Abstract
In the context of global climate change, the frequency of waterlogging is increasing. Therefore, to elucidate the effects of waterlogging under real precipitation conditions on the physiological characteristics of peanuts and the underlying mechanics and to provide a theoretical basis for timely protective measures, this study involved a waterlogging disaster simulation experiment in the field environment and a waterlogging stress control experiment in the potting environment. It was found that sufficient water had a positive effect on the growth and development of peanuts (Arachis hypogaea L.) during the 3–5 days period at the beginning of waterlogging. However, as the duration of waterlogging increased, excess water inhibited the growth of peanuts, with a stronger inhibitory effect on the development of pods. A comparison of the two different experimental models found that in the potting environment, water circulation was not smooth, and the intensity of waterlogging was higher than in the field environment experiment, resulting in the effect of waterlogging being advanced by one observation stage (2 days) in the potting environment. Furthermore, using a novel fluorescence imaging system, an analysis of variations in the physiological characteristics of leaf sections demonstrated that the chlorophyll fluorescence in the leaves of the peanut plant exhibited a specific pattern in response to waterlogging stress.
1. Introduction
Since the middle of the 20th century, there has been abundant evidence of dramatic changes in the global climate [1,2,3,4]. The Sixth Assessment Report of the IPCC has unequivocally stated that climate change is now widespread, rapid, and intense, with magnitudes that have not been recorded for thousands of years [5]. Temperatures have increased more rapidly than the Earth’s natural cyclical patterns [6], resulting in a higher frequency of extreme catastrophic events [7,8]. In recent decades, the number of flooding events has increased worldwide and has had a considerable impact on agricultural production due to the waterlogging phenomenon [9,10]. Approximately 12% of the world’s agricultural land is severely affected by waterlogging stress, leading to significant reductions in crop yields [11,12]. China is a largely agricultural country, and its arable land accounts for 7% of the world’s arable land [13]. However, frequent flooding has greatly limited the development of agriculture in China [14]. Such extreme flooding disasters have a serious impact on food security. For the majority of major grain-producing provinces, the probability of a 10% reduction in grain output is greater than 90% in the context of a 100-year flood disaster [15].
Peanut (Arachis hypogaea L.) is an important food and oil crop worldwide, cultivated on approximately 26 million hectares across 120 countries, yielding 35 to 40 million tons of peanut pods annually [16]. According to the Food and Agriculture Organization (FAO), global peanut production in 2022 is projected to exceed 54 million tons, with an average yield of about 1.8 tons per hectare. The leading peanut exporters are China, India, the United States, and Argentina [17]. Among the development stages of peanuts, the podding stage is the growth period of pods and seed kernels. At this stage, excessive water can hinder ovary development and pod expansion, making it the most susceptible stage to waterlogging [18,19]. For most crops, waterlogging can restrict root growth, reduce dry matter accumulation, cause premature leaf senescence, increase wilting and the production of sterile flowers, and reduce grain weight and yield [20,21,22]. For peanuts in the podding stage, waterlogging stress also significantly reduces the number and weight of pods per plant [23,24]. As the duration of waterlogging increases, the pods may develop discoloration, deterioration, and mold, ultimately leading to a decrease in peanut productivity [18,25].
When plants are subjected to environmental stress, various photosynthetic and chlorophyll fluorescence parameters are more effective for detecting the processes and status of growth and development during the stress period [26,27]. Medrano et al. [28] found that when plants were stressed, the stomatal conductance and net photosynthetic rate were affected and that the intercellular carbon dioxide concentration determined the rate of photosynthesis. Zhao et al. [29] also found that when wheat was subjected to stresses such as drought, a range of photosynthetic parameters were reduced, alongside significant reductions in wheat’s plant height, biomass, and grain number. Changes in the function of chlorophyll often precede changes in the chlorophyll content, and when environmental stresses are present, changes in chlorophyll function can be observed using chlorophyll fluorescence techniques long before the leaves are altered [30]. Rao et al. [31] employed an indoor simulated flooding method to study the morphology of mulberry seedling leaves and the changes in leaf chlorophyll fluorescence parameters and fluorescence imaging under different flooding times and depths and concluded that mulberry has high flooding tolerance due to a combination of morphological and physiological responses. By observing and measuring root morphology, chlorophyll fluorescence, chlorophyll content, and leaf gas exchange, Zhang et al. [32] found that the initial fluorescence and variable fluorescence increased under waterlogging stress, the maximum fluorescence did not change significantly, and the electron transport rate, photochemical quenching, and actual quantum yield of PSII decreased. Therefore, photosynthetic parameters and fluorescence parameters can provide a clearer and more comprehensive understanding of the basic physiological responses of peanuts during waterlogging at the podding stage [19].
To date, there have been few studies involving simulation experiments investigating the effects of short-term waterlogging disasters on peanuts during the podding period. Therefore, in this study, we simulated the short-term waterlogging environment triggered by extreme precipitation by setting up a waterlogging disaster simulation experiment in the field environment and a waterlogging stress control experiment in the potting environment according to the characteristics of extreme precipitation in this region (Henan Province, China). The correlations among variables, such as the net photosynthetic rate, stomatal conductance, and real quantum efficiency of PSII, were analyzed by measuring the photosynthetic and chlorophyll fluorescence parameters of peanuts to determine the changes in their physiological characteristics under waterlogging. The effects of short-term waterlogging on the biomass of various parts of peanut were also determined, and the relationship between the biomass of each part in response to changes in the photosynthetic and chlorophyll fluorescence characteristics was analyzed. Meanwhile, the variations in fluorescence in different sections of peanut leaves were analyzed to explore the response mechanism of photosynthesis in different sections of the leaves to waterlogging stress. Finally, the effects of short-term waterlogging caused by extreme precipitation on the growth status of peanuts were elucidated, laying the foundation for the subsequent establishment of waterlogging disaster indexes and a reliable integrated method of evaluating peanut’s tolerance to waterlogging.
2. Materials and Methods
2.1. Study Site and Background
This study was conducted in a peanut (Arachis hypogaea L.) demonstration field at the National Observatory in Zhengzhou, Henan Province. Henan is located in the middle of China and is one of the main peanut-producing areas. Since Henan is in the subtropical monsoon climate zone, precipitation increases significantly in July and August each year, and extreme precipitation can cause a large number of peanut fields to be flooded, affecting the growth and development of peanuts. Peanuts sown in summer have a fertility period of approximately 113 days and a podding period of approximately 45 days. In this experiment, 10 days of the podding period were selected for the experiment, namely, from 29 August 2023 to 7 September 2023. As illustrated in Figure 1, the experiment was conducted under conditions of normal weather.
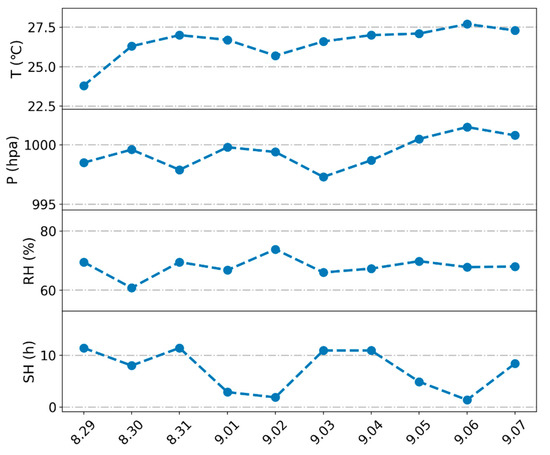
Figure 1.
Variations in individual meteorological elements during the waterlogging experiments. The abbreviation T denotes temperature, P denotes pressure, RH denotes relative humidity, and SH denotes sunshine hours.
2.2. Plant Material and Experimental Treatments
The material was the main variety of peanuts planted in Henan, namely, pearl bean-type peanut (Yu Hua 22, Zhengzhou, China), which has been bred for high and stable yield, drought and flood resistance, multi-resistant properties, and wide adaptability. This variety has been planted in areas across China, including Hebei, Anhui, Jiangxi, and other provinces with significant peanut production.
To more comprehensively analyze the effects of different days of waterlogging on the physiological characteristics of peanuts at the podding stage, two experimental protocols were designed in this study. One was a waterlogging disaster simulation experiment in a field environment, and the other was a waterlogging stress control experiment in a potting environment. The first experiment simulated the waterlogging disaster environment caused by extreme precipitation in a real field environment, while the control experiment waterlogging stress controlled the duration of waterlogging and created waterlogging stress in a traditional potting environment.
①: Waterlogging disaster simulation experiment in the field environment
The field environment plots were rectangular in shape, with a length of 4 m and a width of 3 m. Each plot was separated by a 2.5 m deep plastic film, and 3 replicated plots were set up for each treatment level. Each plot was sown in 10 rows of 18 holes, with 2 seeds per hole, for a total of 150,000 seeds per hectare, and the sowing depth was 3–5 cm. Experimental groups with different durations of waterlogging were set up, namely, the control check (CK, 0 days), 3 days, 5 days, 7 days, and 9 days, based on the precipitation (Table 1). In the control treatment group, the soil moisture was maintained at 65%. In the remaining groups of waterlogging experiments, the waterlogging treatment was achieved by maintaining a depth of accumulated water exceeding 2 cm at the root of the peanut plant through daily watering and the use of shade nets to block light during the waterlogging treatment to simulate the process of sustained precipitation. Once the waterlogging treatment ceased, watering was terminated, and the shade net was opened to align the growing environment with that of the control. For example, in the 3-day waterlogging experiment, watering was ceased on September 1st, without treatment, to allow the peanuts to recuperate autonomously in a manner that simulated the environmental conditions that prevail when precipitation ceases. Once the soil moisture content reached 65%, the plots were treated in the same manner as the control plots.

Table 1.
The number of consecutive rainy days at 119 meteorological stations in Henan Province from 1993 to 2023.
②: Waterlogging stress control experiment in the potting environment
Potting experiments were conducted in which round pots with a diameter of 30 cm and a height of 30 cm were buried in rectangular plots with a length of 6.8 m and a width of 1.6 m. Each of the different waterlogging treatment experiments involved burying 9 pots in square plots where the length of each side was 1 m. The pots were sown with 2 holes, with 2 seeds per hole, while 2 rows totaling 0.3 m of protected rows were planted around each set of potting plots. The presence of protective rows avoided the influence of the surrounding environment. The potting environment isolated the plants’ roots and prevented them from interfering with each other. The soil environment and nutrient status used for the potting environment were consistent with the field environment. In the control experiment of waterlogging stress in the potting environment, the experiment was also carried out with five different sets of waterlogging days, namely, the control CK (0 days), 3 days, 5 days, 7 days, and 9 days, consistent with the waterlogging disaster simulation experiment in the field environment. The control and each of the experimental waterlogging groups were treated consistently with the field environment.
In all experiments, the sowing time of peanuts was 27 May 2023. This was chosen because the podding period of Yuhua 22 takes approximately 45 days. Therefore, in this experiment, we selected the point when kernels had begun to enter the period of fullness in the podding period as the experimental time. That is, the waterlogging treatment started on August 29th. In the case of precipitation or rainy weather, the treatment plots would be covered with rainproof cloth, but there was no precipitation during this trial.
2.3. Determination of Photosynthesis Parameters
To ascertain the photosynthetic rate of peanut leaves, a sunny and cloudless day was selected for the use of the LI-6400 portable photosynthesizer from LI-COR, Tucson, AZ, USA. The light response curve was quantified by the integrated LED red and blue light source at a natural CO2 concentration and temperature, with the gas flow rate controlled at 0.5 L·min−1 and the photosynthetically active radiation set at 1200 mol·m−2. The objective of the experiment was to determine the parameters, including the net photosynthetic rate (Pn), conductance to H₂O (stomatal conductance, Gs), intercellular CO₂ concentration (Ci), and transpiration rate (Tr). The experimental measurement time was 09:00–11:00 a.m. (Beijing time), five consecutive plants were selected in each experimental group, and the third leaf from the top to the bottom on the lateral branch of each peanut plant was taken to measure various photosynthetic parameters.
2.4. Determination of Chlorophyll Fluorescence Parameters
In recent years, the chlorophyll fluorescence method has been widely used for detecting the physiological status of plants under different environmental stress conditions [26,27,33,34]. In this investigation, the novel M-Series IMAGING-PAM fluorescence imaging system was used. The most significant advantage of IMAGING-PAM is its capacity to identify the photosynthetic activity of each pixel in the leaf area. Additionally, it reflects the physiological status of leaves under waterlogging stress through fluorescence imaging. These functions provide the ability to study the fluorescence response of different sections of the leaf blade. For all experiments, measurements were taken at 10 p.m. (the crop was sufficiently dark-adapted at this time), and five consecutive plants were selected from each set of treatment trials. The samples were then transported to the laboratory for chlorophyll fluorescence measurements on the third leaf from the top of each peanut’s lateral branches. The real quantum efficiency of PSII Y(II), electron transfer rate ETR, non-photochemical burst Y(NPQ), and photochemical burst Y(NO) of the leaves were determined using this instrument. In the present study, it was observed that the degree of yellowing of the leaves after waterlogging stress was different in each section of the leaf. Therefore, Y(II) was determined in different sections of the leaf, as described in Section 3.2. According to the variations in the degree of leaf yellowing with the duration of waterlogging stress, the leaves were divided into five sections, from top to bottom, with the top section designated as the first section and the bottom section designated as the fifth.
2.5. Determination of Biomass of Various Parts of Peanut
During the ripening and harvesting period, 10 plants were randomly and consecutively sampled from each plot at the treatment level, and the samples were divided into aboveground parts (including stems and leaves) and belowground parts (root mass and pod weights), and then placed in an electric oven at 85 °C until a constant weight was reached, and then the dry weights of each part of the peanut were measured, which were converted to the weight per square meter by combining these values with planting density. The pod weight, plump pod weight, and kernel weight also were measured to determine the effect of waterlogging on pods.
2.6. Statistical Analysis
For the data analysis, preparation of graphs, and measuring average or standard error, Python 3.8 version was used. ANOVA and post hoc analyses were performed in IBM SPSS Statistics 28.0.0.0. All data in this study were the average of five biological replicates selected randomly from all control and treated plots.
3. Results
3.1. Variations in the Photosynthesis of Peanuts under Different Durations of Waterlogging
Since the leaves are the site of photosynthetic reactions, variations in the photosynthetic parameters are the first to show the effects of waterlogging on peanuts. As illustrated in Figure 2, peanuts were subjected to waterlogging for durations of 3, 5, 7, and 9 days in both the disaster simulation experiment in the field environment and the control experiment of waterlogging stress in the potting environment. In Figure 2a, the Pn of peanut increased and then decreased as the duration of waterlogging increased in both experimental scenarios. At 3 and 5 days of waterlogging, Pn exceeded the value of the control. The highest Pn observed in the field environment experiment was on the fifth day, reaching 17.8 (), while the highest Pn observed in the potting environment experiment was on the third day, with a value of 20.5 (). A rapid decline in photosynthesis was observed from the fifth to the seventh day in both experimental scenarios, with a similar but relatively flat decline occurring from the seventh to the ninth day. It was demonstrated that in the early stages of waterlogging caused by extreme precipitation, a large amount of water promoted photosynthesis in peanuts, resulting in an initial upward trend in Pn.
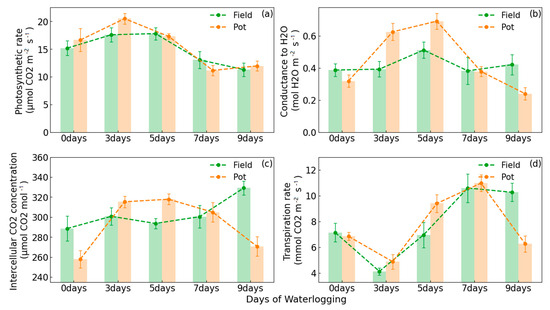
Figure 2.
Variation in the photosynthetic parameters of peanuts with the number of days of waterlogging. The dashed green line represents the field environment experiment, and the dashed orange line represents the potting environment experiment. (a) illustrates the variation in net photosynthetic rate, (b) illustrates the variation in conductance to H₂O, (c) illustrates the variation in intercellular CO₂ concentration, and (d) illustrates the variation in transpiration rate.
As shown in Figure 2b, the trend of Gs also increased and then decreased in the potting environment experiment, but it increased from the beginning of waterlogging to the fifth day of waterlogging and decreased from the fifth day to the ninth day, with a peak observed on the fifth day. At the beginning of waterlogging, photosynthesis requires significant quantities of carbon dioxide and water, and the opening of the stomata allows for the optimal state exchange of gases and water within and between the leaf cells. In the disaster simulation experiment in the field environment, there was little overall variation in Gs, with an increase on the fifth day of waterlogging and then a rapid decrease. This phenomenon was due to the fact that, in the simulation of precipitation-induced waterlogging in the field environment, peanuts were grown in an environment closer to the real atmosphere, with smooth air circulation, and the stomata on the leaves did not need to be opened very wide to satisfy their photosynthetic needs. The stomata opened wider only when Pn reached its highest point on the fifth day, accelerating the rate of gas exchange to meet its photosynthetic needs.
The trend of Ci (Figure 2c) in the potting environment experiment was analogous to Pn and Gs. It also increased and then decreased, reaching a maximum on the fifth day. The variations in Ci are mainly influenced by Pn and Gs. Stomatal opening facilitates the uptake of CO2 into the cell. Conversely, Gs decreases, and CO2 cannot be replenished while photosynthesis continues in peanuts, and this will result in less CO2 consumption and a decrease in content. In the field environment experiment, the Ci exhibited an increasing trend across the duration of the waterlogging period. However, there was a decreasing trend in Ci from the third to the fifth day, which was because, on the fifth day of waterlogging, Pn reached its maximum value and more CO2 was consumed, which then led to a decrease in Ci. This was followed by a rapid increase in Ci from the seventh to the ninth day of waterlogging. The reason is that Pn began to decrease with the increase in the number of days of waterlogging, which led to a slow consumption of CO2 during this period, increasing Ci.
Figure 2d illustrates the variation in Tr in response to the number of days of waterlogging. In Figure 2d, it can be observed that Tr exhibited a downward trend, followed by an upward trend in the two experimental scenarios. The lowest point was reached on the third day, while the highest peak value was observed on the seventh day. This situation showed the opposite trend to Pn. This can be attributed to plant transpiration, which is the expulsion of excess water from the cell, whereas if Pn were higher, it would require a significant consumption of water by the peanuts for photosynthesis. This would lead to a reduction in transpiration. However, in the later stages of waterlogging stress, the Pn decreased while the peanut was in a watery environment, and the Tr increased to discharge the excess water for normal growth and development.
3.2. Variations in the Chlorophyll Fluorescence of Peanuts under Different Days of Waterlogging
When environmental stresses are present, changes in chlorophyll function can be observed via chlorophyll fluorescence techniques long before changes occur in the leaves, so this is a common means of investigating physiological changes in plants. Figure 3a shows how the chlorophyll fluorescence parameters in peanuts varied with days of waterlogging in the field environment experiment. This result revealed that the variation in Y(II) and ETR were similar in the field environment experiment. The trend observed in Y(II) was an increasing trend from the beginning of waterlogging to the fifth day of waterlogging and a decreasing trend from the fifth day to the ninth day. This trend was consistent with the Pn of peanuts. However, the values of Y(II) and ETR on the seventh day remained higher than those of the control, indicating that, at this time, the waterlogging environment continued to exert a promotional effect on peanut growth, albeit to a relatively weak extent. On the ninth day of waterlogging stress, both Y(II) and ETR exhibited a significant reduction, indicating that photosynthesis in peanuts was significantly affected and the photochemical reaction was significantly weakened. This unfavorable condition was detrimental to the peanut’s growth and development. The trend in Y(NO) relative to the number of days of waterlogging demonstrated an increasing and subsequently decreasing variation. However, Y(NPQ) exhibited a contrasting trend, displaying an initial decrease and a subsequent increase. This observation suggests that upon initial exposure to waterlogging, the peanut plants were capable of absorbing and utilizing a substantial amount of light energy for photochemical reactions. Conversely, a smaller quantity of light energy was converted to heat energy and subsequently dissipated. However, due to the alterations in the potting environment and the presence of stress in the peanut, some of the light energy was not applied to the photochemical reaction and was not dissipated as heat energy. As this portion of light energy was not completely absorbed and consumed by the peanut, it resulted in an elevated Y(NO). During the period between the seventh and ninth day of waterlogging stress, the peanuts were subjected to an excess of light energy due to a reduction in the efficiency of actual photosynthesis. To protect themselves, the peanuts converted this excess light energy into heat, increasing Y(NPQ). In this period, Y(NO) decreased, indicating that the peanuts had adapted to the waterlogged environment through the previous waterlogging stress. This adaptation enables peanuts to convert the excess light energy into heat energy, thereby avoiding damage.
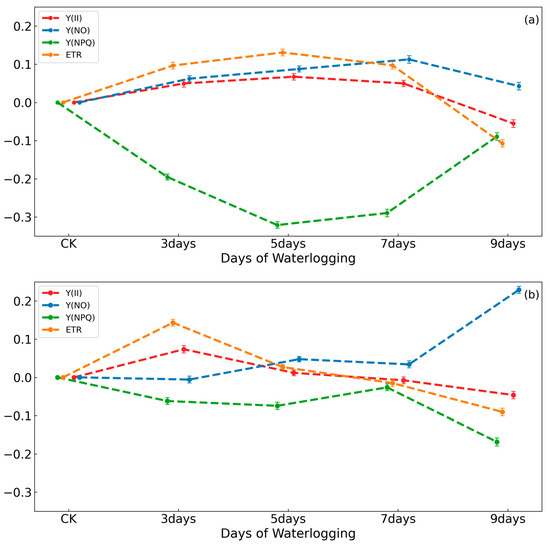
Figure 3.
Variations in the chlorophyll fluorescence parameters of peanuts versus the number of days of waterlogging. (a) indicates the field environment experiment, and (b) indicates the potting environment experiment.
Figure 3b illustrates the variations in each fluorescence parameter in peanut plants under waterlogging stress within the potting environment experiment. The value of ETR exhibited a pattern consistent with that of Y(II), and the overall trend was also an increase and a subsequent decrease. In the potting environment experiment, the maximum value of Y(II) was reached on the third day of waterlogging, after which it began to decrease. By the fifth day, it was already close to the control, and by the seventh and ninth days, it was significantly lower than the control. This phenomenon indicates that 3 days of waterlogging may enhance photosynthesis in peanuts in the potting environment, whereas more than 3 days may exert an inhibitory effect. A comparison of Y(NPQ) and Y(NO) in the potting environment experiment revealed that Y(NPQ) also decreased when Y(II) increased. At this point, the value of Y(NO) also remained consistent with that of the control, indicating that the leaves did not yet exhibit any signs of damage. During Days 3–5 of waterlogging, the levels of Y(II) and ETR began to decrease, and the light reaction in PSII weakened, resulting in excess light energy. At this time, the photoprotective mechanisms had not yet been fully activated, and the values of Y(NPQ) were low, leading to the beginning of damage and elevated Y(NO), which is detrimental to the peanuts’ development. During Days 5–7 of waterlogging, the light response in PSII continued to decrease, with a significant excess of light energy. However, the Y(NPQ) was elevated at this time, allowing Y(NO) to remain the same as in the previous period. During Days 7–9 of waterlogging, the levels of Y(II) and ETR remained consistently low, indicating that photosynthesis was severely affected. This resulted in the accumulation of excess light energy, as indicated by the decrease in Y(NPQ) and the increase in Y(NO). At this time, the peanuts’ cells were damaged, preventing them from converting more light energy into heat.
Figure 3a,b reveals that the variations in the chlorophyll fluorescence of peanuts subjected to waterlogging exhibited some differences between the two experiments (the waterlogging disaster simulation experiment in the field environment and the waterlogging stress control experiment in the potting environment). In the field environment experiment, the value of Y(II) was found to be greater than that of the control on the third, fifth, and seventh days. In the potting environment experiment, the value of Y(II) was found to be greater than that of the control only on the third day. It can be observed that the maximum value of Y(II) in the two different experimental scenarios exceeded the control by approximately 0.07. In conclusion, it can be demonstrated that the photosynthetic efficiency of peanuts was augmented during the beginning of waterlogging. In comparison to the field environment experiment, the effect of waterlogging on the promotion of photosynthesis in the potting environment experiment was shorter in duration. In the field environment experiment, Y(NPQ) decreased and then increased throughout the 9 days of waterlogging, but in the potting environment experiment, it showed a decrease and then an increase from the beginning to the seventh day, and a rapid decrease was observed from the seventh to the ninth day of waterlogging. This was consistent with the previous conclusion that the variations in all photosynthetic parameters were advanced in the control experiment of waterlogging stress in the potting environment. Furthermore, Y(NO) also demonstrated the same variation as that observed in the field environment from the beginning of waterlogging to the seventh day of waterlogging. From the seventh to the ninth day, there was a rapid increase, indicating that by this time, the waterlogging environment had already caused physiological damage to the peanut.
3.3. Variations in the Y(II) Characteristics of Different Sections of Peanut Leaves with Different Periods of Waterlogging
In these experiments, it was observed that when peanuts were subjected to waterlogging stress, the leaf cells exhibited a more pronounced response, manifesting as visible yellowing of the leaves. The process of photosynthesis, which occurs primarily in the leaves, involves the absorption of light energy and the subsequent execution of light reactions within the leaf cells. Therefore, it is necessary to analyze the chlorophyll fluorescence characteristics of the leaves. It was demonstrated that the actual photosynthetic efficiency, Y(II), can serve as a reliable indicator of the light response characteristics of peanut plants subjected to waterlogging stress. As presented in this section, we analyzed the Y(II) of different leaf sections to determine the most susceptible leaf sections when waterlogging stress occurs. This analysis will provide a reliable theoretical basis for subsequent disaster control.
In Figure 4, the upper half depicts the distribution of Y(II) in the leaves of peanut plants subjected to varying degrees of waterlogging stress in the field environment. The lower half represents the distribution of Y(II) in the leaves of potted peanuts. In order to facilitate the clear detection of the variation in the features at different locations, the leaves were divided into five sections. Figure 5 illustrates the variations in the discrepancies in the Y(II) values of the five sections of leaf and their mean values in the field and potting environments.
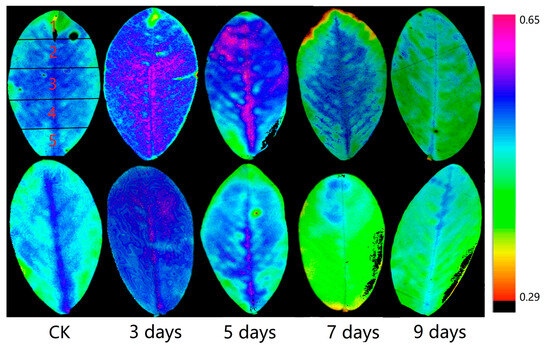
Figure 4.
Variations in the Y(II) of various sections of peanut leaves under different waterlogging durations, with the first row representing the variations in the field environment experiment and the second row representing the variations in the potting environment experiment. Each leaf was divided into five sections, from the top to the bottom, with the first section located at the top of the leaf and the fifth section at the bottom.
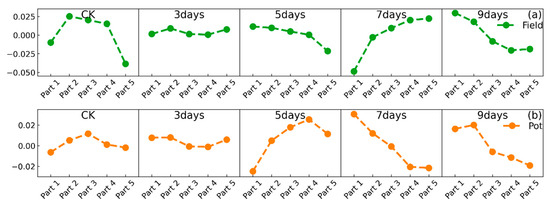
Figure 5.
Variations in the difference between the Y(II) values of the five sections of the leaf and the mean of the Y(II) of a whole leaf for different numbers of days of waterlogging, with (a) representing the variations in the field environment experiment and (b) representing the variations in the potting environment experiment.
According to these figures, it can be observed that when peanuts were not experiencing waterlogging stress in the field environment experiment, the leaf sections with the highest Y(II) values were primarily located in the second, third, and fourth sections, indicating that under normal conditions, peanut leaves perform photosynthesis in the second, third, and fourth sections. At the beginning of the waterlogging stress (3 days), Y(II) was elevated in all sections of the leaf, with minimal discrepancies among the five sections. This suggests that 3 days of waterlogging stress is conducive to photosynthesis in peanuts, in line with the conclusions previously drawn in this article. In the field environment, on the fifth day of waterlogging, the Y(II) in all sections of leaves was also slightly higher than that of the control. At this time, the primary elevation in Y(II) was in the first to fourth sections. On the seventh day of waterlogging, a significant decrease in Y(II) was seen in all sections of the leaf. As shown in Figure 5, the Y(II) in the first and second sections of the leaf was significantly lower than the Y(II) in several other sections. On the ninth day of waterlogging, the Y(II) of the whole leaf also decreased, but at this time, the Y(II) of the third, fourth, and fifth parts of the leaf was significantly lower than the Y(II) of the first and second sections. This phenomenon indicates that when photosynthesis began to weaken, the first and second sections of the leaf blade were the main sections weakening, and the magnitude of the weakening was larger, while the third, fourth, and fifth sections of the leaf were weakened to a lesser extent. As the duration of waterlogging stress increased, the Y(II) in the third, fourth, and fifth sections of the leaves also began to show a significant decrease.
In the potting environment experiment, the Y(II) in all sections of the leaf was elevated on the third day of waterlogging. However, Y(II) showed a significant decrease on the fifth day, and the characteristics of the leaf were consistent with the seventh day of waterlogging in the field environment experiment. The variation on the seventh day of waterlogging in the potting environment experiment was consistent with that on the ninth day in the field environment experiment. This phenomenon could prove that the characteristics of the variations had advanced, and these conclusions are in agreement with those in the previous section.
The variations in photosynthesis and chlorophyll fluorescence in each section of the peanut leaf blades undergoing waterlogging stress exhibited certain patterns. In the absence of waterlogging stress, the second, third, and fourth sections of the leaf primarily engaged in photosynthesis. However, when the waterlogging stress began, the Y(II) of each section increased, and the difference between the sections was not significant. Over time, as the number of days of waterlogging stress increased, the differences between the sections became more pronounced. As the duration of waterlogging stress increased, the photosynthetic and chlorophyll fluorescence capabilities of each section of the leaf blade declined. The Y(II) in the first and second sections of the leaf was the first to decline on the seventh day in the field environment experiment and the fifth day in the potting environment experiment. As the duration of waterlogging stress increased, the Y(II) in the third, fourth, and fifth sections of the leaves also began to show a significant decrease.
3.4. Variations in the Response of Peanut Biomass in Different Parts to Photosynthesis and Chlorophyll Fluorescence in the Field Environment Experiment
The results of earlier research indicated that excess water in the field experiment flowed horizontally and vertically in the soil of the field. Furthermore, the air circulation was smoother than that observed in the potted environment. This kind of simulation experiment is more closely aligned with the continuous waterlogging environment that is caused by actual atmospheric precipitation. Consequently, in this section of the study, the relationship between biomass and both photosynthesis and fluorescence was investigated by examining the effects of waterlogging on the biomass of each part of the peanut. Due to the lack of sufficient surviving samples, the potting experiments were not considered in this section. Figure 6 illustrates two sections: one displaying variations in the aboveground weight and belowground weight, and the other displaying variations in the biomass of pods and kernels. In this figure, the aboveground weight and belowground weight exhibited an initial increase, followed by a subsequent decline. In detail, they had an upward trend on the third and fifth days of waterlogging and reached their maximum levels on the fifth day. Thereafter, they exhibited a downward trend, reaching their lowest levels on the ninth day. This phenomenon indicated that water stress may facilitate the growth of roots, stems, and leaves of peanuts during the beginning of waterlogging. However, on the seventh day of waterlogging, the aboveground and belowground weights were significantly lower than those observed on the previous days. This indicated that the peanuts were no longer able to adapt to the waterlogging environment at this point in time and showed signs that they were about to decline. The variation observed between the seventh and ninth days of waterlogging was not significant. This indicates that the effect of waterlogging on peanut’s roots, stems, and leaves has a threshold, as beyond a certain time and intensity, they will decline. Consequently, the continuation of waterlogging will not have a great impact.
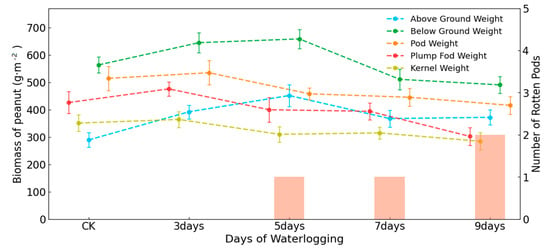
Figure 6.
Variations in the biomass of various parts of peanut versus the number of days of waterlogging duration in the field environment experiment.
The variations in pod and kernel biomass with the number of days of waterlogging stress were not quite the same as those of root, stem, and leaf biomass. From the beginning of waterlogging to the third day, the values of pod weight, plump pod weight, and kernel weight rose slightly and reached their maximum levels on the third day, recording 534.5 g·m−2, 475.5 g·m−2, and 363.8 g·m−2, respectively. However, from the third day of waterlogging to the ninth day, there was a notable decline in all the pod-related parameters. This phenomenon indicated that waterlogging stress beyond a certain duration had a profound effect on the peanut pods. In contrast to the effects of waterlogging stress on root weight, leaf weight, and stem weight, the positive effects of waterlogging stress on the growth of peanut pods were relatively minor. Conversely, the negative effects were pronounced, and there were two declines in pod weight, plump pod weight, and kernel weight. As the duration of waterlogging increased, the number of rotted pods exhibited a corresponding increase. On Day 3 of waterlogging, no pods exhibited signs of rot, while on Days 5–7, one plant exhibited signs of rot; at this point, the plump pod weight and kernel weight started to decline. On Day 9 of waterlogging, the number of rotten pods increased once more, accompanied by a significant reduction in the number and weight of plump pods, with a weight reduction of 92 g·m−2.
Correlation analyses can be employed to gain a more detailed understanding of the relationships among variations in the biomass of various parts of the peanut and variations in the photosynthetic and chlorophyll fluorescence traits of peanuts. As previously demonstrated, Pn and Y(II) are reliable indicators of physiological traits that provide insight into the impact of waterlogging on peanut’s photosynthetic characteristics. Therefore, this section will analyze the correlations using the parameters of Pn and Y(II) as indicators.
As illustrated in Figure 7, aboveground weight had a high correlation with belowground weight, but it showed a negative correlation with the other parameters. The correlation between pod weight and plump pod weight, the correlation between pod weight and kernel weight, and the correlation between plump pod weight and kernel weight were high, with values of 0.92, 0.99, and 0.94, respectively. The correlation between Pn and aboveground weight was 0.41, while the correlation with belowground weight was 0.99. The correlation with belowground weight was significantly greater than that with aboveground weight. Meanwhile, Y(II) showed the same result, with a correlation of 0.5 with aboveground weight and 0.7 with belowground weight. This suggested that photosynthesis is influenced by the number of days of waterlogging, with the greatest impact on belowground weight. The correlation between Pn and the three parameters of pod weight, plump pod weight, and kernel weight was 0.7, 0.8, and 0.64, respectively, and the correlation between Y(II) and these three parameters was 0.38, 0.7, and 0.41, respectively, indicating that variations in photosynthesis mainly affected variations in the pods. The correlation with plump pod weight was the highest, higher than that of pod weight and kernel weight, indicating that the most serious impact was on the plump pod weight. In conjunction with the results in Figure 6, it can be observed that when waterlogging persists, the plump pod weight decreases to a greater extent, and concurrently, rotten pods begin to appear. This indicates that prolonged periods of waterlogging have a detrimental impact on photosynthesis, which, in turn, affects the quality of the pods.
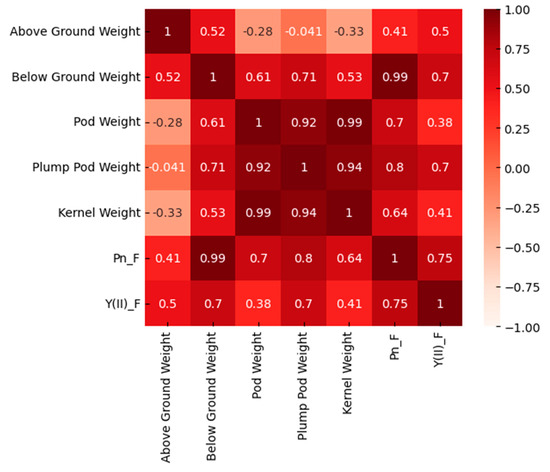
Figure 7.
Correlations among the biomass of various parts of peanuts, Pn, and Y(II) under waterlogging in the field environment experiment.
4. Discussion
This article presents an investigation of the changes in the photosynthetic, chlorophyll fluorescence, and biomass characteristics of peanuts under different durations of waterlogging by conducting field simulation experiments and pot experiments of waterlogging. The present study demonstrated that the growth status of peanuts exhibited an increase and a subsequent decrease with an increase in the number of days of waterlogging in both experimental modes. The results were consistent with those of previous studies. Zeng et al. [18] conducted a series of experiments treating three different ecotypes of peanut varieties with varying durations of waterlogging at specific stages of growth. The results indicated that 5 days of waterlogging promoted the accumulation of stem and leaf dry matter and photosynthesis, while 15 days of waterlogging was detrimental to the accumulation of plant dry matter. Qingrong et al. [35] investigated the impact of the waterlogging duration on peanut yield. The findings revealed that 3–5 days of waterlogging had a beneficial effect, promoting the accumulation of dry matter. However, as the duration of waterlogging increased, there was a notable decline in the accumulation of dry matter. Under conditions of waterlogging, ethylene accumulates in large quantities and plays a pivotal role in the plant’s response to the associated stress. Under normal conditions, gaseous ethylene is produced in the plant’s body and diffuses rapidly into the environment, thus ensuring a low level of ethylene in the body. However, the low solubility of ethylene in water leads to the accumulation of ethylene under waterlogging stress, which is difficult to eliminate from the body, and it rapidly reaches physiological saturation [36]. The accumulation of the ethylene response factor (ERF) serves to further activate the expression of downstream target genes, thereby promoting the plant’s response and adaptation to stress [37,38]. This physiological feature was probably responsible for promoting the elevated photosynthesis observed in peanuts on Day 3. Sharma et al. [19] used long-term waterlogging experiments on peanuts and revealed a significant reduction in total dry mass, total leaf area, total leaf number, and various fluorescence parameters. With the prolongation of waterlogging, the plants exhibited signs of leaf wilting, yellowing, and abscission, and the reduction in photosynthesis became more pronounced. The phenomenon in question can be attributed to the fact that prolonged waterlogging leads to a decrease in the oxygen content of the soil, which can easily cause damage to plant cells. This damage can then accelerate and deepen the damage to plant organs, potentially leading to irreversible functional disorders.
In the field environment experiments and potting environment experiments, several physiological parameters exhibited different variations with an increase in the number of days of waterlogging. If we compare the Pn of peanuts in the field environment and in pots, the time of the extreme point of the Pn was not exactly the same. The reason for this is that in the field environment experiment, the water in the soil can be transferred to other parts, and the soil will have a larger oxygen content. On the contrary, in the pot environment experiment, the physical isolation of the pots prevented the transfer of water and evaporation, resulting in relatively weak water infiltration of the soil, which led to a higher waterlogging intensity than in the field environment. This fact was reflected by how the saturation point of photosynthesis in the potting environment appeared earlier than that in the field environment. The Gs in the field environment experiment was also significantly different from that in the potting environment experiment. This was due to the fact that the potting environment was more occluded, the air flow rate was lower, and the plants needed to open their stomata wider and increase the gas exchange rate more quickly in order to meet their photosynthetic demand. A comparable outcome was observed in wheat. Lee [39] discovered through his study that waterlogging during the filling period enhanced the photosynthetic capacity of wheat leaves. This phenomenon was attributed to the rise in leaf Gs and chlorophyll content under the waterlogging scenario, which effectively augmented the Pn and the efficiency of carbon dioxide utilization. The Ci in the two experimental scenarios showed different trends of variation, where the Ci in the field environment experiment was mainly influenced by Pn, and the Ci in the potting environment experiment was mainly influenced by Gs. The reason for this phenomenon was that in the field environment experiment, Gs did not change much, and it was only on the fifth day that it suddenly increased and rapidly decreased, which resulted in the stomatal conductance not being able to influence the concentration of intercellular carbon dioxide. However, in the potting environment experiment, Gs changed significantly and was the main factor influencing Ci. Tr showed the same trends in both of the experiments. However, in the potting environment experiment, from the seventh to ninth day of waterlogging stress, the Tr also started to decrease rapidly because of the significant decrease in Gs. In the field environment experiment, the Tr did not change, and this was because the Gs did not change either. This phenomenon was also recognized by Jackson [40] in 1979.
Chlorophyll fluorescence technology is a valuable tool for rapidly understanding the changes in the physiological characteristics of crops subjected to waterlogging stress by offering insights into multiple parameters [41,42]. In this study, several chlorophyll fluorescence parameters (Y(II), Y(NPQ), Y(NO), and ETR) were selected to characterize the physiological variations in peanut plants exposed to waterlogging stress. Among these parameters, Y(II) and ETR demonstrated the greatest sensitivity to variations in the photosynthetic reaction rate of peanut plants subjected to waterlogging. This same regulation was also observed in the studies by Martinazzo et al. [43] and Janka et al. [44]. As the intensity and duration of waterlogging increased, plant cells were damaged. The value of Y(NO) can be used to indicate the extent of cell damage caused by excess light energy when waterlogging occurs. When a plant is subjected to stress, it initiates a protective mechanism that converts excess light energy into heat energy for dissipation. This process, known as non-photochemical quenching Y(NPQ), reflects the variation in the strength of the plant’s self-protective mechanism [45]. The two experimental models also showed some differences in the changes in chlorophyll fluorescence. In the field environment experiment, the value of Y(II) was found to be greater than that of the control on the third, fifth, and seventh days. In the potting environment experiment, the value of Y(II) was found to be greater than that of the control only on the third day. In comparison with the field environment experiment, the effect of waterlogging on the promotion of photosynthesis in the potting environment experiment was shortened in duration. This phenomenon can be attributed to the fact that in the field environment experiments, the water in the soil can be transferred to other areas, resulting in a relatively low intensity of waterlogging.
In this study, we analyzed the variations in the physiological characteristics of different sections of the leaf by observing the variations in the Y(II) of different sections of the leaf during the occurrence of waterlogging. It was found that under normal conditions, peanut leaves mainly photosynthesize in the second, third, and fourth sections. When the waterlogging stress started, the photosynthetic efficiency of each section was elevated, and the differences among the sections were not significant. Then, with an increase in the duration of waterlogging, the Y(II) of each section of the leaf began to decrease. The order of the decrease in Y(II) started in the first and second sections, then the third, fourth, and fifth sections. Similarly, an examination of the results of the experiments conducted in two distinct modes revealed that that photosynthesis in peanut plants reached saturation at a more rapid rate in experiments conducted in potting environments with an elevated waterlogging intensity. Furthermore, this variation occurred one stage earlier than in the field environment experiment. As illustrated in Figure 4 and Figure 5, the change observed in the field environment after five days coincided with the change observed in the potted environment after three days. Similarly, the change observed in the field environment after seven days coincided with the change observed in the potted environment after five days, and the change observed in the field environment after nine days coincided with the change observed in the potted environment after seven days.
Photosynthates are transported to storage organs mainly in the form of sucrose via the phloem [46]. It is evident that any impairment to the photosynthetic process of a crop will inevitably result in a decline in the biomass of the crop [47,48]. Zeng et al. [18] demonstrated that the impact of waterlogging stress on yield increased with the duration of waterlogging. The yield loss was primarily attributed to a reduction in the number of total and full pods. Stasnik et al. [49] found that waterlogging significantly reduced the number and weight of pods per plant, which ultimately led to a decline in the productivity of peanuts. This phenomenon can be attributed to the fact that under stress, the photosynthetic products synthesized by plant leaves may not be efficiently transported to the pods, resulting in a reduction in the number of pods [23,24]. In addition, prolonged waterlogging conditions can lead to pods rotting in the soil or needle failure, resulting in a significant reduction in the quality of peanuts [50]. The findings of this study indicated that the variations in root, stem, and leaf biomass under waterlogging stress were not quite the same as those of pod and kernel biomass. During the beginning of the waterlogging period, the presence of excess water promoted the growth of peanut roots, stems, and leaves. However, as the duration of waterlogging increased, the promoting effect changed to an inhibiting effect, which negatively impacted the growth and development of peanuts. However, the effect of waterlogging on pods was inhibitory from the outset. At 5 days of waterlogging, the number of rotten pods began to increase, accompanied by a significant decrease in the weight of plump pods at this stage. Such a phenomenon became increasingly severe with the duration of waterlogging.
5. Conclusions
The variations in the photosynthesis, chlorophyll fluorescence, and biomass characteristics of peanuts under different durations of waterlogging were investigated through a waterlogging disaster simulation experiment in the field environment and a waterlogging stress control experiment in the potting environment. It was observed that when waterlogging occurred in the early stages of growth, it had a beneficial effect on the development of the peanut plant. However, as the duration of waterlogging increased, the physiological indexes of the peanut plant, including its photosynthetic rate, began to decline. This resulted in impaired growth, with the effect of waterlogging on pods being particularly pronounced. Differences between the two different experimental models were found, as the circulation of water in the potting environment was not smooth, so the waterlogging intensity was higher than in the field environment experiments, which resulted in the effect of waterlogging being advanced by one stage in the potting environment experiment. Furthermore, this study found variations in the physiological characteristics of leaf sections under waterlogging conditions by measuring the Y(II) in different sections of the leaf with the novel M-Series IMAGING-PAM fluorescence imaging system. The research in this article can help us better understand the mechanism of waterlogging damage, clarify the regulatory feedback processes of peanuts, and provide a theoretical basis for disaster prevention and mitigation.
Author Contributions
Conceptualization, Y.W. and Q.M.; Methodology, Y.W.; Software, Y.W.; Formal analysis, Y.W.; Investigation, Y.W., Z.Z., R.C. and C.H.; Resources, Q.M.; Data curation, Y.W., Q.M., R.C. and C.H.; Writing—original draft, Y.W. and Q.M.; Writing—review & editing, Q.M., Z.Z., R.C. and C.H.; Visualization, Z.Z.; Supervision, Q.M.; Funding acquisition, Q.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Henan Key Laboratory of Agrometeorological Support and Applied Technique grant number AMF202202.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Age, L.I.; Anomaly, M.C. Global Signatures and Dynamical Origins of the. J. Clim. 2008, 21, 2283. [Google Scholar]
- Screen, J.A.; Simmonds, I. The central role of diminishing sea ice in recent Arctic temperature amplification. Nature 2010, 464, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Böhm, R.; Jones, P.D.; Hiebl, J.; Frank, D.; Brunetti, M.; Maugeri, M. The early instrumental warm-bias: A solution for long central European temperature series 1760–2007. Clim. Change 2010, 101, 41–67. [Google Scholar] [CrossRef]
- Rohde, R.; Muller, R.; Jacobsen, R.; Muller, E.; Perlmutter, S.; Rosenfeld, A.; Wurtele, J.; Groom, D.; Wickham, C. A new estimate of the average Earth surface land temperature spanning 1753 to 2011. Geoinfor Geostat Overv. 2013, 1, 1–7. [Google Scholar]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; The Australian National University: Canberra, Australia, 2023. [Google Scholar]
- NASA. Global Temperature. New York, USA. Available online: https://climate.nasa.gov (accessed on 19 March 2021).
- Kuglitsch, F.G.; Toreti, A.; Xoplaki, E.; Della-Marta, P.M.; Zerefos, C.S.; Türkeş, M.; Luterbacher, J. Heat wave changes in the eastern Mediterranean since 1960. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Fu, H.-Z.; Waltman, L. A large-scale bibliometric analysis of global climate change research between 2001 and 2018. Clim. Chang. 2022, 170, 36. [Google Scholar] [CrossRef]
- Mirza, M.M.Q. Climate change, flooding in South Asia and implications. Reg. Environ. Chang. 2011, 11, 95–107. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kim, H.; Kanae, S. Global flood risk under climate change. Nat. Clim. Chang. 2013, 3, 816–821. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, X.; Singh, V.P.; Liu, L.; Kong, D. Flood-induced agricultural loss across China and impacts from climate indices. Glob. Planet. Change 2016, 139, 31–43. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, Q.; Zhou, A.-L.; Ran, H. Assessment of flood catastrophe risk for grain production at the provincial scale in China based on the BMM method. J. Integr. Agric. 2013, 12, 2310–2320. [Google Scholar]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Peanut (Arachis hypogaea L.): A prospective legume crop to offer multiple health benefits under changing climate. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1325–1338. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nation (FAO). FAO Statistical Database. 2023. Available online: https://www.fao.org/statistics/en (accessed on 15 June 2024).
- Zeng, R.; Chen, L.; Wang, X.; Cao, J.; Li, X.; Xu, X.; Xia, Q.; Chen, T.; Zhang, L. Effect of waterlogging stress on dry matter accumulation, photosynthesis characteristics, yield, and yield components in three different ecotypes of peanut (Arachis hypogaea L.). Agronomy 2020, 10, 1244. [Google Scholar] [CrossRef]
- Sharma, S.; Bhatt, U.; Sharma, J.; Darkalt, A.; Mojski, J.; Soni, V. Effect of different waterlogging periods on biochemistry, growth, and chlorophyll a fluorescence of Arachis hypogaea L. Front. Plant Sci. 2022, 13, 1006258. [Google Scholar] [CrossRef]
- Zhang, H.; Turner, N.; Poole, M.; Simpson, N. Crop production in the high rainfall zones of southern Australia—Potential, constraints and opportunities. Aust. J. Exp. Agric. 2006, 46, 1035–1049. [Google Scholar] [CrossRef]
- Hossain, M.A.; Araki, H.; Takahashi, T. Poor grain filling induced by waterlogging is similar to that in abnormal early ripening in wheat in Western Japan. Field Crops Res. 2011, 123, 100–108. [Google Scholar] [CrossRef]
- Hossain, M.A.; Uddin, S.N. Mechanisms of waterlogging tolerance in wheat: Morphological and metabolic adaptations under hypoxia or anoxia. Aust. J. Crop Sci. 2011, 5, 1094–1101. [Google Scholar]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Setter, T.L.; Schortemeyer, M. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, N.; Krishnamoorthy, H. Effect of waterlogging and gibberellic acid on leaf gas exchange in peanut (Arachis hypogaea L.). J. Plant Physiol. 1992, 139, 503–505. [Google Scholar] [CrossRef]
- Soliman, W.S.; Fujimori, M.; Tase, K.; Sugiyama, S.I. Oxidative stress and physiological damage under prolonged heat stress in C3 grass Lolium perenne. Grassl. Sci. 2011, 57, 101–106. [Google Scholar] [CrossRef]
- Soliman, W.S.; Fujimori, M.; Tase, K.; Sugiyama, S.-I. Heat tolerance and suppression of oxidative stress: Comparative analysis of 25 cultivars of the C3 grass Lolium perenne. Environ. Exp. Bot. 2012, 78, 10–17. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Chlorophyll fluorescence effects on vegetation apparent reflectance: II. Laboratory and airborne canopy-level measurements with hyperspectral data. Remote Sens. Environ. 2000, 74, 596–608. [Google Scholar] [CrossRef]
- Rao, L.; Li, S.; Cui, X. Leaf morphology and chlorophyll fluorescence characteristics of mulberry seedlings under waterlogging stress. Sci. Rep. 2021, 11, 13379. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K.; Wang, Y.; Zhang, Z.; Lu, F.; Yu, H.; Zou, J. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and waterlogging stress. Photosynthetica 2019, 57, 1156–1164. [Google Scholar] [CrossRef]
- Mohammed, G.; Binder, W.; Gillies, S. Chlorophyll fluorescence: A review of its practical forestry applications and instrumentation. Scand. J. For. Res. 1995, 10, 383–410. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Qingrong, M.; Xuan, Z.; Chengda, H.; Lin, C.; Tongxiao, L. Effects of waterlogging on photosynthetic characteristics and yield of summer peanut. J. Appl. Meteorol. Sci. 2021, 32, 479–490. [Google Scholar]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; van Veen, H.; Yeung, E.; Voesenek, L.A. Signal dynamics and interactions during flooding stress. Plant Physiol. 2018, 176, 1106–1117. [Google Scholar] [CrossRef]
- Kendrick, M.D.; Chang, C. Ethylene signaling: New levels of complexity and regulation. Curr. Opin. Plant Biol. 2008, 11, 479–485. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Alonso, J.M. Ethylene signaling and response: Where different regulatory modules meet. Curr. Opin. Plant Biol. 2009, 12, 548–555. [Google Scholar] [CrossRef]
- Lee, H.S.; Hwang, W.H.; Jeong, J.H.; Ahn, S.H.; Baek, J.S.; Jeong, H.Y.; Park, H.K.; Ku, B.I.; Yun, J.T.; Lee, G.H. Analysis of the distribution of assimilation products and the characteristics of transcriptomes in rice by submergence during the ripening stage. BMC Genom. 2019, 20, 18. [Google Scholar] [CrossRef]
- Jackson, M.B. Rapid injury to peas by soil waterlogging. J. Sci. Food Agric. 1979, 30, 143–152. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a fluorescence kinetics of mung bean (Vigna radiata L.) grown under artificial continuous light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef]
- Bhatt, U.; Singh, H.; Kumar, D.; Strasser, R.J.; Soni, V. Severe leaf-vein infestation upregulates antioxidant and photosynthetic activities in the lamina of Ficus religiosa. Acta Physiol. Plant. 2022, 44, 15. [Google Scholar] [CrossRef]
- Martinazzo, E.G.; Ramm, A.; Bacarin, M.A. The chlorophyll a fluorescence as an indicator of the temperature stress in the leaves of Prunus persica. Braz. J. Plant Physiol. 2012, 24, 237–246. [Google Scholar] [CrossRef]
- Janka, E.; Körner, O.; Rosenqvist, E.; Ottosen, C.-O. High temperature stress monitoring and detection using chlorophyll a fluorescence and infrared thermography in chrysanthemum (Dendranthema grandiflora). Plant Physiol. Biochem. 2013, 67, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumar, P.; Gupta, V.; Tyagi, B.; Singh, C.; Sharma, A.K.; Singh, G. Multivariate approach to identify and characterize bread wheat (Triticum aestivum) germplasm for waterlogging tolerance in India. Field Crops Res. 2018, 221, 81–89. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, T.; Wang, X.; Cao, J.; Li, X.; Xu, X.; Chen, L.; Xia, Q.; Dong, Y.; Huang, L. Physiological and expressional regulation on photosynthesis, starch and sucrose metabolism response to waterlogging stress in peanut. Front. Plant Sci. 2021, 12, 601771. [Google Scholar]
- Stasnik, P.; Großkinsky, D.K.; Jonak, C. Physiological and phenotypic characterization of diverse Camelina sativa lines in response to waterlogging. Plant Physiol. Biochem. 2022, 183, 120–127. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Physiological and proteomic mechanisms of waterlogging priming improves tolerance to waterlogging stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 132, 175–182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).