Effects of Pre-Treatments on Seed Dormancy and Germination of Endemic Muscari bourgaei Baker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Material
2.3. Germination Conditions and Tests
2.4. Preliminary Treatments for the Breaking of Dormancy
2.5. Main Experiment: Prolonged Moist Chilling, Scarification, Responses to GA3, and Seed Morphological Characteristics
2.6. Statistical Analysis
3. Results
3.1. Preliminary Treatments for the Breaking of Dormancy
3.2. Main Experiment: Prolonged Moist Chilling, Scarification, Responses to GA3, and Seed Morphological Characteristics
3.3. Scarification and GA3 Pre-Treatment
3.4. Seed Morphological Characteristics
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donohue, K.; Foster, D.R.; Motzkin, G. Effects of the past and the present on species distribution: Land-use history and demography of wintergreen. J. Ecol. 2000, 88, 303–316. [Google Scholar] [CrossRef]
- Qaderi, M.M. Environmental regulation of weed seed dormancy and germination. Seeds 2023, 2, 259–277. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar] [CrossRef]
- Schwienbacher, E.S.; Marcante, B.; Erschbamer, B. Alpine species seed longevity in the soil in relation to seed size and shape—A 5-year burial experiment in the Central Alps. Flora Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 19–25. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Clemente, A.S.; Müller, J.V.; Almeida, E.; Costa, C.A.; Dias, S.L.; Brehm, J.M.; Martins-Loução, M.A. What can routine germination tests in seed banks tell us about the germination ecology of endemic and protected species? Botany 2017, 95, 673–684. [Google Scholar] [CrossRef]
- Francisco-Ortega, J.; Santos-Guerra, A.; Kim, S.C.; Crawford, D.J. Plant genetic diversity in the Canary Islands: A conservation perspective. Am. J. Bot. 2000, 87, 909–919. [Google Scholar] [CrossRef]
- Estrelles, E.; Pena, C.; Sebastian, A.; Ibars, A.M. Nymphaea alba L. behaviour regarding seed germination and ex situ conservation. In Proceedings of the 4th European Conference on the Conservation of Wild Plants, Planta Europa, Valencia, Spain, 17–20 September 2004. [Google Scholar]
- Flores, J.; Jurado, E.; Himenez-Bremont, J.F. Breaking seed dormancy in specially protected Turbinicarpus lophophoroides and Turbinicarpus pseudopectinatus (Cactaceae). Plant Species Biol. 2008, 23, 43–46. [Google Scholar] [CrossRef]
- Çığ, A.; Başdoğan, G. In vitro propagation techniques for some geophyte ornamental plants with economic value. Int. J. Second. Metab. 2015, 2, 27–49. [Google Scholar]
- Özgen, Y.; Arslan, N. Farklı gibberellik asit dozlarının ve uygulama sürelerinin Muscari azureum Fenzi (Keşişbaşı) tohumlarının çıkışına etkileri. In Proceedings of the VI. Süs Bitkileri Kongresi Tam Metin Bildiri Kitabı, Antalya, Türkiye, 19–22 April 2016; pp. 313–318. [Google Scholar]
- Ekim, T.; Koyuncu, M.; Vural, M.; Duman, H.; Aytaç, Z.; Adıgüzel, N. Türkiye Bitkileri Kırmızı Kitabı; Barışkan Offset: Ankara, Türkiye, 2000. [Google Scholar]
- Davis, P.H.; Stuart, D.C. Muscari Mill. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1984; Volume 8, pp. 245–263. [Google Scholar]
- Eker, İ.; Yıldırım, H.; Armağan, M. Türkiye Florası için yeni bir müşkürüm kaydı: Muscari pallens. Bağbahçe Bilim Dergisi 2019, 6, 45–53. [Google Scholar]
- Eker, İ.; Yıldırım, H. Muscari inundatum (Asparagaceae, Scilloideae), a new species from southern Anatolia. Phytotaxa 2021, 484, 181–194. [Google Scholar] [CrossRef]
- Qian, D.; Li, Q.; Guo, X.; Fan, B.; Lan, Y.; Si, M.; Cao, G. Ecosystem services relationship characteristics of the degraded alpine shrub meadow on the Qinghai-Tibetan Plateau. Ecol. Evol. 2023, 13, e10351. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.A.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Doussi, M.A.; Thanos, C.A. Ecophysiology of seed germination in Mediterranean geophytes. 1. Muscari spp. Seed Sci. Res. 2002, 12, 193–201. [Google Scholar] [CrossRef]
- Çatav, Ş.S.; Küçükyavuz, K.; Tavşanoğlu, Ç.; Akbaş, K. Effects of aqueous smoke and nitrate tratments on germination of 12 Eastern Mediterranean Basin plants. Ann. Bot. Fenn. 2015, 52, 93–100. [Google Scholar] [CrossRef]
- Salmeri, C.; Trubia, M. Seed germination reports for coastal sand dune species from Sicily. Flora Mediterr. 2019, 29, 277–287. [Google Scholar]
- Labbaf, N.; Rohollahi, I.; Naj, A.M. Muscari germination enhancement by using sulphuric acid stratification priming. Genet. Resour. Crop Ev. 2023, 2, 171–180. [Google Scholar] [CrossRef]
- Eroğlu, S.; Karaismailoğlu, M.C.; Pınar, S.M.; Fidan, M. Seed micromorphology and anatomy of 36 Muscari (Asparagaceae) taxa from Turkey with notes on their systematical importance. Acta Bot. Croat. 2021, 80, 146–152. [Google Scholar] [CrossRef]
- Uysal, T.; Bozkurt, M.; Sezer, E.N.Ş.; Aksoy, A.; Ertuğrul, K. Karyomorphological Studies of Six Species of Turkish Muscari (Asparagaceae). Cytologia 2021, 86, 351–357. [Google Scholar] [CrossRef]
- Özhatay, N.; Byfield, A.; Atay, S. Türkiye’nin 122 Önemli Bitki Alanı; WWF Türkiye: Istanbul, Türkiye, 2005. [Google Scholar]
- Kırmızı, S. Dormancy and germination requirements of five species from Brassicaceae. Iğdır Univ. J. Inst. Sci. Technol. 2017, 7, 21–29. [Google Scholar] [CrossRef]
- Simbaña, W.; Tye, A. Reproductive biology and responses to threats and protection measures of the total population of a critically endangered Galápagos plant, Linum cratericola (Linaceae). Bot. J. Linn. Soc. 2009, 161, 89–102. [Google Scholar] [CrossRef]
- Martínez-García, F.; Guerrero-García, S.; Pérez-García, F. Evaluation of reproductive success and conservation strategies for Senecio coincyi (Asteraceae), a narrow and threatened endemic plant of Central Western Spain. Arch. Biol. Sci. 2012, 64, 1001–1016. [Google Scholar] [CrossRef]
- Yücel, G.; Erken, K. Optimal germination methods, ornamental plant features, and ex situ conservation of endemic Campanula grandis Fisch and CA Mey. J. Environ. Eng. Landsc. Manag. 2023, 31, 132–141. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Kabiel, H.F.; Boulos, L.; Sharashy, O.S. Conservation approach to the demography and dynamics of protected and unprotected populations of the endemic Ebenus armitagei in the Western Mediterranean Coast of Egypt. J. Nat. Conserv. 2010, 18, 151–158. [Google Scholar] [CrossRef]
- Ayele, T.B.; Gailing, O.; Finkeldey, R. Assessment and integration of genetic, morphological and demographic variation in Hagenia abyssinica (Bruce) J.F. Gmel to guide its conservation. J. Nat. Conserv. 2011, 19, 8–17. [Google Scholar] [CrossRef]
- Lozano, F.D.; Moreno Saiz, J.C.; Ollero, H.S. Biological properties of the endemic and threatened shrub in Iberia Vella pseudocytisus subsp. paui Gomez Campo (Cruciferae) and implications for its conservation. J. Nat. Conserv. 2005, 13, 17–30. [Google Scholar] [CrossRef]
- Jang, G.H.; Chung, J.M.; Rhie, Y.H.; Lee, S.Y. Seed Dormancy Class and Ecophysiological Features of Veronicastrum sibiricum (L.) Pennell (Scrophulariaceae) Native to the Korea Peninsula. Plants 2022, 11, 160. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds, Ecology Biogeography and Evolution of Dormancy and Germination; Academic Press: London, UK, 1998. [Google Scholar]
- Cavieres, L.A.; Arroyo, M.T. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae)–altitudinal variation in the Mediterranean Andes of central Chile. Plant Ecol. 2000, 149, 1–8. [Google Scholar] [CrossRef]
- Cerabolini, B.; De Andreis, R.; Ceriani, R.M.; Pierce, S.; Raimondi, B. Seed germination and conservation of endangered species from the Italian Alps: Physoplexis comosa and Primula glaucescens. Biol. Conserv. 2004, 117, 351–356. [Google Scholar] [CrossRef]
- Deng, S.; Deng, Z.; Wong, X.; Lio, H.; Xue, H. Effects of temperature, scarification, stratification, phytohormones and afterripening on the dormancy and germination of Eucommia ulmoides Oliv. seeds. Forests 2021, 12, 1593. [Google Scholar] [CrossRef]
- Billings, W.D.; Mooney, H.A. The ecology of arctic and alpine plants. Biol. Rev. 1968, 43, 481–529. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Gutterman, Y. Germination strategies of some Allium species of the subgenus Melanocrommuyum from arid zone of central Asia. J. Arid Environ. 2000, 45, 61–71. [Google Scholar] [CrossRef]
- Specht, C.E.; Keller, E.R.J. Temperature requirements for seed germination in species of the genus Allium L. Genet. Resour. Crop Ev. 1997, 44, 509–517. [Google Scholar] [CrossRef]
- Leubner-Metzger, G. Brassinosterolids and gibberellins promote tobacco seed germination by distinct pathways. Planta 2001, 213, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Payal, K.; Maikhuri, R.K.; Rao, K.S.; Kandari, L.S. Effect of gibberellic acid and water based presoaking treatments under different temperatures and photoperiods on the seed germination of Allium stracheyi Baker: An endangered alpine species of central Himalaya, India. Plant Biosyst. 2013, 148, 1075–1084. [Google Scholar] [CrossRef]
- Kaya, M.D.; Kulan, E.G.; Gümüşçü, G.; Gümüşçü, A. Factors affecting germination performance of four endemic Sideritis species in Turkey. Tarım Bilimleri Dergisi 2015, 21, 406–413. [Google Scholar]
- Jaganathan, G.K.; Dalrymple, S.E.; Baolin, L. Towards an understanding of factors controlling seed Bank composition and longevity in the alpine environment. Bot. Rev. 2015, 81, 70–103. [Google Scholar] [CrossRef]
- Chambers, J.C.; MacMahon, J.A.; Haefner, J.H. Seed entrapment in alpine ecosystems: Effects of soil particle size and diaspore morphology. Ecology 1991, 72, 1668–1677. [Google Scholar] [CrossRef]
- Thompson, K.; Bakker, J.P.; Bekker, R.M. The Soil Seed Banks of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Sedláková, V.; Hanáček, P.; Grulichová, M.; Zablatzká, L.; Smýkal, P. Evaluation of seed dormancy, one of the key domestication traits in chickpea. Agronomy 2021, 12, 2292. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Breaking seed dormancy during dry storage: A useful tool or major problem for successful restoration via direct seedling? Plants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Kırmızı, S. Biyoçeşitliliğin Korunmasında Tohum Bankasının Rolü. In Biyoloji Alanında Teorik ve Uygulamalı Akademik Çalışmalar; Türkoğlu, Ş., Ed.; İKSAD (ISPEC) Yayınevi: Ankara, Türkiye, 2022; pp. 29–40. [Google Scholar]
- European Council and Planta Europa. European plant conservation strategies. In Proceedings of the Conference of the Parties to the Convention Biological Diversity, 6th Meeting, The Hague, The Netherlands, 7–9 April 2002. [Google Scholar]

| GA3 Concentration (mg/L−1) | % Germination | MGT |
|---|---|---|
| Distilled water | 30.4 a ± 4.7 | 4.9 a ± 0.8 |
| 250 | 31.0 a ± 3.8 | 5.3 a ± 0.5 |
| 500 | 21.0 a ± 8.9 | 5.1 a ± 0.8 |
| 1000 | 21.0 a ± 1.0 | 5.0 a ± 0.2 |
| Temperature | Moist Chilling Duration | Darkness | Photoperiod |
|---|---|---|---|
| 20/10 °C | 0 m | 00.00 dD ± 00.00 | 00.00 dD ± 00.00 |
| 3 m | 26.00 cC ± 06.80 | 09.00 cC ± 03.00 | |
| 6 m | 81.00 abAB ± 07.30 | 80.00 bB ± 07.80 | |
| 9 m | 90.00 abB ± 03.50 | 95.00 aA ± 03.00 | |
| 12 m | 96.00 aA ± 01.60 | 99.00 aA ± 01.00 | |
| 15/10 °C | 0 m | 00.00 dD ± 00.00 | 00.00 cD ± 00.00 |
| 3 m | 40.00 cCB ± 04.60 | 25.00 bC ± 03.40 | |
| 6 m | 85.00 abAB ± 07.10 | 87.00 aB ± 02.50 | |
| 9 m | 83.00 abAB ± 01.00 | 89.00 aB ± 03.80 | |
| 12 m | 98.00 aA ± 02.00 | 94.00 aA ± 03.90 | |
| 25/15 °C | 0 m | 00.00 dD ± 00.00 | 00.00 cC ± 00.00 |
| 3 m | 13.00 cC ± 01.90 | 18.60 bB ± 03.50 | |
| 6 m | 80.00 abAB ± 02.30 | 93.00 aA ± 01.90 | |
| 9 m | 97.00 aA ± 03.00 | 98.00 aA ± 02.00 | |
| 12 m | 98.00 aA ± 01.50 | 94.00 aA ± 03.50 |
| Factor | Germination Percentage | ||||
|---|---|---|---|---|---|
| Darkness | Photoperiod | ||||
| df | F | p | F | p | |
| Temperature (A) | 2 | 2.017 | 0.145 | 0.650 | 0.527 |
| Duration (B) | 4 | 378.899 | 0.000 | 148.212 | 0.000 |
| A × B | 8 | 6.122 | 0.000 | 0.855 | 0.561 |
| Error | 45 | ||||
| Moist Chilling Duration | Temperature | Darkness | Photoperiod |
|---|---|---|---|
| 3 months | 20/10 °C | 13.6 ± 1.9 bD | 3.9 ± 0.9 bB |
| 15/10 °C | 10.6 ± 1.2 bCB | 7.5 ± 3.2 bB | |
| 25/15 °C | 21.1 ± 0.9 bB | 17.1 ± 3.9 aA | |
| 6 months | 20/10 °C | 9.3 ± 1.1 aA | 27.1 ± 3.3 bB |
| 15/10 °C | 11.6 ± 2.3 aB | 15.8 ± 1.2 bB | |
| 25/15 °C | 13.3 ± 0.5 aB | 12.7 ± 0.3 bA | |
| 9 months | 20/10 °C | 22.5 ± 0.5 abC | 8.7 ± 0.1 aA |
| 15/10 °C | 7.2 ± 0.8 abCB | 6.0 ± 1.4 abB | |
| 25/15 °C | 2.0 ± 0.0 abD | 2.0 ± 0.0 cB |
| Factor | Germination Percentage | ||||
|---|---|---|---|---|---|
| Darkness | Photoperiod | ||||
| df | F | p | F | p | |
| Temperature (A) | 2 | 5.260 | 0.012 | 29.751 | ˂0.001 |
| Duration (B) | 2 | 13.453 | ˂0.001 | 4.268 | 0.25 |
| A × B | 4 | 45.586 | ˂0.001 | 21.640 | ˂0.001 |
| Error | 27 | ||||
| Temperature | GA3 Concentration (mg/L−1) | % Germination | MGT | ||
|---|---|---|---|---|---|
| Darkness | Photoperiod | Darkness | Photoperiod | ||
| 15/10 °C | Control | 00.00 c ± 00.00 | 00.00 c ± 00.00 | nd | nd |
| 250 | 16.60 ab ± 03.33 | 14.60 b ± 05.33 | 09.20 b ± 02.81 | 12.10 ± 01.26 | |
| 500 | 18.00 b ± 01.30 | 22.60 a ± 03.50 | 21.40 a ± 02.70 | 19.30 ± 03.66 | |
| 1000 | 28.00 a ± 02.30 | 05.30 ab ± 01.30 | 06.10 b ± 00.80 | nd | |
| Factor | Germination Percentage | MGT | |||
|---|---|---|---|---|---|
| Darkness | Photoperiod | ||||
| df | F | p | F | p | |
| Light (A) | 1 | 2.006 | 0.170 | 78.067 | ˂0.001 |
| Hormone (B) | 3 | 18.801 | ˂0.001 | 35.109 | ˂0.001 |
| A × B | 3 | 24.153 | ˂0.001 | 21.076 | ˂0.001 |
| Error | 24 | ||||
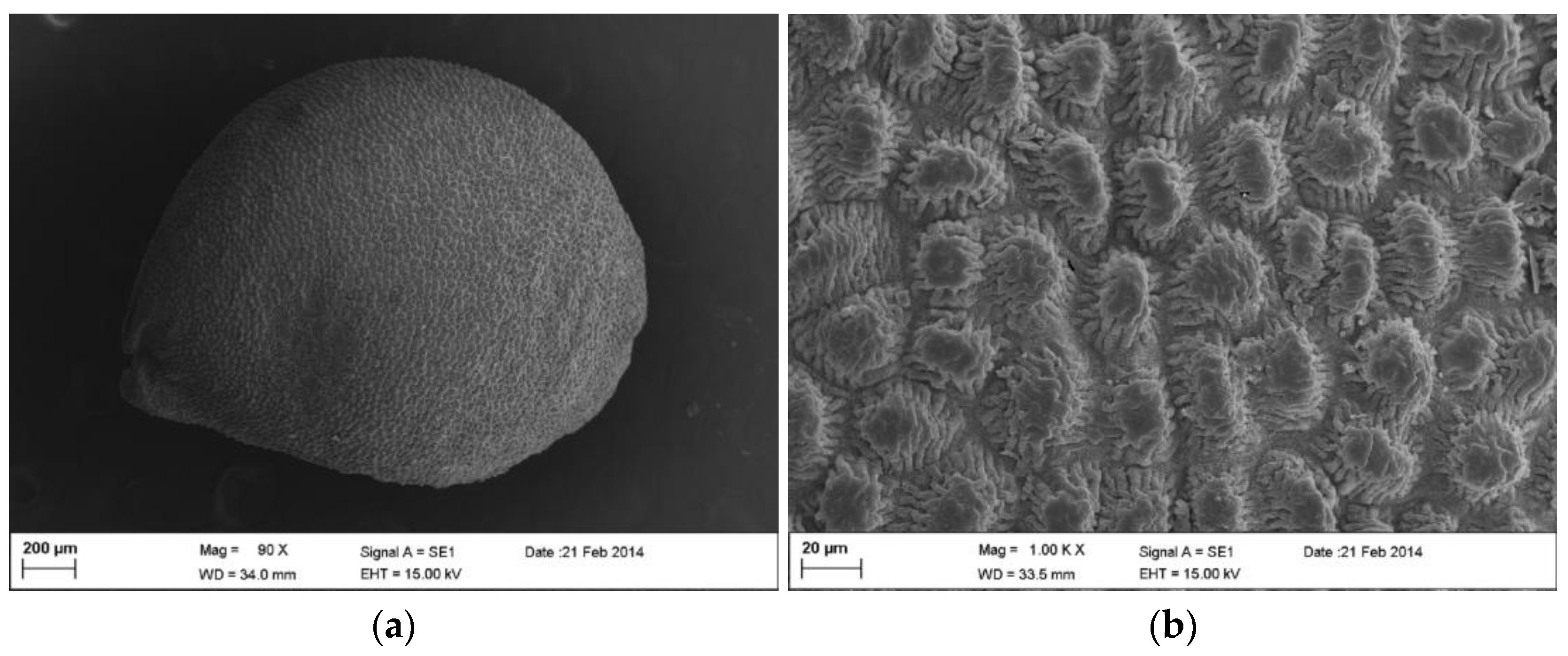
| Color | 1000 Seed Weight (g) | Length (mm) | Width (mm) | Weight (g) | Shape | Shape of Testa Cells |
|---|---|---|---|---|---|---|
| Black | 03.20 ± 00.23 | 01.17 ± 00.03 | 1.04 ± 00.02 | 0.0034 ± 0.0001 | orbicular | Stellate-grooved |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kırmızı, S. Effects of Pre-Treatments on Seed Dormancy and Germination of Endemic Muscari bourgaei Baker. Agronomy 2023, 13, 2438. https://doi.org/10.3390/agronomy13092438
Kırmızı S. Effects of Pre-Treatments on Seed Dormancy and Germination of Endemic Muscari bourgaei Baker. Agronomy. 2023; 13(9):2438. https://doi.org/10.3390/agronomy13092438
Chicago/Turabian StyleKırmızı, Serap. 2023. "Effects of Pre-Treatments on Seed Dormancy and Germination of Endemic Muscari bourgaei Baker" Agronomy 13, no. 9: 2438. https://doi.org/10.3390/agronomy13092438
APA StyleKırmızı, S. (2023). Effects of Pre-Treatments on Seed Dormancy and Germination of Endemic Muscari bourgaei Baker. Agronomy, 13(9), 2438. https://doi.org/10.3390/agronomy13092438







