Abstract
The cultivated peanut (Arachis hypogaea L.) is a main cash crop globally, providing oil, protein, and various beneficial phytochemicals, with high-oleic peanut offering enhanced health benefits and oxidative stability. Despite these advantages, many widely cultivated peanut varieties remain normal-oleic, and the conversion of these varieties to high-oleic types without compromising yield and adaptability is of significant interest. This study evaluated the feasibility of using Pingyangmycin, a chemical mutagen, to induce high-oleic mutations in the popular peanut variety 308 through floral organ injection. The results showed that this method effectively generated high-oleic mutants with oleic acid content exceeding 75%. The mutants yielded more pods and kernels than the parental variety. Genotypic analysis confirmed mutations in the FAD2A and FAD2B genes, associated with the high-oleic phenotype. This novel approach, which reduces seed and reagent requirements and accelerates the breeding timeline, holds promise for enhancing peanut breeding programs and the development of high-oleic cultivars with superior quality and yield.
Keywords:
high-oleic peanut; Pingyangmycin; mutagenesis; FAD2A; FAD2B; floral organ injection; mutagenesis; breeding; productivity 1. Introduction
The cultivated peanut (Arachis hypogaea L.) is a globally significant crop, grown in over 100 countries across Asia, Africa, Oceania, and the Americas [1]. As a rich source of culinary oil, highly digestible protein, and a diverse array of health-beneficial phytochemicals, including amino acids, phenolic acids, and vitamins [2,3,4], peanut plays a crucial role in human nutrition and rural economy.
High-oleic peanut, compared to its normal-oleic counterpart, boasts a higher oleic acid content (no less than 75%) and a lower linoleic acid content (less than 8%) [5]. Consumption of high-oleic peanut has been linked to numerous health benefits, including improved cardiovascular and liver health, reduced low-density cholesterol levels, better glucose control and weight management, slowed aging, and enhanced cognitive function [6]. Furthermore, high-oleic peanut exhibits superior oxidative stability, as oleic acid oxidizes at approximately one-tenth the rate of linoleic acid. This enhanced stability not only benefits raw peanut materials, oil, and food products but also extends to biodiesel and seed storage, reducing spoilage and facilitating more efficient production in the food processing sector [7]. In addition, the use of high-oleic peanut in food processing aligns with consumer demand for clear and additive-free labeling.
Despite the advantages and the growing global demand for high-oleic peanut, many widely cultivated peanut varieties in China, India, and other regions remain normal-oleic. These cultivars are favored for their high yields, regional adaptability, and processing suitability, making them highly valued by growers, processors, and consumers. Small-seeded, normal-oleic peanut cultivar 308 is a case in point in China. Northeast China (38°–54° N, 119°–135° E) is a key region for high-quality peanut, characterized by a short frost-free period and a cool climate. Peanut from this region is renowned for meeting the stringent aflatoxin standards of the European Union. Cultivar 308 is particularly well suited for this region, with a duration of approximately 120 days and yields typically ranging from 4500 to 6000 kg/ha. With national fame for its high-quality seed peanut, Qingdao (35°35′–37°09′ N, 119°30′–121° E) has been a key seed supplier to northeastern growers for years. In Qingdao, 308 may achieve yields exceeding 7500 kg per hectare under favorable conditions. Converting these normal-oleic cultivars to high-oleic variants, without compromising their yield, adaptability, or processing quality, would provide significant benefits across the production chain.
Chemical mutagenesis has emerged as a viable approach for inducing high-oleic traits. Traditional techniques involve treating seeds with chemical mutagens such as sodium azide or ethyl methanesulfonate (EMS) to produce high-oleic mutants [5,6,7]. However, these methods require large quantities of mutagens, generate substantial chemical waste during soaking and rinsing processes, and necessitate extensive planting of derived progenies to identify desirable mutants, with high-quality mutants typically not detectable until the M2 generation. The mutagenesis process demands protective measures for the operators handling these chemicals. Improper disposal of chemical waste can result in leaching into groundwater, posing risks to human health and ecosystems. Sustainable practices must be considered to mitigate these risks, as the long-term consequences of chemical mutagenesis extend beyond immediate agricultural benefits. Addressing these issues is essential for ensuring that the benefits of chemical mutagenesis do not come at the cost of environmental integrity and public health.
To this end, we have developed a novel chemical mutagenesis technique for peanut that involves injecting chemical mutagens into floral organs, significantly reducing the amount of mutagen needed [8,9,10]. Two high-yielding, normal-oleic peanut varieties, Huayu 40 and Huayu 9610, were bred by our research team via floral organ injection of EMS and diethyl sulfate (DES) [9,10]. An increase in chlorophyll content in leaflets was observed in a high-yielding peanut mutant [8]. However, no high-oleic peanut mutants have been obtained through this innovative approach.
Pingyangmycin has demonstrated effectiveness as a chemical mutagen in several crops [11,12]. In peanut, Pingyangmycin-induced mutants with drought and salt tolerance or high oil content have been developed through in vitro mutagenesis [13,14]. The potential of Pingyangmycin in high-oleic peanut breeding has yet to be fully explored.
This study aimed to evaluate the feasibility of floral organ injection with Pingyangmycin to induce high-oleic acid mutants from the widely cultivated normal-oleic peanut variety 308 in a manner that minimizes negative impacts on both operators and the environment. Where possible, comprehensive assessments of mutant-derived lines were conducted to provide valuable references for accelerating high-oleic peanut breeding.
2. Materials and Methods
2.1. Peanut Cultivar for Chemical Mutagen Injection and Its Cultivation
The popular Spanish-type peanut cultivar 308, widely cultivated in the northeastern peanut-producing region of China, was used as the parent for chemical mutagenesis. To facilitate operations, the peanut plants intended for injections were grown on an elevated platform typically used for hybridization. In early May 2020, peanut was sown under polythene mulch at the Shandong Peanut Research Institute (SPRI) Laixi Experimental Station (36.85° N, 120.39° E). The region features a temperate semi-humid monsoon climate and brown soil. Routine agronomic practices were followed [15]. During land preparation, Poly-Magnesium Fertilizer for Continuous Cropping Resistance and Plant Survival Enhancement (N 16%, P2O5 10%, K 6%; Qingdao Lilihui Biotech, Laixi, China) was applied at a rate of 1125 kg/ha. Prior to sowing, Wood Vinegar Growth Activator (Shandong Yisheng Biotech, Laocheng, China) was sprayed onto the soil surface at 1.13 kg/ha. After sowing, herbicides including Acetochlor (1.5 kg/ha; Jinan Tianbang Chemicals, Jinan, China) and Haloxyfop-P-methyl (1.8 kg/ha; Nanjing Zhongqi, Nanjing, China) were applied for weed control, followed by mulching with polythene film. During the flowering and pegging phase, a critical period for water sensitivity, sprinkler irrigation was used to maintain sufficient soil moisture. Diseases were managed by spraying Tebuconazole plus Prochloraz (0.75 kg/ha; Shandong Shangnong Arotech, Heze, China). For pest control, several insecticides were applied, including alpha-Cypermethrin plus Emamectin Benzoate (0.98 kg/ha; Qingdao Haina Biotech, Laixi, China), Bifenazate plus Etoxazole (0.375 kg/ha; Shandong Wanhao Agrotech, Laixi, China), and Imidacloprid (0.3 kg/ha; Qingdao Taiyuan, Laixi, China). Peanut was harvested at the end of August of the same year.
2.2. Floral Injection of Chemical Mutagen Solution and Harvesting of M1 Seeds
The chemical mutagen used was PulaiPingYang® Pingyangmycin hydrochloride for injection (8 mg per vial) (Harbin Bolaitong Pharmaceutical, Harbin, China). During the full flowering phase, on the day before or on the day of anthesis, Pingyangmycin at concentrations of 0.75–5.00 mg/L was injected into the calyx tube or inside the keel/corolla at a rate of approximately 0.1 mL per flower (Table 1, Figure 1). Since there was no precedent for injecting pingyangmycin into peanut floral organs, and given that these organs are delicate with germ cells and zygotes potentially being more sensitive to the compound, the upper limit of pingyangmycin concentration was set at approximately one-tenth of the soaking concentration used for seeds of other crops. Additional concentrations were determined based on recommendations from DesignExpert 13.0 software (Stat Ease Inc., Minneapolis, MN, USA). The selection of the injection site and timing was guided by our prior experience with injecting other chemical mutagens, along with our knowledge of peanut floral organ structure, the fertilization process, and embryo development. The considerations behind injecting chemical mutagens into the peanut flower organs are as follows: Injecting the chemical mutagen into the corolla is straightforward, as both the stamens and pistils are located within it. On the other hand, injecting the chemical mutagen into the calyx tube has a different rationale, as the peanut ovary is located at the base of the calyx tube. The pollen tube must pass through the calyx tube to reach the ovary; in other words, the calyx tube is a necessary pathway. Identifying the young floral buds a day before anthesis is not difficult for hybridization breeders, and this may increase the opportunity for inducing mutations in early reproductive cells.

Table 1.
Pingyangmycin concentration, injection time and injection site.

Figure 1.
Injection of Pingyangmycin into peanut corolla (a,c) and calyx tube (b,d) on the day of anthesis (a,b) and the day before (c,d).
The injection procedures were conducted with proper safety measures, including chemical-resistant masks, protective goggles, gloves and long-sleeved laboratory coats. This mutagenesis method did not generate waste liquid. A total of twenty-four injection treatments were tested in the study, varying across six mutagen concentrations, two injection times, and two injection sites (Table 1). Each treatment included seventy flowers, and all injections were completed by 10 July 2020. Treated peanut flowers and resultant pegs were marked, and pods from treated and untreated flowers were harvested and sun-dried separately.
2.3. Planting of M1 Plants
The cultivation practices were the same as those for peanut used for injection, except that Wood Vinegar Growth Activator and pesticides were not applied, and the planting method involved ridge cultivation instead of flat planting. In 2021, the M1 seeds were sown in spring, and M1 plants were harvested in autumn. After drying the peanut pods from individual single M1 plants, typical pods were photographed to document their characteristics.
2.4. Quality Analysis
Bulk seed samples from individual peanut plants were analyzed for biochemical quality, including oleic acid, linoleic acid, oil, protein, sucrose and Vitamin E content, using a near-infrared (NIR) spectrometer (Model Matrix-I, Bruker Optics, Ettlingen, Germany) and near-infrared spectroscopy (NIRS) models developed at our laboratory [16,17,18].
To confirm the high-oleic phenotype in chemical mutants, the main fatty acid content of peanut seeds was further determined by gas chromatography (GC) [19]. The cotyledonary tissue at the distal end of the embryo in a single peanut seed, weighing no more than 30 mg, was excised and finely chopped into powders (1–2 mm in diameter) using a scalpel. Approximately 5 mg of the chopped seed tissue was placed into a 5 mL centrifuge tube, followed by the addition of 1 mL lipid extraction solution (benzene: petroleum ether = 1:1, v/v). After gentle shaking, the mixture was left undisturbed for 5 min. Subsequently, 1.5 mL of sodium methoxide–methanol solution (0.5 M) was added, and the mixture was vortexed. Esterification was carried out at room temperature for 10 min, after which 2 mL of saturated sodium chloride solution was added to terminate the reaction. The mixture was thoroughly mixed, allowed to separate, and 100 μL of the upper phase was pipetted into a tube. Then, 300 μL of petroleum ether (boiling range: 30–60 °C) was added. The diluted solution was transferred into Agilent autosampler vials. Fatty acid profiles were analyzed using an Agilent 7890A gas chromatograph equipped with a flame ionization detector (FID) and a DB-WAX silica capillary column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, Santa Clara, CA, USA). The gas chromatographic program was as follows: the column temperature was initially set at 210 °C (held for 9 min), then increased to 230 °C at a rate of 20 °C/min, and held for 8 min. The gas flow rates were 1.3 mL/min for nitrogen (carrier gas), 40 mL/min for hydrogen, and 400 mL/min for air. The injection volume was 2 μL, with a split ratio of 5:1 at an inlet temperature of 250 °C. Peak areas were integrated, and the percent composition of fatty acids was calculated using an Agilent 7890A integrator. Individual fatty acid peaks were identified by comparing retention times with those of fatty acid methyl ester standards (Sigma-Aldrich, MO, USA).
For varieties/lines under yield evaluation, quality was predicted using the NIRS method based on two replicates.
2.5. FAD2A and FAD2B Genotyping
FAD2 (fatty acid desaturase 2) is an enzyme in the fatty acid biosynthesis pathway that catalyzes the desaturation of oleic acid at the 12th carbon position (counting from the carboxyl end), forming a double bond and converting oleic acid into linoleic acid. The gene encoding this enzyme, FAD2, is the major gene controlling the high-oleic trait in peanut. Cultivated peanut is an allotetraploids, formed through the hybridization of two diploid wild species, followed by chromosome doubling or the retention of unreduced chromosomes. As a result, cultivated peanut possess two oleate desaturase genes derived from their wild ancestors: FAD2A and FAD2B, originating from the A and B subgenomes, respectively. To achieve the high-oleic phenotype, both genes must be inactivated [7]. Therefore, it is necessary to examine FAD2A and FAD2B in high-oleic mutants.
The FAD2A and FAD2B genotypes of the parent and high-oleic mutants were assayed by Sanger sequencing (Sangon Biotech, Shanghai, China) following PCR amplification with gene-specific primers (aF19/R1 for FAD2A and bF19/R1 for FAD2B) (Table 2) [20] and template DNA extracted from cotyledon slices of 308 and individual single M2 seeds [21]. The PCR reaction mixture (50 μL) included 25 μL of Tiangen 2×Taq Platinum Master Mix (Tiangen Biotech, Beijing, China), 5 μL of DNA template, and 2 μL of forward and reverse primer each (10 μM). The PCR program began with an initial denaturation at 95 °C for 6 min, followed by 35 cycles consisting of 30 s at 94 °C for denaturation, 1 min at 53 °C for annealing, and 2 min at 72 °C for extension. A final extension step at 72 °C for 4 min was then carried out. The amplified products of both FAD2A and FAD2B were expected to be 1140 bp in length for wild-type peanut. The PCR products were directly sequenced using the same primer pairs (Table 2) as utilized for amplification.

Table 2.
Primer sequence information.
2.6. Productivity Evaluation of Mutant Lines
During 2023–2024, yield assessments of mutant-derived lines were conducted. The 2023 yield evaluation was conducted with two replicates, with each entry assigned to six rows per replicate. The 2024 yield evaluation was conducted with three replicates, with each entry also assigned to six rows per replicate. Both trials followed a randomized block design. The cultivation was performed under polythene mulch on two-row raised seedbeds 85 cm wide and 4 m long, 30 hills per row. Concurrently, the control variety 308 was planted under identical conditions for comparison. The weed and pest management practices were the same as those for planting M1 plants.
2.7. Statistical Analysis
Analysis of variance and Tukey’s multiple comparison were performed using the DPS 14.50 package [22].
3. Results
3.1. Identification of High-Oleic M1 Plants Using NIR Screening
The M1 seeds from the 2020 floral injections were planted in the field in spring 2021, with pods harvested in autumn of that year. NIR analysis identified high-oleic mutants from only four treatments (Table 3). Among these, three treatments involved injection into the calyx tube, while one used injection inside the keel petal. Effective mutagenesis was achieved both on the day of anthesis and the day prior (Table 3). Treatments EJ-3, EJ-11, EJ-13, and EJ-15 yielded 11, 19, 4, and 5 M1 plants, respectively, from which 6, 14, 4, and 2 high-oleic plants were identified (Table 3). The oleic acid content of these high-oleic mutants was at least 76.45%, with an oleic-to-linoleic (O/L) ratio above 9.48, compared to the parental variety 308, which had less than 47% oleic acid and an O/L ratio below 1.5 (Table 3).

Table 3.
Oleic and linoleic acid contents of wild-type 308 and bulk seed samples from 26 high-oleic M1 mutant individual single plants as determined by NIRS, number of seeds per plant and seed mass per plant.
3.2. FAD2A/FAD2B Genotyping of High-Oleic Mutants
Sanger sequencing of the FAD2A and FAD2B genes in high-oleic M2 seeds derived from the four treatments and their parental variety 308 confirmed that all high-oleic mutants carried the F435 type mutations, as previously reported (Figure 2) [7]. The 448G>A mutation in the FAD2A gene results in a D150N substitution, leading to a significant reduction in enzymatic activity. The 441_442insA mutation in the FAD2B gene causes a frameshift mutation, resulting in premature termination of protein synthesis [16]. The mutations were associated with increased oleic acid levels, affirming the effectiveness of Pingyangmycin-induced mutagenesis in altering fatty acid profiles in peanut.
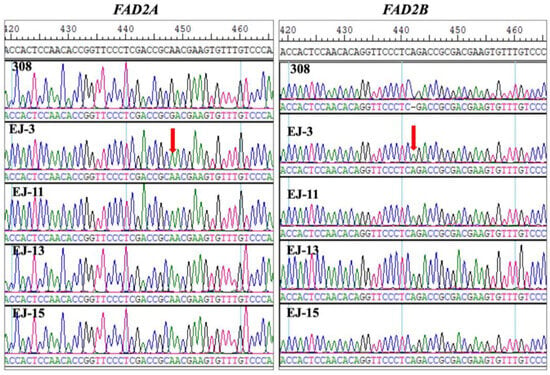
Figure 2.
Typical F435-type FAD2 mutations (FAD2A G448A, FAD2B 442A) in high-oleic M2 seeds representing four treatments (EJ-3, EJ-11, EJ-13, EJ-15), with red arrows indicating the mutated bases.
3.3. Pod Morphology and Preliminary Productivity Evaluation of M1 Plants
Distinct morphological differences were observed between the pods of high-oleic M1 plants and the parental variety 308, particularly in pod constriction (Figure 3). This suggests that the mutagenesis treatment impacted not only the fatty acid composition but also the pod morphology. The high-oleic M1 plants listed in Figure 4 exhibited greater productivity than 308. As showed in Table 2, the seed mass per plant of 308 was under 28 g, while that of the best high-oleic mutants exceeded 50 g. These findings highlight the potential for developing high-oleic, high-yielding peanut varieties from 308-derived mutants.
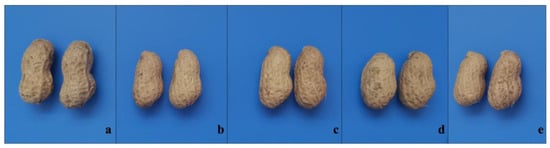
Figure 3.
Representative pods from four high-oleic M1 single plants resulting from four treatments and their parent variety 308. (a): 308, (b): EJ-3, (c): EJ-11, (d): EJ-13, (e): EJ-15.

Figure 4.
Seeds from four high-oleic M1 single plants representing four treatments (a–d) and their parent variety (308), bar size = 2 cm. (a): 308, (b): EJ-3, (c): EJ-11, (d): EJ-13, (e): EJ-15.
3.4. Quality Evaluation, Field Performance and Selection of M2 Plants
Viable seeds from the high-oleic M1 plants were sown in fields on 4 May 2022, with single-plant harvesting conducted at the end of August 2022. NIRS quality analysis indicated that all harvested M2 single plants maintained an oleic acid content above 75%. Main fatty acids in seeds from four randomly selected M2 single plants from the four treatments were analyzed by GC. The oleic acid content in these mutants ranged from 76.21% to 80.58%, with O/L ratios (oleate-to-linoleate ratios) between 25.37 and 57.89, as compared to 39.85% oleic acid and an O/L ratio of 1.42 in the original 308 cultivar (Table 4). These results confirmed the high-oleic phenotype in the selected M2 lines, with substantial increases in oleic acid and O/L ratio relative to the parental variety.

Table 4.
Major fatty acid content (%, percentage of total) of peanut seeds harvested from four M2 single mutant plants representing four treatments and wild-type 308 in 2022 as determined by GC method.
The four representative M2 high-oleic plants from the respective treatments were shown in Figure 5. Among them, the mutant plant from the EJ-3 treatment (20EJD-7A) demonstrated notably superior productivity leading to the advancement of three lines from this treatment to preliminary yield trials in 2023 (Table 5).

Figure 5.
Four randomly selected high-oleic M2 plants representing four treatments (a–d), bar size = 10 cm. (a): EJ-3, (b): EJ-11, (c): EJ-13, (d): EJ-15.

Table 5.
Key biochemical quality traits of four high-oleic peanut mutant lines (2023 Crop) as predicted by NIRS.
3.5. Quality and Yield Evaluation of the High-Oleic Mutant Lines
In 2023, the four high-oleic peanut mutant lines displayed superior biochemical quality, with oleic acid contents between 75.5% and 82.0%, significantly exceeding the levels in the normal-oleic control 308. Oil content in these mutants ranged from 50.7% to 52.6%, while protein content varied from 24.6% to 25.0% (Table 5). Based on the results in Table 5, the injection of mutagens into peanut flowers not only affected the contents of oleic acid and linoleic acid but also had significant impacts on oil content, vitamin E content, and sucrose content at the 0.01 or 0.05 probability levels.
Yield performance data (Table 6) revealed a clear advantage of the mutants over the CK in both 2023 and 2024. In 2023, pod yields for the mutants ranged from 4851 to 5702 kg/ha, with line HO308-4 achieving the highest yield—an impressive 75.2% increase over the CK’s 3254.9 kg/ha. Seed yields for the mutants were also enhanced, between 3606.2 and 4389.6 kg/ha, compared to the CK’s 2140.2 kg/ha.

Table 6.
Pod and seed characteristics and yield performance of four high-oleic acid peanut mutants (2023 and 2024 cropping seasons).
In 2024, despite an overall reduction in yields, the mutant lines maintained a performance advantage. Pod yields ranged from 2629.1 to 3289.2 kg/ha, and seed yields were between 1885.8 and 2446.2 kg/ha. Line HO308-3 led in 2024 pod yield, 45.7% higher than the CK.
4. Discussion and Conclusions
Our previous work optimized a protocol for chemical mutagenesis in peanut seeds that included a pre-soaking step to increase sensitivity to mutagens. This improvement allowed us to reduce the number of seeds needed for mutagenesis from 1000 to 120. However, the approach had limitations in early trait selection due to the physiological rather than genetic basis of some altered traits observed in early generations. Additionally, at this stage, key target genes may not yet be homozygous, complicating reliable early selection [6]. Despite these limitations, we were able to develop high-oleic mutants from six normal-oleic cultivars using this modified soaking technique [5,6,7].
The current study introduced an innovative approach, injecting chemical mutagens directly into peanut floral organs, which substantially reduces both seed and reagent requirements and eliminates the need to manage waste liquid. In the seed-soaking treatment, each seed generally requires 1.5–2 mL of chemical mutagen. In contrast, when injecting floral organs, only 0.1 mL of chemical mutagen is used per flower. Compared to seed-soaking methods, chemical mutagen injection into floral organs is an environmentally friendly technology that supports the principles of sustainable development. From the perspective of worker protection, since there is no need to handle large amounts of hazardous chemicals, the ethical burden on researchers is considerably lighter. By targeting early germline cells, this method significantly lowers or even eliminates chimerism risk. Screening bulk seeds from M1 single plants using NIRS allowed for early identification of high-oleic individuals. This floral injection technique, compared to seed soaking, advances the breeding timeline for high-oleic peanut by at least one generation. Importantly, the high-oleic trait in mutants generated by this method was stably inherited in the next generation, further demonstrating the technique’s efficiency. A similar in planta approach has enabled us to obtain high-oleic T1 peanut seeds that stably passed on this trait to subsequent generations [23].
It should be noted that the relatively low yields observed for the peanut variety/lines in this study are due to the specific conditions of yield assessment trials used by Chinese peanut breeders, which differ from commercial production practices. In these trials, plant overgrowth is not controlled, and no fungicides are applied. Consequently, when the same variety is grown by farmers under typical field conditions, the yield is much higher. Additionally, although some may consider the simultaneous genetic alterations in both FAD2A and FAD2B genes unlikely, this outcome is indeed feasible due to the high sequence similarity between the two genes (only 11 bp difference between FAD2A and FAD2B in the coding region). This possibility has been validated by both our study and other research efforts [6]. Peanut FAD2A and FAD2B showed different mutation hot spots, indicating that factors other than coding sequences of genes of interest may have some influence on mutation site [6].
In this study, the chemical mutagen Pingyangmycin contributed to the successful induction of high-oleic peanut mutants. While our earlier efforts using other chemical mutagens for floral organ injection were conducted without NIRS screening due to lack of equipment, it would be premature to conclude that those mutagens were ineffective in inducing high-oleic mutations in peanut.
Developing high-oleic peanut varieties through hybridization or backcrossing is time-intensive. Yield evaluation in hybridization programs typically cannot commence until F6 generation, while backcrossing requires at least three successive cycles to introgress the desired trait. In contrast, the highly efficient floral organ injection-based chemical mutagenesis technique introduced in this study offers a means to substantially accelerate the development of high-oleic peanut. However, some prominent U.S. processing companies remain cautious about adopting high-oleic peanut as raw materials, primarily due to concerns that high-oleic alternatives may change flavor profiles and affect consumer acceptance (Dr. Charles Y. Chen, Professor of Auburn University, Personal communication). The technique developed here provides a viable strategy for inducing high-oleic mutations while retaining the favorable characteristics of the original varieties, thereby addressing these challenges and broadening the potential for market adoption.
Badigannavar and Mondal compiled a list of induced peanut mutants and their derivatives exhibiting tolerance to diseases, pests, drought, acidic soils, heat, and cold [24]. In our previous work, peanut mutants resistant to invasion by Aspergillus flavus or A. niger were identified from the progeny of susceptible peanut varieties treated with EMS through seed soaking (G. Yuan and J. Yu, unpublished data). In this study, in addition to traits such as fatty acid composition and yield, further investigation is needed to determine whether the mutants differ from wild-type peanut in their responses to several major diseases.
In summary, this study presents a novel method using Pingyangmycin injection into floral organs to induce high-oleic peanut mutants. This approach allows for the identification of mutants as early as the M1 generation, offering significant advantages in terms of seed, time, and reagent savings, as well as avoiding waste disposal and the need to plant large progeny populations. While, in this study, our findings with a single cultivar show promise, encouragingly, in a separate recent unpublished study, we have successfully obtained high-oleic mutants of five other peanut cultivars using this approach. It is expected that this method will greatly enhance breeding programs by enabling the rapid conversion of competitive normal-oleic cultivars into high-oleic types and facilitating the development of peanut mutant libraries [25]. Its application scope should naturally extend beyond high-oleic mutants; the potential application of this method to other agronomically and economically important traits warrants further investigation. The mutagenesis method of injecting chemical mutagens into floral organs may also have certain reference value for other leguminous plants.
Author Contributions
C.J., responsible for data collection and analysis, resource provision, drafting the initial version of the paper, and revising the paper. Z.W., in charge of experimental methods, resource provision, and drafting the initial version of the paper. H.S., responsible for data collection and analysis, and drafting the initial version of the paper. S.Y., responsible for data collection and analysis, and drafting the initial version of the paper. H.H., responsible for data collection and analysis, and drafting the initial version of the paper. Z.Y., responsible for data collection and analysis, resource provision, and drafting the initial version of the paper. M.Z., responsible for conceiving the paper and drafting the initial version of the paper. G.Y., responsible for data collection and analysis, and drafting the initial version of the paper. J.Y., responsible for data collection and analysis, and drafting the initial version of the paper. C.W., responsible for conceiving the paper, experimental methods, resource provision, revising the paper, obtaining project funding, and supervising the implementation of the research. All authors have agreed to the final version of the manuscript and are willing to take responsibility for the accuracy and authenticity of the entire research work to ensure that any issues related to the accuracy or integrity of any part of the manuscript are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agricultural Research System (grant No. CARS-13), Key Research & Development Program of Shandong Province (grant No. 024TSGC0532), and Shandong Peanut Breeding Key Laboratory (grant No. PKL2024B02).
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
The authors whose names are listed immediately below certify that they have no conflicts of interest to declare: Chunjiao Jiang. Zhiwei Wang, Haojie Sun, Shutao Yu, Hongwei Han, Zhen Yang, Guangdi Yuan, Jing Yu, Chuantang Wang. Author Ming Jun Zhang was employed by the company Shandong Rainbow Agri-Tech Co., Ltd. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Reddy, P. Groundnut; Publications and Information Division, Indian Council of Agricultural Research, Krishi Anusandhan Bhavan, Pusa: New Delhi, India, 1988. [Google Scholar]
- Smartt, J. The Groundnut Crop: A Scientific Basis for Improvement; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Lusas, E.W.; Erickson, D.R.; Nip, W.-K. Food Uses of Whole Oil and Protein Seeds; Amer Oil Chemists Society: Champaign, IL, USA, 1989. [Google Scholar]
- Łozowicka, B.; Kaczyński, P.; Iwaniuk, P.; Rutkowska, E.; Socha, K.; Orywal, K.; Farhan, J.A.; Perkowski, M. Nutritional compounds and risk assessment of mycotoxins in ecological and conventional nuts. Food Chem. 2024, 458, 140222. [Google Scholar] [CrossRef] [PubMed]
- Nkuna, R.T.; Wang, C.T.; Wang, X.Z.; Tang, Y.Y.; Wang, Z.W.; Zhang, J.C. Sodium azide induced high-oleic peanut (Arachis hypogaea L.) mutant of Virginia type. Genet. Resour. Crop Evol. 2021, 68, 1759–1767. [Google Scholar] [CrossRef]
- Han, H.W.; Wang, Z.W.; Wang, X.Z.; Sun, X.S.; Fang, C.Q.; Wang, C.T. Identification of high-oleic peanut chemical mutants and functional analysis of mutated FAD2B gene. Plant Genet. Resour. 2022, 20, 15–21. [Google Scholar] [CrossRef]
- Wang, C.T.; Yu, S.L.; Zhu, L.G. High Oleic Acid Peanuts in China; Shanghai Science and Technology Press: Shanghai, China, 2021. [Google Scholar]
- Wang, C.T.; Wang, X.Z.; Tang, Y.Y.; Chen, D.X.; Zhang, J.C.; Cui, F.G.; Yu, S.L. High yielding mutants achieved by injecting EMS into peanut flower organs. J. Nucl. Agric. Sci. 2010, 24, 239–242. [Google Scholar]
- Wang, C.T.; Wang, X.Z.; Tang, Y.Y.; Zhang, J.C.; Chen, D.X.; Xu, J.Z.; Yang, X.D.; Song, G.S.; Cui, F.G. Huayu 40, a groundnut cultivar developed through EMS mutagenesis. J. SAT Agric. Res. 2011, 9, 1–2. [Google Scholar]
- Wang, C.T.; Tang, Y.Y.; Wang, X.Z.; Wu, Q.; Sun, Q.X.; Gong, Q.X.; Yang, Z.; Song, G.S.; Wang, Z.W. Breeding of Huayu 9610, a high-yielding export-type conventional large-podded peanut cultivar, through chemical mutagenesis combined with intersectional hybridization. Seeds 2016, 35, 100–101. [Google Scholar]
- International Rice Research Institute. Genetic Manipulation in Crops: Proceedings of the International Symposium on Genetic Manipulation in Crops: The 3rd International Symposium on Haploidy, the 1st International Symposium on Somatic Cell Genetics in Crops, Beijing, October 1984; Natural Resources and the Environment Series; Cassell Tycooly: London, UK, 1988. [Google Scholar]
- Zhang, R.; Li, W.; Pan, S.; Dai, L.; Liu, S. Application of chemical mutagenesis in improving germplasm resource. Mol. Plant Breed. 2017, 15, 5189–5196. [Google Scholar]
- Sui, J.; Wang, Y.; Wang, P.; Qiao, L.; Sun, S.; Hu, X.; Chen, J.; Wang, J. Generation of peanut drought tolerant plants by pingyangmycin-mediated in vitro mutagenesis and hydroxyproline-resistance screening. PLoS ONE 2015, 10, e0119240. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, L.; Liu, Y.; Zhao, M.; Wang, X.; Qiao, L.; Sui, J.; Li, G.; Zhu, H.; Yu, S. Development of peanut varieties with high oil content by in vitro mutagenesis and screening. J. Integr. Agric. 2020, 19, 2974–2982. [Google Scholar] [CrossRef]
- Wan, S.B. Peanut Cultivation Science in China; Shanghai Sci & Tech Press: Shanghai, China, 2003. [Google Scholar]
- Wang, C.T.; Zhang, J.C. Peanut Genetic Improvement; Shanghai Sci & Tech Press: Shanghai, China, 2013. [Google Scholar]
- Tang, Y.Y.; Wang, X.Z.; Liu, T.; Wu, Q.; Sun, Q.X.; Wang, Z.W.; Zhang, X.; Wang, C.T.; Shao, J.F. A near infrared spectroscopy model for predicting sucrose content of sun-dried peanut seeds. Shandong Agric. Sci. 2018, 50, 159–162. [Google Scholar]
- Liu, T.; Wang, C.T.; Tang, Y.Y.; Hu, D.Q.; Wang, X.Z.; Wu, Q.; Sun, Q.X.; Wang, Z.W.; Song, G.S.; Shi, C.R.; et al. A near infrared spectroscopy model for predicting vitamin E content in sun-dried peanut seeds. Shandong Agric. Sci. 2018, 50, 163–166. [Google Scholar]
- Yang, C.D.; Guan, S.Y.; Tang, Y.Y.; Wang, X.Z.; Wu, Q.; Gong, Q.X.; Wang, C.T. Rapid non-destructive determination of fatty acids in single groundnut seeds by gas chromatography. J. Peanut Sci. 2012, 41, 21–26. [Google Scholar]
- Patel, M.; Jung, S.; Moore, K.; Powell, G.; Ainsworth, C.; Abbott, A. High-oleate peanut mutants result from a MITE insertion into the FAD2 gene. Theor. Appl. Genet. 2004, 108, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.T.; Wang, C.T.; Yu, S.L.; Wang, X.Z.; Tang, Y.Y.; Chen, D.X.; Zhang, J.C. Simple method to prepare DNA templates from a slice of peanut cotyledonary tissue for Polymerase Chain Reaction. Electron. J. Biotechnol. 2010, 13, 9. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Zhang, C.-X. Data Processing System (DPS) Software with Experimental Design, Statistical Analysis and Data Mining Developed for Use in Entomological Research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Han, H.W.; Yu, S.T.; Wang, Z.W.; Yang, Z.; Jiang, C.J.; Wang, X.Z.; Sun, X.S.; Wang, C.T. In planta genetic transformation to produce CRISPRed high-oleic peanut. Plant Growth Regul. 2023, 101, 443–451. [Google Scholar] [CrossRef]
- Badigannavar, A.M.; Mondal, S. Advances in Mutation Breeding of Groundnut (Arachis hypogaea L.). In Mutation Breeding for Sustainable Food Production and Climate Resilience; Penna, S., Jain, S.M., Eds.; Springer Nature: Singapore, 2023; pp. 487–519. ISBN 9789811697203. [Google Scholar]
- Chen, T.; Huang, L.; Wang, M.; Huang, Y.; Zeng, R.; Wang, X.; Wang, L.; Wan, S.; Zhang, L. Ethyl methyl sulfonate-induced mutagenesis and its effects on peanut agronomic, yield and quality traits. Agronomy 2020, 10, 655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).