Abstract
Although organic amendment has been widely accepted to be capable of facilitating soil agglomeration in coastal salt-affected soils, quantitative characterization with respect to how abiotic and biotic components drive the formation and stabilization of soil aggregates remains largely unexplored and poorly understood. In the current study, wet-sieving, Miseq sequencing, etc., were employed to study the impacts of different application amounts of sewage sludge on soil aggregates, physicochemical properties, enzyme activities, and microbial core microbiomes in coastal saline soils. The results indicated that sewage sludge was conducive to soil agglomeration, abiotic constraint alleviation, microbial activity enhancement, and bacterial and fungal community stabilization and functionalization. The results derived from variation partitioning analysis and the structural equation model showed that elevated soil organic carbon and mitigated salinization were dominant abiotic factors that directly drove the stabilization and functionalization of bacterial and fungal microbiomes. In addition, bacterial families (e.g., Xanthomonadaceae, Rhodospirillaceae, and Micrococcaceae) and fungal genera (e.g., Trichoderma, Cephaliophora, Mortierella, and Penicillium) were potential functional microbial populations related to soil agglomeration in organic amended coastal salt-affected soils. Together, these abiotic and biotic agents jointly drove soil agglomeration and totally explained 87% of the variations in soil aggregates. Collectively, this study highlighted the approach and effectiveness of the impacts of organic amendment on soil agglomeration in coastal salt-affected land based on qualitative and quantitative analysis, which would enhance our knowledge with respect to coastal salt-affected soil quality indication and development.
1. Introduction
Widely distributed coastal saline–alkali land is a significant potential resource of farming land; hence, rational exploitation and utilization of these salt-affected lands have been increasingly considered efficient agricultural practices to relieve cultivated land scarcity as well as world food shortage [1]. Because of long-time seawater immersion and intense soil evaporation, newly reclaimed coastal mudflats are usually characterized by underdeveloped soil with numerous abiotic and biotic constraints, such as poor soil structure, salinization, soil organic carbon (SOC) shortage, nutritional deficiency, limited microbial activity, and undiversified microflora, which severely restrict field performance of subsequent cultivations [2]. Consequently, overcoming the obstacle factors mentioned above is essential for soil environmental condition improvement and subsequent high-yield cultivations in coastal salinized regions. Nevertheless, it is full of challenges as the abiotic and biotic constraints are concomitants and interact with each other in complex ways.
Salinization alleviation has been recognized as a significant prerequisite for the successful amendment of coastal saline soils [3]. However, due to the rudimentary soil structure and intense soil evaporation, salts in the deep soil can easily move upward by siphon action, with salt accumulation occurring frequently in the newly reclaimed coastal salt-affected soils [4]. Against this background, soil agglomeration has been considered the key approach to alleviate salinization in coastal salt-affected areas [5]. The reasons lie in the fact that more macroaggregates directly contribute to soil saline–alkali stress alleviation by blocking the capillary rise of upward-moving salt [6]. In addition, improved aggregation status is deeply involved in multitudinous soil functions, such as the maintenance of soil structure, cycling of nutrients, and diversification of the microbial community [7]. However, due to the adverse environmental constraints mentioned above, soil agglomeration in coastal areas is widely believed to be an extremely slow process. Therefore, revealing mechanisms of soil agglomeration and exploring effective agronomic measurements have important implications for us to better exploit and utilize coastal salt-affected soils.
Soil aggregates, which serve as essential building blocks of soil, are intricately related to soil quality and agricultural sustainability [5]. Soil aggregates are primarily composed of cementation of mineral particles and colloidal organic particles, and they are significantly affected by various physical, chemical, and microbiological agents [8]. For example, SOC has been extensively proven to be one significant factor that affects the degree of soil agglomeration, as they are the major cementing substance involved in the formative process from smaller-particle-size aggregates to larger ones [9]. Instead, it has been shown that pH and salinity negatively affect the stabilization of newly formed aggregates due to their flocculating and destructive effects on these aggregates [7,10,11]. Recently, soil microbial community diversity and functionality have gained prominence in the underlying mechanism disclosure with respect to soil aggregate formation and stabilization alongside the traditional physical and chemical attributes [12]. Specifically, soil aggregation status is closely associated with abundances and diversities of potential functional microbial groups, such as extracellular polymeric substances producing bacteria and arbuscular mycorrhizal fungi [13]. These microbial populations directly participate in soil agglomeration through multiple ways, such as electrostatic forces [14], organic cementing substance (e.g., extracellular polysaccharides) production, and hyphal enmeshment. Additionally, soil microbiomes can also indirectly influence soil aggregation status by regulating the dynamic equilibrium of input and output soil organic matter [15,16,17]. Of note, Zhang et al. [18] found that bacterial community complexity and stability together with core bacterial species with multifunctionalities dominated the differentiated responses of macroaggregates and microaggregates to soil restorations. It is worth noting that soil aggregation status is affected by the interactive effects of physical, chemical, and microbiological factors rather than the individual ones mentioned above; the mechanisms and respective importance of these drivers involved in the process of soil agglomeration under high pH and high salinity conditions remain rudimentary. Consequently, it is crucial to thoroughly inquire about the mechanisms and respective significance of these environmental factors involved in soil agglomeration in coastal salt-affected areas.
At present, agricultural methods employed to improve coastal salt-affected soils primarily encompass physical, biological, and chemical amendments. Physical approaches such as natural leaching and artificial irrigation require extensive human effort and material and freshwater resources. In terms of biological methods, such as salt-tolerant crop breeding and cultivation, these are characterized by being time-consuming and having slow effects. By contrast, chemical ameliorants are extensively applied on account of their adaptable formulations, rapid efficacy, and straightforward operation [3,19]. As for chemical methods, exogenous organic ameliorant application has been increasingly demonstrated to be conducive to soil quality and productivity enhancement. Among the numerous organic ameliorants, sewage sludge is considered to be a cost-effective soil amendment because of its sufficient sources and high nutrient content, which includes abundant carbon, phosphorus, and nitrogen. Recently, sewage sludge has been increasingly demonstrated to be an efficient agricultural practice for saline–alkali stress alleviation, SOC sequestration, and microbiome reassembly. In particular, sewage sludge was found to be capable of promoting soil aggregation status in coastal salt-affected soils [19,20,21]. Nevertheless, mechanisms by which abiotic and biotic environmental agents contribute to soil aggregate formation and stability remain poorly understood. In the present study, field trials consisting of different sewage sludge application rates were conducted to (i) evaluate the influences of organic amendment on soil aggregate composition and stability, (ii) disclose the variations in abiotic constraints and responses of microbial communities to exogenous ameliorant applications, and (iii) quantitatively elucidate the regulatory pathways and respective effectiveness of biotic and abiotic agents driving soil agglomeration in coastal saline–alkali soils.
2. Materials and Methods
2.1. Study Area Location, Experimental Design, and Soil Sampling
The field experimental site is situated in Dong’tai City (E 120°56′51″, N 32°49′56″), Jiangsu Province, China, with an altitude of 1.4~5.1 m. This area had a northern subtropical continental monsoon climate, with an average annual precipitation of 1417 mm (mainly concentrated between June and September) and an annual average temperature of 16.5 °C (minimum and maximum yearly average temperature occurred in January and July). The dominant soil type is categorized as Solonchaks [22] with sodium chloride as a main component, and Cl− accounted for 70~90% of the total amount of anions, followed by sulfate and bicarbonate. The predominant planting system in this region involves a rice–wheat rotation, with wheat being cultivated in November and then rotated with rice in the following June. Due to long-term seawater immersion, newly reclaimed soil in this area harbored typical characteristics of high saline–alkali, low SOC, and nutrient contents. Specifically, main chemical properties of soils in this area were as follows: pH (soil/water ratio of 1:5) of 9.02, electrical conductivity (EC) of 1.38 mS cm−1, SOC content of 1.97 g kg−1, total nitrogen (TN) content of 0.28 g kg−1, total phosphorus (TP) content of 0.51 g kg−1, alkaline nitrogen (AN) content of 17.08 mg kg−1, and available phosphorus (AP) content of 6.99 mg kg−1.
In the current study, carbon- and nutrient-rich sewage sludge (Chunguang composting plant, Yancheng, China) was chosen as exogenous organic ameliorants for coastal salt-affected soil amendment. The basic chemical properties of sewage sludge used in this study were as follows: pH (soil/water ratio of 1:5) of 6.32, electrical conductivity (EC) of 32.9 mS cm−1, SOC content of 216.2 g kg−1, TN content of 51.2 g kg−1, TP content of 5.51 g kg−1, AN content of 3440 mg kg−1, and AP content of 813 g kg−1. Field experiments comprised four treatments, namely, soils amended by exogenous materials at rates of 0 (CK), 50 (S50), 100 (S100), and 200 (S200) t ha−1. Every treatment was composed of three randomly distributed microplots (4 m × 4 m). In October 2019, sewage sludge was applied once into each plot on a dry weight basis according to the corresponding design application rates and mixed uniformly with the topsoil (0~20 cm) using a rototiller (SFXGJ-20, Honggong Machinery Co., Ltd., Changzhou, China). Subsequently, each microplot was irrigated and soaked with a depth of approximately 8~10 cm of water. After seven days of deposition, a rotary tiller was used to level the surface soil puddling. In October 2020, a total of twelve soil samples were obtained (five random soil samples at each microplot, which were mixed to form a composite individual sample) from twelve microplots (0~20 cm). The collected soils were carefully fragmented into smaller sections along natural break points and homogenized using a 5 mm sieve (GN-PVC, Yangtai Instrument Co., Ltd., Yangzhou, China). One batch of the soil samples was stored at 4 °C for soil aggregate fractions. Other soil samples were sieved (2 mm) and stored at 4 °C and −80 °C and oven-dried for subsequent enzyme activity measurements, microbial analyses, and physical and chemical property determinations, respectively. Particularly, soil aggregate fractions and enzyme activity determinations were completed within 15 days and 7 days after the soils arrived at the lab, respectively.
2.2. Physicochemical Property and Microbial Enzyme Activity Measurements
Soil physical and chemical properties, including bulk density (BD), pH (soil/water ratio of 1:5), EC, and contents of SOC, TN, TP, AN, and AP, were measured according to Lu [23]. In this study, impacts of organic amendment on soil microbial enzyme activities closely link to soil total microbial activity (fluorescein diacetate hydrolysis, FDA) and cycles of carbon (sucrase, SUC), nitrogen (urease, URE), and phosphorus (alkaline phosphatase, ALP) were measured using colorimetric methods described in detail by Li et al. [21].
2.3. Soil Water-Stable Aggregate Fraction
Soil water-stable aggregates were determined with a wet-sieving device following the established methods adapted from Li et al. [20]. Briefly, a total of 100 g of moist soil samples (<5 mm, dry weight basis) were placed atop a series of sieves (2 mm, 1 mm, 0.5 mm, 0.25 mm, and 0.106 mm) and then submerged in a wet-sieving machine (S-2018, Dadi Fengke Co., Ltd., Nanjing, China) full of deionized water. The sieve assembly was shaken up and down for 300 s (amplitude, 3.0 cm; frequency, 30 times min−1). Afterward, soil aggregate fractions remaining on different-mesh-aperture sieves were dried at 50 °C. The proportions of aggregate with different particle sizes to the total soil mass were calculated. In addition, soil aggregate formation and stability parameters, including macroaggregates (particle size ≥ 0.25 cm, R0.25), mean weight diameter (MWD), geometric average diameter (GMD), and fractal dimension D, were analyzed according to Hong et al. [24].
2.4. Amplicon Sequencing and Bioinformatics Processing
Soil genomic DNA was isolated, quality-controlled, amplified, and sequenced following the established methods of Li et al. [17,18]. A DADA2 plugin [25] in the QIIME2 (version 2023.5) pipeline [26] was applied for sequence denoising. The Naïve Bayes consensus taxonomy classifier available in QIIME2 was performed for the taxonomic classification of amplicon sequence variants (ASVs), and databases of SILVA 16S rRNA (v138) “https://www.arb-silva.de (accessed on 21 July 2024)” and UNITE 8.0 “https://pan.baidu.com/share/init?surl=_4o1Dk5fKISbcPgR2HmtqA&pwd=7u82 (accessed on 23 July 2024)” were employed for the classification of bacterial and fungal taxa, respectively. Afterward, bacterial and fungal sequences across treatments were rarefied to 68,000 and 40,000 for subsequent bacterial and fungal community diversity analysis, respectively [20,21]. Compositional diversity indices and the average variation degree (AVD) of soil bacterial and fungal communities were calculated to characterize the differentiations in community composition and stability across treatments. Principal coordinate analysis (PCoA) accompanied by permutational multivariate analysis of variance (PERMANOVA) with 999 permutations was applied to compare the dissimilarities in community structural diversity among treatments based on BC distance matrices [21]. Particularly, core ASVs and unique ASVs were analyzed by retaining ASVs that presented in all replicates of each treatment to disclose treatment-shared effects and treatment-unique effects on soil microbiomes.
2.5. Statistical Analysis
A one-way analysis of variance (ANOVA) and multiple comparative analyses (Duncan) were performed for difference significance tests (p < 0.05) of soil physical and chemical properties, aggregate distribution and stability, community diversity indices, and community taxonomies across all treatments. Principal component analysis (PCA) was conducted to reveal the dominant components accounting for the dissimilarity of soil aggregation, bacterial community, and fungal community across different treatments. Mantel test analysis and heatmap analysis based on Spearman’s rank-order correlation analysis were performed by using the “ggcor (v0.9.3.1)” and “vegan (v2.6-4)” packages of the R language to explore the correlation among soil physical and chemical properties, enzyme activities, and core microbial groups (p < 0.05) [27]. Variation partitioning analysis (VPA) [28] was conducted to estimate the respective importance of physical, chemical, and microbiological components driving the variation in aggregation stability (MWD) in coastal salt-affected soils, physical and chemical properties, enzyme activities, and compositional and structural diversity indices of bacterial and fungal communities. Afterward, the lavaan (v0.6-19) R package was used to construct a structural equation model (SEM) to explore the correlations among soil aggregation status and environmental driving factors, which was derived from the VPA results mentioned above. The goodness of fit of the model was assessed using the chi-square test (χ2, p > 0.05), the goodness-of-fit index (GFI), and the root mean square error of approximation (RMSEA) [29]. In addition, based on results obtained from normality tests (Shapiro–Wilk), Spearman’s rank-order correlation analysis and linear regression analysis were performed to reveal the potential core bacterial and fungal populations closely linked to microbial community stability and macroaggregate formation, as well as aggregate stability.
3. Results
3.1. Soil Aggregates
In general, sewage sludge amendment exhibited significant (p < 0.05) facilitation on aggregation status improvement in coastal salt-affected soils (Figure 1). Remarkably, the proportion of macroaggregates (R0.25) in S100 and S200 treatments was significantly higher than in the control soil (Figure 1a). In terms of aggregate stability, organic amended soils harbored notably higher MWDs and GMDs (Figure 1b) but lower fractal dimension D (Figure 1c) values in comparison to those in unamended soils, which implied that aggregate stability in coastal salt-affected soils was enhanced and caused by sewage sludge amendment. Additionally, PCA showed sewage sludge amendments drastically altered soil aggregate structures along the applied amount gradient of sewage sludge (Figure 1d). Particularly, larger-class aggregates with higher stabilities were the primary factors contributing to the differences observed between organic-treated soils and unamended soils.
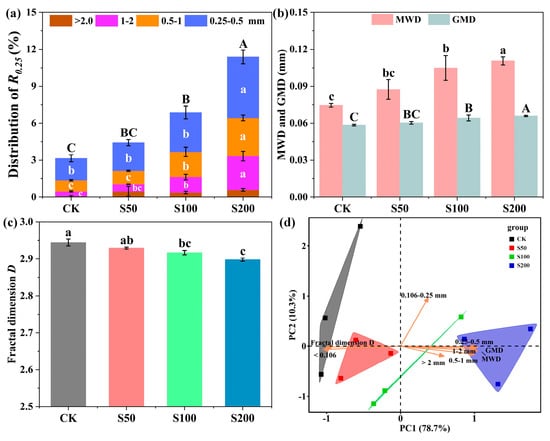
Figure 1.
Effects of sewage sludge amendments aggregate formation and stability in coastal salt-affected soils. (a) represents particle distribution of macroaggregates; (b,c) represent aggregate stability indices of the MWD, GMD, and fractal dimension D, respectively; (d) represents the PCA of aggregate structures based on aggregate composition and stability across treatments. Error bars denote standard errors. Different lowercase letters denote significant differences within aggregate particles with sizes of 0.25–0.5, 0.5–1, 1–2 mm (a), MWD (b), and fractal dimension D (c) among treatments, respectively. Different capital letters denote significant differences of aggregate particles larger than 0.25 mm (a) among treatments. (Duncan’s test, p < 0.05).
3.2. Soil Physicochemical Property and Microbial Enzyme Activity
Overall, sewage sludge amendments significantly (p < 0.05) promoted physicochemical constraint alleviation in coastal saline soils (Table 1). Specifically, in comparison to unamended soil, organic amendments improved soil structure (BD), ameliorated salinization (pH and EC), and enhanced SOC storage as well as nutrient supply (TN, TP, AN, and AP). Particularly, sewage sludge amendments with higher application amounts exhibited more pronounced impacts on physical and chemical attribute alleviation as compared to the lower ones, with the lowest BD, pH, and EC measurements and highest values of SOC, TN, TP, AN, and AP detected in the S200 treatment in comparison to those in other treatments. As for microbial enzyme activity, the results showed that sewage sludge amendments were conducive to the increment of microbial activity with significantly higher FDA, SUC, URE, and ALP measurements detected in amended soils in comparison to the control soil (Table 2). Similarly, enzyme activities increased with an increase in the applied amount of sewage sludge, with the highest values observed in S200.

Table 1.
Effects of sewage sludge applications on soil physical and chemical properties.

Table 2.
Effects of sewage sludge applications on soil enzyme activities.
3.3. Diversity and Stability of Soil Microbial Community
Applying sewage sludge results in significant (p < 0.05) alterations in the diversity and stability of bacterial and fungal communities in coastal saline soil (Figure 2). In general, both bacterial and fungal communities in amended soils processed more ASVs (richness) but lower average variation degrees (AVDs) as compared to those in the untreated soil (Figure 2a,b). Additionally, compositional diversity indices mentioned above were dosage-dependent with the highest values of richness and the lowest values of AVD observed in the S200 treatment compared to different treatments. As for structural diversity, the results derived from PCoA revealed that organic amendment significantly (PERMANOVE, p < 0.01) altered soil bacterial and fungal community structures (Figure 2c,d). The first and second principal components of PCoA ordination plots expressed, in total, 78.0% and 87.8% of the variations in bacterial communities and fungal communities across the four treatments, respectively. Similar results were shown by PCA that bacterial and fungal communities in amended soils comparatively separated from the control soil, with dominant bacterial phyla (e.g., Proteobacteria, Chloroflexi, Bacteroidetes, Acidobacteria, Gemmatimonadetes, etc.) and fungal phyla (e.g., Ascomycota, Chytridiomycota, Rozellomycota, Blastocladiomycota, Basidiomycota, etc.) accounting for the dissimilarity across treatments (Figure 2e,f).
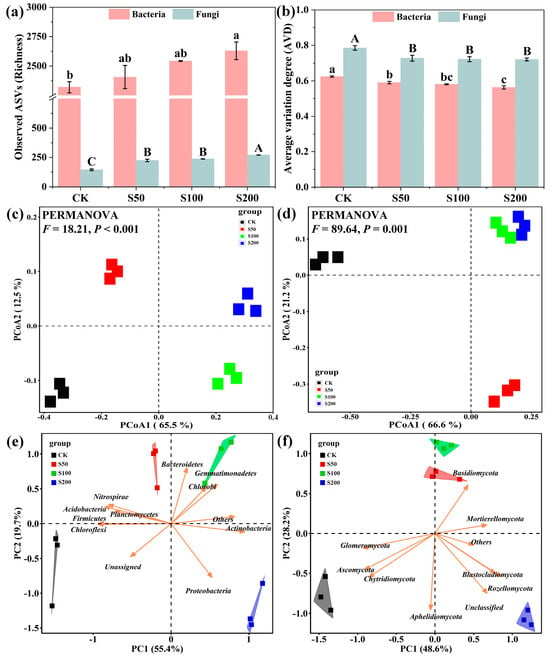
Figure 2.
Effects of sewage sludge applications on diversity and stability of soil microbial communities. (a,b) represent richness and AVD of bacterial and fungal communities, respectively; (c,d) denote structural diversities of bacterial and fungal communities, respectively; (e,f) represent PCA of taxonomic compositions (phyla level) of bacterial and fungal communities, respectively. Error bars denote standard errors, and different letters denote significant differences among treatments (Duncan’s test, p < 0.05).
3.4. Core and Unique Microbiomes of Soil Bacterial and Fungal Communities
Apparently, organic amendment significantly (p < 0.05) modulated core and unique taxonomic compositions (ASV levels) of soil bacterial and fungal microbiomes with differential core and unique compositional profiles observed in amended soils in comparison to the control soil (Figure 3a–d). For bacteriomes, a large proportion (46.3–60.7%) of the core ASVs were categorized into 15 bacterial families. ASVs affiliated with Pelobacteraceae (8.8%), Sinobacteraceae (7.0%), Thermomonosporaceae (7.8%), and Xanthomonadaceae (16.7%) were the predominant bacterial families in CK, S50, S100, and S200, respectively. Relative abundances (RAs) of dominant core bacterial families across treatments, including Xanthomonadaceae, Rhodospirillaceae, Micrococcaceae, and Pirellulaceae, were significantly higher in organic amended soils than those in the control treatment (Figure 3a). For unique ASVs, 27.6–56.4% of the unique ASVs were categorized into 20 families, and predominant unique ASVs changed from Pelobacteraceae in unamended soil to Chitinophagaceae (S50), Flavobacteriaceae (S100), and Xanthomonadaceae (S200) (Figure 3c). In terms of fungal microbiomes, the majority (91.4–98.2%) of the core ASVs were categorized into 15 fungal genera. ASVs affiliated with Stachybotrys (81.3%), Mortierella (19.5%), Trichoderma (25.0%), and Trichoderma (32.5%) were the predominant fungal genera in CK, S50, S100, and S200, respectively. In particular, dominant core ASVs assigned to the genera Trichoderma, successively followed by Fusarium, Mortierella, and Myriococcum, were significantly enriched in all amended soils, while Stachybotrys and Acremonium were depleted in amended soils in comparison to the control soil (Figure 3b). For unique ASVs, 69.8–89.0% of the unique ASVs were categorized into 20 genera, and predominant unique ASVs changed from Phialophora in unamended soil to Periconia (S50), Waitea (S100), and Coprinellus (S200) (Figure 3d). Of note, correlation analysis indicated that BD, pH, and EC exhibited significant relationships with contents of carbon, nitrogen, and phosphorus together with microbial enzyme activity (e.g., FDA, SUC, URE, and ALP). Additionally, Mantel test results showed bacterial and fungal core microbiomes were significantly related to the physical, chemical, and enzymatic attributes mentioned above in coastal salt-affected soils (Figure 3e).
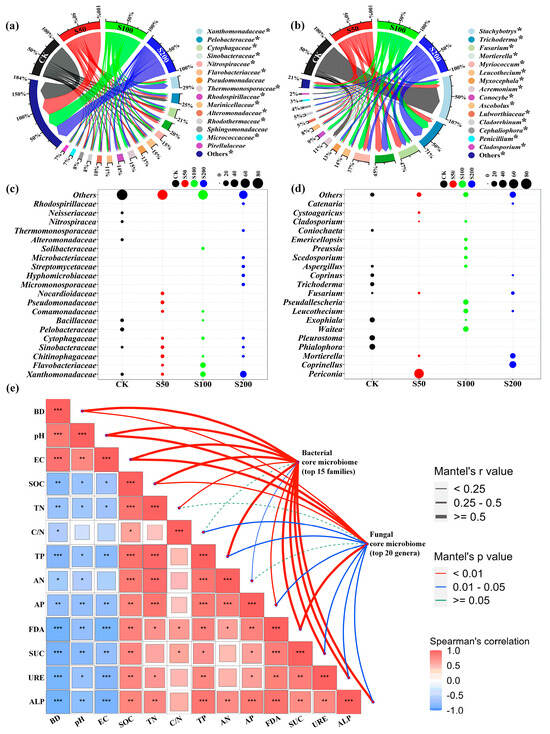
Figure 3.
Effects of sewage sludge applications on taxonomic compositions at ASV levels of soil microbial communities. (a,b) represent RAs of dominant core ASVs assigned to bacterial family and fungal genera across treatments, respectively; (c,d) represent RAs of dominant and unique ASVs assigned to bacterial family and fungal genera across treatments, respectively; “*” denotes significant differences among treatments (Duncan’s test, p < 0.05). (e) Core bacterial and fungal microbiomes are related to soil physical and chemical properties and enzyme activities using a partial Mantel test. “*”, “**”, and “***” in the box indicate significant correlations at 0.05, 0.01, and 0.001 levels according to Spearman’s rank correlation analysis. The width of the edges is the representation of Mantel’s r values between distance correlations, and the color of the edges is the representation of statistical significance based on 999 permutations.
3.5. Linking Physical, Chemical, and Microbial Properties to Aggregation Status in Coastal Salt-Affected Soils
VPA results showed that chemical properties (e.g., pH, EC, and SOC), enzyme activities (FDA and SUC), and average variation degree of bacterial and fungal communities were dominant components that significantly (PERMUTATIONS, p < 0.01) drove the variations in aggregation status in coastal salt-affected soils (Figure 4a). Overall, these abiotic and biotic variables explained 91% of the total variations in MWD, with the highest individual explanatory power observed in SUC (21.6%) successively followed by SOC (18.4%), bacterial AVD (13.1%), pH (12.9%), FDA (11.9%), EC (11.1%), and fungal AVD (8.1%).
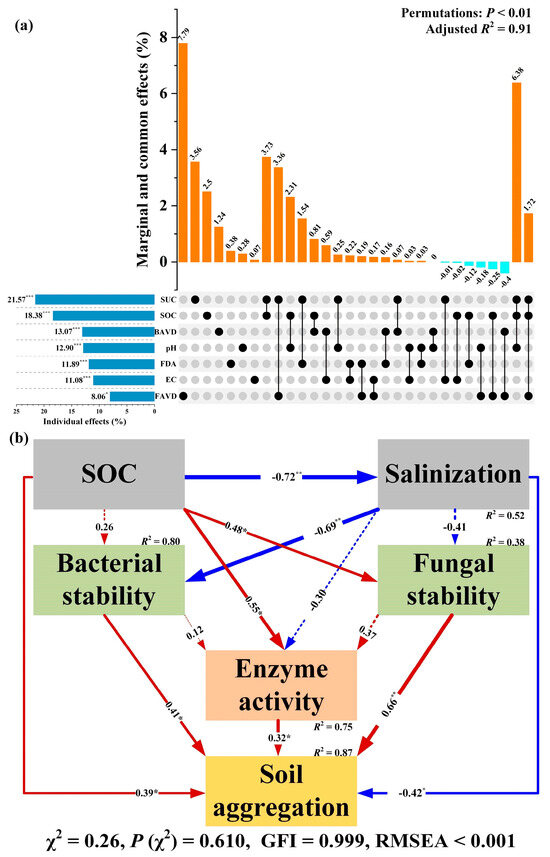
Figure 4.
Linking of soil physical and chemical factors and microbial communities to aggregation status in coastal saline soils. (a) represents the explanatory power of measured abiotic and biotic attributes with respect to variations in aggregation stability under different treatments. “BAVD” and “FAVD” indicate the average variation degree of bacterial and fungal communities, respectively. (b) SEMs exhibiting the correlations among soil chemical property, enzyme activity, microbial community, and aggregation status. Red and blue arrows denote positive and negative correlations, respectively. Arrow width corresponds to the magnitude of the association. Values adjacent to the arrows denote standardized path coefficients. R2 denotes the explained variance for induced variables. “*”, “**”, and “***” denote significant correlations at p < 0.05, p < 0.01, and p < 0.001, respectively.
Additionally, SEM analysis exhibited a good fit for the SOC, salinization (pH and EC), stability of bacterial and fungal communities (1-AVD), enzyme activity (FDA), and aggregation status (MWD) in coastal salt-affected soils (χ2 = 0.26, p (χ2) = 0.610, GFI = 0.999, RMSEA < 0.001) (Figure 4b). Specifically, SOC was found to be significantly and negatively (standardized path coefficient = −0.72, p < 0.001) correlated with soil salinization, which explained 52% of variations in salinization. Subsequently, microbial community stability was found to be positively related to SOC but negatively related to salinization, and the two variables totally explained 80% and 38% of variations in bacterial stability and fungal stability, respectively. Then, microbial enzyme activity was found to positively respond to the variations in SOC accompanied by bacterial stability and fungal stability, whereas it negatively responded to salinization variation. Finally, soil aggregation status was found to be significantly and positively related to SOC, bacterial and fungal community stabilities, and enzyme activity, but significantly and negatively related to salinization. Together, these abiotic and biotic driving factors jointly explained 87% of the variations in soil aggregation in coastal salt-affected soils.
Spearman’s rank-order correlation accompanied by linear regression analysis to further disclose potential microbial groups drove the improvement of microbial community stability and aggregation status (Table 3). The results showed that both bacterial and fungal core microbiomes (11 bacterial families and 10 fungal genera) exhibited significant correlations with microbial community stability as well as aggregation formation and stability. In particular, enriched core bacterial families in sewage sludge-amended soils, including Xanthomonadaceae, Rhodospirillaceae, and Micrococcaceae, and fungal genera, including Trichoderma, Myxocephala, and Penicillium, were found to be significantly and positively related to microbial stability and aggregation status simultaneously.

Table 3.
Correlations between core microbial populations, microbial community stability, and soil aggregate formation and stabilization.
4. Discussion
4.1. Organic Amendment Significantly Promoted Soil Agglomeration
Organic-rich ameliorant application has been increasingly demonstrated to be an effective agricultural regime for soil structure improvement [30,31]. Organic amended soils had more and larger aggregates (Figure 1a), reflecting that organic amendment is conducive to the formation of macroaggregates in coastal salt-affected areas. This was in accordance with our previous investigations that found that applying organic ameliorants, such as sewage sludge, manure, and vermicompost, is capable of facilitating the cementation of aggregate particles from smaller ones to larger ones in coastal salt–alkali soils [32]. This was likely attributed to the organic-induced alleviation of abiotic constraints (e.g., decreased pH and salinity or elevated SOC stock) and diversification of microbial community and functioning (e.g., increased richness or enhanced enzyme activity), as these environmental factors are deeply involved in macroaggregate formation [33]. Additionally, higher MWD and GMD, but lower fractal dimension D measurements (Figure 1b,c), revealed that organic amendments enhanced soil aggregate stability, which was in line with previous observations that showed the positive impacts of sewage sludge application on soil aggregate stabilization [34,35]. The reasons for this lie in the fact that soil aggregate stability is typically found to be strongly associated with aggregate particle sizes, exhibiting an increase in stability corresponding to a higher proportion of macroaggregates. It is likely that organic amendments facilitate the cementation of soil microaggregates, thereby promoting macroaggregate formation, which is expected to exhibit greater stability and resilience against dispersive forces [20]. It is noteworthy that the aforementioned conclusions are solely inferences derived from particle size measurements. To comprehensively elucidate the causes and mechanisms behind the improved aggregate stability in organic amended coastal salt-affected soils, it is imperative to conduct further investigations based on the determinations of the hierarchical order of aggregate breakdown, scanning electron microscopy observations of particle structures with various sizes, and the analysis of functional groups [5,36]. Interestingly, impacts of sewage sludge on aggregate formation and stabilization exhibited a dosage-dependent tendency with a more pronounced promotion of macroaggregate proportions and stability observed in the larger-amount ameliorant-treated soils in comparison to those in the smaller-amount ones (Figure 1). Consistent with previous studies in the literature, this phenomenon might be partially attributed to the scarcity of binding agents that participate in soil agglomeration (e.g., carbohydrates, proteins, mineral particles, microbial metabolites, and functional microbes) in soils amended by inadequate sewage sludge [37]. Collectively, these results implied that organic amendment was a feasible and effective agricultural regime for soil agglomeration in coastal mudflat areas, which provides a reliable guarantee for the improvement of the soil structure of coastal saline–alkali land.
4.2. Organic Amendment Facilitated Microhabitat Amelioration and Microbial Community Diversification and Stabilization
The application of organic ameliorants has been extensively recognized as an effective agricultural strategy for mitigating adverse factors in coastal salt-affected soils affected by salinity. The present study revealed that soils amended with sewage sludge exhibited significantly lower pH and salinity levels, alongside increased SOC stock and nutrient retention (Table 1), which aligns with previous investigations indicating that the organic amendments can effectively alleviate environmental constraints in salt-affected coastal lands [7]. The observed reduction in pH in amended soils may be due to the neutralizing effects of the lower-pH sewage sludge. Additionally, released acidic compounds during the decomposition of the input organic materials may also contribute to the observed reduction in soil pH [38]. Applying sewage sludge exhibited positive effects on SOC stock, which is mainly due to the abundant organic substances introduced by exogenous organic materials. Moreover, more aggregates with larger particle sizes and enhanced aggregate stability might also partially contribute to the SOC elevation, as they have been proven to be conducive to SOC sequestration and protection [39]. Additionally, enriched core and unique microbial species in bacterial and fungal microbiomes, such as Xanthomonadaceae and Mortierella (Figure 3a–d), might also participate in SOC storage due to their functions associated with carbon turnover [40,41]. In terms of soil salinity reduction, improved soil structure with more non-capillary pores might facilitate the downward leaching of salts within the plow layer [42].
In addition to the alleviation of abiotic constraints, it is widely recognized that soil biological characteristics, particularly microbial communities, exhibit positive responses to changes in microhabitats induced by organic amendments [43,44,45]. In the current study, organic amendment significantly altered soil bacterial and fungal communities’ compositional and structural diversities (Figure 2 and Figure 3), suggesting that organic amendment was conducive to the reassemblage of microbiomes in coastal salt-affected soils. Specifically, results derived from the Mantel test revealed that both bacterial and fungal core microbiomes were significantly related to physical and chemical properties measured in the current study (Figure 3e), indicating that soil core microbiomes were profoundly influenced by their surrounding microhabitat conditions [43,46]. For instance, pH and salinity are increasingly viewed as dominant abiotic agents driving soil microbial community, and correspondingly, alleviated salt–alkali stress has been proven to enable the facilitation of the flourishment of microbial populations with relatively narrow pH and salinity spectra in coastal salt-affected soils [21]. Simultaneously, significant correlations among microbial communities and contents of carbon, nitrogen, and phosphorus implied that abundant carbon substrates, energy substances, and nutrients contributed to the growth and proliferation of microorganisms, which eventually facilitated the evolution of microbial communities in organic amended coastal salt-affected soils [6,7]. It is worth noting that a significant decline in average variation degree (AVD) of bacterial and fungal microbiomes was found in organic amended soils (Figure 2b), suggesting that both bacterial and fungal communities’ stability enhanced as a result of organic amendment, which might partially be attributed to the specific enrichment and strengthened connections of dominant microbial groups in soils amended by sewage sludge (Figure 2e,f and Table 3). Of note, distinct microbial community structures were observed in soils amended by sewage sludge with different applications (Figure 2c,d). One potential explanation is that varying application rates of exogenous organic materials resulted in differences in the degree of soil microbial enrichment. Conversely, the utilization patterns of the imported carbon sources by the differentially enriched microorganisms were inconsistent, leading to variations in the organic compositions within the soil and ultimately resulting in differentiation of the microbial community structure. Regrettably, from the limited data available, we cannot see evidence for organic composition differences within soils across treatments; more investigations are essential with respect to the organic compositions of different exogenous organic ameliorants and related soils. Overall, these outcomes corroborated the idea that organic-induced ameliorations in microhabitat conditions enabled the facilitation of the diversification and stabilization of microbial communities in coastal areas.
4.3. Organic Amendments Promoted Soil Agglomeration Mainly via Alleviating Abiotic Constraints and Stabilizing and Functionalizing Microbiomes
Soil chemical factors, such as SOC, pH, and salinity, have been widely demonstrated to be crucial abiotic components driving aggregation status [7,10,11]. Specifically, results derived from VPA accompanied by SEM (Figure 4) showed that SOC exerted predominant positive impacts on aggregate stability when compared to other abiotic determinants, which was likely due to the fact that organic substances originating from input organic ameliorants contribute to soil agglomeration via reducing aggregate wettability and promoting cementation of organic matters and mineral particles [36,47,48,49]. Soil pH and salinity are generally recognized as flocculating and dispersive factors in soil aggregate destruction, and, correspondingly, alleviation in pH and salinization enabled the formation and stabilization of macroaggregates in organic amended coastal salt-affected soils [7,10]. It is noteworthy that the alleviated abiotic factors mentioned above can not only directly facilitate soil aggregation status but also indirectly promote soil agglomeration via driving reassemblages of soil bacterial and fungal microbiomes possessing versatile functional microbial species [50]. In this study, SOC- and salinization-induced enzyme activity and microbial community stability were found to be significantly correlated with soil aggregation status (Figure 4), which was in accordance with earlier investigations that found that soil agglomeration is a microbiome-driven process [51,52]. Particularly, microbial enzyme activities exhibited a significant and positive influence on soil aggregation, indicating that enhanced microbial activity in amended soils might directly participate in aggregate formation and stabilization (Table 2 and Table 3 and Figure 4). Additionally, core bacterial and fungal populations specifically and positively respond to the modifications in SOC, and salinization might be the most responsible for the higher microbial community stability observed in amended soils, which has been increasingly considered one of the most important ecologically associated bio-indicators in soil structure [15]. Of these microbial species, significantly enriched ASVs affiliated with bacterial families Xanthomonadaceae, Rhodospirillaceae, and Micrococcaceae have been reported to be closely related to a higher production of exopolysaccharides (EPSs) and lipopolysaccharides (LPSs) [53,54,55], which play crucial roles during the binding of organo-mineral complexes [56]. Similarly, ASVs belonging to fungal genera Trichoderma, Cephaliophora, Mortierella, and Penicillium might be vital pioneer microbial populations that participate in relevant functions associated with soil agglomeration [40,57,58,59]. Together, our results suggested that abiotic constraint alleviation (e.g., SOC elevation and salinization mitigation) was conducive to the stabilization and functionalization of soil bacterial and fungal microbiomes, which jointly contributed to soil agglomeration in organic amended coastal salt-affected soils.
5. Conclusions
The results in the current study suggested that ameliorated microhabitat conditions with elevated SOC storage and mitigated salinization were conducive to the stabilization and functionalization of bacterial and fungal microbiomes, and the abiotic and biotic components jointly drove aggregate formation and stabilization in sewage sludge-amended coastal salt-affected soils. Specifically, the impacts of exogenous organic ameliorants on abiotic constraint alleviation and soil agglomeration, as well as reassemblage of microbial communities, exhibited dosage-dependent tendencies with the more pronounced effectiveness observed in higher-amount sewage sludge-amended soils. Particularly, potential exopolysaccharide- and lipopolysaccharide-producing bacterial populations belonging to Xanthomonadaceae, Rhodospirillaceae, and Micrococcaceae, together with agglomeration-facilitating fungal groups affiliated with Trichoderma, Cephaliophora, Mortierella, and Penicillium, might directly participate in forming and stabilizing aggregates in organic amended coastal salinized soils. These outcomes will offer valuable guidance for improving coastal salt-affected soil by applying organic amendments. Subsequent research should be conducted to investigate the effects of applying organic ameliorants with different organic source types on their potential in agricultural production in coastal salt-affected areas.
Author Contributions
Conceptualization, Y.S. and Y.B.; methodology, L.J. and Z.H.; software, M.W., M.F. and X.L.; validation, W.Z.; formal analysis, L.J. and Z.H.; investigation, M.W., M.F. and X.L.; data curation, M.W., M.F. and X.L.; project administration, J.Y. and Y.Y.; resources, Y.Y.; writing—original draft preparation, Y.L.; writing—review and editing, Y.L.; visualization, Y.L.; supervision, J.Y. and W.Z.; funding acquisition, Y.S. and Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA0440402), the Research Fund for Agricultural Science and Technology of Jiangsu Province (CX[23]1019), and the Research Fund for Jiangsu Agricultural Industry Technology System (JATS[2023]317 and JATS[2023]318) for Yanchao Bai.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, J.; Liu, D.Y.; Gong, H.R.; Liu, Z.; Zhang, Y.T. Crop yield increments will enhance soil carbon sequestration in coastal arable lands by 2100. J. Clean. Prod. 2023, 432, 139800. [Google Scholar] [CrossRef]
- Su, X.S.; Wang, Y.Q.; Wang, G.X.; Zhang, Y.; Gong, X.L.; Yu, J.; Gou, F.G.; Lyu, H. Assessment and prediction of coastal saline soil improvement effects combining substrate amendments and salt barrier materials in typical region of the Yangtze River Delta. Soil Tillage Res. 2022, 223, 105483. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Srivastava, A.K.; Pandey, G.K.; Suprasanna, P. Halophytes in biosaline agriculture: Mechanism, utilization, and value addition. Land Degrad. Dev. 2018, 29, 1081–1095. [Google Scholar] [CrossRef]
- Li, Y.L.; Chen, W.F.; Xu, Y.W.; Yu, J.; Zuo, W.G.; Shan, Y.H.; Bai, Y.C. Environmental constraints mitigation directly drove the diversifications of fungal community and functional profile in amended coastal salt-affected soils. Agronomy 2024, 14, 2772. [Google Scholar] [CrossRef]
- Bai, Y.C.; Xue, W.J.; Yan, Y.Y.; Zuo, W.G.; Shan, Y.H.; Feng, K. The challenge of improving coastal mudflat soil: Formation and stability of organo-mineral complexes. Land Degrad. Dev. 2018, 29, 1074–1080. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, J.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till. Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Merino-Martín, L.; Stokes, A.; Gweon, H.S.; Moragues-Saitua, L.; Staunton, S.; Plassard, C.; Griffiths, R.I. Interacting effects of land use type, microbes and plant traits on soil aggregate stability. Soil Biol. Biochem. 2021, 154, 108072. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Y.Z.; Zhou, Y.Y.; Zhou, X.; Zhou, W.J. Soil organic carbon and soil aggregate stability associated with aggregate fractions in a chronosequence of citrus orchards plantations. J. Environ. Manag. 2021, 293, 112847. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.J.; Chen, Q.F.; Wu, L.F.; Yang, H.J.; Xu, J.K.; Zhang, Y.P. Coastal saline soil aggregate formation and salt distribution are affected by straw and nitrogen application: A 4-year field study. Soil Till. Res. 2020, 198, 104535. [Google Scholar] [CrossRef]
- Feng, H.J.; Wang, S.Y.; Gao, Z.D.; Pan, H.; Zhuge, Y.P.; Ren, X.Q.; Hu, S.W.; Li, C.L. Aggregate stability and organic carbon stock under different land uses integrally regulated by binding agents and chemical properties in saline-sodic soils. Land Degrad. Dev. 2021, 32, 4151–4161. [Google Scholar] [CrossRef]
- Wu, L.P.; Wang, Y.D.; Zhang, S.R.; Wei, W.L.; Kuzyakov, Y.; Ding, X.D. Fertilization effects on microbial community composition and aggregate formation in saline-alkaline soil. Plant Soil 2021, 463, 523–535. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.S.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Guhra, T.; Stolze, K.; Totsche, K.U. Pathways of biogenically excreted organic matter into soil aggregates. Soil Biol. Biochem. 2022, 164, 108483. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.; Blouin, M.; Brown, G.; Decaëns, T.; Grimaldi, M.; Jiménez, J.J.; McKey, D.; Mathieu, J.; Velasquez, E.; et al. Ecosystem engineers in a self-organized soil: A review of concepts and future research questions. Soil Sci. 2016, 181, 91–109. [Google Scholar] [CrossRef]
- Hale, L.; Curtis, D.; Leon, N.; McGiffen, M., Jr.; Wang, D. Organic amendments, deficit irrigation, and microbial communities impact extracellular polysaccharide content in agricultural soils. Soil Biol. Biochem. 2021, 162, 108428. [Google Scholar] [CrossRef]
- Landeweert, R.; Hoffland, E.; Finlay, R.D.; Kuyper, T.W.; van Breemen, N. Linking plants to rocks: Ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 2001, 16, 248–254. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.Y.; Liang, Y.Q.; Liang, Y.Y.; Zhao, Y.T.; Wang, Z.C.; Li, Y.Z.; Liu, W.C.; Wang, X.; Yang, G.H.; et al. The multifunctionality of soil aggregates is related to the complexity of aggregate microbial community during afforestation. Catena 2024, 236, 107737. [Google Scholar] [CrossRef]
- Wu, Q.C.; Chen, Y.; Dou, X.H.; Liao, D.X.; Li, K.Y.; An, C.C.; Li, G.H.; Dong, Z. Microbial fertilizers improve soil quality and crop yield in coastal saline soils by regulating soil bacterial and fungal community structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef]
- Li, Y.L.; Shen, C.; Wang, Y.M.; Xu, L.; Zhao, Y.L.; Yi, S.Q.; Zuo, W.G.; Yao, R.J.; Zhang, X.; Gu, C.H.; et al. Alleviated environmental constraints and restructured fungal microbiome facilitate aggregate formation and stabilization in coastal mudflat saline soil amended by sewage sludge. Land Degrad. Dev. 2023, 34, 3064–3075. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, Y.M.; Gu, C.H.; Shen, C.; Xu, L.; Zhao, Y.L.; Yi, S.Q.; Zuo, W.G.; Shan, Y.H.; Zhang, Z.Q.; et al. Differential effects of organic ameliorants on the reassembly of bacterial communities in newly amended coastal mudflat salt-affected soil. Agronomy 2022, 12, 2525. [Google Scholar] [CrossRef]
- IUSS Working Group WRB (International Union of Soil Science Working Group on World Reference Base). World Reference Base for Soil Resources 2006 (First Update 2007); World Soil Resources Reports No. 103; FAO: Rome, Italy, 2007. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agricultural Chemical Analysis Method; China Agricultural Science Technology Press: Beijing, China, 2000. [Google Scholar]
- Hong, Y.H.; Zhao, D.; Zhang, F.Z.; Shen, G.N.; Yuan, Y.; Gao, Y.M.; Yan, L.; Wei, D.; Wang, W.D. Soil water-stable aggregates and microbial community under long-term tillage in black soil of Northern China. Ecotoxicology 2021, 30, 1754–1768. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.J.N. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.F.; Cui, X.S.; Zhu, N.; Zhu, X.Q.; Wang, X.L.; Wang, Y.G. Characterization of root microbial communities associated with Astragalus membranaceus and their correlation with soil environmental factors. Rhizosphere 2023, 25, 100656. [Google Scholar] [CrossRef]
- Chen, Q.L.; An, X.L.; Li, H.; Su, J.Q.; Ma, Y.B.; Zhu, Y.G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92, 1–10. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Caravaca, F.; Alguacil, M.M.; Azcón, R.; Roldán, A. Formation of stable aggregates in rhizosphere soil of Juniperus oxycedrus: Effect of AM fungi and organic amendments. Appl. Soil Ecol. 2006, 33, 30–38. [Google Scholar] [CrossRef]
- Liu, M.L.; Wang, C.; Wang, F.Y.; Xie, Y.J. Vermicompost and humic fertilizer improve coastal saline soil by regulating soil aggregates and the bacterial community. Arch. Agron. Soil Sci. 2019, 65, 281–293. [Google Scholar] [CrossRef]
- Hanay, A.; Büyüksönmez, F.; Kiziloglu, F.M.; Canbolat, M.Y. Reclamation of saline-sodic soils with gypsum and MSW compost. Compost Sci. Util. 2004, 12, 175–179. [Google Scholar] [CrossRef]
- Xiao, R.; Guo, Y.T.; Zhang, M.X.; Pan, W.B.; Wang, J.J. Stronger network connectivity with lower diversity of soil fungal community was presented in coastal marshes after sixteen years of freshwater restoration. Sci. Total Environ. 2020, 744, 140623. [Google Scholar] [CrossRef]
- Curci, M.; Lavecchia, A.; Cucci, G.; Lacolla, G.; Corato, U.D.; Crecchio, C. Short-term effects of sewage sludge compost amendment on semiarid soil. Soil Syst. 2020, 4, 48. [Google Scholar] [CrossRef]
- Ferreras, L.; Gómez, E.; Toresani, S.; Firpo, I.; Rotondo, R. Effect of organic amendments on some physical, chemical and biological properties in a horticultural soil. Bioresource Technol. 2006, 97, 635–640. [Google Scholar] [CrossRef]
- Oades, J.M.; Waters, A.G. Aggregate hierarchy in soils. Soil Res. 1991, 29, 815–828. [Google Scholar] [CrossRef]
- Sandoval-Estrada, M.; Celis-Hidalgo, J.; Stolpe-Lau, N.; Capulín-Grande, J.J.A. Effect of sewage sludge and salmon wastes amendments on the structure of an Entisol and Alfisol in Chile. Agrociencia 2010, 44, 503–515. [Google Scholar] [CrossRef]
- Rashid, I.; Murtaza, G.; Zahir, Z.A.; Farooq, M. Effect of humic and fulvic acid transformation on cadmium availability to wheat cultivars in sewage sludge amended soil. Environ. Sci. Pollut. Res. 2018, 25, 16071–16079. [Google Scholar] [CrossRef]
- Aminiyan, M.M.; Sinegani, A.A.S.; Sheklabadi, M. Aggregation stability and organic carbon fraction in a soil amended with some plant residues, nanozeolite, and natural zeolite. Int. J. Recycling Org. 2015, 4, 11–22. [Google Scholar] [CrossRef]
- Zhang, L.M.; Luo, Y.H.; Wang, Y.; Zhang, C.F.; Cai, G.J.; Su, W.C.; Yu, L.F. Key microorganisms influencing mineral-protected organic carbon formation in soils with exogenous carbon addition. Agronomy 2024, 14, 2333. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Redmile-Gordon, M.; Zhang, J.B.; Zhang, C.Z.; Ning, Q.; Li, W. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad. Dev. 2018, 29, 1642–1651. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijakowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindi, S.; Abed, R.M. Effect of biostimulation using sewage sludge; soybean meal; and wheat straw on oil degradation and bacterial community composition in a contaminated desert soil. Front. Microbiol. 2016, 7, 240. [Google Scholar] [CrossRef] [PubMed]
- Mattana, S.; Petrovičová, B.; Landi, L.; Gelsomino, A.; Cortés, P.; Ortiz, O.; Renella, G. Sewage sludge processing determines its impact on soil microbial community structure and function. Appl. Soil Ecol. 2014, 75, 150–161. [Google Scholar] [CrossRef]
- Guevara, R.; Ikenaga, M.; Dean, A.L.; Pisani, C.; Boyer, J.N. Changes in sediment bacterial community in response to long-term nutrient enrichment in a subtropical seagrass-dominated estuary. Microb. Ecol. 2014, 68, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 4, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.; Johnson, M.G. Advances in understanding the molecular structure of soil organic matter: Implications for interactions in the environment. Adv. Agron. 2010, 106, 77–142. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. Sorptive stabilization of organic matter by microporous goethite: Sorption into small pores vs. surface complexation. Eur. J. Soil Sci. 2007, 58, 45–59. [Google Scholar] [CrossRef]
- Aksakal, E.L.; Sari, S.; Angin, I. Effects of vermicompost application on soil aggregation and certain physical properties. Land Degrad. Dev. 2016, 27, 983–995. [Google Scholar] [CrossRef]
- Li, P.; Kong, D.N.; Zhang, H.J.; Xu, L.Y.; Li, C.K.; Wu, M.C.; Jiao, J.G.; Li, D.M.; Xu, L.; Li, H.X.; et al. Different regulation of soil structure and resource chemistry under animal-and plant-derived organic fertilizers changed soil bacterial communities. Appl. Soil Ecol. 2021, 165, 104020. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil aggregate microbial communities: Towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Tong, L.H.; Zhu, L.; Lv, Y.Z.; Zhu, K.; Liu, X.Y.; Zhao, R. Response of organic carbon fractions and microbial community composition of soil aggregates to long-term fertilizations in an intensive greenhouse system. J. Soil. Sediment. 2020, 20, 641–652. [Google Scholar] [CrossRef]
- Yao, J.C.; Yao, G.J.; Wang, Z.H.; Yan, X.J.; Lu, Q.Q.; Li, W.; Liu, Y.D. Bioaugmentation of intertidal sludge enhancing the development of salt-tolerant aerobic granular sludge. J. Environ. Manag. 2023, 325, 116394. [Google Scholar] [CrossRef] [PubMed]
- Asif, T.; Javed, U.; Zafar, S.B.; Ansari, A.; Qader, S.A.U.; Aman, A. Bioconversion of colloidal chitin using novel chitinase from Glutamicibacter uratoxydans exhibiting anti-fungal potential by hydrolyzing chitin within fungal cell wall. Waste Biomass Valor. 2020, 11, 4129–4143. [Google Scholar] [CrossRef]
- Adia, M.H.; Jacques, M.A.; Koebnik, R. Adhesion mechanisms of plant-pathogenic Xanthomonadaceae. In Advances in Experimental Medicine and Biology; Bacterial Adhesion; Springer: Dordrecht, The Netherlands, 2011; Volume 715, pp. 71–89. [Google Scholar] [CrossRef]
- Vuko, M.; Cania, B.; Vogel, C.; Kublik, S.; Schloter, M.; Schulz, S. Shifts in reclamation management strategies shape the role of exopolysaccharide and lipopolysaccharide-producing bacteria during soil formation. Microb. Biotechnol. 2020, 13, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.X.; Cao, M.M.; Sang, C.C.; Li, T.X.; Zhang, Y.J.; Chang, Y.X.; Li, L.L. Trichoderma bio-fertilizer decreased C mineralization in aggregates on the southern North China Plain. Agriculture 2022, 12, 1001. [Google Scholar] [CrossRef]
- Kinsbursky, R.; Levanon, D.; Yaron, B. Role of fungi in stabilizing aggregates of sewage sludge amended soils. Soil Sci. Soc. Am. J. 1989, 53, 1086–1091. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Chen, L.; Zhu, F.; Wang, Y.J.; Jiang, J.; Chen, K.B.; Xue, S.G. Stable aggregate formation and microbial diversity resilience in soil formation of bauxite residue: Roles of extracellular polymeric substances secreted by Penicillium oxalicum. ACS EST Eng. 2023, 3, 1758–1769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).