Abstract
Phytotoxicity refers to the capacity of chemical substances or environmental factors to have a negative impact on plants. This is a crucial issue in both the context of crop cultivation and environmental protection. The research results were based on a 3-year field experiment conducted at an experimental station in Jadwisin (52°28′ N, 21°02′ E) on loamy soil. The experiment was set up using a randomized sub-block design in a split–split–plot arrangement with three replications. The first-order factor consisted of potato cultivars, while the second-order factors were weed control methods: (1) without protection; (2) mechanical weed control, extensive mechanical treatments to close rows; (3) Sencor 70 WG—pre-emergence (PRE) of potatoes; (4) Sencor 70 WG + Titus 25 WG + Trend 90 EC—PRE of potatoes; (5) Sencor 70 WG—post-emergence (POST) of potatoes; (6) Sencor 70 WG + Titus 25 WG + Trend 90 EC—POST of potatoes; (7) Sencor 70 WG + Fusilade Forte 150 EC—POST of potatoes; and (8) Sencor 70 WG + Apyros 75 WG + Atpolan 80 SC—POST of potatoes. The phytotoxic effects of herbicides on potato plants and weeds were assessed every 7 days, starting from the date when the first signs of damage appeared until they stabilized or disappeared. Phytotoxic damage to potato and weed plants was caused by the chemical weed control methods used. The response of potato plants to herbicides was significantly related to the genetic traits of the cultivars and meteorological conditions in the years of research. Phytotoxicity is an important aspect in both agriculture and environmental protection. Research on its mechanisms and impact will enable the development of effective plant protection strategies and the preservation of ecosystem balance.
1. Introduction
The potato is an important and widely recognized food product worldwide. It is particularly recommended by the United Nations Food and Agriculture Organization as a plant that supports food security, especially in the face of continuous population growth and associated challenges in food access [1,2]. Potatoes are low in calories but rich in starch, protein, vitamins (C and B-group), and minerals such as potassium, magnesium, zinc, and manganese. They are the most commonly consumed vegetable in Europe and North America, simultaneously serving as the primary source of antioxidants in the human diet. Therefore, technologies and cultivation methods aimed at improving the nutritional quality of potatoes can significantly impact public health [3]. To achieve success in potato cultivation and maintain food security, herbicides are often used to control weeds [4,5,6,7,8]. Alternative methods of weed control are used in organic farming [9]. Diversifying approaches to weed control can contribute to more sustainable potato cultivation, which is crucial for maintaining the supply of this essential carbohydrate source and dietary component for people worldwide [8,10].
Phytotoxicity refers to the ability of chemical substances or environmental factors to induce negative effects on plants. This includes substances like pesticides, herbicides, heavy metals, and mineral salts, as well as environmental factors such as air pollution, UV radiation, and climate change [10,11]. In agriculture, phytotoxicity is significant due to the extensive use of chemical substances for pest, disease, and weed control. However, the improper use or excessive application of these substances can lead to plant damage, reduced yields, and a loss of production value. Adverse weather conditions, such as heavy rainfall or drought, can also increase phytotoxicity, especially in certain soil types and susceptible potato cultivars [11]. Phytotoxicity is an important aspect that must be considered in agriculture to ensure effective plant protection and maintain crop productivity while minimizing the environmental impact [11,12].
Phytotoxic effects on plants can manifest as leaf necrosis, growth inhibition, deformations, and changes in plant tissue structure. These effects can have a negative impact on plant development and crop quality. Research on phytotoxicity is essential for evaluating the impact of various substances and factors on plants. This could help develop guidelines and regulations regarding the use of chemical substances in agriculture. Phytotoxicity can also have a detrimental impact on the natural environment, including aquatic and terrestrial ecosystems. Therefore, it is crucial to address the risks associated with the release of phytotoxic substances into the environment and the necessity of controlling them to protect nature.
The threat of weed infestations in potato plantations, particularly from herbicides, is significant and continues to grow. Potatoes have a low competitive ability against weeds, stemming from their slow initial growth. Factors contributing to weed infestation in potato cultivation include the increasing share of cereals in crop structure, simplifying crop rotations, organic fertilization, and no-till and poorly conducted maintenance practices [10,11,12,13,14,15]. The introduction of simplifications in crop cultivation typically results in increased weed infestation. However, currently, replacing mechanical treatments with appropriate herbicides and their mixtures greatly simplifies maintenance. Properly selected herbicides provide nearly complete destruction of most weed species in potato plantations and are fully selective for the protected crop [4,13,14,15].
When selecting herbicides for potato cultivation, consideration should be given not only to the spectrum of targeted weeds but also to the phytotoxic effects of the substances on the cultivated plant [5,11,16,17,18]. Phytotoxic reactions most commonly occur when herbicides are applied after potato emergence. This reaction is particularly significant in seed production as it can hinder or even prevent proper negative selection through difficulties in identifying virus diseases. In commercial production, this can lead to reduced yields, the production of smaller tubers, increased damage, and a decline in quality. This is most noticeable in cultivars with the shortest growing periods, as they have limited time for chlorophyll regeneration [10,19,20,21].
The phytotoxicity of herbicides is largely determined by the genetic tolerance of cultivars and soil–climate factors [11,12,22,23]. The phytotoxic effect of herbicides also increases under conditions of low rainfall, poor preparation of the herbicide, and cold, high precipitation years [24,25,26].
Phytotoxic symptoms on potato plants are usually transient and persist, depending on the sensitivity of a particular cultivar, for 14 to 28 days following treatment [7,27,28,29,30]. Prolonged symptom persistence can impede the regeneration of the photosynthetic surface, affecting yield accumulation and quality [31,32].
Earlier research has not yielded a definitive answer regarding the response of plants to herbicides used in potato cultivation and their selectivity towards the cultivated plant. The aim of the conducted research was to assess the phytotoxic effects of herbicides on two selected potato varieties, weeds, their fresh and dry mass, and their overall and commercial tuber yield. Additionally, relationships between phytotoxic damage to potato plants and their overall and commercial tuber yield were investigated. The study formulated two alternative hypotheses, stating that the application of herbicides or their mixtures, such as: (a) metribuzin − PRE; (b) metribuzin + rimsulfuron + isodecyl ethoxylated alcohol − PRE; (c) metribuzin − POST; (d) metribuzin + rimsulfuron + isodecyl ethoxylated alcohol − PRE; (e) metribuzin + fluazifop-P-butyl − POST; and (f) metribuzin + sulfosulfuron + SN oil − POST:
- will provide a broader range of herbicidal action and may cause more significant damage to weeds, simultaneously preventing phytotoxic damage to crop plants, compared to mechanically limiting weed infestation;
- will allow for the reduction of environmental pollution and ensure an improvement in the effectiveness of chemical treatments by using smaller herbicide doses, compared to the null hypothesis, which assumes no differences between variants of herbicide application or herbicide mixture and untreated control objects or the combination of the experiment with mechanical weed control.
2. Materials and Methods
The research results were based on a field experiment conducted in 2007–2009 at the Institute of Plant Breeding and Acclimatization—National Research Institute in Jadwisin, Poland (52°28′ N, 21°02′ E).
2.1. Field Research
The experiment was designed using the method of randomized sub-blocks in a dependent layout, a split-plot design, with three replications. The study investigated two factors: the first-order factor comprised potato cultivars—moderately early ‘Irga’ and moderately late ‘Fianna’, while the second-order factors were weed control methods: (1) control object—without protection; (b) mechanical weed control, extensive mechanical treatments (every 2 weeks) from planting until row closure; (3) Sencor 70 WG—1 kg∙ha−1—before potato emergence; (4) Sencor 70 WG—1 kg∙ha−1 + Titus 25 WG—40 g∙ha−1 + Trend 90 EC—0.1% before potato emergence (PRE); (5) Sencor 70 WG—0.5 kg∙ha−1 after potato emergence (POST); (6) Sencor 70 WG—0.3 kg∙ha−1 + Titus 25 WG—30 g∙ha−1 + Trend 90 EC—0.1% after potato emergence (POST); (7) Sencor 70 WG—0.3 kg∙ha−1 + Fusilade Forte 150 EC—2 dm∙ha−1 after potato emergence (POST); ad (8) Sencor 70 WG—0.3 kg∙ha−1 + Apyros 75 WG—26.5 g∙ha−1 + Atpolan 80 SC—1 dm∙ha−1 after potato emergence (POST). The control object in the experiment is an area with natural weed infestation without human intervention, which allows for the assessment of the impact of controlled activities, such as herbicide use or cultural practices, on the number and fresh and dry weight of weeds, the soil cover with crops and weeds, and the overall structure of vegetation in the area. The objective of mechanical cultivation is to assess the effectiveness of weed control using mechanical treatments, involving extensive mechanical work (every 2 weeks) from planting until row closure of the cultivated crop. In the context of the experiment, this comparison aims to uncover the impact of this specific weed control method on potato varieties and the overall vegetation structure in the studied area. The use of herbicides and their mixtures in the experiment, applied both before and after the emergence of potatoes, was aimed at ensuring a wider range of herbicide action and causing significant damage to weeds while preventing phytotoxic damage to the crop, compared to mechanical weed control and the absence of any interference in the architecture of the potato field. The intention was to reduce environmental pollution and ensure better effectiveness of chemical treatment by using lower doses of herbicides compared to a variant of the experiment without weed protection and treatment with mechanical control of weeds.
Herbicides were applied using 300 dm∙ha−1 of water. Winter rye was the preceding crop, and after its harvest, white mustard was sown as a cover crop for plowing. After winter rye harvest, nitrogen fertilization at a rate of 50 kg N·ha−1 was applied, followed by subsoiling and sowing of white mustard (20 kg·ha−1). In the autumn of the year preceding potato planting, phosphorus–potassium fertilization was applied (39.3 kg P·ha−1 and 116.2 kg K·ha−1), followed by autumn ploughing. Nitrogen fertilizers were applied in the spring (100 kg N·ha−1), mixed with the soil using a cultivation tool with a coil harrow. Potato tubers were planted in the third decade of April with a spacing of 75 × 33 cm. The seed material was classified as C/A, according to EU standards. An accumulator sprayer equipped with flat fan nozzles with a flow rate of 0.35–0.65 dm·min−1 and a pressure of 0.1–0.2 MPa was used for the spraying. Potato protection against diseases and pests was carried out according to IOR recommendations. Preparations such as Carial Star 500 SC 0.6 dm·ha−1, Altima 500 SC—0.4 dm·ha−1, Cabrio Duo 112 EC 2.5 dm·ha−1, and Ridomil Gold MZ Pepite 67.8 WG—2.5 kg·ha−1 were used for protection against late blight and early blight. Insecticides were applied to reduce Colorado potato beetle infestation, including Nuprid 200 SC—0.15 dm·ha−1, Cyperkil Max 500 EC—0.06 dm·ha−1, Calypso 480 SC—0.75 dm·ha−1, and Mospilan 20 SP at 0.05 kg·ha−1. All pesticides were applied following IOR-PIB recommendations [33,34].
2.2. Characteristics of Cultivars
The tested potato cultivars are presented in Table 1.

Table 1.
Characteristics of the tested potato cultivars.
2.3. Herbicidal Active Substances and Adjuvants
2.3.1. Sulfosulfuron
Chemical name: sulfosulfuron. IUPAC name: 1-(4,6-dimethoxypyrimidin-2-yl)-3-(2-ethylsulfonylimidazo[1,2-a]pyridin-3-yl)sulfonylurea (Figure 1) [36]. Molecular formula: not provided. Sulfosulfuron belongs to the sulfonourea group, characterized by the chemical structure represented in Figure 1. Its molecular formula, registry number, molecular weight, and GHS classification can be found in publications [36,37].
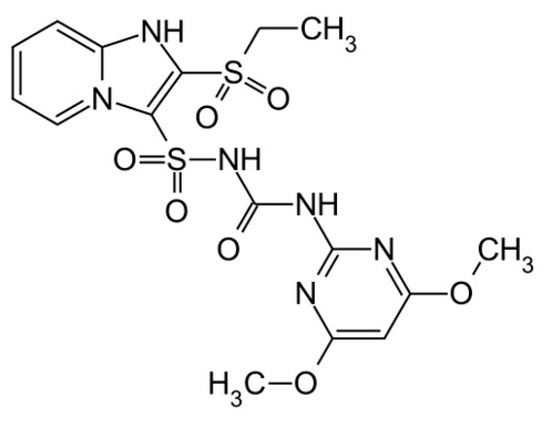
Figure 1.
Structural formula of sulfosulfuron, 1-(4,6-dimethoxypyrimidin-2-yl)-3-(2-ethylsulfonylimidazo [1,2-a]pyridin-3-yl)sulfonylurea. Source: https://zwalczamychwasty.pl/sulfosulfuron/, accessed on 18 October 2023.
2.3.2. Rimsulfuron
Chemical name: rimsulfuron. IUPAC name: 1-(4,6-dimethoxypyrimidin-2-yl)-3-(3-ethylsulfonylpyridin-2-yl)sulfonylurea (Figure 2). Molecular formula: C14H17N5O7S2. Registry number: 122931-48-0. Chemical group: sulfonylurea herbicide used in agriculture [36].
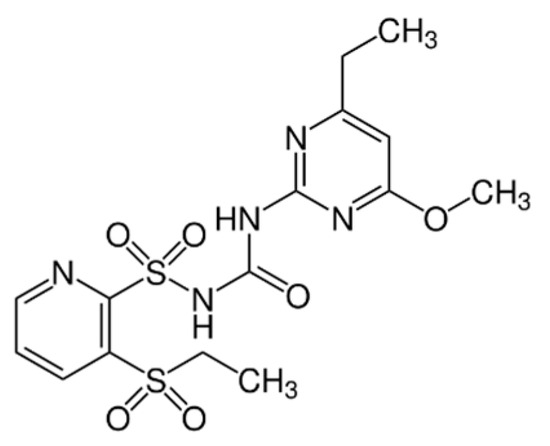
Figure 2.
Structural formula of rimsulfuron, N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-ethylsulfonyl-2-Pyridinesulfonamide. Source: https://zwalczamychwasty.pl/rimsulfuron, accessed on 18 October 2023.
Rimsulfuron is a selective herbicide that inhibits amino acid biosynthesis to impair weed growth. It is available as granules, liquid solutions, and mixtures with other active substances. The herbicide works by disrupting metabolic processes in weeds. Safety regulations apply for both crop and environmental protection during its use [36].
2.3.3. Metribuzin
Chemical name: metribuzin. Molecular formula: C8H14N4OS. Registry number: 21087-64-9. Metribuzin, a colorless crystalline solid, falls under 1,2,4-triazines, with an amino group at position 4, a tert-butyl group at position 6, and a methylsulfonyl group at position 3 (Figure 3). It is a xenobiotic, herbicide, and agrochemical agent, classified under 1,2,4-triazines, organic sulfones, and cyclic ketones [36,37]. Functioning as a selective herbicide, metribuzin impacts plant photosynthesis, hindering weed solar energy absorption. This weakens and eventually kills the plants, causing leaves to turn yellow or white. Metribuzin is effective both pre- and post-weed emergence. Caution and adherence to safety and environmental regulations are vital during its use to prevent adverse effects on crops and the environment [37].
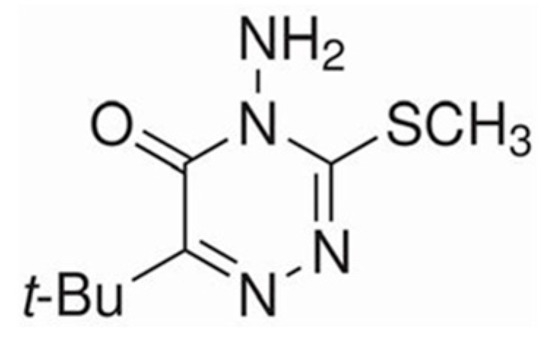
Figure 3.
4-Amino-6-tert-butyl-3-(methylation)-1,2,4-triazin-5(4H)-one structural formula. Source: own based on https://www.chembk.com/en/chem/4-Amino-6-tert-butyl-3-%28methylthio%29-1,2,4-triazin-5%284H%29-one, accessed on 18 October 2023.
2.3.4. Fluasyfop-P-butyl
Synonyms: Fluazifop-P-Butyl, Fusillade Super, Fusillade 2000, Fusillade S, Fusillade DX, Fusillade II, and Fluazyfop-P-butyl [ISO]. Chemical formula: C19H20F3NO4. CAS number: 79241-46-6. Fluazifop-P-Butyl, also known by various trade names, is represented by the chemical formula C19H20F3NO4. It is a selective herbicide that inhibits weed growth. The compound’s systematic name is (2R)-2-[4-[5-(trifluoromethyl)pyridin-2-yloxy]phenoxy]propanoic acid butyl ester (Figure 4) [36,37].
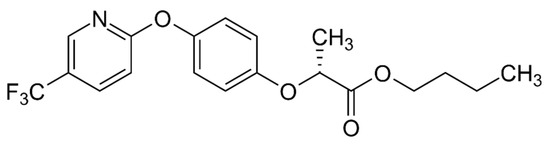
Figure 4.
Structural formula of Fluazyfop-P-butyl. Source: own based on https://commons.wikimedia.org/wiki/File:Fluazifop-P-butyl.svg, accessed on 18 October 2023.
Fluazifop-P-butyl is a selective herbicide, categorized as a derivative of aryloxy phenoxy alkanoic acid. It effectively manages weed growth in both root and above-ground systems, ensuring the preservation of cultivated crops. Essential details include its CAS number—79241-46-6, molecular weight—383.36, MDL number—MFCD06199153, and substance identifier—329753893 [34,38] (Table 2).

Table 2.
Characteristics of the herbicides used in the experiment.
2.4. Phytotoxicity Assessment
The phytotoxic effects of herbicides on potato plants were assessed every 7 days, starting from the date when the first signs of damage appeared (such as leaf discoloration, yellowing, or browning) and continuing until they stabilized or disappeared (for a total of six assessments) on the EWRC scale (Table 3).

Table 3.
EWRC Scale for assessing the impact of investigated herbicides on target plants (1–9°).
The first assessment of the plant’s condition and weed infestation was conducted when weeds emerged in the control plots while the potatoes were at BBCH stage 12 (the development of successive leaves). The subsequent assessment was carried out seven days later—at BBCH stage 20 (beginning of lateral branching) and the final one when the rows were closing (BBCH 40). The degree of phytotoxicity of the preparation was assessed using the 9-point scale (EWRC) [39].
At the stage of technical maturity, the potato crop was harvested using a potato elevator. The tuber yield and its structure were determined, and, on this basis, the marketable tuber yield was calculated [22,40].
2.5. Soil Assessment
Annually, prior to commencing the experiment, in accordance with the PN-R-04031 [41] standard, 20 soil samples were collected from the arable layer (0–20 cm) to create a composite sample weighing approximately 0.5 kg. These samples were analyzed to determine the soil’s particle size composition, the availability of phosphorus, potassium, and magnesium, and the soil pH in accordance with the Mocek [42]. The chemical and physicochemical properties of the soil were determined in a certified laboratory at the District Chemical and Agricultural Station in Wesoła, near Warsaw, using the following methods: soil particle size composition was determined by laser diffraction [43]; pH was measured in a suspension of 1 mol KCl dm−3 and in a water suspension using the potentiometric method [44]; organic carbon content (Corg.) was determined using the Tiurin method [42]; available magnesium content was determined using the Schachtschabel method [45]; and the content of absorbable forms of phosphorus and potassium was measured using the Egner–Riehm method [46,47].
The experiment was carried out on loamy, sandy, and clay soil [48]. The share of sand, silt, and clay was 66.98%, 30.57%, and 2.45%, respectively (Table 4).

Table 4.
Soil granulometric composition.
The results of the soil analyses were confronted with standard values provided by the Soil Science and Plant Cultivation—National Research Institute [49].
In the physicochemical analysis, the content of assimilable macronutrients in soil, dry matter, pH value, and organic matter content in the soil were considered. The content of assimilable phosphorus (P) in 2007 was 104.2 mg kg−1, which can be classified as moderately high. In 2008, the content of this element decreased to 42.8 mg P kg−1, and in 2009, it further decreased to 17.1 mg kg−1, classifying the soil as low in phosphorus. For potassium (K), the content of assimilable potassium in 2007 was high, at 183.7 mg kg−1. In 2008, it was 139.2 mg kg−1, and in 2009, it decreased to 61.1 mg kg−1, making the soil potassium-deficient. The magnesium (Mg) content in 2007 was 121.3 mg kg−1, which is considered high [49]. In 2008, this value decreased to 92.9 mg/kg, and in 2009, it was only 35.7 mg kg−1. The soil pH (KCl) was found to be acidic, ranging from 4.9 in 2007 to 5.3 pH in 2009. The content of organic matter (Corg) in the soil was from 7.2 to 7.5 g kg−1. Loamy soils, due to their nature, often contain less organic matter than forest soils or peatlands. Therefore, a value of 7.4 g kg−1 for loamy soil can be considered moderately low. These data are essential for assessing soil suitability for crop cultivation (Table 5).

Table 5.
Physical and chemical properties of soil in Jadwisin (2007–2009).
2.6. Meteorological Conditions
The weather conditions during the growing season in 2007–2009 were characterized by changeable air temperatures and rainfalls (Figure 5, Table 6).
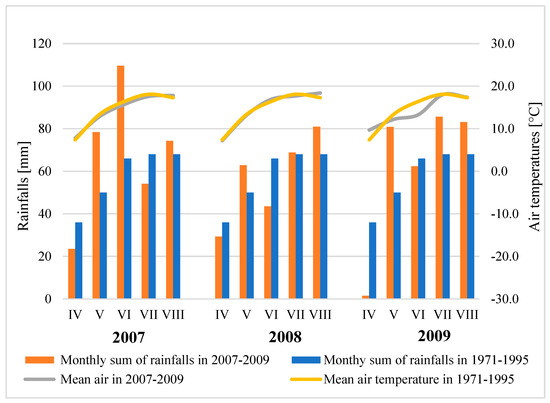
Figure 5.
Rainfalls and air temperature during the growing season of potato by the weather station IHAR-PIB in Jadwisin (2007–2009) against the multiannual average.

Table 6.
Selyaninov’s hydrothermal coefficients for Jadwisin (2007–2009).
In the years 2007–2009, the growing period conditions in Jadwisin exhibited varying temperatures and precipitation levels (Figure 5). In 2007, the year could be described as relatively dry, 2008 as dry, and 2009 as having the most optimal moisture and temperature conditions for potato growth.
During the first year of the study, the average temperature from April to September was 13.7 °C, which was 0.6 °C lower than the long-term average. The total precipitation during this period was 436 mm, which was 165% of the long-term norm (Figure 1).
In 2008, the weather was unusual. Precipitation in May and August exceeded the long-term average, while June and July were dry, with water shortages observed in other months. The average temperature from April to September was 14.2 °C, 0.3 °C lower than the long-term average (Figure 5).
The meteorological conditions in 2009 were diverse, but the main characteristic was drought at the beginning. The average temperature from April to September was 15.3 °C, within the long-term norm, while the total precipitation during this period was 360 mm, which was 4.3 mm lower than the long-term average. Precipitation in the second half of the growing period was well-distributed over time (Figure 5).
The values of Selyaninov’s hydrothermal coefficient are calculated from the formula [50]:
where:
P—sum of monthly precipitation in mm;
Σt—monthly total air temperature. This is the sum of precipitations and temperatures in the period when the temperature has not been lower than 10 °C.
According to Selyaninov’s hydrothermal coefficient, the potato growing period was classified as wet (2007), dry (2008), and optimal (2009). In 2007, drought was recorded in April and July, while the remaining months were humid. The year 2008 was characterized by an optimal moisture content, but in June, during the period of intensive harvesting, dry conditions prevailed. In 2009, during potato planting and harvest, drought was recorded, while the remaining months of the growing season were moist (Table 6).
2.7. Statistical Calculations
The statistical calculations were conducted using SAS statistical software version 9.2. [51]. The statistical analyses were based on a three-factor model (years × cultivars × maintenance) of analysis of variance (ANOVA) as well as multiple t-Tukey tests (or confidence intervals). The significance level was set at p ≤ 0.05. The significance of sources of variability was assessed using the Fisher–Snedecor test, known as the “F” test. Tukey’s multiple comparison tests allowed for detailed comparative analyses of means by identifying statistically homogeneous groups and determining the so-called Least Significant Differences (LSD) for means, which are denoted by HSD (Tukey’s Honest Significant Difference) in Tukey’s tests.
For variables expressed in percentages that were close to 0 or 100, normalizing transformations were applied using the natural logarithm (ln(x)). After the calculations, the data were retransformed. The logarithmic transformation of a random variable x is described by the formula:
where g(x) = ln(x) [52].
Y = ln(x),
In practice, logarithmic transformations are often used to adjust a distribution of data to meet statistical assumptions, especially when the data exhibit a nonlinear relationship or a skewed distribution. The results of a statistical analysis using these methods can help in better understanding the relationships between variables and assessing the significance of differences between groups or conditions. Moreover, descriptive statistics [53] were used.
The relationships between the analyzed variables (traits) were examined using Pearson correlation coefficients (calculating correlation coefficients and associated p-values from Student’s t-test functions for assessing significance by comparing them with the adopted significance level).
3. Results
3.1. Coverage of Soil with Crops and Weeds
The soil coverage by potato plants averaged 95.5%; monocot weeds accounted for 2.4%; and dicot weeds for 12.1% (Table 7).

Table 7.
Coverage of soil with crops and weeds, depending on cultivars, methods of care, and years of cultivation.
Varietal characteristics and the study years did not significantly differentiate the soil coverage by the crop. Significantly higher plant soil coverage was observed with Sencor 70 WG + Fusilade Forte 150 EC (treatment 7) compared to the control. However, this treatment tended to be higher than the others. In the field of the “Irga” potato cultivar, a higher degree of soil coverage by both monocot- and dicot weeds was observed, and the crop coverage was lower compared to the field of the “Fianna” cultivar. The response of potato plants to the applied herbicides was not significantly related to the genetic properties of the studied cultivars (Table 7).
3.2. Damage to Potato Plants
Herbicide damage to potato plants was predominantly influenced by the chemical weed control method applied in the experiment (Table 8). Greater changes in leaf blade damage were observed after the POST application of herbicides compared to the PRE application for the potatoes. There was a significant interaction across years × treatment method. Specifically, in 2009, the highest level of damage was recorded after the application of Sencor 70 WG POST herbicide at a concentration of 0.5 kg ha−1 (treatment 5). On the other treatment plots, the values remained at a similar level and were homologous in terms of the examined characteristic. The PRE use of the herbicide mixture Sencor 70 WG + Titus 25 WG + Trend 90 EC (treatment 4) resulted in more significant discoloration of leaf blades compared to the application of a single active herbicide substance such as metribuzin (Table 8, Tables S3–S8 in Supplementary Materials).

Table 8.
Damage to potato plants caused by herbicides, depending on cultivars, care operations, and years of cultivation, on the EWRC scale (9°) (average of 6 connection dates).
The years × cultivars × care interaction turned out to be insignificant in the case of this feature (Table 8).
3.3. Damage to Weeds
The average degree of damage to dicot weeds was 3.1° on the 9° EWRC scale (Table 9). The genetic characteristics of the examined cultivars and weed control methods did not significantly differentiate the extent of damage to this group of weeds. Instead, the weather conditions during the study years had the most significant impact on the damage to dicot weeds in the crop field. The highest effectiveness in reducing dicot weed damage was achieved in 2009, while the lowest was observed in 2008. This was mainly due to the weather conditions during the potato growing season (Table 9, Tables S10, S11 and S14–S16 in Supplementary Materials).

Table 9.
Dicotyledonous weed damage caused by herbicides according to EWRC (9° ***) during the growing period (average of 6 observation periods).
A significant interaction between maintenance methods and years was also observed. Only in maintenance methods 4 to 8 (Sencor PRE and POST in different combinations with herbicides) were significant differences in weed reduction identified during the study years. The strongest herbicidal effect was observed in the optimal year of 2009, while the weakest was found in the dry year of 2008. The interaction between years, cultivars, and care was found to be insignificant concerning the damage of dicotyledonous weeds (Table 9).
The average degree of damage to monocot weeds was 2.1° on the 9° EWRC scale (Table 10). Potato maintenance had the most significant impact on the damage to this weed class. All mechanical–chemical maintenance methods increased the damage compared to mechanical maintenance methods. The most significant phytotoxic damage was observed in monocot weeds after the application of the herbicide mixture Sencor + Apyros + Atpolan (treatment 8), followed by the use of preparations Sencor + Fusilade Forte (treatment 7), while the least damage was caused by mechanical–chemical maintenance involving the Sencor PRE preparation (treatment 3) (Table 10). The meteorological and soil conditions during the study years also influenced the degree of damage to monocot weeds. The most significant symptoms of phytotoxic damage in this group of weeds were observed in 2009, a year characterized by a very wet May with the highest pH and the highest organic carbon content in the soil, while the least damage was observed in 2008, a year with a warm and dry period during plant emergence. The meteorological and soil conditions during the potato growing period modified the damage to monocot weeds only in weed control methods from 5 to 8. The most substantial reduction in weed infestation was achieved in 2009, which was optimal for potato yields, while the lowest reduction was observed in the dry year of 2008. The interaction between years, cultivars, and care was found to be insignificant concerning the damage of monocotyledonous weeds (Table 10, Tables S12–S14 in Supplementary Materials).

Table 10.
Damage to monocotyledonous weeds caused by herbicides according to the EWRC (9˚ scale) during the growing season (average of 6 observation periods).
3.4. Yield of Tubers
The total and commercial yields of tubers were determined. The commercial yield accounted for 93.4% of the total yield. The genetic characteristics of the studied cultivars only differed in the commercial potato yield. The moderately late cultivar “Fianna” proved to be more productive than the moderately early cultivar “Irga” (Table 11).

Table 11.
Influence of potato cultivars, care methods, and growing seasons on total and commercial yields.
The methods of potato cultivation influenced both the total yield and the commercial yield of the tubers. The best yield results in both cases were achieved by using the Sencor 70 WG preparation PRE (treatment 3) at the recommended dose (1 kg ha−1). In the case of the commercial yield, all other combinations with herbicides were comparable to the PRE application of the Sencor preparation (treatment 3), as well as with mechanical potato cultivation (treatment 2) (Table 11, Tables S1 and S2 in Supplementary Materials).
Regarding the total yield, objects 2 to 4 and 6 to 8 showed homogeneity in terms of this trait, while object 5, with the application of Sencor POST at a reduced dose (0.5 kg hm2), exhibited a significantly lower total yield but a significantly higher yield compared to the control object and the mechanically cultivated object (Table 11).
The values of the total and commercial yield were primarily influenced by the meteorological and soil conditions during the years of the study. The highest values of these traits were obtained in 2009, an optimal year in terms of moisture and thermal conditions during the potato grooving period, with the highest pH and the biggest organic carbon content in the soil. Homogeneous values for the total and commercial yield were achieved in 2008, characterized by a favorable period shortly before and after potato emergence, along with better meteorological conditions in the second half of the growing period. The lowest yield for both traits was obtained in 2007, a flood year with excessive rainfall in June and September (three times higher than the long-term average) (Table 11).
Only in the case of the commercial yield did the tested cultivars exhibit a varied response to meteorological conditions during the study years. The cultivar “Irga” achieved the highest yield in 2008, while the mid–late cultivar “Fianna” achieved its best yield in 2009, a year that was optimal in terms of moisture and thermal conditions. Both cultivars, however, produced the lowest yield in the flood year of 2007 (Table 11).
3.5. Descriptive Characteristics of Potato Plant Yields and Phytotoxic Damage
Table 12 offers a comprehensive view of the descriptive statistics related to potato yield and phytotoxic damage. It encompasses both dependent and independent variables. Dependent variable y1 (total yield): The average total potato yield stands at approximately 30.2 t hm2 with a standard error of 0.77. The median is 28.80, while the standard deviation is 9.28 t hm2. The total yield data exhibit slight negative skewness (0.02), and the kurtosis is −0.51. The total productivity ranges from 9.48 to 51.84 t hm2, and the coefficient of variation is 30.74%, indicating relatively high stability in the value of this feature. Practically, this means that the total potato yield values deviate by approximately 30.74% from the average. A larger coefficient of variation implies greater data variability (Table 12).

Table 12.
Descriptive statistics of total and commercial yield and phytotoxic damage to potatoes.
Similarly, marketable yield (y2): The average marketable yield is 28.27 t hm2, with a standard error of 0.79. The median market yield is 26.89 units, and the standard deviation is 9.49. Market yield data also exhibit slight negative skewness (−0.01), with a kurtosis of −0.50. The marketable yield ranges from 6.30 to 49.37 t hm2, and the coefficient of variation for this feature is 33.56%. A coefficient of variation (CV) of 33.56% indicates significant variability concerning its average value. In the dataset, the marketable potato yield may vary due to factors such as growing conditions, soil, diseases, or pests. A high coefficient of variation can imply greater yield-related risk, impacting farmers’ incomes. To stabilize yields and incomes, measures can be taken to minimize this variability (Table 12).
Independent variables (x1 to x6) (phytotoxic damage at different time points): These variables represent phytotoxic damage levels at varying time intervals (7, 14, 21, 28, 35, and 42 days). The phytotoxic damage to cultivated plants decreased over time, with x1 having the highest mean (2.42) and x6 having the lowest mean (0.79). The standard deviations also increased, indicating greater variability. The positive skewness values suggest right-skewed distributions, with x4 being the most positively skewed (skewness 1.98). The kurtosis values vary, with relatively high kurtosis values for x5 (4.84) and x6 (0.11), indicating heavier tails in their distributions. The ranges of phytotoxic damage values also expand over time (Table 12).
In summary, the descriptive statistics offer a comprehensive view of the total and marketable potato yield and the progression of phytotoxic damage over different time points. These statistics reveal insights into central tendencies, variability, and data distributions for each parameter.
3.6. The Relationship between Potato Yield and Phytotoxic Damage in Plants
Figure 6 presents Pearson’s correlation coefficients between various variables, including the total and marketable potato yield and the degree of phytotoxic damage to potato plants at different time intervals after herbicide application.
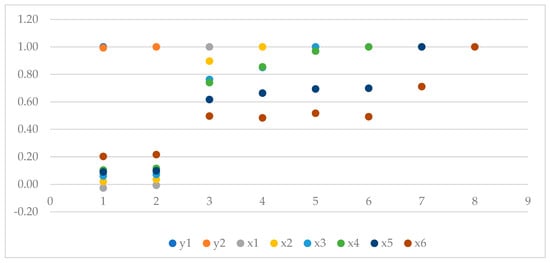
Figure 6.
Pearson’s correlation coefficients between total and marketable potato yields and phytotoxic damage to the crop plant; y1—total yield, y2—trade yield; x1—phytotoxic damage after 7 days; x2—phytotoxic damage after 14 days; x3—phytotoxic damage after 21 days; x4—phytotoxic damage after 28 days; x5—phytotoxic damage after 35 days; x6—phytotoxic damage after 42 days.
For the total potato yield (y1), the correlation between the total yield and the marketable yield was r = 0.99, indicating a strong positive correlation, suggesting that changes in one of these parameters go hand in hand with similar changes in the other (Figure 6).
The correlation between the total yield and the degree of phytotoxic damage to potato plants at various time intervals is very weak and close to zero (ranging from −0.03 to 0.20). This suggests that there is no clear correlation between the total potato yield and the degree of phytotoxic damage in the observed periods, except for damage observed after 42 days from the first herbicide application. For the marketable potato yield (y2), the correlation with the degree of phytotoxic damage is also very weak and close to zero (Figure 6).
Regarding the degree of phytotoxic damage (x1 to x6), the correlation between different time intervals of phytotoxic damage is generally positive and moderate, indicating that the degree of plant damage increases over time (Figure 6).
In summary, the results indicate a strong correlation between the total and marketable potato yields, as expected, given that both variables should be closely related. However, the lack of a clear correlation between the yield and the degree of phytotoxic damage suggests that phytotoxicity has a limited impact on yield within the observed range.
4. Discussion
In conditions of intensive potato cultivation technology, plants are exposed to the influence of various stressful conditions that often hinder the realization of physiological processes at the potential capacity of this species. It is known that herbicides can translocate from leaves and stems to fruits, seeds, and tubers, and accumulate within them, altering their physiological, biochemical, and consumable properties [14,19,25,54,55,56]. Herbicides can induce enduring or transient alterations in the morphology of potato plants [10,57,58]. The extent of damage is not necessarily linked to a spectacular appearance of damage symptoms.
Our study confirmed the alternative hypothesis that the use of metribuzin post-emergence allows for the reduction of environmental pollution and improves the effectiveness of chemical treatments by using half the dose of this active substance, contrary to the null hypothesis, which assumes no differences between variants of this herbicide and variants without protection of potatoes against weeds or with mechanical weed control.
4.1. Phytotoxicity of Herbicides and Its Effects
The consequences of herbicide phytotoxicity for yields, as stated by numerous authors [10,11,19,27,58,59], can be better assessed based on the time of herbicide application and the duration of symptoms rather than their intensity. The response of potato plants to applied herbicides is also dependent on various factors, such as the genetic characteristics of cultivars, the timing of application, the air temperature during application, post-application precipitation, and the soil organic matter content [10,16,19,54,58,60,61]. In the conducted research, POST herbicide applications (such as metribuzin) resulted in more significant changes in the potato plants, visible on leaf blades, than those used for PRE weed control. The application of the herbicide Sencor 70 WG at a concentration of 0.5 kg ha−1 had a decisive impact on the level of damage to the potato plants. On the remaining objects, the damage values were at a similar level and turned out to be homologous in terms of the assessed feature. A higher discoloration of leaf blades was also observed in an experiment carried out on a site where a mixture of Sencor 70 WG + Titus 25 WG + Trend 90 EC preparations was used to reduce weed infestation before potato emergence compared to the use of a single herbicide active substance, such as metribuzin. According to Lichtenthaler [57,62,63,64], herbicides disrupt the course of photosynthesis and enzymatic processes, damage chlorophyll, induce excessive transpiration, and inhibit cell division. The phytocide changes subsided after about 6 weeks but caused irreversible damage to the plant’s assimilation apparatus. According to Skórska and Swarcewicz [11,65,66], the active substances in herbicides can easily penetrate chloroplasts, causing damage to photosystem II and the light-harvesting complex (LHC). According to these authors, herbicides also disrupt the chlorophyll a:b ratio and reduce the activity of electron carriers. As a result, changes in chlorophyll fluorescence parameters occur [62,64,65,66]. In the conducted research, the most significant phytotoxic damage to the potato plants was found in cases where metribuzin was the active ingredient, applied after potato emergence. The level of herbicide damage after the application of metribuzin after emergence was two on the EWRC scale out of nine and was higher compared to objects where this substance was used pre-emergence. The most severe phytotoxic symptoms on potato plants, as well as a lower level of soluble solids and reduced potato yields after metribuzin, dichlozoline, and imazethapyr application, were also reported by Fonseca et al. [7]. Therefore, the herbicides they examined were considered less selective. Linuron and clomazone had no effect on the level of soluble solids in their research and did not reduce potato yields; thus, they were considered more selective for this species. Singh et al. [67] examined the effectiveness of weed control in sweet potato cultivation and the phytocide effects of using bentazon. However, they did not observe any phytotoxic symptoms on the sweet potato plants after applying this preparation. An evaluation of POST damage caused, among others, by metribuzin in Romania was conducted by Hermeziu et al. [68]. However, they did not observe any post-herbicide damage to potato plants. Other herbicides did not show any significant damage at any time after application compared to the control treatment. The sensitivity of potato cultivars to metribuzin and fomesafen applied before potato emergence was studied by Tkach and Golubev [58]. They observed phytotoxic symptoms only in the early cultivars (‘Udacha’ and ‘Nevsky’). For the Avrora cultivar, they only found a negative impact on plant height due to metribuzin and formesafen applications, resulting in a significant growth delay. Despite the observed phytotoxic symptoms, these authors did not demonstrate any negative effects on the yield of the tested potato cultivars. Phytotoxic symptoms caused by urea-based herbicides include chlorotic changes that subsequently transform into necroses [10,11,56,58,69,70].
Currently, research on phytotoxicity focuses on identifying target processes shaped by allelochemicals present in acceptor plants or isolating specific chemical compounds from donor plants. Despite the numerous advantages of advanced biotechnological and omics techniques, they have not been widely utilized for a comprehensive understanding of phytotoxicity. While some genetic studies on allelopathy and phytotoxicity have been conducted [71,72], only a few have focused on identifying the fundamental genetic mechanisms and global gene expression changes related to these processes [72,73]. To date, there is a lack of research aimed at determining the genetic or molecular basis of the benefits arising from positive allelopathic interactions.
An RNA sequencing analysis revealed that low-phytotoxicity offspring exhibited an increased expression of genes related to flavanol/3-hydroxylase synthesis, influencing potato plant growth. This demonstrates that metabolic changes in potato offspring can affect various physiological responses in the recipient plant, including white mustard [73]. Phytotoxicity is not solely related to the quantity of glycoalkaloids but also to their composition and the presence of other metabolites, including flavonoids. Consequently, it is suggested that diverse factors, including glycoalkaloids and flavonoids, may influence plant phytotoxicity [72,74]. This is a key aspect of agriculture that contributes to maintaining the balance of ecosystems. Research on the mechanisms of phytotoxicity and its impact allows for the development of effective strategies for plant and environmental protection.
4.2. Mechanisms of Phytotoxic Action
Herbicides from various chemical groups exhibit diverse mechanisms of phytotoxic action. They interact with different plant life processes [75]. According to Praczyk and Skrzypczak [57], urea herbicides are more readily absorbed through roots than leaves and move within a plant, disrupting the process of photosynthesis. Selective systemic herbicides like Sencor move through the xylem and interfere with photosynthesis, affecting a broad spectrum of both monocot and dicot weed species. Luz et al. [29] found that the active ingredients in these herbicides hinder the early stages of photosynthesis by inhibiting water photolysis. By acting as electron transport inhibitors in the light phase of photosynthesis, they generate active oxygen species, which react with the lipid–protein components of plasma membranes, ultimately damaging chloroplast structures.
Rimsulfuron, an active ingredient with systemic action, is absorbed through the leaves and swiftly moves throughout a plant, inhibiting weed growth by disrupting the biosynthesis of amino acids. This active substance is selective to potatoes, making it relatively safe for this crop. Its herbicidal effect becomes noticeable after 7–20 days post-application. Rimsulfuron operates through systemic selectivity, which means that the potato plant breaks it down into inactive compounds [28,33,76]. According to Alebrahim et al. [77], rimsulfuron is commonly used for controlling Chenopodium album L. and Amaranthus retroflexus L. in potato fields. Investigating the absorption and metabolic patterns of rimsulfuron between these two weed species and potatoes can provide valuable insights for optimizing herbicide application in the field. Redroot pigweed (A. retroflexus L.), the most sensitive species in their study, showed the highest absorption rate and the lowest herbicide metabolism rate. Potatoes proved to tolerate rimsulfuron well. The combination of the active substances rimsulfuron (Titus 25 WG) and metribuzin (Sencor 70 WG) in the study resulted in more severe damage to both potato plants and weeds when applied POST rather than PRE. Boydston [28] and Alebrahim et al. [77] observed similar effects. This combination was intended to enhance the control of monocot weeds in potato cultivation. Rimsulfuron interrupted lipid processes, whereas metribuzin disrupted photosynthesis. Together, these active substances seemed to act synergistically, achieving more effective weed control compared to each one applied individually.
Apyros 75 WG, containing the active substance sulfosulfuron, is absorbed through both roots and leaves, moving throughout a plant, where it acts as an amino acid biosynthesis inhibitor. Amino acids like valine, isoleucine, and leucine are vital for plant growth and development [6,78]. By interfering with the production of these amino acids, sulfosulfuron hinders cell growth and leads to a decline in plant yield. In the study, sulfosulfuron caused more damage to the potato plants of the “Irga” cultivar compared to “Fianna”. It also induced more damage in monocot weeds than dicot weeds. The existing leaf blades of monocotyledonous weeds were 3.5° and those of dicotyledonous weeds were 2.8° on the 9° EWRC scale.
Fluazifop-P-butyl (Fusilade Forte 150 EC), an aryloxyphenoxypropionate herbicide, actively moves within a plant and accumulates in root stems and rhizomes. It disrupts various biochemical processes in plants, particularly inhibiting lipid production, which is crucial for monocot weeds compared to cultivated dicot plants [36,57,75]. Metribuzin, on the other hand, impacts the photosynthesis process, leading to plant damage [57]. In the conducted study, combining fluazifop-P-butyl with metribuzin led to the most damage to weeds compared to the mechanical–chemical control group. The potato cultivar “Irga” proved more sensitive to this combination than the relatively late-maturing cultivar “Fianna”. This synergy helped control monocot weeds more effectively, contributing to better crop yield and quality [68,77].
Dittmar et al. [79] assessed the toxicity of metribuzin on potato plants and recorded reversible damage at 8%. Stressed conditions like prolonged drought, excessive rainfall, flooding, heavy metals, or soil salinity can cause chloroplast membrane disorganization, directly affecting the efficiency of photosystem PS II. In such cases, reparation is facilitated by the short-lived nature of stress and the early growth phase of potato plants [40].
The active substance metribuzin can affect plants in various ways, such as inhibiting photosynthesis, disturbing metabolic processes, inhibiting cell division, impeding the movement of water and nutrients, and acting both PRE and POST. Metribuzin’s impact may differ depending on its concentration, the weed species, and environmental conditions.
4.3. Impact of Environmental Conditions on Herbicide Phytotoxicity
Environmental conditions significantly influenced the risk of herbicide toxicity. The existing relationship between weather patterns and the sensitivity of plants of this species to herbicides indicates that it is largely determined by post-application habitat conditions in unfavorable meteorological and soil conditions. These observations, concerning potato plants, are supported by many authors [10,11,54,59]. According to Gugała and Zarzecka [19], increased herbicide phytotoxicity concerning potatoes may occur in wet and cool years when plants are less resilient to adverse weather conditions. In the opinion of many authors [13,25,54,80], low temperatures and low precipitation may create less favorable conditions for herbicide degradation in the soil, thereby increasing their phytotoxicity. The above statements were reflected in our research. The highest phytotoxic impact of the preparations used was visible in 2009, where only during the potato planting and harvesting period was there drought, while the remaining months were wet. The least damage was recorded in 2008, which was classified as dry according to the Sielaninov hydrothermal coefficient. Significant differences were also observed in the interaction: years × cultivar—0.2 and years × care method—0.9. The interaction of summer × care methods × cultivar has not been statistically proven. Conversely, high levels of rainfall during potato planting, emergence, and vigorous vegetative growth can increase their sensitivity to herbicides. According to Edwards [11], strategic deep soil tillage increases damage caused by certain herbicides, including those containing metribuzin as the active substance. In the opinion of Davies et al. [81], often different yield constraints occur simultaneously and can appear on both the topsoil and subsoil. While some substrate constraints reflect the inherent nature of the soil, others occurring in the upper 0.5 m of the soil profile, such as soil acidity or compaction resulting from machinery practices, result from agricultural management.
Prudent herbicide use in potato production is crucial because their improper use induces stress in plants, potentially leading to growth and development disruptions. The extent of the stress depends on the type of active substance, application timing, conditions, fertilization, and the genetic properties of the cultivated plants.
4.4. The Impact of Varietal Traits on Phytotoxic Damage in Potatoes
The genetic makeup of potato cultivars plays a significant role in determining their susceptibility to phytotoxicity. Some cultivars may possess genetic traits that make them more resistant to the effects of herbicides, while others may be more sensitive. Research has demonstrated genetic diversity among potato genotypes, which can explain variations in phytotoxic responses [19,54,56,59,60,80]
Cultivars with different growth habits, such as determinate or indeterminate growth, may exhibit varying sensitivities to herbicides. Determinate cultivars tend to have limited vegetative growth and may be less affected by herbicides that target vegetative growth processes [22]. Varietal traits related to tuber formation, such as the number, size, and depth of tubers, can affect how potatoes respond to herbicides. Cultivars with deeper or larger tubers may be less vulnerable to herbicide damage because the tubers are further below the soil surface [11].
Differences in leaf structure and morphology among potato cultivars can impact their susceptibility to herbicides. Cultivars with thicker or waxier leaves may provide some protection against herbicide absorption, reducing phytotoxic effects [19,72]. Urbanowicz [19] examined the influence of the number of stomata on the damage to potato plants after POST metribuzin application. He demonstrated that the leaf structure of the studied potato cultivars had a significant effect on the intensity of phytotoxic symptoms and the pace of their reduction.
Early-maturing and late-maturing potato cultivars may respond differently to herbicides. The growth stage at which herbicides are applied can affect the extent of damage. Early-maturing cultivars may be more sensitive to herbicides applied during the early growth stages [54,80,82]. In the conducted studies, the mid–late cultivar “Fianna” demonstrated a better response to stress tolerance compared to the mid–early cultivar “Irga”. The highest phytocidal effect on the tested potato cultivar was visible after the application of metribuzin after the emergence of potatoes.
Cultivars that are more stress-tolerant may recover more effectively from herbicide-induced stress. Some cultivars exhibit better resilience to adverse environmental conditions and herbicide-related stress [14,26,62,63,72,82]. Varietal differences in metabolic processes can influence how herbicides are processed and detoxified within a plant. In the case of our own research, the cultivar ‘‘Fianna’’ was characterized by a faster rate of metabolism than “Irga”. Cultivars with efficient metabolic pathways may be less affected by herbicides [4,82].
Cultivars with variations in nutrient uptake and utilization may respond differently to herbicides. Adequate nutrient levels can enhance a plant’s ability to recover from herbicide stress [14,26,72]. In the conducted research, various fertilizers were utilized, including the foliar fertilizer, which included phosphorus, potassium, and acetate ions and had a strongly alkaline pH (pH 14.5). This alkaline pH hinders pathogen development and reduces the potato’s response to stress, which could have contributed to enhancing the resistance of the studied cultivars to phytocides stress.
Understanding the influence of these varietal traits on phytotoxic damage is crucial for selecting appropriate potato cultivars and implementing effective herbicide management strategies. Different cultivars may respond differently to herbicide treatments and environmental conditions, so choosing the right cultivar for specific growing conditions is essential to minimize phytotoxic effects and maximize potato yields.
The herbicide resistance of potato cultivars is a highly valuable attribute, denoting the capacity of certain cultivars to withstand the phytotoxic effects of herbicides. This resistance can be attributed to specific genetic characteristics that enhance these cultivars’ ability to endure herbicide applications more effectively, enabling precise weed control without causing substantial crop damage. Extensive research on potato cultivar resistance to the active substance metribuzin has been conducted by many authors [19,54,56,59,80]. Based on our own research and that of other authors [19,22,56,59], it has been established that the fundamental aspects of potato cultivar resistance to herbicides encompass the following.
The herbicide resistance of potato cultivars is genetically determined. Certain potato cultivars possess inherent genetic traits that render them less susceptible to the toxic effects of herbicides. These traits are often inherited and transmitted through the breeding process [59,72]. Some potato cultivars have been developed or selected specifically for their herbicide resistance, and these are referred to as herbicide-tolerant cultivars [74]. Resistance mechanisms operate at the genetic and biochemical levels and may encompass reduced herbicide uptake, enhanced herbicide detoxification, modified target site sensitivity, or a combination of these factors [74,83]. Herbicide-resistant potato cultivars are typically developed for use with specific herbicides that effectively control problematic weeds while having minimal impact on potato yields. This selective approach permits efficient weed management without harming the potato crop [72]. Growers employing herbicide-resistant potato cultivars must continually monitor weed populations and adapt their weed control strategies. This practice helps prevent the development of herbicide-resistant weeds and maintains the long-term efficacy of herbicides [34].
In summary, the herbicide resistance of potato cultivars is a valuable tool for effective weed management while minimizing damage to potato crops. It is the result of genetic traits and extensive research, empowering farmers to use herbicides more efficiently and sustainably in potato cultivation. However, prudent herbicide resistance management is crucial to ensuring its long-term effectiveness and sustainability.
4.5. Dependence of Yield on Phytotoxic Damage
The results presented in this manuscript provide valuable insights into the characteristics of potato yields and the degree of phytotoxic damage caused by herbicide applications. Phytotoxic damage decreases over time. Over the observed time intervals (x1–x6), the data showed a positive yet moderate correlation between time and the extent of plant damage. This suggests that as time progresses, phytotoxic damage tends to decrease.
The strong correlation between total and marketable yield: A significant finding was the very strong correlation observed between total and marketable potato yields, with a Pearson correlation coefficient (r) of 0.99. This strong positive correlation indicates that variations in one of these yield parameters closely correspond to similar variations in the other.
The limited correlation between yield and phytotoxic damage: Conversely, the correlation between the total yield (y1) and phytotoxic damage at various time intervals exhibited very weak correlations, ranging from −0.03 to 0.20. This implies little to no clear relationship. The same trend was observed for the marketable yield (y2) (r = 0.01 to 0.22). These results suggest that phytotoxic damage does not significantly impact yield within the observed range. A similar correlation between phytotoxic damage and potato yield was observed by [13,14,21,25,26,80]. The statistical analysis of the research results regarding the total and marketable yield also showed the highest significance in the interaction between cultivar × care methods and variety × year (but only for marketable yield). The interaction between care methods and years of research and cultivar × care method × years of research was not statistically proven.
Our research revealed several significant interactions between years, cultivation methods, cultivars, and care concerning weed damage. An interaction between the years of the study and weed management methods was also observed. In 2009, the highest level of damage occurred after applying the Sencor 70 WG POST herbicide at a dose of 0.5 kg·ha−1 (treatment 5). The potato yields on plots treated with methods other than Sencor POST at a reduced dose remained at a similar level and were homogeneous concerning the examined feature. This suggests that the effectiveness of herbicide treatment varied significantly in different years, emphasizing the importance of considering random factors in weed management.
The lack of significance in the cultivar × year × care interaction (Table 8) indicates that the combination of years, cultivars, and care did not have a significant impact on weed damage. This may suggest that the selected cultivars and care practices had a consistent effect across the study years.
Significant differences in weed reduction were identified only in maintenance methods 4 to 8 (Sencor PRE and Sencor POST in different combinations with herbicides). The strongest herbicidal effect was observed in the optimal year of 2009, while the weakest occurred in the dry year of 2008. This underscores the significant influence of potato care methods on weed control, depending on the meteorological conditions of the respective year.
In the case of monocot weeds, there was no significant dependence between years, cultivars, and potato care methods. This suggests that the interaction between years, cultivars, and care did not have a significant impact on the damage caused by the dicot weeds. The effectiveness of the applied cultivars and care practices seems consistent across the years, concerning the control of dicotyledonous weeds. Similarly, the interaction between years, cultivars, and potato care concerning monocot weeds was found to be insignificant. This implies a consistent impact of cultivars and care practices on damage caused by monocotyledonous weeds across the study years. According to Skowera et al. [50] and Kalbarczyk [84], the main cause of the decline in potato yield in Poland is agrophenological factors, and particularly a delay in the potato planting date, a delay in emergence, and a delay in tuberization and flowering may contribute to a decline in potato yield of 10 to 16% in relation to that for a long-term crop. While the observed interactions provide valuable insights, further investigation and consultation with experts in this field are necessary to validate and contextualize the results. Collaboration and a comprehensive review of research on weed management methods will contribute to a more in-depth understanding of the complex mechanisms influencing weed damage in potato cultivation.
The analysis results indicate a strong correlation between the total and marketable potato yields, which aligns with expectations since these variables are inherently related. However, there is limited evidence supporting a clear correlation between potato yields and the degree of phytotoxic damage caused by herbicides. This suggests that, within the observed range, herbicide-induced phytotoxicity has a limited impact on potato yield. The robust correlation between the total and marketable yield can be beneficial for farmers, as it allows for more accurate predictions of the marketable yield based on the total yield. Additionally, the data highlight that phytotoxic damage becomes less visible over time, providing essential insights into the potential effects of herbicide applications on crop health. It is important to note that these findings are specific to the dataset and conditions under examination, and further research may be necessary to extend these conclusions to different scenarios.
The analysis of Pearson’s correlation coefficients reveals some important findings regarding the relationship between potato yield and phytotoxic damage in plants. While a strong positive correlation exists between the total and marketable potato yields, suggesting that changes in one parameter are closely associated with similar changes in the other, the degree of phytotoxic damage shows a very weak correlation with potato yield. This indicates that the phytotoxic damage to potato plants, observed at different time intervals after herbicide application, has a limited impact on the overall yield within the observed range. Potato growers and researchers should be aware that the effects of phytotoxicity on yield are relatively minor in comparison to other factors that influence potato production.
5. Towards the Future
In studies of herbicide phytotoxicity in the context of potato cultivation, significant aspects regarding plant reactions to these substances and the influence of environmental conditions on phytotoxicity risk have been emphasized. Here is a summary and a challenge for the future.
The phytotoxicity of herbicides and genetic variation: Research has shown that different potato cultivars exhibit varying sensitivity to applied herbicides. There is a need for further research to identify the genes and genetic mechanisms influencing this sensitivity and to use this knowledge in breeding potato cultivars with greater herbicide tolerance.
The impact of environmental conditions: Weather and soil conditions are crucial for the influence of herbicides on potato plants. Studies demonstrate that low temperatures, low rainfall, or excessive rainfall can increase herbicide phytotoxicity. It is worthwhile to continue researching this aspect to better understand how different environmental conditions affect plant reactions to herbicides.
The role of biostimulants and secondary substances: Research into interactions between herbicides and other chemical compounds in potato plants, such as glycoalkaloids and flavonoids, is essential. It is valuable to investigate how these substances affect herbicide phytotoxicity and how their impact can be managed.
The optimization of herbicide applications: Studies on the timing and dosages of herbicide applications are significant, particularly in the context of minimizing phytotoxicity risk and maximizing weed control effectiveness. This research can contribute to the development of improved herbicide application practices in potato cultivation.
Integrated farming approach: In the context of herbicide application optimization, it is valuable to promote an integrated approach that considers various factors, such as plant genetics, environmental conditions, herbicide type, and application timing. This approach can contribute to more sustainable potato cultivation.
As agriculture faces challenges related to environmental protection and increased production efficiency, research on herbicide phytotoxicity in potato cultivation remains a significant research area. Knowledge in this area can contribute to the development of more efficient and sustainable agricultural practices.
6. Conclusions
The use of herbicides, especially in POST applications, resulted in significant leaf damage to potatoes compared to PRE applications, especially when the herbicide Sencor 70 WG was applied POST at a dose of 0.5 kg ha−1.
The atmospheric conditions during the study years had a more pronounced impact on weed damage than genetic factors or weed control methods.
The best results in terms of both overall and marketable potato yields were obtained by using the Sencor 70 WG herbicide PRE at the recommended dose. However, using this herbicide POST, even at a reduced dose, led to a reduction in the overall yield compared to the control object and mechanical care.
The Apyros 75 WG herbicide, which contains sulfosulfuron as its active ingredient, can be a valuable tool for controlling monocot weeds but may carry the risk of damaging potato plants. Further research on this herbicide’s impact on different plant cultivars is valuable, and strategies should be developed for its effective use in agriculture while minimizing crop damage.
The impact of herbicides on potato yield turned out to be variable and depended on several factors, including the potato variety, weed control method, and weather conditions. Therefore, it is essential for farmers to consider these factors in their agricultural practices and make informed decisions to optimize potato yield.
The potato cultivar can influence its sensitivity to the herbicide’s effects, and further research is needed to investigate the mechanisms behind this difference in sensitivity.
The weather and soil conditions during the growing season had a substantial effect on both the total and marketable potato yields. The highest yields were achieved in a year with optimal humidity and thermal conditions, whereas the lowest yields were observed during a dry year. This underscores the critical role of weather conditions in potato cultivation.
The study highlights the dynamic nature of weed management, where the effectiveness of treatment methods, cultivars, and care practices is shaped by the variability of climatic conditions in different years. Understanding these interactions is crucial for optimizing weed control strategies, and the results emphasize the need for tailored approaches based on specific environmental conditions.
The alternative hypothesis has been confirmed in the study, demonstrating that the application of herbicides and their mixtures, such as (a) metribuzin—PRE; (b) metribuzin + rimsulfuron + ethoxylated isodecyl alcohol—PRE; (c) metribuzin—POST; (d) metribuzin + rimsulfuron + ethoxylated isodecyl alcohol—PRE; (e) metribuzin + fluazifop-P butyl—POST; and (f) metribuzin + sulfosulfuron + SN oil—POST emergence:
- Provides a broader range of herbicidal action and inflicts more substantial damage to weeds while simultaneously preventing phytotoxic damage to crop plants when compared to mechanical weed control and their elimination.
- Allows for a reduction in environmental pollution and ensures improved chemical treatment efficacy by employing smaller herbicide doses, contrary to the null hypothesis that posits no differences between herbicide or herbicide mixture variants and variants without weed protection or with mechanical control.
In studies of herbicide phytotoxicity in the context of potato cultivation, significant aspects concerning plant responses to these substances and the impact of environmental conditions on phytotoxicity risk have been highlighted. Here is a summary and a challenge for the future: Phytotoxicity is a crucial factor in both agriculture and environmental conservation. Investigating its mechanisms and effects will enable the development of effective plant protection strategies and the maintenance of ecological equilibrium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14010085/s1, Table S1: ANOVA table for total tuber yield. Table S2: ANOVA table for comercial tuber yield. Table S3: ANOVA table for herbicide damage—7 days after herbicide application. Table S4: ANOVA table for herbicide damage—14 days after herbicide application. Table S5: ANOVA table for herbicide damage—21 days after herbicide application. Table S6: ANOVA table for herbicide damage—28 days after herbicide application. Table S7: ANOVA table for herbicide damage—35 days after herbicide application. Table S8: ANOVA table for herbicide damage—42 days after herbicide application. Table S9: Table Analysis of variance ANOVA of number of monocotyledonous weeds before short circuit of potato rows. Table S10: Table Analysis of variance ANOVA of number of dicotyledonous weeds before short circuit of potato rows. Table S11: Table Analysis of variance ANOVA of number of monocotyledonous and dicotyledonous weeds before short circuit of potato rows. Table S12: ANOVA table of monocotyledonous weeds before potato harvest. Table S13: ANOVA table of broadleaf weeds before potato harvest. Table S14: Table of variance analysis of ANOVA of number of monocotyledonous and dicotyledonous weeds before harvesting. Table S15: Table of variance analysis ANOVA of fresh weeds mass. Table S16: Table of variance analysis ANOVA of dry weed mass.
Author Contributions
Conceptualization: P.B. and B.S.; methodology: P.B., M.P. and B.S.; software: D.S. and P.P.; validation, P.B., M.P. and D.S.; formal analysis: P.B.; investigation: P.B.; resources: P.B. and P.P.; data curation: P.B. and D.S.; writing—original draft preparation: P.B. and P.P.; writing—review and editing: P.B. and B.S.; visualization: M.P. and D.S.; supervision: B.S.; project administration: P.B. and M.P.; funding acquisition: B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We would like to thank the Institute of Plant Breeding and Acclimatization—National Research Institute branch in Jadwisin for administrative and technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PRE | pre-emergence |
| POST | post-emergence |
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 12 October 2023).
- Anonymous. Chapter 2. Food security and nutrition around the world. In The State of Food Security and Nutrition in the World. Urbanization, Agrifood Systems Transformation and Healthy Diets across the Rural–Urban Continue; Food and Agriculture Organization of the United Nations International Fund for Agricultural Development, United Nations Children’s Fund World Food Programmer, World Health Organization: Rome, Italy, 2023; Available online: https://www.fao.org/3/cc3017en/cc3017en.pdf (accessed on 10 October 2023).
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for Sustainable Global Food Security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Alebrahim, M.T.; Majd, R.; Rashed Mohassel, M.H.; Wilcockson, S.; Baghestani, M.A.; Ghorbani, R.; Kudsk, P. Evaluating the efficacy of pre- and post-emergence herbicides for controlling Amaranthus retroflexus L. and Chenopodium album L. in potato. Crop Prot. 2012, 42, 345–350. [Google Scholar] [CrossRef]
- Hussain, Z.; Munsif, F.; Marwat, K.B.; Ail, K.; Afridi, R.A.; Bibi, S. Studies on efficacy of different herbicides against weeds in potato crop in Peshawar. Pak. J. Bot. 2013, 45, 487–491. [Google Scholar]
- Singh, S.P.; Rawal, S.; Dua, V.K.; Sharma, S.K. Effectiveness of sulfosulfuron herbicide in controlling weeds in potato crops. Potato J. 2017, 44, 110–116. [Google Scholar]
- Zawiślak, K.; Janczak-Tabaszewska, D.; Adamiak, J. Phytotoxicity of soil herbicides towards some potato varieties and weeds. Zesz. Nauk. ART Olszt. 1985, 41, 126–138. (In Polish) [Google Scholar]
- Herrera-Murillo, F.; Picado-Arroyo, G. Evaluation of pre-emergent herbicides for weed control in sweet potato. Agron. Costarric. 2023, 47, 59–71. [Google Scholar]
- Wójtowicz, A.; Mrówczyński, M. (Eds.) Metodyka Integrowanej Ochrony Ziemniaka dla Doradców. Opracowanie Zbiorowe; IOR-PIB: Poznań, Poland, 2017; 248p, ISBN 978-83-64655-32-6. (In Polish) [Google Scholar]
- Biswas, U.; Kundu, A.; Labar, A.; Datta, M.K.; Kundu, C.K. Bioefficacy and phytotoxicity of 2, 4-D Dimethyl Amine 50% SL for weed control in potato and its effect on succeeding crop greengram. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1261–1267. [Google Scholar] [CrossRef]
- Edwards, T.J.; Davies, S.L.; Yates, R.J.; Rose, M.; Howieson, J.G.; O’Hara, G.; Steel, E.J.; Hall, D.J.M. The phytotoxicity of soil-applied herbicides is enhanced in the first-year post strategic deep tillage. Soil Tillage Res. 2023, 231, 105734. [Google Scholar] [CrossRef]
- Felipe, J.M.; Martins, D.; Da Costa, N.V. Seletividade de herbicidas aplicados em pré-emergência sobre cultivares de batata. Bragantia Camp. 2006, 5, 615–621. [Google Scholar] [CrossRef]
- Gugała, M.; Zarzecka, K. The effectiveness and the selectivity of herbicides in weed control on the potato plantation. Biul. IHAR 2011, 262, 103–110. (In Polish) [Google Scholar] [CrossRef]
- Mystkowska, I.; Zarzecka, K.; Baranowska, A.; Gugała, M. An effect of herbicides and their mixtures on potato yielding and efficacy in potato crop. Prog. Plant Prot./Postępy Ochr. Roślin 2017, 57, 21–26. [Google Scholar]
- Abdallah, I.S.; Atia, M.A.; Nasrallah, A.K.; El-Beltagi, H.S.; Kabil, F.F.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of new pre-emergence herbicides on quality and yield of potato and its associated weeds. Sustainability 2021, 13, 9796. [Google Scholar] [CrossRef]
- Dvořak, P.J.; Remešowă, I. Assessment of metribuzin effects on potatoes using a method of very rapid fluorescence induction. Rast. Výr. 2002, 48, 107–117. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Boydston, R.A.; Ransom, C.V.; Tonks, D.J.; Beutler, B.R. Potato variety tolerance to flumioxazin and sulfentrazone. Weed Technol. 2005, 19, 683–696. [Google Scholar] [CrossRef]
- Brooke, M.J.; Stenger, J.; Svyantek, A.W.; Auwarter, C.; Hatterman-Valenti, H. ‘Atlantic’ and ‘Dakota Pearl’ chipping potato responses to glyphosate and dicamba simulated drift. Weed Technol. 2022, 36, 15–20. [Google Scholar] [CrossRef]
- Urbanowicz, J. Reaction of potato cultivars with diverse leaf morphology to metribuzin applied post emergence. Biul. Inst. Hod. Aklim. Roślin 2012, 265, 15–28. (In Polish) [Google Scholar]
- Dias, R.C.; Barbosa, A.R.; Silva, G.S.; De Lourdes Pereira Assis, A.C.; Melo, C.A.D.; Silva, D.V.; Reis, M.R. Efeito de herbicidas no crescimento, produtividade e qualidade de tubérculos de batata. Magistra Cruz Almas BA 2017, 29, 71–79. [Google Scholar]
- Ginter, A.; Zarzecka, K.; Gugała, M.; Mystkowska, I. Impact of the method of plantation care on weeds on the production and economics results of three cultivars of edible potato. Prog. Plant Prot./Postępy Ochr. Roślin 2023, 63, 41–47. [Google Scholar]
- Barbaś, P. Morphology changes of Chenopodium album in conditions of metribuzin and metribuzin mix with sulfosulfuron, rimsulfuron and fluazyfop used in potato cultivation. Ann. Univ. Mariae Curie-Skłodowska. Sect. E Agric. 2008, 63, 71–80. (In Polish) [Google Scholar] [CrossRef]
- Reader, A.J.; Lyon, D.; Harsh, J.; Burke, I. How Soil pH Affects the Activity and Persistence of Herbicides; Washington State University: Pullman, WA, USA, 2015; Available online: https://research.libraries.wsu.edu:8443/xmlui/bitstream/handle/2376/6097/FS189E.pdf (accessed on 18 October 2023).
- Ramsey, R.J.L.; Stephenson, G.R.; Hall, J.C. A review of the effects of humidity, humectants, and surfactant composition on the absorption and efficacy of highly water-soluble herbicides. Pestic. Biochem. Physiol. 2005, 82, 162–175. [Google Scholar] [CrossRef]
- Gugała, M.; Zarzecka, K.; Sikorska, A. Evaluation of herbicide efficiency and their influence on potato marketable yield. Biul. IHAR 2013, 270, 75–84. (In Polish) [Google Scholar] [CrossRef]
- Mystkowska, I.; Zarzecka, K.; Baranowska, A.; Gugała, M. Weed infestation of potato cultivars depending on weed control methods and weather conditions. Acta Agrophysica 2017, 24, 111–121. (In Polish) [Google Scholar]
- Siblani, W.; Haidar, M.A. Reduced Rates of Metribuzin and Time of Hilling Controlled Weeds in Potato. Am. J. Plant Sci. 2017, 8, 3207–3217. [Google Scholar] [CrossRef][Green Version]
- Boydston, R.A.; Felix, J.; Al-Khatib, K. Preemergence Herbicides for Potential Use in Potato Production. Weed Technol. 2012, 26, 731–739. [Google Scholar] [CrossRef]
- Correia, N.M.; Carvalho, A.D. Herbicide selectivity for potato crop. Hortic. Bras. 2019, 37, 302–308. [Google Scholar] [CrossRef]
- Luz, J.M.Q.; Fonseca, L.F.; Duarte, I.N. Selectivity of pre-emergence herbicides in potato cv. Innovator. Hortic. Bras. 2018, 36, 223–228. [Google Scholar] [CrossRef]
- Hoza, G.; Enescu, B.G.; Becherescu, A. Research regarding the effect of applying herbicides to combat weeds in quickly-potato cultures. Sci. Pap. Ser. B Hortic. 2015, 59, 219–223. [Google Scholar]
- Ma, H.; Lu, H.; Han, H.; Yu, Q.; Powles, S. Metribuzin resistance via enhanced metabolism in a multiple herbicide resistant Lolium rigidum population. Pest Manag. Sci. 2020, 76, 3785–3791. [Google Scholar] [CrossRef]
- Plant Protection Recommendations for 2018/2019. Instytut Ochrony Roślin—Państwowy Instytut Badawczy. Części I–IV. Opracowanie Zbiorowe; IOR-PIB: Poznań, Poland, 2018. (In Polish)
- Korbas, M. (Ed.) Potato Protection Program; IOR-PIB: Poznań, Poland, 2017; Available online: https://www.ior.poznan.pl/plik,2913program-ochrony-ziemniaka-pdf. (accessed on 23 September 2023). (In Polish)
- Nowacki, W. (Ed.) Characteristics of the National Register of Potato Cultivars, 22nd ed.; IHAR—PIB: Jadwisin, Poland, 2019; 45p. (In Polish) [Google Scholar]
- PubChem Compound Summary for CID 70551071, CID 70551071. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/70551071. (accessed on 12 October 2023).
- CAS Databases 2023. Available online: https://www.cas.org/support/documentation/cas-databases (accessed on 8 October 2023).
- EPA. Fluazifop-P-butyl; Pesticide Tolerances. Resolution of the Environmental Protection Agency of April 27, 2023; Environmental Protection Agency (EPA): Washington, DC, USA, 2023. Available online: https://www.federalregister.gov/documents/2023/04/27/2023-08939/fluazifop-p-butyl-pesticide-tolerances (accessed on 16 December 2023).
- Badowski, M.; Domaradzki, K.; Filipiak, K.; Franek, M.; Gołębiewska, H.; Kieloch, R.; Kucharski, M.; Rola, H.; Rola, J.; Sadowski, J.; et al. Methodology of Biological Evaluation Experiments of Herbicides, Bioregulators and Adjuvants. Part I. Field Experiments; Wyd. IUNG: Puławy, Poland, 2001; 167p. (In Polish) [Google Scholar]
- Pszczółkowski, P.; Sawicka, B.; Skiba, D.; Barbaś, P.; Noaema, A.H. The Use of Chlorophyll Fluorescence as an Indicator of Predicting Potato Yield, Its Dry Matter and Starch in the Conditions of Using Microbiological Preparations. Appl. Sci. 2023, 13, 10764. [Google Scholar] [CrossRef]
- PN-R-0403:1997; Chemical and Agricultural Analysis of Soil. Sampling. Polish Committee for Standardization: Warszawa, Poland, 1997. (In Polish)
- Mocek, A. Gleboznawstwo, 1st ed.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2015; 589p, ISBN 978-83-01-18795-8. (In Polish) [Google Scholar]
- Ryżak, M.; Bartmiński, P.; Bieganowski, A. Methods for determination of particle size distribution of mineral soils. Acta Agroph. Theses Monogr. 2009, 175, 1–97. [Google Scholar]
- Lützenkirchen, J.; Preočanin, T.; Kovačević, D.; Tomišić, V.; Lövgren, L.; Kallay, N. Potentiometric titrations as a tool for surface charge determination. Croat. Chem. Acta 2012, 85, 391–417. [Google Scholar] [CrossRef]
- PN-R-04020:1994+AZ1:2004; Chemical and Agricultural Analysis of Soil. Polish Committee for Standarization: Warszawa, Poland, 2004. (In Polish)
- PN-R-04023:1996; Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils. Polish Committee for Standarization: Warszawa, Poland, 1996. (In Polish)
- PN-R-04022:1996+AZ1:2002; Chemical and Agricultural Analysis of Soil. Determination of Available Potassium Content in Mineral Soils. Polish Committee for Standardization: Warszawa, Poland, 2002. (In Polish)
- World Reference Database for Soil Resources. WRB. 2014. Available online: https://www.fao.org/3/ai3794e.pdf (accessed on 8 June 2020).
- Nawrocki, S. Fertilizer Recommendations. Part. I. Limit Numbers for Valuation of Soils in Macro- and Microelements; IUNG: Puławy, Poland, 1990; p. 44. (In Polish) [Google Scholar]
- Skowera, B.; Kopcińska, J.; Kopeć, B. Changes in thermal and precipitation conditions in Poland in 1971–2010. Ann. Wars. Univ. Life Sci. Land Reclam. 2014, 46, 153–162. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT®9.2. User’s Guide; SAS Institute: Cary, NC, USA, 2008. [Google Scholar]
- Koronacki, J.; Mielniczuk, J. Statystyka dla Studentów Kierunków Technicznych i Przyrodniczych; WNT: Warszawa, Poland, 2006; 456p. (In Polish) [Google Scholar]
- IBM. SPSS Statistics 28 User’s Guide—IBM Core System. Available online: https://www.ibm.com/docs/en/SSLVMB_28.0.0 (accessed on 18 October 2023).
- Sawicka, B. The response of 44 cultivars of potato to metribuzin. Rocz. Nauk. Rol. Ser. E–Ochr. Roślin 1994, 23, 103–123. (In Polish) [Google Scholar]
- Hermeziu, M.; Nitu, S.; Hermeziu, R. Studies of efficacy of different herbicides against weeds in potato crop in central part of Romania. Ann. Univ. Craiova 2020, 25, 388–393. [Google Scholar]
- Urbanowicz, J. Skuteczność zwalczania chwastów w ziemniaku za pomocą wybranych herbicydów przedwschodowych. Ziemn. Pol. 2021, 1, 24–33. (In Polish) [Google Scholar]
- Praczyk, T.; Skrzypczak, G. (Eds.) Properties and use of herbicides. In Herbicides; PWRiL: Poznań, Poland, 2004; pp. 17–66. ISBN 83-09-01786-3. (In Polish) [Google Scholar]
- Tkach, A.S.; Golubev, A.S. Sensitivity of potato cultivars to former safe and metribuzin. Potato J. 2022, 49, 17–26. [Google Scholar]
- Urbanowicz, J. The influence of post-emergence application of metribuzin on the yield of selected potato varieties. Prog. Plant Prot./Postępy Ochr. Roślin 2010, 50, 837–841. (In Polish) [Google Scholar]
- Sawicka, B.; Skalski, J. Potato weeds under the conditions of using the herbicide Sencor 70 WP. Vol. II. Herbicidal effectiveness of the herbicide. Rocz. Nauk Rol. 1996, A-112, 169–182. (In Polish) [Google Scholar]
- Riethmuller-Haage, I.; Bastiaans, L.; Kempenaar, C.; Smutny, V.; Kropff, M.J. Are pre-spraying growing conditions a major determinant of herbicide efficacy? Weed Res. 2007, 47, 415–424. [Google Scholar] [CrossRef]
- Lichenthaler, H.K. Vegetation stress an introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–11. [Google Scholar] [CrossRef]
- Starck, Z. Effect of stress conditions on coordination of photosynthetic production and resources allocation. Post. Nauk Roln. 2010, 1, 9–26. (In Polish) [Google Scholar]
- Sawicka, B.; Michałek, W.; Pszczółkowski, P. Dependence of chemical composition of potato (Solanum tuberosum L.) tubers on physiological indicators. Zemdirb.-Agric. 2015, 102, 41–50. [Google Scholar] [CrossRef][Green Version]
- Skórska, E.; Swarcewicz, M. Use of chlorophyll fluorescence for estimation of some adjuvants efficacy in a mixture with atrazine. Inżynieria Roln. 2005, 64, 253–259. (In Polish) [Google Scholar][Green Version]
- Sawicka, B.; Michałek, W.; Skiba, D. Susceptibility of plants of Helianthus tuberosus L. on clomazone. Post. Ochr. Roślin 2007, 47, 365–370. (In Polish) [Google Scholar][Green Version]
- Singh, S.P.; Rawal, S.; Dua, V.K.; Roy, S.; Sharma, S.K. Evaluation of post emergence herbicide bentazon in potato crop. Int. J. Chem. Stud. 2019, 7, 2816–2820. [Google Scholar][Green Version]
- Hermeziu, M.; Niţu, S.; Hermeziu, R. Efficacy of some herbicides on weed growth and yield of potato in climatic conditions of barsa county. Sci. Pap. Ser. A Agron. 2022, 65, 349–353. [Google Scholar][Green Version]
- Monjezi, N.; Razmjo, J.; Karimmojeni, H. Valerian (Valeriana officinalis L.) tolerance to some post-emergence herbicides. J. Plant Prot. Res. 2015, 55, 415–420. [Google Scholar] [CrossRef]
- Baker, N.R.; Bowyer, J.R. (Eds.) Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; BIOS Scientific Publishers: Oxford, UK, 1994; ISBN 1-872748-3-1. [Google Scholar]
- Chung, I.M.; Ham, T.H.; Cho, G.W.; Kwon, S.W.; Lee, Y.; Seo, J.; An, Y.-J.; Kim, S.-Y.; Kim, S.-H.; Lee, J. Study of quantitative trait loci (QTLs) associated with the allelopathic trait in rice. Genes 2020, 11, 470. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, S.; Zhang, Q.; Wang, P.; Wang, H.; He, H. Comparative analysis on metabolites and gene expression difference of various allelopathic potential rice under weed stress. Weed Res. 2022, 62, 340–346. [Google Scholar] [CrossRef]
- Szajko, K.; Smyda-Dajmund, P.; Ciekot, J.; Marczewski, W.; Sołtys-Kalina, D. Glycoalkaloid Composition and Flavonoid Content as Driving Forces of Phytotoxicity in Diploid Potato. Int. J. Mol. Sci. 2023, 24, 1657. [Google Scholar] [CrossRef]
- Bakhsh, A.; Hussain, T.; Rahamkulov, I.; Demirel, U.; Çalışkan, M.E. Transgenic potato lines expressing CP4-EPSP synthase exhibit resistance against glyphosate. Plant Cell Tissue Organ Cult. 2020, 140, 23–34. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The e-Pesticide Manual: A World Compendium. Version 5.2; British Crop Production Council: Alton Hampshire, UK, 2011. [Google Scholar]
- Zalecenia Ochrony Roślin Rolniczych na 2020 rok. Instytut Ochrony Roślin—Państwowy Instytut Badawczy (Tom III. Rośliny Oleiste, Okopowe, Bobowate i Zielarskie). Opracowanie Zbiorowe; IOR-PIB: Poznań, Poland, 2020; Available online: https://www.ior.poznan.pl/plik,3801,tom-3-chwasty-ziemniak-pdf.pdf (accessed on 8 October 2023). (In Polish)
- Alebrahim, M.T.; Majd, R.; Abdollahi, F.; Zangoueinejad, R.; Dayan, F.E.; Mathiassen, S.K.; Kudsk, P. Absorption and Metabolism of Foliar-Applied Rimsulfuron in Potato (Solanum tuberosum L.), Common Lambsquarters (Chenopodium album L.) and Redroot Pigweed (Amaranthus retroflexus L.). Potato Res. 2021, 64, 635–648. [Google Scholar] [CrossRef]
- Paporisch, A.; Laor, Y.; Rubin, B.; Achdari, G.; Eizenberg, H. Application Timing and Degradation Rate of Sulfosulfuron in Soil Co-affect Control Efficacy of Egyptian broomrape (Phelipanche aegyptiaca) in Tomato. Weed Sci. 2018, 66, 780–788. [Google Scholar] [CrossRef]
- Dittmar, P.J.; Batts, R.B.; Jennings, K.M.; Bellinder, R.R.; Meyers, S.L. Reduced Metribuzin preharvest interval on potato yield and tuber quality. Weed Technol. 2015, 29, 335–339. [Google Scholar] [CrossRef]
- Gruczek, T. Sensitivity of potato cultivars to metribuzine. Biul. IHAR 2004, 232, 193–199. (In Polish) [Google Scholar]
- Davies, S.; Armstrong, R.; Macdonald, L.; Condon, K.; Petersen, E. Soil Constraints: A Role for Strategic Deep Tillage Australian Agriculture in 2020: From Conservation to Automation; Agronomy Australia: South Perth, Australia, 2019; pp. 117–135. [Google Scholar]
- Wichrowska, D.; Wojdyła, T.; Rogozińska, I. Concentrations of some macro elements in potato tubers stored at 4 °C and 8 °C. J. Elem. 2009, 14, 373–382. [Google Scholar]
- Sherman, T.D.; Vaughn, K.C.; Duke, S.O. Mechanisms of action and resistance to herbicides. In Herbicide-Resistant Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 13–35. [Google Scholar]
- Kalbarczyk, R. Relationship between the level of yielding and the agrophenology of late potato cultivars in Poland. Acta Sci. Pol. Agric. 2003, 2, 83–92. (In Polish) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).