Development and Investigation of HRM Markers to Discriminate Two Ogura Cytoplasmic Male Sterility Restorer Genes in Radish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Male Sterility Evaluation
2.2. Total DNA Isolation and the PCR Assays
2.3. Genetic Mapping of Restorer-of-Fertility Genes
2.4. Development of Molecular Markers
2.5. HRM Analysis for Fine-Mapping
3. Results
3.1. Genetic Mapping of Rft and Rfo
3.2. Development of Rft Gene-Related HRM Molecular Markers
3.3. Development of HRM Molecular Markers Related to the Rfo Gene
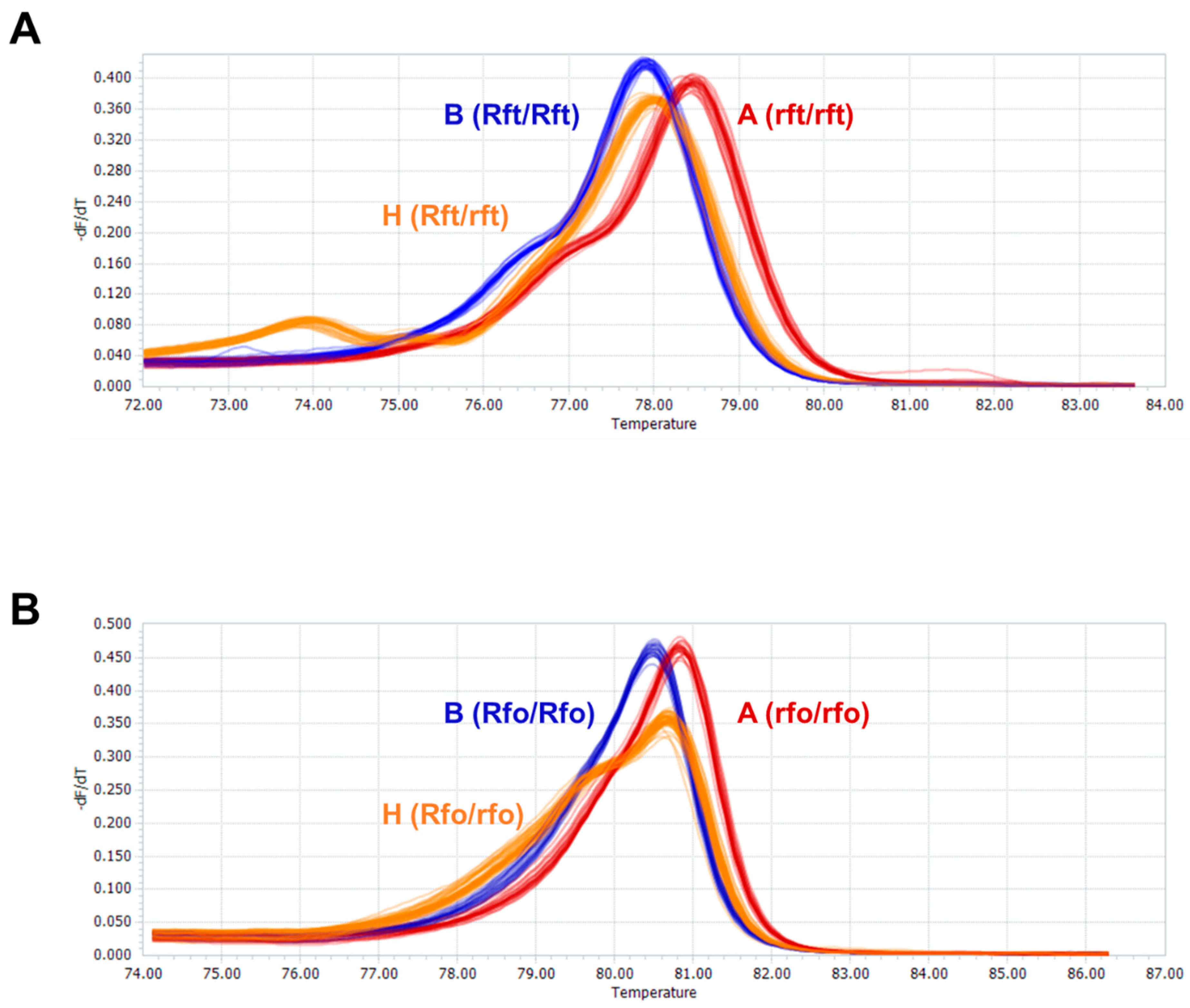
3.4. Validation of HRM Markers Linked to Rft and Rfo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogura, H. Studies on the new male-sterility in Japanese radish, with special references to the utilization of this sterility towards the practical raising of hybrid seeds. Mem. Fac. Agric. Kagoshima Univ. 1968, 6, 39–78. [Google Scholar]
- Ren, W.; Si, J.; Chen, L.; Fang, Z.; Zhuang, M.; Lv, H.; Wang, Y.; Ji, J.; Yu, H.; Zhang, Y. Mechanism and Utilization of Ogura Cytoplasmic Male Sterility in Cruciferae Crops. Int. J. Mol. Sci. 2022, 23, 9099. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, H.; Terachi, T. Molecular and biological studies on male-sterile cytoplasm in the Cruciferae. III. Distribution of Ogura-type cytoplasm among Japanese wild radishes and Asian radish cultivars. Theor. Appl. Genet. 1996, 93, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Uyttewaal, M.; Arnal, N.; Quadrado, M.; Martin-Canadell, A.; Vrielynck, N.; Hiard, S.; Gherbi, H.; Bendahmane, A.; Budar, F.; Mireau, H. Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. Plant Cell 2008, 20, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, H.; Park, S.; Cho, K.H.; Sung, S.K.; Oh, D.G.; Kim, K.T. Identification of a novel mitochondrial genome type and development of molecular markers for cytoplasm classification in radish (Raphanus sativus L.). Theor. Appl. Genet. 2007, 115, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, M.; Koizuka, N.; Fujimoto, H.; Sakai, T.; Imamura, J. Identification and expression of the kosena radish (Raphanus sativus cv. Kosena) homologue of the ogura radish CMS-associated gene, orf138. Plant Mol. Biol. 1999, 39, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Koizuka, N.; Imai, R.; Fujimoto, H.; Hayakawa, T.; Kimura, Y.; Kohno-Murase, J.; Sakai, T.; Kawasaki, S.; Imamura, J. Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J. 2003, 34, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Nahm, S.H.; Lee, H.J.; Lee, S.W.; Joo, G.Y.; Harn, C.H.; Yang, S.G.; Min, B.W. Development of a molecular marker specific to a novel CMS line in radish (Raphanus sativus L.). Theor. Appl. Genet. 2005, 111, 1191–1200. [Google Scholar] [CrossRef]
- Yamagishi, H.; Hashimoto, A.; Fukunaga, A.; Terachi, T. Appearance of male sterile and black radishes in the progeny of cross between Raphanus raphanistrum and Raphanus sativus. Breed. Sci. 2020, 70, 637–641. [Google Scholar] [CrossRef]
- Lee, Y.P.; Park, S.; Lim, C.; Kim, H.; Lim, H.; Ahn, Y.; Sung, S.K.; Yoon, M.K.; Kim, S. Discovery of a novel cytoplasmic male-sterility and its restorer lines in radish (Raphanus sativus L.). Theor. Appl. Genet. 2008, 117, 905–913. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.P.; Lee, J.; Choi, B.S.; Kim, S.; Yang, T.J. Complete mitochondrial genome sequence and identification of a candidate gene responsible for cytoplasmic male sterility in radish (Raphanus sativus L.) containing DCGMS cytoplasm. Theor. Appl. Genet. 2013, 126, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.P.; Lim, H.; Ahn, Y.; Sung, S.K. Identification of highly variable chloroplast sequences and development of cpDNA-based molecular markers that distinguish four cytoplasm types in radish (Raphanus sativus L.). Theor. Appl. Genet. 2009, 119, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Delourme, R.; Eber, F. Linkage between an isozyme marker and a restorer gene in radish cytoplasmic male sterility of rapeseed (Brassica napus L.). Theor. Appl. Genet. 1992, 85, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.G.; Formanova, N.; Jin, H.; Wargachuk, R.; Dendy, C.; Patil, P.; Laforest, M.; Zhang, J.; Cheung, W.Y.; Landry, B.S. The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J. 2003, 35, 262–272. [Google Scholar] [CrossRef]
- Desloire, S.; Gherbi, H.; Laloui, W.; Marhadour, S.; Clouet, V.; Cattolico, L.; Falentin, C.; Giancola, S.; Renard, M.; Budar, F.; et al. Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep. 2003, 4, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Terachi, T.; Yamagishi, H. A novel Rf gene controlling fertility restoration of Ogura male sterility by RNA processing of orf138 found in Japanese wild radish and its STS markers. Genome 2009, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; De Wang, C.; Gao, L.; Mei, S.Y.; Zhou, Y.; Xiang, C.P.; Wang, T. Heterozygous alleles restore male fertility to cytoplasmic male-sterile radish (Raphanus sativus L.): A case of overdominance. J. Exp. Bot. 2013, 64, 2041–2048. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.-P.; Lim, H.; Han, T.; Sung, S.-K.; Kim, S. Identification of Rfd1, a novel restorer-of-fertility locus for cytoplasmic male-sterility caused by DCGMS cytoplasm and development of simple PCR markers linked to the Rfd1 locus in radish (Raphanus sativus L.). Euphytica 2010, 175, 79–90. [Google Scholar] [CrossRef]
- Lee, Y.-P.; Cho, Y.; Kim, S. A high-resolution linkage map of the Rfd1, a restorer-of-fertility locus for cytoplasmic male sterility in radish (Raphanus sativus L.) produced by a combination of bulked segregant analysis and RNA-Seq. Theor. Appl. Genet. 2014, 127, 2243–2252. [Google Scholar] [CrossRef]
- Wang, X.; An, Y.; Xu, P.; Xiao, J. Functioning of PPR Proteins in Organelle RNA Metabolism and Chloroplast Biogenesis. Front. Plant Sci. 2021, 12, 627501. [Google Scholar] [CrossRef]
- Qin, X.; Warguchuk, R.; Arnal, N.; Gaborieau, L.; Mireau, H.; Brown, G.G. In vivo functional analysis of a nuclear restorer PPR protein. BMC Plant Biol. 2014, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Marcial, M.; Pacheco-Arjona, R.; Góngora-Castillo, E.; De-la-Peña, C. Chloroplastic pentatricopeptide repeat proteins (PPR) in albino plantlets of Agave angustifolia Haw. reveal unexpected behavior. BMC Plant Biol. 2022, 22, 352. [Google Scholar] [CrossRef] [PubMed]
- Grüttner, S.; Nguyen, T.-T.; Bruhs, A.; Mireau, H.; Kempken, F. The P-type pentatricopeptide repeat protein DWEORG1 is a non-previously reported rPPR protein of Arabidopsis mitochondria. Sci. Rep. 2022, 12, 12492. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kim, J.; Lee, S.Y.; Lee, J. Diallelic SNP marker development and genetic linkage map construction in octoploid strawberry (Fragaria × ananassa) through next-generation resequencing and high-resolution melting analysis. Hortic. Environ. Biotechnol. 2020, 61, 371–383. [Google Scholar] [CrossRef]
- Kim, J.; Manivannan, A.; Kim, D.S.; Lee, E.S.; Lee, H.E. Transcriptome sequencing assisted discovery and computational analysis of novel SNPs associated with flowering in Raphanus sativus in-bred lines for marker-assisted backcross breeding. Hortic. Res. 2019, 6, 120. [Google Scholar] [CrossRef]
- Van Ooijen, J.W. JoinMap® 4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Plant Research International BV and Kayazma BV: Wageningen, The Netherlands, 2006. [Google Scholar]
- Kosambi, D.D. The Estimation of Map Distances from Recombination Values. In D.D. Kosambi: Selected Works in Mathematics and Statistics; Ramaswamy, R., Ed.; Springer: New Delhi, India, 2016; pp. 125–130. [Google Scholar]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Shirasawa, K.; Hirakawa, H.; Fukino, N.; Kitashiba, H.; Isobe, S. Genome sequence and analysis of a Japanese radish (Raphanus sativus) cultivar named ‘Sakurajima Daikon’ possessing giant root. DNA Res. 2020, 27, dsaa010. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Shirasawa, K.; Hirakawa, H.; Fukino, N.; Kitashiba, H.; Isobe, S. Genome sequence analysis of a giant-rooted ‘Sakurajima daikon’ radish (Raphanus sativus). bioRxiv 2020. [Google Scholar] [CrossRef]
- Bellaoui, M.; Grelon, M.; Pelletier, G.; Budar, F. The restorer Rfo gene acts post-translationally on the stability of the ORF138 Ogura CMS-associated protein in reproductive tissues of rapeseed cybrids. Plant Mol. Biol. 1999, 40, 893–902. [Google Scholar] [CrossRef]
- Bellaoui, M.; Martin-Canadell, A.; Pelletier, G.; Budar, F. Low-copy-number molecules are produced by recombination, actively maintained and can be amplified in the mitochondrial genome of Brassicaceae: Relationship to reversion of the male sterile phenotype in some cybrids. Mol. Gen. Genet. 1998, 257, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lezhneva, L.; Arnal, N.; Quadrado, M.; Mireau, H. The radish Ogura fertility restorer impedes translation elongation along its cognate CMS-causing mRNA. Proc. Natl. Acad. Sci. USA 2021, 118, e2105274118. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-M.; Kim, N.; Ahn, B.O.; Oh, M.; Chung, W.-H.; Chung, H.; Jeong, S.; Lim, K.-B.; Hwang, Y.-J.; Kim, G.-B.; et al. Elucidating the triplicated ancestral genome structure of radish based on chromosome-level comparison with the Brassica genomes. Theor. Appl. Genet. 2016, 129, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Gudi, S.; Atri, C.; Goyal, A.; Kaur, N.; Akhtar, J.; Mittal, M.; Kaur, K.; Kaur, G.; Banga, S.S. Physical mapping of introgressed chromosome fragment carrying the fertility restoring (Rfo) gene for Ogura CMS in Brassica juncea L. Czern & Coss. Theor. Appl. Genet. 2020, 133, 2949–2959. [Google Scholar] [CrossRef]
- Yamagishi, H.; Jikuya, M.; Okushiro, K.; Hashimoto, A.; Fukunaga, A.; Takenaka, M.; Terachi, T. A single nucleotide substitution in the coding region of Ogura male sterile gene, orf138, determines effectiveness of a fertility restorer gene, Rfo, in radish. Mol. Genet. Genom. 2021, 296, 705–717. [Google Scholar] [CrossRef]
- Kim, S.; Lim, H.; Cho, K.; Park, P.; Park, S.; Sung, S.; Oh, D.; Kim, K. Development of gene-based markers for the allelic selection of the restorer-of-fertility gene, Rfo, in radish (Raphanus sativus). Korean J. Breed. Sci. 2009, 41, 194–204. [Google Scholar]

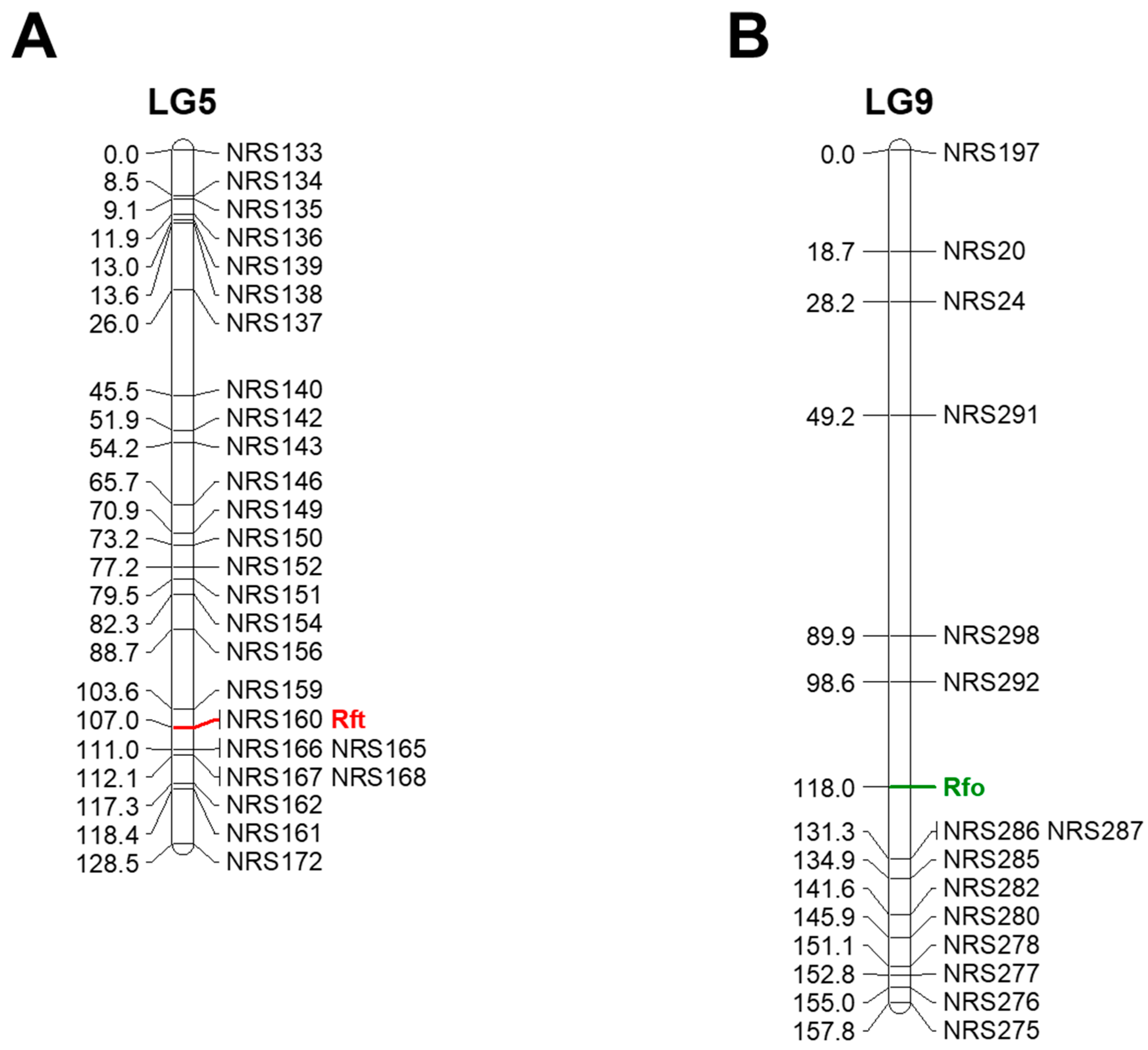
| Locus. | Chr. | Number of SNPs | Position (Mbp) | Designed Marker | Average Distance between Markers |
|---|---|---|---|---|---|
| Rft | R5 | 451 | 30.4–36.8 | 18 | 355 kbp |
| 32.8–35.2 | 79 | 30 kbp | |||
| 33.0–33.5 | 20 | 25 kbp | |||
| Rfo | R9 | 510 | 11.39–11.41 | 17 | 1 kbp |
| R0 | 310 | 28.45–28.46 | 10 | 1 kbp |
| Population | No. of Individuals | Marker | Genotype | Phenotype | No. of Recombinants | |
|---|---|---|---|---|---|---|
| Fertile | Sterile | |||||
| Rft segregating population; ‘Bokjeong’ × ‘Bakdal sn’ F2 | 180 | Rsc17 | A | 0 | 43 | 0 |
| B | 45 | 0 | ||||
| H | 92 | 0 | ||||
| Total | 137 | 43 | ||||
| RSRF27 | A | 36 | 13 | 62 | ||
| B | 29 | 6 | ||||
| H | 61 | 20 | ||||
| No call | 11 | 4 | ||||
| Total | 137 | 43 | ||||
| Rfo segregating population; ‘OharuA’ × ‘Bakdal’ F2 | 183 | Rsc17 | A | 42 | 13 | 75 |
| B | 29 | 15 | ||||
| H | 66 | 18 | ||||
| Total | 137 | 46 | ||||
| RSRF27 | A | 1 | 42 | 5 | ||
| B | 49 | 1 | ||||
| H | 87 | 3 | ||||
| Total | 137 | 46 | ||||
| (‘Bokjeong’ × ‘Bakdal sn’ F1) × ‘Gwandong summer radish’ | 199 | Rsc17 | A | 34 | 12 | 66 |
| B | 60 | 17 | ||||
| H | 61 | 15 | ||||
| Total | 155 | 44 | ||||
| RSRF27 | A | 1 | 41 | 3 | ||
| B | 46 | 0 | ||||
| H | 108 | 2 | ||||
| No call | 0 | 1 | ||||
| Total | 155 | 44 | ||||
| Primer | Sequence | SNP Position (bp) | Gene | Position in Gene | Function | |
|---|---|---|---|---|---|---|
| Rft | RSc15F | TACAATCATGTGGCAAAGCACA | 33,336,477 | RSAskr_r1.0R5g59291 | intron | PentatricoPeptide Repeat |
| RSc15R | CGGAATCATCGTCTACCAGGTT | |||||
| RSb13F | CATGAAGTGTGATTTGTATTGGT | 33,340,197 | RSAskr_r1.0R5 | intergenic region | - | |
| RSb13R | GTGTCATCGTTCACTATACATTCT | |||||
| RSc17F | ACAAGTTCGTATTGAGGAGCGT | 33,366,537 | RSAskr_r1.0R5g59296 | exon | Leucine Rich Repeat | |
| RSc17R | TCAGAGAGACCATCCAAAGCTG | |||||
| RS017F | CAATCTTGGCTGTAAACTTGTGAA | 33,903,431 | RSAskr_r1.0R5g59414 | exon | N-acetyltransferase 9-like protein | |
| RS017R | TAGGAAAGGAATCTGTGTTGATGA | |||||
| Rfo | RSRF27F | TCTCAAACATACAGCTGGAAAGC | 28,459,573 /28,459,588 | RSAskr_r1.0R9 | intergenic region | - |
| RSRF27R | ACCGTCGTGTTATTGGCTACC | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.-I.; Han, K.; Yang, H.-B.; Lee, E.S.; Lee, Y.-R.; Kim, J.; Park, H.Y.; Kim, D.-S. Development and Investigation of HRM Markers to Discriminate Two Ogura Cytoplasmic Male Sterility Restorer Genes in Radish. Agronomy 2024, 14, 43. https://doi.org/10.3390/agronomy14010043
Ahn H-I, Han K, Yang H-B, Lee ES, Lee Y-R, Kim J, Park HY, Kim D-S. Development and Investigation of HRM Markers to Discriminate Two Ogura Cytoplasmic Male Sterility Restorer Genes in Radish. Agronomy. 2024; 14(1):43. https://doi.org/10.3390/agronomy14010043
Chicago/Turabian StyleAhn, Hong-Il, Koeun Han, Hee-Bum Yang, Eun Su Lee, Ye-Rin Lee, Jinhee Kim, Han Yong Park, and Do-Sun Kim. 2024. "Development and Investigation of HRM Markers to Discriminate Two Ogura Cytoplasmic Male Sterility Restorer Genes in Radish" Agronomy 14, no. 1: 43. https://doi.org/10.3390/agronomy14010043
APA StyleAhn, H.-I., Han, K., Yang, H.-B., Lee, E. S., Lee, Y.-R., Kim, J., Park, H. Y., & Kim, D.-S. (2024). Development and Investigation of HRM Markers to Discriminate Two Ogura Cytoplasmic Male Sterility Restorer Genes in Radish. Agronomy, 14(1), 43. https://doi.org/10.3390/agronomy14010043







