis | Inside calyx tube |
| EJ-15 | 2.45 | A day before anthesis | Inside calyx tube |
| EJ-16 | 3.30 | A day before anthesis | Inside calyx tube |
| EJ-17 | 4.15 | A day before anthesis | Inside calyx tube |
| EJ-18 | 5.00 | A day before anthesis | Inside calyx tube |
| EJ-19 | 0.75 | A day before anthesis | Inside corolla |
| EJ-20 | 1.60 | A day before anthesis | Inside corolla |
| EJ-21 | 2.45 | A day before anthesis | Inside corolla |
| EJ-22 | 3.30 | A day before anthesis | Inside corolla |
| EJ-23 | 4.15 | A day before anthesis | Inside corolla |
| EJ-24 | 5.00 | A day before anthesis | Inside corolla |
| Primer | Sequence (5′to 3′) | Use |
|---|---|---|
| aF19 | gattactgattattgactt | Amplification of FAD2A |
| bF19 | cagaaccattagctttg | Amplification of FAD2B |
| R1 | ctctgactatgcatcag | Amplification of FAD2A/FAD2B |
| Treatment No./Cultivar | Plant Serial No. | Oleic (O) (%) | Linoleic (L) (%) | O/L | Number of Seeds per Plant | Seed Mass per Plant (g) |
|---|---|---|---|---|---|---|
| 308 (CK) | 20ECK-10 D4.2 | 46.64 | 31.57 | 1.48 | 42 | 27.87 |
| 308 (CK) | 20ECK-10 D4.3 | 46.34 | 32.24 | 1.44 | 40 | 22.59 |
| EJ-3 | 20EJD-7A D11.0 | 76.58 | 6.28 | 12.19 | 41 | 22.67 |
| EJ-3 | 20EJD-7A D11.1 | 78.35 | 5.20 | 15.07 | 51 | 33.90 |
| EJ-3 | 20EJD-7A D11.2 | 77.13 | 6.89 | 11.19 | 96 | 64.89 |
| EJ-3 | 20EJD-7A D11.3 | 79.03 | 4.15 | 19.04 | 47 | 29.84 |
| EJ-3 | 20EJD-7A D11.5 | 78.66 | 4.73 | 16.63 | 66 | 41.91 |
| EJ-3 | 20EJD-7A D11.8 | 78.00 | 4.85 | 16.08 | 65 | 45.16 |
| EJ-11 | 20EJD-8 D19.0 | 82.75 | 3.50 | 23.64 | 44 | 30.78 |
| EJ-11 | 20EJD-8 D19.1 | 80.68 | 4.35 | 18.55 | 113 | 77.23 |
| EJ-11 | 20EJD-8 D19.12 | 76.48 | 8.07 | 9.48 | 60 | 42.19 |
| EJ-11 | 20EJD-8 D19.13 | 80.05 | 4.48 | 17.87 | 74 | 44.82 |
| EJ-11 | 20EJD-8 D19.14 | 81.70 | 3.98 | 20.53 | 44 | 30.40 |
| EJ-11 | 20EJD-8 D19.15 | 83.06 | 3.67 | 22.63 | 78 | 46.14 |
| EJ-11 | 20EJD-8 D19.16 | 78.61 | 5.48 | 14.34 | 92 | 65.59 |
| EJ-11 | 20EJD-8 D19.2 | 79.45 | 5.93 | 13.40 | 87 | 56.97 |
| EJ-11 | 20EJD-8 D19.3 | 78.63 | 5.33 | 14.75 | 47 | 29.35 |
| EJ-11 | 20EJD-8 D19.4 | 78.31 | 6.42 | 12.20 | 99 | 60.49 |
| EJ-11 | 20EJD-8 D19.5 | 80.43 | 4.57 | 17.60 | 100 | 66.10 |
| EJ-11 | 20EJD-8 D19.6 | 81.75 | 3.30 | 24.77 | 76 | 53.24 |
| EJ-11 | 20EJD-8 D19.7 | 79.80 | 5.09 | 15.68 | 49 | 30.57 |
| EJ-11 | 20EJD-8 D19.9 | 78.41 | 6.50 | 12.06 | 59 | 38.08 |
| EJ-13 | 20EJD-9A D4.0 | 81.10 | 4.47 | 18.14 | 89 | 62.56 |
| EJ-13 | 20EJD-9A D4.1 | 80.87 | 4.56 | 17.73 | 63 | 39.49 |
| EJ-13 | 20EJD-9A D4.2 | 78.94 | 6.08 | 12.98 | 40 | 27.93 |
| EJ-13 | 20EJD-9A D4.3 | 81.27 | 3.99 | 20.37 | 39 | 24.97 |
| EJ-15 | 20EJD-1B D5.0 | 79.31 | 4.94 | 16.05 | 74 | 50.31 |
| EJ-15 | 20EJD-1B D5.1 | 78.90 | 4.86 | 16.23 | 64 | 47.11 |
| Fatty Acid Parameter | 308 (ck) | Four M2 Single Plants Representing Four Treatments | |||
|---|---|---|---|---|---|
| EJ-3 | EJ-11 | EJ-13 | EJ-15 | ||
| Palmitic acid (%) | 19.05 | 9.16 | 9.16 | 8.36 | 8.95 |
| Stearic acid (%) | 6.13 | 4.52 | 3.24 | 5.41 | 3.55 |
| Oleic acid (O/) | 39.85 | 76.21 | 78.66 | 79.89 | 80.58 |
| Linoleic acid (L) | 28.10 | 2.37 | 3.10 | 1.38 | 2.26 |
| O/L | 1.42 | 32.16 | 25.37 | 57.89 | 35.65 |
| Line/Cultivar | Treatment No. | Origin (Plant Serial No.) | Oleic Acid (%) | Linoleic Acid (%) | Oil (%) | Protein (%) | Vitamin E (mg/100 g) | Sucrose (%) |
|---|---|---|---|---|---|---|---|---|
| HO308-1 | EJ-3 | 20EJD-7A D11.2 | 80.93A | 4.70B | 50.71C | 24.62 | 12.67ab | 6.44A |
| HO308-2 | EJ-11 | 20EJD-8 D19.1 | 82.03A | 3.61B | 51.93B | 25.01 | 13.05ab | 5.92AB |
| HO308-3 | EJ-3 | 20EJD-7A D11.0 | 75.52A | 7.01B | 51.67B | 24.93 | 11.96b | 5.99AB |
| HO308-4 | EJ-3 | 20EJD-7A D11.5 | 77.06A | 2.40B | 52.61B | 24.93 | 12.76b | 5.32B |
| 308 (CK) | - | - | 47.19B | 32.48A | 54.55A | 24.34 | 14.02a | 4.37C |
| Line | 2023 Crop | 2024 Crop | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100-Pod Weight (g) | 100-Seed Mass (g) | Shelling Outturn (%) | Pod Yield (kg/ha) | Seed Yield (kg/ha) | 100-Pod Weight (g) | 100-Seed Mass (g) | Shelling Outturn (%) | Pod Yield (kg/ha) | Seed Yield (kg/ha) | |
| HO308-1 | 160.00 | 73.33 | 77.11 A | 4953.00 ab | 3819.30 AB | 138.33 b | 61.67 B | 73.84 | 2882.35 AB | 2128.20 AB |
| HO308-2 | 178.33 | 81.67 | 74.34 A | 4851.00 ab | 3606.15 AB | 145.00 b | 66.67 B | 71.73 | 2629.05 AB | 1885.80 AB |
| HO308-3 | 167.50 | 82.50 | 77.50 A | 5110.35 ab | 3960.75 AB | 151.67 ab | 70.00 AB | 74.33 | 3289.21 A | 2446.20 A |
| HO308-4 | 177.50 | 75.00 | 76.98 A | 5702.25 a | 4389.60 A | 146.67 b | 63.33 B | 71.42 | 2856.15 AB | 2040.00 AB |
| 308(CK) | 175.00 | 85.00 | 65.75 B | 3254.90 b | 2140.20 B | 177.50 a | 80.00 A | 69.67 | 2257.35 B | 1572.75 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Wang, Z.; Sun, H.; Yu, S.; Han, H.; Yang, Z.; Zhang, M.; Yuan, G.; Yu, J.; Wang, C. Rapid Conversion of the Popular Normal-Oleic Peanut Cultivar 308 to High-Oleic Variants via Floral Organ Injection of Pingyangmycin. Agronomy 2024, 14, 2928. https://doi.org/10.3390/agronomy14122928
Jiang C, Wang Z, Sun H, Yu S, Han H, Yang Z, Zhang M, Yuan G, Yu J, Wang C. Rapid Conversion of the Popular Normal-Oleic Peanut Cultivar 308 to High-Oleic Variants via Floral Organ Injection of Pingyangmycin. Agronomy. 2024; 14(12):2928. https://doi.org/10.3390/agronomy14122928
Chicago/Turabian StyleJiang, Chunjiao, Zhiwei Wang, Haojie Sun, Shutao Yu, Hongwei Han, Zhen Yang, Mingjun Zhang, Guangdi Yuan, Jing Yu, and Chuantang Wang. 2024. "Rapid Conversion of the Popular Normal-Oleic Peanut Cultivar 308 to High-Oleic Variants via Floral Organ Injection of Pingyangmycin" Agronomy 14, no. 12: 2928. https://doi.org/10.3390/agronomy14122928
APA StyleJiang, C., Wang, Z., Sun, H., Yu, S., Han, H., Yang, Z., Zhang, M., Yuan, G., Yu, J., & Wang, C. (2024). Rapid Conversion of the Popular Normal-Oleic Peanut Cultivar 308 to High-Oleic Variants via Floral Organ Injection of Pingyangmycin. Agronomy, 14(12), 2928. https://doi.org/10.3390/agronomy14122928






