Challenges and Alternatives of Herbicide-Based Weed Management
Abstract
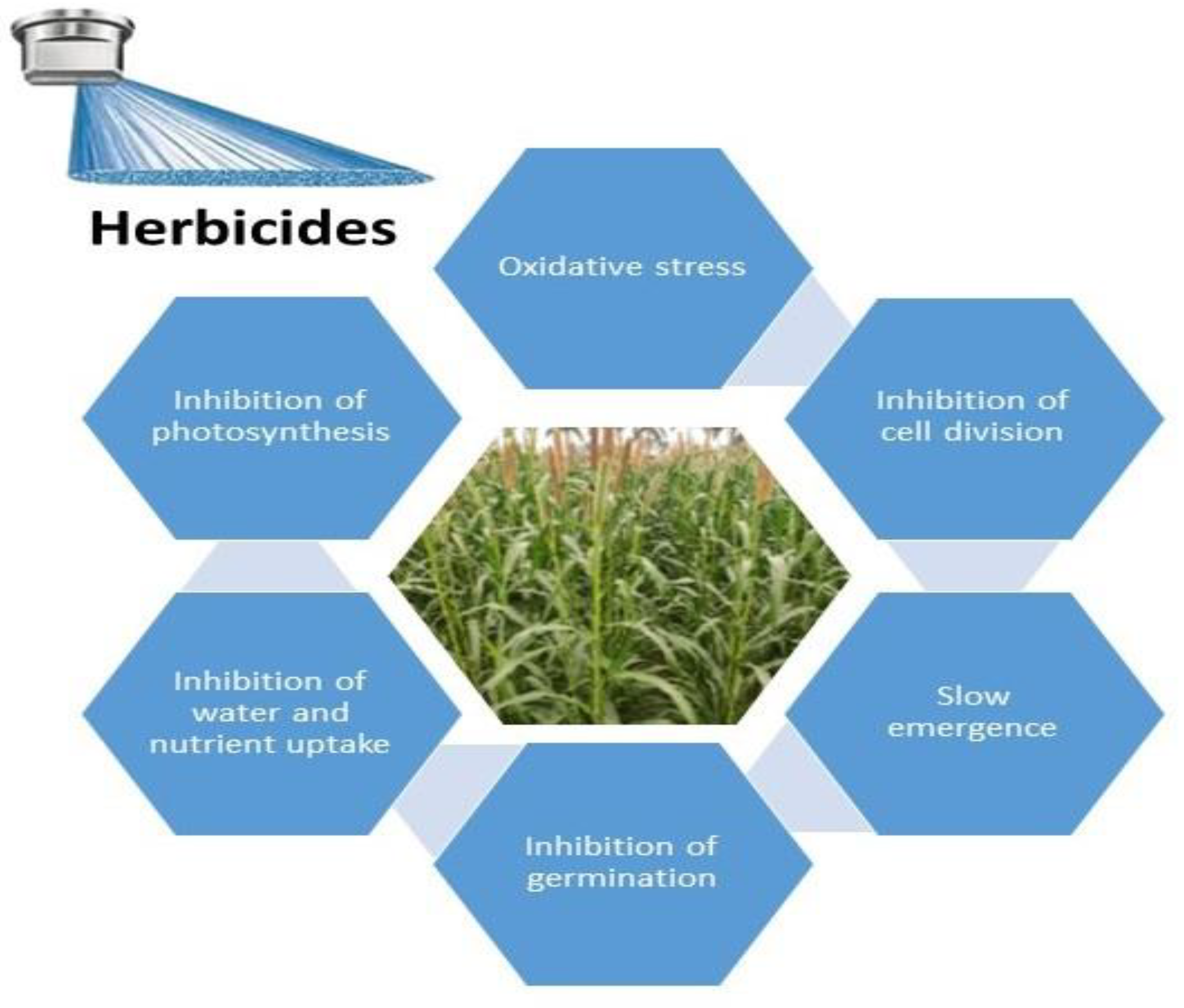
1. Background
2. Negative Consequences of Chemical Weed Management
2.1. Toxicology
2.2. Herbicide Residue(s)
2.3. Herbicide Persistency and Degradation in the Soil
2.4. Herbicide Resistance
2.5. Food Hazards
2.6. Water Pollution
2.7. Soil Pollution
3. Ecological Weed Management/Functions
3.1. Principles of Ecological Weed Management
3.2. Preventive Measures rather Than Eradication
3.3. Weed Surveillance
3.4. Economic Threshold of Weeds
3.5. Weed Seedbank Depletion
3.6. Competitive Crops
3.7. Weeds Predation
3.8. Allelopathy
4. System Based Approaches for Non-Chemical Weed Management
4.1. Cropping System Approaches for Ecological Management
4.2. Good Agronomic Practices
4.2.1. Soil Solarization
4.2.2. Stale Seedbed Technique
4.2.3. Crop Establishment Methods
4.2.4. Adjusting the Crop Planting Date
4.2.5. Adjusting the Crop Density
4.2.6. Fertilizer Management
4.2.7. Water Management (Irrigation and Drainage)
4.3. Cover Crops
4.4. Intercropping
4.5. Mulching
4.6. Crop Diversification
4.7. Conservation Agriculture (CA)
4.7.1. Minimum Soil Disturbance/Zero Tillage (ZT)
4.7.2. Permanent Soil Cover/Surface Residue Retention
4.8. Physical Weed Management
4.8.1. Weed Control by Hot Water and Hot Foam
4.8.2. Weed Control by Flaming
4.8.3. Weed Control by Abrasive Grit
4.9. Biological Control
4.10. Artificial Intelligence and Robotics Application
5. Recommendations or Concepts for Future Research
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.G.; Mahoney, K.J.; Sikkema, P.H.; Swanton, C.J. The importance of light quality in crop–weed competition. Weed Res. 2009, 49, 217–224. [Google Scholar] [CrossRef]
- Rao, A.N.; Singh, R.G.; Mahajan, G.; Wani, S.P. Weed research issues, challenges, and opportunities in India. Crop Prot. 2020, 134, 104451. [Google Scholar] [CrossRef]
- Bell, C. A Historical View of Weed Control Technology. 2015. Available online: https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=17593 (accessed on 1 July 2023).
- Timmons, F.L. A history of weed control in the United States and Canada. Weed Sci. 2017, 18, 294–307. [Google Scholar] [CrossRef]
- Zimdahl, R.L. A History of Weed Science in the United States; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Bonny, S. Genetically modified herbicide-tolerant crops, weeds, and herbicides: Overview and impact. Environ. Manag. 2016, 57, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Székács, A.; Zaller, J.G. Herbicides: Brief history, agricultural use, and potential alternatives for weed control. In Herbicides; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Moss, S.; Ulber, L.; Hoed, I.D. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Singh, R.G.; Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Prot. 2012, 38, 57–65. [Google Scholar] [CrossRef]
- Swanton, C.J.; Shrestha, A.; Knezevic, S.Z.; Roy, R.C.; Ball-Coelho, B.R. Influence of tillage type on vertical weed seed bank distribution in a sandy soil. Can. J. Plant Sci. 2000, 80, 455–457. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2012, 67, 493–506. [Google Scholar]
- Das, T.K.; Yaduraju, N.T. Optimization of metribuzin use for controlling isoproturon-resistant Phalaris minor Retz. in wheat. Pestic. Res. J. 2002, 14, 47–56. [Google Scholar]
- Nath, C.P.; Hazra, K.K.; Kumar, N.; Singh, S.S.; Praharaj, C.S.; Singh, U.; Singh, N.P.; Nandan, R. Impact of crop rotation with chemical and organic fertilization on weed seed density, species diversity, and community structure after 13 years. Crop Prot. 2022, 153, 105860. [Google Scholar] [CrossRef]
- Sosnoskie, L.M.; Herms, C.P.; Cardina, J. Weed seed bank community composition in a 35-year-old tillage and rotation experiment. Weed Sci. 2006, 54, 263–273. [Google Scholar] [CrossRef]
- Sondhia, S. Herbicide residues, persistence and degradation: An Indian viewpoint. In Fifty Years of Weed Science Research in India; Sushilkumar, Mishra, J.S., Eds.; Indian Society of Weeds Science: Jabalpur, India, 2018. [Google Scholar]
- Strange, M.L. UC Master Gardener. Master Gardener Newspaper Articles. University of California Cooperative Extension. 2012. Available online: https://ucanr.edu/sites/UC_Master_Gardeners/http___ucanredu_sites_UC_Master_Gardeners_Newspaper_Articles_254_/ (accessed on 1 July 2023).
- Sondhia, S.; Singh, P.K. Bioefficacy and fate of pendimethalin residues in soil and mature plants in chickpea Field. J. Res. Weed Sci. 2018, 1, 28–39. [Google Scholar]
- Paul, R.; Sharma, R.; Kulshrestha, G.; Singh, S.B. Analysis of metsulfuron–methyl residues in wheat field soil a comparison of HPLC and bioassay technique. Pest Manag. Sci. 2009, 65, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Sondhia, S.; Dwivedi, A.K. Dissipation and persistence of imazethapyr in soybean soil under application of long-term fertilizers in Typic Hapuustert. In Proceedings of the Abstract in Biennial Conference on Recent Advances in Weed Science Research, Raipur, India, 25–26 February 2010; p. 139. [Google Scholar]
- Sondhia, S.; Varshney, J.G. Herbicides; Satish Serial Publication House: New Delhi, India, 2010; p. 567. [Google Scholar]
- Sondhia, S. Liquid chromatographic determination of Imazosulfuron resin food commodity, water and soil using photo diode array detector. Asian J. Chem. 2016, 28, 1021–1023. [Google Scholar] [CrossRef]
- Sondhia, S. Evaluation of imazethapyr leaching in soil under natural rainfall conditions. Indian J. Weed Sci. 2013, 45, 58–61. [Google Scholar]
- Sondhia, S. Determination of imazosulfuron persistence in rice crop and soil. Environ. Monitor. Assess. 2007, 137, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Maheswari, S.T. Dissipation of alachlor in cotton plant soil and water and its bioaccumulation in fish. Chemosphere 2004, 54, 547–652. [Google Scholar] [CrossRef]
- Aktar, M.W.; Gupta, A.; Gade, V.; Bhattacharyya, A. Fate and behavior of benthiocarb a herbicide in transplanted paddy under East–Indian Climatic Condition. Bull. Enviro. Contam. Toxicol. 2007, 79, 646–649. [Google Scholar] [CrossRef]
- Sondhia, S. Leaching behaviour of metsulfuron in two texturally different soils. Environ. Moni. Assess. 2009, 154, 111–115. [Google Scholar] [CrossRef]
- Aktar, M.W.; Gupta, D.; Alam, S.; Chowdhury, A. Degradation dynamics of a di–nitro aniline herbicide (trifluralin) in/on Black gram (Vigna mungo) under East–Indian climatic condition. J. Environ. Agric. Food Chem. 2009, 8, 1172–1177. [Google Scholar]
- Sondhia, S. Herbicides residues: Monitoring in soil, water, plants and non targeted organisms and human health implications: An Indian perspective. In Proceedings of the Biennial Conference of Indian Society of Weed Science, Jabalpur, India, 15–17 February 2014; p. 15. [Google Scholar]
- Kaur, R.; Gill, B. Analysis of herbicide residues in celery seeds. Indian J. Ecol. 2012, 39, 258–260. [Google Scholar]
- Heap, I. The International Survey of Herbicide Resistant Weeds. 2010. Available online: www.weedscience.com (accessed on 31 July 2023).
- Tafoya-Razo, J.A.; Oregel-Zamudio, E.; Velázquez-Márquez, S.; Torres-García, J.R. 10,000-times diluted doses of ACCase-inhibiting herbicides can permanently change the metabolomic fingerprint of susceptible Avena fatua L. plants. Plants 2019, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Délye, C. Weed resistance to acetyl coenzyme A carboxylase inhibitors: An update. Weed Sci. 2005, 53, 728–746. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. 2017. Available online: www.weedscience.com (accessed on 15 November 2017).
- Beckie, H.J.; Heap, I.M.; Smeda, R.J.; Hall, L.M. Screening for herbicide resistance in weeds. Weed Technol. 2000, 14, 428–445. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Domínguez-Martínez, P.A.; da Silveira, H.M.; Cruz-Hipólito, H.E.; Palma-Bautista, C.; Vázquez-García, J.G.; Domínguez-Valenzuela, J.A.; de Prado, R. Management of glyphosate-resistant weeds in Mexican citrus groves: Chemical alternatives and economic viability. Plants 2019, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Survey of Herbicide-Resistant Weeds. 2023. Available online: http://www.weedscience.com (accessed on 7 December 2023).
- Duke, S.O. Why have no new herbicide mode of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef]
- Reddy, K.N.; Nandula, V.K. Herbicide resistant crops: History, development and current technologies. Indian J. Agron. 2012, 57, 1–7. [Google Scholar]
- Chhokar, R.S.; Sharma, R.K. Multiple herbicide resistance in little seed canary grass (Phalaris minor); A threat to wheat production in India. Weed Biol. Manag. 2008, 8, 112–123. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Ervin, D.; Jussaume, R. Integrating social science into managing herbicide-resistant weeds and associated environmental impacts. Weed Sci. 2014, 62, 403–414. [Google Scholar] [CrossRef]
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef]
- Shallis, R.M.; Gore, S.D. Agent Orange and dioxin-induced myeloid leukemia: A weaponized vehicle of leukemogenesis. Leuk. Lymphoma 2022, 63, 1534–1543. [Google Scholar] [CrossRef]
- Michalopoulos, S. EurActiv.com (Updated: 12 July 2016). Available online: http://www.euractiv.com/section/agriculture–food/news/eu–agrees–ban–on–glyphosate–co–formulant.2016 (accessed on 31 October 2023).
- Ustuner, T.; Sakran, A.; Almhemed, K. Effect of herbicides on living organisms in the ecosystem and available alternative control methods. Int. J. Sci. Res. Publ. 2020, 10, 633–641. [Google Scholar]
- Newton, I. The recent declines of farmland bird populations in Britain: An appraisal of causal factors and conservation actions. Int. J. Avian Sci. 2004, 146, 579–600. [Google Scholar] [CrossRef]
- Hayes, T.B.; Collins, A.; Lee, M. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Nat. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.F.; Qi, H.Y.; Zhang, H.X. The impact of acetochlor on the bacterial diversity in soil. Acta Microbiol. Sin. 2004, 44, 519–522. [Google Scholar]
- Tilak, K.S.; Veeraiah, P.; Thathaji, M.S.; Butchiram, M.S. Toxicity studies of butachlor to the freshwater fish Channa punctata (Bloch). J. Environ. Biol. 2007, 28, 485–487. [Google Scholar]
- Kole, R.K.; Banerjee, H.; Bhattacharya, A. Monitoring of market fish samples for endosulfan and hexachlorocyclohexane residues in and around Calcutta. Bull. Environ. Contam. Toxicol. 2001, 67, 554–559. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Sannino, M.; Coviella, C.; Momo, F. Glyphosate sublethal effects on the population dynamics of the earthworm Eisenia fetida (savigny, 1826). Water Air Soil Pollut. 2014, 225, 2207. [Google Scholar]
- Ouyang, W.; Zhao, X.; Tysklind, M.; Hao, F. Typical agricultural diffuse herbicide sorption with agricultural waste derived biochars amended soil of high organic matter content. Water Res. 2016, 92, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W.; Peer, W.A.; Conteh, A.; Diggs, A.R.; Cooper, B.R.; Glickman, N.W. Detection of herbicides in the urine of pet dogs following home lawn chemical application. Sci. Total Environ. 2013, 456, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Le Bellec, F.; Velu, A.; Foumier, P.; Le Squin, S.; Michels, T.; Tendero, A.; Bockstaller, C. Helping farmers to reduce herbicide environmental impacts. Ecol. Indic. 2015, 54, 207–216. [Google Scholar] [CrossRef]
- Hasenbein, S.; Peralta, J.; Lawler, S.P.; Connon, R.E. Environmentally relevant concentrations of herbicides impact non-target species at multiple sublethal endpoints. Sci Total Environ. 2017, 607, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Bhuiyan, T.F.; Anee, T.I.; Masud, A.A.C.; Nahar, S. Phytotoxicity, environmental and health hazards of herbicides: Challenges and ways forward. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 55–99. [Google Scholar]
- Zain, N.M.M.; Mohamad, R.B.; Sijam, K.; Morshed, M.M.; Awang, Y. Effects of selected herbicides on soil microbial populations in oil palm plantation of Malaysia: A microcosm experiment. Afr. J. Microbiol. Res. 2013, 7, 367–374. [Google Scholar]
- Adhikary, P.; Shil, S.; Patra, P.S. Effect of herbicides on soil microorganisms in transplanted chilli. Environ. Technol. Innov. 2014, 13, 304–317. [Google Scholar]
- Undabeytia, T.; Sopena, F.; Sanchez-Verdejo, T.; Vilaverde, J.; Nir, S.; Morillo, E. Performance of slow release formulations of alachlor. Soil Sci. Soc Am. J. 2010, 74, 898–905. [Google Scholar] [CrossRef]
- Sidoli, P.; Baran, N.; Angulo-Jaramillo, R. Glyphosate and AMPA adsorption in soils: Laboratory experiments and pedotransfer rules. Environ. Sci. Pollut. Res. 2016, 23, 5733–5742. [Google Scholar] [CrossRef]
- Silva, V.; Montanarell, L.; Jones, A.; Fernandez-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J. Distribution of glyphosate and aminomethyl phosphonic acid (AMPA) in agricultural topsoil of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef]
- Garcia-Perez, J.A.; Alacron-Gutierrez, E.; Perroni, Y.; Barois, I. Earthworm communities and soil properties in shaded coffee plantations with and without application of glyphosate. Appl. Soil Ecol. 2014, 83, 230–237. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.I. Assesing the toxicity of herbicide isoproturon on Aporrectodea caliginosa (Oligochaeta) and its fate in soil ecosystem. Environ. Toxicol. 2008, 24, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Bastiaans, L.; Paolini, R.; Baumann, D.T. Focus on ecological weed management: What is hindering adoption? Weed Res. 2008, 48, 481–491. [Google Scholar] [CrossRef]
- Norris, R.F. Ecological implications of using thresholds for weed management. In Expanding the Context of Weed Management; CRC Press: Boca Raton, FL, USA, 2020; pp. 31–58. [Google Scholar]
- Bàrberi, P. Ecological weed management in sub-Saharan Africa: Prospects and implications on other agroecosystem services. Adv. Agron. 2019, 156, 219–264. [Google Scholar]
- Bahadur, S.; Verma, S.K.; Prasad, S.K.; Madane, A.J.; Maurya, S.P.; Gaurav, V.V.; Sihag, S.K. Eco-friendly weed management for sustainable crop production—A review. J. Crop Weed 2015, 11, 181–189. [Google Scholar]
- Varshney, J.G.; Babu, M.B.B.P. Future scenario of weed management in India. Indian J. Weed Sci. 2008, 40, 1–9. [Google Scholar]
- Timmins, S.M.; Braithwaite, H. Early detection of invasive weeds on islands. In Turning the Tide: The Eradication of Invasive Species; IUCN: Gland, Switzerland, 2002; pp. 311–318. [Google Scholar]
- Göktoǧan, A.H.; Sukkarieh, S.; Bryson, M.; Randle, J.; Lupton, T.; Hung, C. A rotary-wing unmanned air vehicle for aquatic weed surveillance and management. J. Intell. Robot. Syst. 2010, 57, 467–484. [Google Scholar] [CrossRef]
- Jones, R.E.; Medd, R.W. Economic thresholds and the case for longer term approaches to population management of weeds. Weed Technol. 2000, 14, 337–350. [Google Scholar] [CrossRef]
- Nath, C.P.; Hazra, K.K.; Praharaj, C.S.; Singh, U.; Singh, S.S.; Kumar, N.; Nandan, R.; Singh, N.P. Long-term impact of legume-based cropping with chemical and integrated fertilisation on viable weed seed density, diversity and community structure. Weed Res. 2021, 61, 360–374. [Google Scholar] [CrossRef]
- Nandan, R.; Singh, V.; Kumar, V.; Singh, S.S.; Hazra, K.K.; Nath, C.P.; Malik, R.K.; Poonia, S.P. Viable weed seed density and diversity in soil and crop productivity under conservation agriculture practices in rice-based cropping systems. Crop Prot. 2020, 136, 105210. [Google Scholar] [CrossRef]
- Verhulst, N.; Govaerts, B.; Verachtert, E.; Castellanos-Navarrete, A.; Mezzalama, M.; Wall, P.; Deckers, J.; Sayre, K.D. Conservation agriculture, improving soil quality for sustainable production systems. In Advances in Soil Science: Food Security and Soil Quality; Lal, R., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 137–208. [Google Scholar]
- Scavo, A.; Mauromicale, G. Crop allelopathy for sustainable weed management in agroecosystems: Knowing the present with a view to the future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Bashar, H.K.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Uddin, M.K.; Asib, N.; Anwar, M.P.; Rahaman, F. A Mystic Weed, Parthenium hysterophorus: Threats, Potentials and Management. Agronomy 2021, 11, 1514. [Google Scholar] [CrossRef]
- Baghel, J.K.; Das, T.K.; Mukherjee, I.; Nath, C.P.; Bhattacharyya, R.; Ghosh, S.; Raj, R. Impacts of conservation agriculture and herbicides on weeds, nematodes, herbicide residue and productivity in direct-seeded rice. Soil Tillage Res. 2020, 201, 104634. [Google Scholar] [CrossRef]
- Shafeeq, P.M.; Aggarwal, P.; Krishnan, P.; Rai, V.; Pramanik, P.; Das, T.K. Modeling the temporal distribution of water, ammonium-N, and nitrate-N in the root zone of wheat using HYDRUS-2D under conservation agriculture. Environ. Sci. Pollut. Res. 2020, 27, 2197–2216. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, T.K.; Nath, C.P.; Bhatia, A.; Biswas, D.R.; Bandyopadhyay, K.K.; Yeasin, M.; Raj, R. Weed seedbank, above-ground weed community and crop yields under conventional and conservation agriculture practices in maize–wheat–mungbean rotation. Weed Res. 2023, 63, 270–281. [Google Scholar] [CrossRef]
- Kumar, N.; Nath, C.P.; Hazra, K.K.; Praharaj, C.S.; Singh, S.S.; Singh, N.P. Long-term impact of zero-till residue management in post-rainy seasons after puddled rice and cropping intensification on weed seedbank, above-ground weed flora and crop productivity. Ecol. Eng. 2022, 176, 106540. [Google Scholar] [CrossRef]
- Kanellou, E.; Papafotiou, M.; Economou, G.; Ntoulas, N. Soil Solarization as an Alternative Weed Control Method for Archaeological Sites in the Mediterranean Region. Sustainability 2023, 15, 11324. [Google Scholar] [CrossRef]
- Muthumanickam, K.; Anburani, A. Soil solarization an effective management practice on weed management and yield of palak (Beta vulgaris var. bengalensis). World Sci. News 2019, 129, 211–221. [Google Scholar]
- Kumar, V.; Ladha, J.K. Direct seeding of rice: Recent developments and future research needs. Adv. Agron. 2010, 111, 297–413. [Google Scholar]
- Renu, S.; Thomas, C.G.; Abraham, C.T. Stale seedbed technique for the management of Sacciolepis interrupta in semi-dry rice. Indian J. Weed Sci. 2000, 32, 140–145. [Google Scholar]
- Walia, U.S.; Brar, L.S.; Jand, S. Integrated effect of planting methods and herbicides on Phalaris minor and wheat. Indian J. Weed Sci. 2003, 35, 169–172. [Google Scholar]
- Melander, B.; Kristiansen, J.K. Soil steaming effects on weed seedling emergence under the influence of soil type, soil moisture, soil structure and heatd uration. Ann. Appl. Biol. 2011, 158, 194–203. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Growth response of direct-seeded rice to oxadiazon and bispyribac-sodium in aerobic and saturated soils. Weed Sci. 2011, 59, 119–122. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B.S. Effects of planting pattern and cultivar on weed and crop growth in aerobic rice system. Weed Technol. 2011, 25, 521–525. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Korres, N.E.; Walsh, M.J.; Powles, S.B. Integrating herbicide programs with harvest weed seed control and other fall management practices for the control of glyphosate-resistant Palmer amaranth. Weed Sci. 2016, 64, 40–550. [Google Scholar] [CrossRef]
- Brockman, J.S.; Wilkins, R.J. Grassland. In Primrose McConnell’s the Agricultural Note Book; Soffe, R., Ed.; Blackwell Science Ltd.: London, UK, 2003; pp. 131–176. [Google Scholar]
- Korres, N.E.; Norsworthy, J.K.; Mauromoustakos, A. Effects of Palmer amaranth (Amaranthus palmeri) establishment time and distance from the crop row on biological and phenological characteristics of the weed: Implications on soybean yield. Weed Sci. 2019, 67, 126–135. [Google Scholar] [CrossRef]
- Anderson, R.L. A multi-tactic approach to manage weed population dynamics in crop rotations. Agron. J. 2005, 97, 1579–1583. [Google Scholar] [CrossRef]
- Chhokar, R.S.; Sharma, R.K.; Jat, G.R.; Pundir, A.K.; Gathala, M.K. Effect of tillage and herbicides on weeds and productivity of wheat under rice-wheat growing system. Crop Prot. 2007, 26, 1689–1696. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Chhokar, R.S.; Malik, R.K.; Brainard, D.C.; Ladha, J.K. Weed management strategies to reduce herbicide use in zero-till rice–wheat cropping systems of the Indo-Gangetic Plains. Weed Technol. 2013, 27, 241–254. [Google Scholar] [CrossRef]
- Chhokar, R.S.; Singh, S.; Sharma, R.K.; Singh, M. Influence of straw management on Phalaris minor Retz. Control. Indian J. Weed Sci. 2009, 41, 150–156. [Google Scholar]
- Singh, S.; Kirkwood, R.C.; Marshall, G. A review of the biology and control of Phalaris minor Retz. (little seed canarygrass) in cereals. Crop Prot. 2014, 18, 1–16. [Google Scholar] [CrossRef]
- Gill, H.S.; Brar, L.S. Importance of weedicides in the agriculture of Punjab and Haryana. Pesticides 1975, 9, 20–24. [Google Scholar]
- Susha, V.S.; Das, T.K.; Nath, C.P.; Pandey, R.; Paul, S.; Ghosh, S. Impacts of tillage and herbicide mixture on weed interference, agronomic productivity and profitability of a maize–Wheat system in the North-western Indo-Gangetic Plains. Field Crops Res. 2018, 219, 180–191. [Google Scholar] [CrossRef]
- Taa, A.; Tanner, D.; Bennie, A.T. Effects of stubble management, tillage and cropping sequence on wheat production in the south-eastern highlands of Ethiopia. Soil Tillage Res. 2004, 76, 69–82. [Google Scholar] [CrossRef]
- Das, T.K.; Ghosh, S.; Nath, C.P. Brown manuring optimization in maize: Impacts on weeds, crop productivity and profitability. J. Agric. Sci. 2020, 157, 599–610. [Google Scholar] [CrossRef]
- Ngwira, A.R.; Aune, J.B.; Thierfelder, C. On-farm evaluation of the effects of the principles and components of conservation agriculture on maize yield and weed biomass in Malawi. Exp. Agric. 2014, 50, 591–610. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, T.K.; Rathi, N.; Rana, K.S.; Biswas, D.R. Productivity, profitability and input-use efficiency of direct-seeded rice (Oryza sativa) under conservation agriculture. Indian J. Agril. Sci. 2020, 90, 1004–1008. [Google Scholar] [CrossRef]
- Rask, A.M.; Kristoffersen, P. A review of non-chemical weed control on hard surfaces. Weed Res. 2007, 47, 370–380. [Google Scholar] [CrossRef]
- Cisneros, J.J.; Zandstra, B.H. Flame weeding effects on several weed species. Weed Technol. 2008, 22, 290–295. [Google Scholar] [CrossRef]
- Rifai, M.N.; Astatkie, T.; Lacko-Bartosova, M.; Gadus, J. Effect of two different thermal units and three types of mulch on weeds in apple orchards. J. Environ. Eng. Sci. 2002, 1, 331–338. [Google Scholar] [CrossRef]
- Forcella, F. Air-propelled abrasive grit for post-emergence in-row weed control in field corn. Weed Technol. 2012, 26, 161–164. [Google Scholar] [CrossRef]
- Wortman, S.E. Air-propelled abrasive grits reduce weed abundance and increase yields inorganic vegetable production. Crop Prot. 2015, 77, 157–162. [Google Scholar] [CrossRef]
- Korres, N.E.; Singh, A.; Nizami, A.S.; Murphy, J.D. Is grass biomethane a sustainable transport biofuel? Biofuels Bioprod. Biorefin. 2010, 4, 310–325. [Google Scholar] [CrossRef]
- Young, S.L.; Meyer, G.E.; Woldt, W.E. Future directions for automated weed management in precision agriculture. In Automation: The Future of Weed Control in Cropping Systems; Young, S.L., Pierce, F.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 249–259. [Google Scholar]
- Bajwa, A.A. Sustainable weed management in conservation agriculture. Crop Prot. 2014, 65, 105–113. [Google Scholar] [CrossRef]
- Merfield, C.N. Robotic weeding’s false dawn? Ten requirements for fully autonomous mechanical weed management. Weed Res. 2016, 56, 340–344. [Google Scholar] [CrossRef]
- Tillett, N.D.; Hague, T.; Grundy, A.C.; Dedousis, A.P. Mechanical within-row weed control for transplanted crops using computer vision. Biosyst. Eng. 2008, 99, 171–178. [Google Scholar] [CrossRef]
- Scotford, I.M.; Miller, P.C.H. Applications of spectral reflectance techniques in northern European cereal production: A review. Biosyst. Eng. 2005, 90, 235–250. [Google Scholar] [CrossRef]
- Pannacci, E.; Lattanzi, B.; Tei, F. Non-chemical weed management strategies in minor crops: A review. Crop Prot. 2017, 96, 44–58. [Google Scholar] [CrossRef]
| Herbicide | Half-Life (Days) | Toxicity Class Based on LD50 |
|---|---|---|
| Atrazine | 13–58 | III |
| Butachlor | 5–24 | III |
| Fluazifop-p-butyl | 8–24 | III |
| Fluchloralin | 12–46 | IV |
| Dithiopyr | 11–25 | IV |
| Imazethapyr | 57–71 | IV |
| Isoproturon | 13–21 | III |
| Chlorsulfuron | 31–93 | IV |
| Chlorimuron | 60 | IV |
| Flufenacet | 9–22.5 | V |
| Metribuzin | 23–49 | III |
| Metolachlor | 8–27 | III |
| Oxyfluorfen | 12–29 | III |
| Pendimethalin | 15–77 | IV |
| Pretilachlor | 10–11 | IV |
| Sulfosulfuron | 3–27 | IV |
| 2,4-D | 7–22 | II–III |
| Metsulfuron-methyl | 70–147 | IV |
| Thiobencarb | 19–24 | III |
| Pyrazosulfuron-ethyl | 16–21 | IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and Alternatives of Herbicide-Based Weed Management. Agronomy 2024, 14, 126. https://doi.org/10.3390/agronomy14010126
Nath CP, Singh RG, Choudhary VK, Datta D, Nandan R, Singh SS. Challenges and Alternatives of Herbicide-Based Weed Management. Agronomy. 2024; 14(1):126. https://doi.org/10.3390/agronomy14010126
Chicago/Turabian StyleNath, Chaitanya Prasad, Ravi Gopal Singh, Vijay K. Choudhary, Debarati Datta, Rajiv Nandan, and Sati Shankar Singh. 2024. "Challenges and Alternatives of Herbicide-Based Weed Management" Agronomy 14, no. 1: 126. https://doi.org/10.3390/agronomy14010126
APA StyleNath, C. P., Singh, R. G., Choudhary, V. K., Datta, D., Nandan, R., & Singh, S. S. (2024). Challenges and Alternatives of Herbicide-Based Weed Management. Agronomy, 14(1), 126. https://doi.org/10.3390/agronomy14010126






