Core Collection Formation in Guatemalan Wild Avocado Germplasm with Phenotypic and SSR Data
Abstract
1. Introduction
2. Materials and Methods
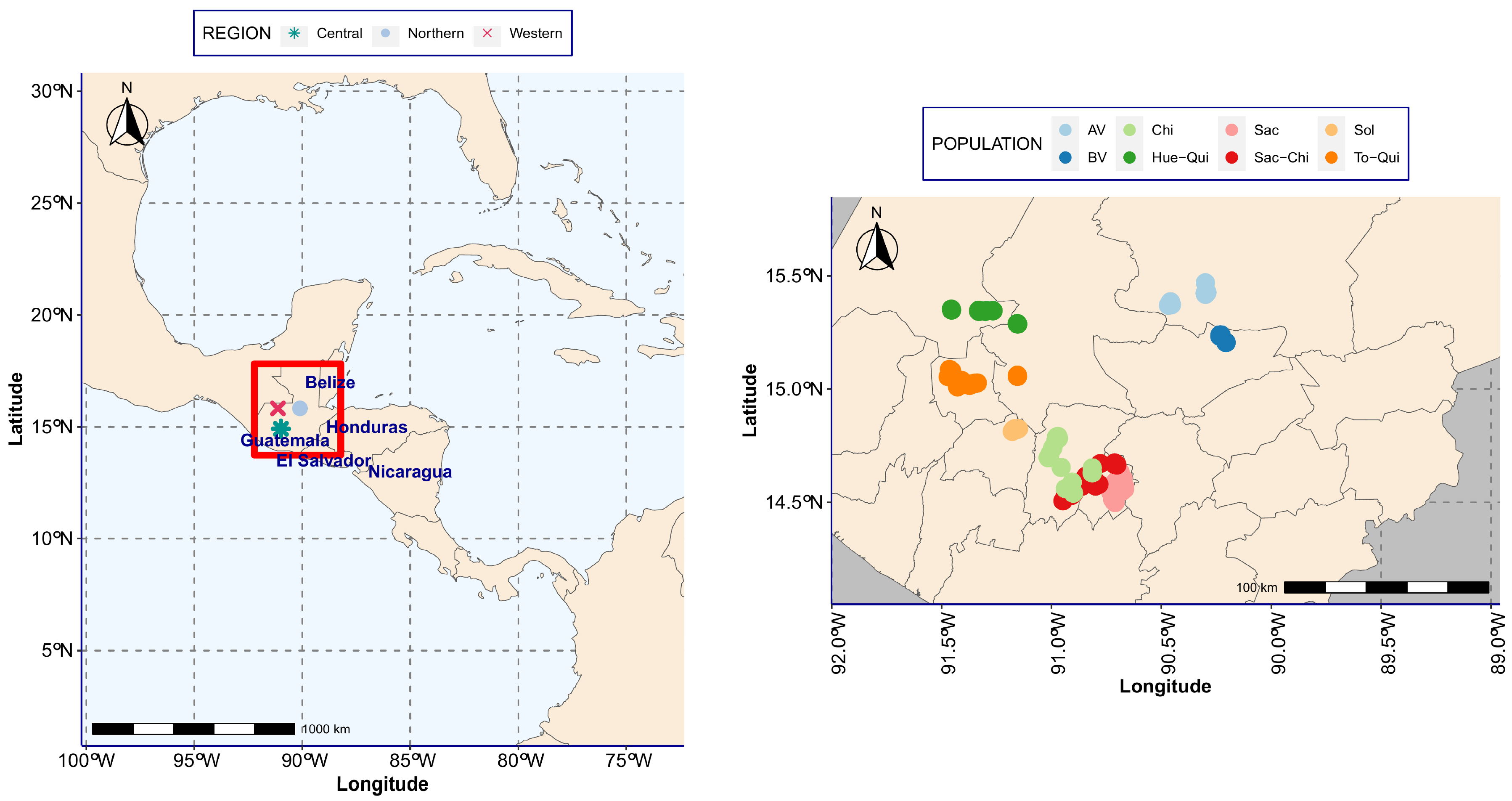
2.1. Study Site and Sampling
2.2. DNA Isolation, SSR Amplification and Genotyping
2.3. Measurement of Quantitative and Qualitative Morphological Traits
2.4. Data Analysis
2.4.1. Population Structure Analysis
2.4.2. Genetic Diversity
2.4.3. Phenotypic Variability
2.4.4. Joint Analysis of Phenotypic and Molecular Data
2.4.5. Development of the Core Collection
- I.
- maximizing E-NE distances (CC 01)
- II.
- maximizing A-NE distance (CC 02)
- III.
- maximizing both E-NE and A-NE with equal weightage of 1:1 (CC 03)
- IV.
- E-NE and A-NE with unequal weightage of 0.3:0.7 (CC 04)
- V.
- E-NE and A-NE with equal weightage of 0.7:0.3 (CC 05)
2.4.6. Evaluation of the Core Collection
3. Results
3.1. Genetic Characterization
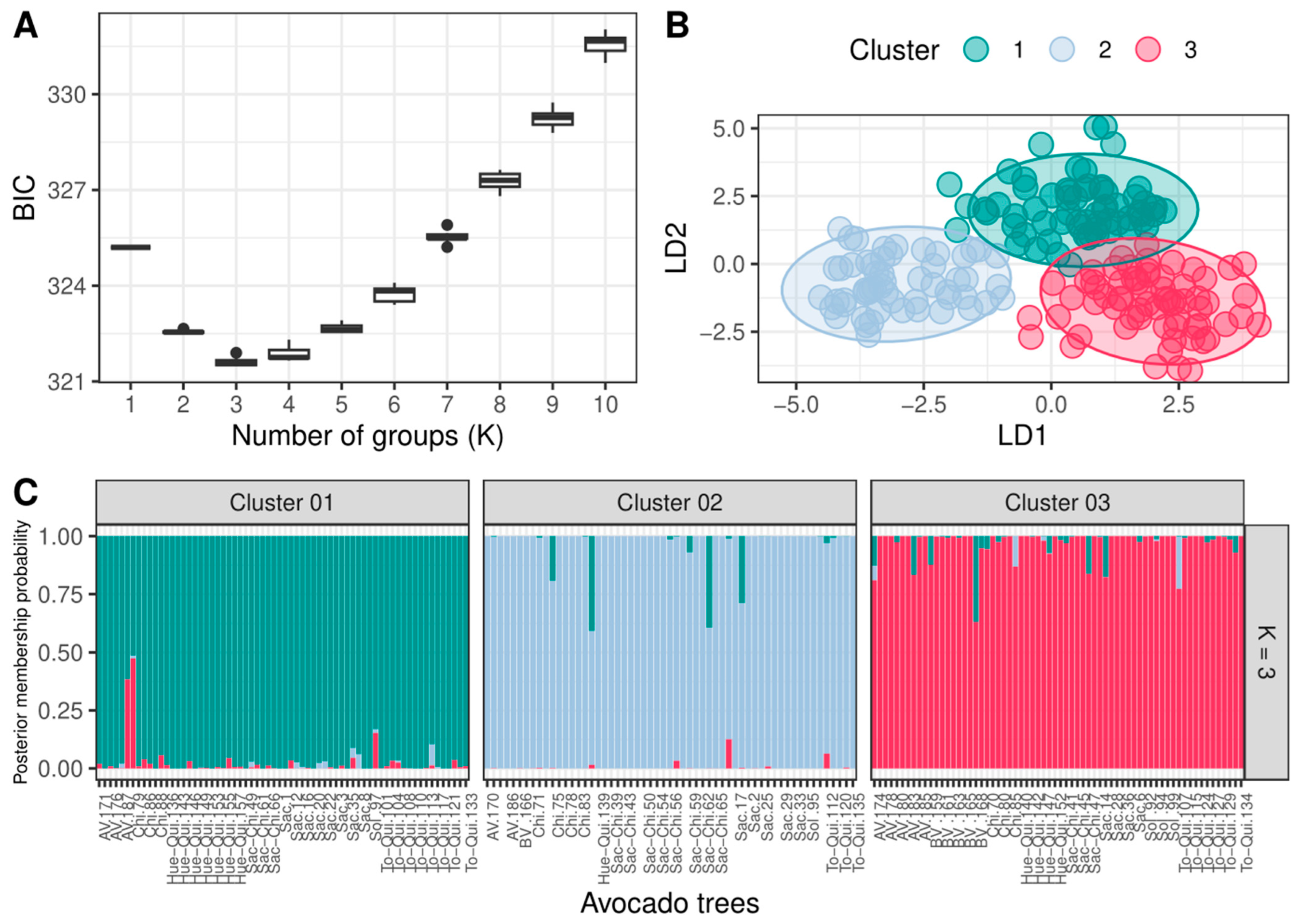
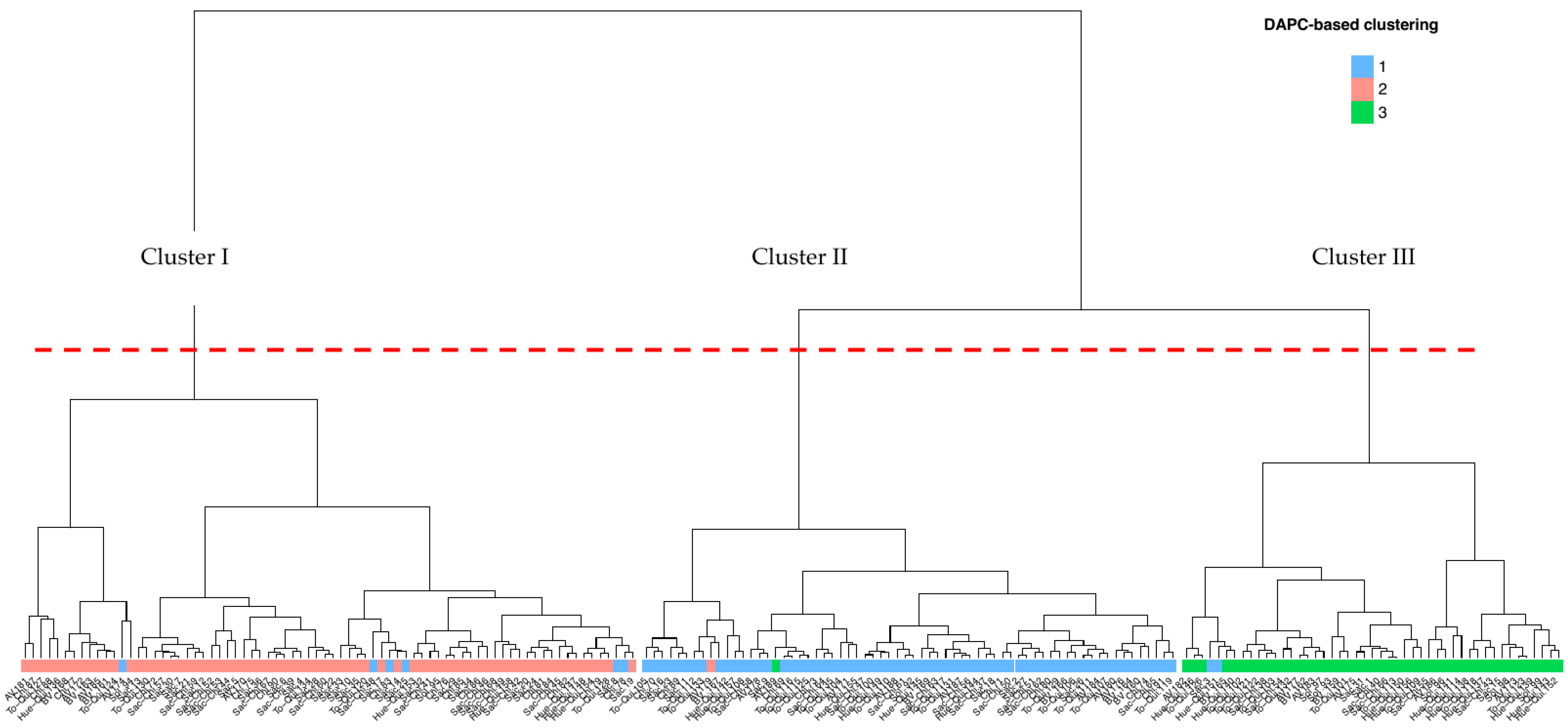
3.1.1. Identification of Genetic Subpopulations (Clusters) and Description of Population Structure
3.1.2. Genetic Diversity among Genetic Clusters
3.1.3. Analysis of Molecular Variance and Population Differentiation
3.2. Morphological Characterization
3.2.1. Quantitative Traits among Genetic Clusters
3.2.2. Qualitative Traits among Genetic Clusters
3.3. Joint Analysis of Phenotypic and Molecular Data
3.4. Selection of a Core Collection of Avocado Genotypes Based on Phenotypic Traits and Molecular Markers
3.4.1. Assembly and Quality Evaluation of the Core Collections
3.4.2. Comparative Evaluation of the Core Collection with the Entire Wild Guatemalan Avocado Germplasm Collection
4. Discussion
4.1. Genetic Characterization
4.2. Morphological Characterization
4.2.1. Quantitative Traits
4.2.2. Qualitative Traits
4.3. Joint Analysis of Phenotypic and Molecular Data
4.4. Core Collection
4.4.1. Quality Assessment of Core Collections
4.4.2. Comparative Evaluation of the Core Collections with the Whole Wild Guatemalan Avocado
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaffer, B.; Wolstenholme, B.; Whiley, A. The Avocado: Botany, Production and Uses, 2nd ed.; CPI Group (UK) Ltd.: Croydon, UK, 2012. [Google Scholar]
- Galindo-Tovar, M.E.; Ogata-Aguilar, N.; Arzate-Fernández, A. Some Aspects of Avocado (Persea americana Mill.) Diversity and Domestication in Mesoamerica. Genet. Resour. Crop Evol. 2008, 55, 441–450. [Google Scholar] [CrossRef]
- Gross-German, E.; Viruel, M.A. Molecular Characterization of Avocado Germplasm with a New Set of SSR and EST-SSR Markers: Genetic Diversity, Population Structure, and Identification of Race-Specific Markers in a Group of Cultivated Genotypes. Tree Genet. Genomes 2013, 9, 539–555. [Google Scholar] [CrossRef]
- Sánchez-González, E.I.; Gutiérrez-Díez, A.; Mayek-Pérez, N. Outcrossing Rate and Genetic Variability in Mexican Race Avocado. J. Am. Soc. Hortic. Sci. 2020, 145, 53–59. [Google Scholar] [CrossRef]
- Borrone, J.; Olano, C.; Kuhn, D.; Brown, J.; Schnell, R.J.; Violi, H. Outcrossing in Florida Avocados as Measured Using Microsatellite Markers. J. Am. Soc. Hortic. Sci. 2008, 133, 255–261. [Google Scholar] [CrossRef]
- Juma, I.; Geleta, M.; Nyomora, A.; Saripella, G.V.; Hovmalm, H.P.; Carlsson, A.S.; Fatih, M.; Ortiz, R. Genetic Diversity of Avocado from the Southern Highlands of Tanzania as Revealed by Microsatellite Markers. Hereditas 2020, 157, 40. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.L.; Hormaza, J.I. Influence of Physical Distance between Cultivars on Yield, Outcrossing Rate and Selective Fruit Drop in Avocado (Persea americana, Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- Boza, E.J.; Tondo, C.L.; Ledesma, N.; Campbell, R.J.; Bost, J.; Schnell, R.J.; Gutiérrez, O.A. Genetic Differentiation, Races and Interracial Admixture in Avocado (Persea americana Mill.), and Persea Spp. Evaluated Using SSR Markers. Genet. Resour. Crop Evol. 2018, 65, 1195–1215. [Google Scholar] [CrossRef]
- Talavera, A.; Soorni, A.; Bombarely, A.; Matas, A.J.; Hormaza, J.I. Genome-Wide SNP Discovery and Genomic Characterization in Avocado (Persea americana Mill.). Sci. Rep. 2019, 9, 20137. [Google Scholar] [CrossRef]
- Guzmán, L.F.; Machida-hirano, R.; Borrayo, E.; Cortés-cruz, M.; Jarret, R.L. Genetic Structure and Selection of a Core Collection for Long Term Conservation of Avocado in Mexico. Front. Plant Sci. 2017, 8, 243. [Google Scholar] [CrossRef]
- Yasir, M.; Das, S.; Kharya, M.D. The Phytochemical and Pharmacological Profile of Persea americana Mill. Pharmacogn. Rev. 2010, 4, 77–84. [Google Scholar] [CrossRef]
- Thormann, C.E.; Ferreira, M.E.; Camargo, L.E.A.; Tivang, J.G.; Osborn, T.C. Comparison of RFLP and RAPD Markers to Estimating Genetic Relationships within and among Cruciferous Species. Theor. Appl. Genet. 1994, 88, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Guerrero, T.; Hernandez-Perez, M.I.; Tabares, M.S.; Marulanda-Tobon, A.; Villanueva, E.; Peña, A. Agroclimatic and Phytosanitary Events and Emerging Technologies for Their Identification in Avocado Crops: A Systematic Literature Review. Agronomy 2023, 13, 1976. [Google Scholar] [CrossRef]
- Juma, I.; Nyomora, A.; Hovmalm, H.P.; Fatih, M.; Geleta, M.; Carlsson, A.S.; Ortiz, R.O. Characterization of Tanzanian Avocado Using Morphological Traits. Diversity 2020, 12, 64. [Google Scholar] [CrossRef]
- López-Galé, Y.; Murcia-Riaño, N.; Romero-Barrera, Y.; Fernando Martínez, M. Morphological Characterization of Seed-Donor Creole Avocado Trees from Three Areas in Colombia. Rev. Chapingo Ser. Hortic. 2022, 28, 93–108. [Google Scholar] [CrossRef]
- Rincón-Hernández, C.A.; Sánchez-Pérez, J.; Espinosa-García, F.J. Caracterización Química Foliar de Los Árboles de Aguacate Criollo (Persea americana Var. Drymifolia) En Los Bancos de Germoplasma de Michoacán, México. Rev. Mex. Biodivers. 2011, 82, 395–412. [Google Scholar]
- Alcaraz, M.L.; Hormaza, J.I. Molecular Characterization and Genetic Diversity in an Avocado Collection of Cultivars and Local Spanish Genotypes Using SSRs. Hereditas 2007, 144, 244–253. [Google Scholar] [CrossRef]
- Bekele, A.; Bekele, E. Overview: Morphological and Molecular Markers Role in Crop Improvement Programs. Int. J. Curr. Res. Life Sci. 2014, 3, 35–42. [Google Scholar]
- Torres, A.M.; Bergh, B. Isozymes as Indicator of Outcrossing among “Pinkerton” Seedlings. Calif. Avocado Soc. Yearb. 1978, 62, 103–110. [Google Scholar]
- Davis, J.; Henderson, D.; Kobayashi, M.; Clegg, M.T.; Michael, T.; Allen, P.C.K. Genealogical Relationships among Cultivated Avocado as Revealed through RFLP Analyses. J. Hered. 1998, 89, 319–323. [Google Scholar] [CrossRef]
- Mhameed, S.; Sharon, D.; Kaufman, D.; Lahav, E.; Hillel, J.; Lavi, U. Genetic Relationships within Avocado (Persea americana Mill) Cultivars and between Persea Species. Theor. Appl. Genet. 1997, 94, 279–286. [Google Scholar] [CrossRef]
- Fiedler, J.; Bufler, G.; Bangerth, F. Genetic Relationships of Avocado (Persea americana Mill.) Using RAPD Markers. Euphytica 1998, 101, 249–255. [Google Scholar] [CrossRef]
- López-Guzmán, G.G.; Palomino-Hermosillo, Y.A.; Balois-Morales, R.; Bautista-Rosales, P.U.; Jiménez-Zurita, J.O. Genetic Diversity of Native Avocado in Nayarit, Mexico, Determined by ISSRs. Cienc. Tecnol. Agropecu. 2021, 22, e1686. [Google Scholar] [CrossRef]
- Ashworth, V.E.T.M.; Kobayashi, M.C.; De La Cruz, M.; Clegg, M.T. Microsatellite Markers in Avocado (Persea americana Mill.): Development of Dinucleotide and Trinucleotide Markers. Sci. Hortic. 2004, 101, 255–267. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, T.; Wu, B.; Tan, L.; Ma, F.; Zou, M.; Chen, H.; Pei, J.; Liu, Y.; Chen, Z.; et al. Genome-Wide Assessment of Avocado Germplasm Determined from Specific Length Amplified Fragment Sequencing and Transcriptomes: Population Structure, Genetic Diversity, Identification, and Application of Race-Specific Markers. Genes 2019, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Eshed, R.; Rozen, A.; Zviran, T.; Kuhn, D.N.; Irihimovitch, V.; Sherman, A.; Ophir, R. Genetic Diversity of Avocado (Persea americana Mill.) Germplasm Using Pooled Sequencing. BMC Genom. 2019, 20, 379. [Google Scholar] [CrossRef]
- Popenoe, W.; Zentmyer, G.A. Early History of the Avocado. Calif. Avocado Assoc. Yearb. 1997, 81, 163–171. [Google Scholar]
- Reyes-Alemán, J.C.; Valadez-Moctezuma, E.; Simuta-Velázco, L.; Barrientos-Priego, A.; Gallegos-Vázquez, C. Distinción de Especies Del Género Persea Mediante RAPD e ISSR de ADN. Rev. Mex. Cienc. Agrícolas 2013, 4, 517–529. [Google Scholar] [CrossRef]
- Brown, A.H.D. The Use of Plant Genetic Resources. In The Case for Core Collections; Brown, A.H.D., Frankel, O.H., Marshall, D.R., Williams, J.T., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 136–156. [Google Scholar]
- Brown, A.H.D. Core Collections: A Practical Approach to Genetic Resources Management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- Odong, T.L.; Jansen, J.; van Eeuwijk, F.A.; van Hintum, T.J.L. Quality of Core Collections for Effective Utilisation of Genetic Resources Review, Discussion and Interpretation. Theor. Appl. Genet. 2013, 126, 289–305. [Google Scholar] [CrossRef]
- Mahmoodi, R.; Dadpour, M.R.; Hassani, D.; Zeinalabedini, M.; Vendramin, E.; Micali, S.; Nahandi, F.Z. Development of a Core Collection in Iranian Walnut (Juglans regia L.) Germplasm Using the Phenotypic Diversity. Sci. Hortic. 2019, 249, 439–448. [Google Scholar] [CrossRef]
- Richards, C.M.; Volk, G.M.; Reeves, P.A.; Reilley, A.A.; Henk, A.D.; Forsline, P.L.; Aldwinckle, H.S. Selection of Stratified Core Sets Representing Wild Apple (Malus sieversii). J. Am. Soc. Hortic. Sci. 2009, 134, 228–235. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Bullock, E.L.; Nolte, C.; Segovia, A.R.; Woodcock, C.E. Ongoing Forest Disturbance in Guatemala’s Protected Areas. Remote Sens. Ecol. Conserv. 2020, 6, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Chután, J.A.; Berdúo-Sandoval, J.E.; Kalousová, M.; Fernández, E.; Žiarovská, J.; Sánchéz-Pérez, A.; Lojka, B. SSRs Markers Reveal High Genetic Diversity and Limited Differentiation among Populations of Native Guatemalan Avocado. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e6134. [Google Scholar] [CrossRef]
- Ruiz-Chután, J.A.; Berdúo-Sandoval, J.E.; Maňourová, A.; Kalousová, M.; Villanueva-González, C.E.; Fernández, E.; Žiarovská, J.; Sánchez-Pérez, A.; Lojka, B. Variability Analysis of Wild Guatemalan Avocado Germplasm Based on Agro-Morphological Traits. Trop. Subtrop. Agroecosyst. 2023, 26, 52. [Google Scholar] [CrossRef]
- Azurdia, C.; Williams, K.; Williams, D.; Van Damme, V.; Jarvis, A.; Castaño, S. Guatemalan Atlas of Crop Wild Relatives. Available online: http://www.ars.usda.gov/Services/docs.html?docid=22225 (accessed on 2 May 2023).
- Gutierrez Caro, B. Consideraciones Para El Muestreo y Colecta de Germoplasma En La Conservación Ex Situ de Recursos Genéticos Forestales. In Conservacion de Recursos Genéticos Forestales: Principios y Prácticas; Gutierrez, B., Ipoinza, R., Barros, S., Eds.; Instituto Forestal: Concepción, Chile, 2015; pp. 179–196. ISBN 978 956 318 108 1. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- IPGRI. Descriptors for Avocado (Persea spp.); International Plant Genetic Resources Institute: Rome, Italy, 1995; 106p. [Google Scholar]
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; GBIF: Vienna, Austria, 2022. [Google Scholar]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Goudet, J.; Jombart, T. Hierfstat: Estimation and Tests of Hierarchical F-Statistics. R Package Version 0.5-11. Available online: https://CRAN.R-project.org/package=hierfstat/ (accessed on 24 May 2023).
- Keenan, K.; McGinnity, P.; Cross, T.F.; Crozier, W.W.; Prodöhl, P.A. DiveRsity: An R Package for the Estimation and Exploration of Population Genetics Parameters and Their Associated Errors. Methods Ecol. Evol. 2013, 4, 782–788. [Google Scholar] [CrossRef]
- Adamack, A.T.; Gruber, B. PopGenReport: Simplifying Basic Population Genetic Analyses in R. Methods Ecol. Evol. 2014, 5, 384–387. [Google Scholar] [CrossRef]
- Gruber, B.; Adamack, A.T. Landgenreport: A New r Function to Simplify Landscape Genetic Analysis Using Resistance Surface Layers. Mol. Ecol. Resour. 2015, 15, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Subirana, I.; Sanz, H.; Vila, J. Building Bivariate Tables: The CompareGroups Package for R. J. Stat. Softw. 2014, 57, 1–16. [Google Scholar] [CrossRef]
- Aravind, J.; Kaur, V.; Wankhede, D.P.; Nanjundan, J. EvaluateCore: Quality Evaluation of Core Collections. R Package Version 0.1.3. Available online: https://aravind-j.github.io/EvaluateCore/ (accessed on 21 June 2023).
- Wotzlaw, A.; Speckenmeyer, E.; Porschen, S. Generalized K-Ary Tanglegrams on Level Graphs: A Satisfiability-Based Approach and Its Evaluation. Discret. Appl. Math. 2012, 160, 2349–2363. [Google Scholar] [CrossRef][Green Version]
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25. [Google Scholar] [CrossRef]
- Pages, J. Analyse Factorielle de Données Mixtes. Rev. Stat. Appliquée 2004, 52, 93–111. [Google Scholar]
- Kenkel, N. On Selecting an Appropriate Multivariate Analysis. Can. J. Plant Sci. 2006, 86, 663–676. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Pages, J. Principal Component Methods—Hierarchical Clustering—Partitional Clustering: Why Would We Need to Choose for Visualizing Data? Appl. Math. Dep. 2010, 17, 1–17. [Google Scholar]
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Muñoz-Pajares, A.J. SIDIER: Substitution and Indel Distances to Infer Evolutionary Relationships. Methods Ecol. Evol. 2013, 4, 1195–1200. [Google Scholar] [CrossRef]
- Bougeard, S.; Dray, S. Supervised Multiblock Analysis in R with the Ade4 Package. J. Stat. Softw. 2018, 86, 1–17. [Google Scholar] [CrossRef]
- Graebner, R.; Cuesta-Marcos, A. GeneticSubsetter: Identify Favorable Subsets of Germplasm Collections. R Package Version 0.8. Available online: https://CRAN.R-project.org/package=GeneticSubsetter/ (accessed on 10 June 2021).
- Brouwer, M.; de Blok, R. CoreCollection: Creating a Core Collection. R Package Version 0.9.5. Available online: https://github.com/PBR/coreCollection/ (accessed on 13 June 2023).
- De Beukelaer, H.; Davenport, G. Corehunter: Multi-Purpose Core Subset Selection. R Package Version 3.2.2. Available online: https://CRAN.R-project.org/package=corehunter/ (accessed on 30 June 2023).
- De Beukelaer, H.; Davenport, G.F.; Fack, V. Core Hunter 3: Flexible Core Subset Selection. BMC Bioinform. 2018, 19, 203. [Google Scholar] [CrossRef]
- Kaur, V.; Aravind, J.; Manju; Jacob, S.R.; Kumari, J.; Panwar, B.S.; Pal, N.; Rana, J.C.; Pandey, A.; Kumar, A. Phenotypic Characterization, Genetic Diversity Assessment in 6778 Accessions of Barley (Hordeum vulgare L. ssp. Vulgare) Germplasm Conserved in National Genebank of India and Development of a Core Set. Front. Plant Sci. 2022, 13, 771920. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, J.; Xu, H.M. Methods of Constructing Core Collections by Stepwise Clustering with Three Sampling Strategies Based on the Genotypic Values of Crops. Theor. Appl. Genet. 2000, 101, 264–268. [Google Scholar] [CrossRef]
- Kim, M.-J.; Hyun, J.-N.; Kim, J.-A.; Park, J.-C.; Kim, M.-Y.; Kim, J.-G.; Lee, S.-J.; Chun, S.-C.; Chung, I.-M. Relationship between Phenolic Compounds, Anthocyanins Content and Antioxidant Activity in Colored Barley Germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef]
- Mantel, N. The Detection of Disease Clustering and Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Newman, D. The Distribution of Range in Samples from a Normal Population, Expressed in Terms of an Independent Estimate of Standard Deviation. Biometrika 1939, 31, 20–30. [Google Scholar] [CrossRef]
- Keuls, M. The Use of the “Studentized Range” in Connection with an Analysis of Variance. Euphytica 1952, 1, 112–122. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Ed.; Stanford Studies in Mathematics and Statistics; Stanford University Press: Redwood City, CA, USA, 1960; pp. 278–292. ISBN 9780804705967. [Google Scholar]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Wilk, M.B.; Gnanadesikan, R. Probability Plotting Methods for the Analysis of Data. Biometrika 1968, 55, 1–17. [Google Scholar] [CrossRef]
- Kullback, S.; Leibler, R.A. On Information and Sufficiency. Ann. Math. Stat. 1951, 22, 79–86. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Juma, I.; Geleta, M.; Hovmalm, H.P.; Nyomora, A.; Saripella, G.V.; Carlsson, A.S.; Fatih, M.; Ortiz, R. Comparison of Morphological and Genetic Characteristics of Avocados Grown in Tanzania. Genes 2021, 12, 63. [Google Scholar] [CrossRef]
- Schnell, R.J.; Brown, J.S.; Olano, C.T.; Power, E.J.; Krol, C.A.; Kuhn, D.N.; Motamayor, J.C. Evaluation of Avocado Germplasm Using Microsatellite Markers. J. Am. Soc. Hortic. Sci. 2003, 128, 881–889. [Google Scholar] [CrossRef]
- Cañas-Gutiérrez, G.P.; Alcaraz, L.; Hormaza, J.I.; Arango-Isaza, R.E.; Saldamando-Benjumea, C.I. Diversity of Avocado (Persea americana Mill.) Cultivars from Antioquia (Northeast colombia) and Comparison with a Worldwide Germplasm Collection. Turkish J. Agric. For. 2019, 43, 437–449. [Google Scholar] [CrossRef]
- Sjöstrand, A.E.; Sjödin, P.; Jakobsson, M. Private Haplotypes Can Reveal Local Adaptation. BMC Genet. 2014, 15, 61. [Google Scholar] [CrossRef]
- Turner, T.L.; Bourne, E.C.; Von Wettberg, E.J.; Hu, T.T.; Nuzhdin, S.V. Population Resequencing Reveals Local Adaptation of Arabidopsis lyrata to Serpentine Soils. Nat. Genet. 2010, 42, 260–263. [Google Scholar] [CrossRef]
- Ashworth, V.E.T.M.; Chen, H.; Clegg, M.T. Wild Crop Relatives: Genomic and Breeding Resources: Tropical and Subtropical Fruits. In Persea; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 173–189. ISBN 978-3-642-20447-0. [Google Scholar]
- Storey, W.; Bergh, B.; Zentmyer, G. The Origin, Indigenous Range and Dissemination of the Avocado. Calif. Avocado Soc. 1986, 70, 127–133. [Google Scholar]
- Bergh, B. The Origin, Nature, and Genetic Improvement of the Avocado. Calif. Avocado Soc. 1992, 76, 61–75. [Google Scholar]
- Colunga, P.; Zizumboo, D. Domestication of Plants in Maya Lowlands. Econ. Bot. 2004, 58, 101–110. [Google Scholar] [CrossRef]
- Chung, M.Y.; Merilä, J.; Li, J.; Mao, K.; López-Pujol, J.; Tsumura, Y.; Chung, M.G. Neutral and Adaptive Genetic Diversity in Plants: An Overview. Front. Ecol. Evol. 2023, 11, 1116814. [Google Scholar] [CrossRef]
- Purugganan, M.D. Evolutionary Insights into the Nature of Plant Domestication. Curr. Biol. 2019, 29, R705–R714. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, R. Variabilidad Genética y Caracterización de Especiesvegetales. In Análisis Estadístico de Datos de Caracterización Morfológica de Recursos Fitogenéticos; Franco, T.L., Hidalgo, R., Eds.; Instituto Internacional de Recursos Fitogenéticos (IPGRI): Cali, Colombia, 2003; p. 226. [Google Scholar]
- López-Guzmán, G.; Medina-Torres, R.; Guillén-Andrade, H.; Ramírez-Guerrero, L.G.; Juárez-López, P.; Ruelas-Hernández, P. Caracterización Morfológica En Genotipos Nativos de Aguacate (Persea americana Mill.) de Clima Tropical En Nayarit, México. Rev. Mex. Cienc. Agrícolas 2015, 6, 2157–2163. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Ashworth, V.; Xu, S.; Clegg, M. Quantitative Genetic Analysis of Growth Rate in Avocado. J. Am. Soc. Hortic. Sci. 2007, 132, 691–696. [Google Scholar] [CrossRef]
- Henao-Rojas, J.C.; Lopez, J.H.; Osorio, N.W.; Ramírez-Gil, J.G. Fruit Quality in Hass Avocado and Its Relationships with Different Growing Areas under Tropical Zones. Rev. Ceres 2019, 66. [Google Scholar] [CrossRef]
- Cañas-Gutiérrez, G.P.; Sepulveda-Ortega, S.; López-Hernández, F.; Navas-Arboleda, A.A.; Cortés, A.J. Inheritance of Yield Components and Morphological Traits in Avocado Cv. Hass from “Criollo” “Elite Trees” via Half-Sib Seedling Rootstocks. Front. Plant Sci. 2022, 13, 843099. [Google Scholar] [CrossRef]
- Mokria, M.; Gebrekirstos, A.; Said, H.; Hadgu, K.; Hagazi, N.; Dubale, W.; Bräuning, A. Fruit Weight and Yield Estimation Models for Five Avocado Cultivars in Ethiopia. Environ. Res. Commun. 2022, 4, 075013. [Google Scholar] [CrossRef]
- Scora, R.; Bergh, B. Origin of the Taxonomic Relationships within the Genus Persea. In Proceedings of the II World Avocado Congress, Orange, CA, USA, 21–26 April 1991; pp. 505–514. [Google Scholar]
- Pino, J.A.; Marbot, R.; Martí, M.P. Leaf Oil of Persea americana Mill. Var. Drymifolia Cv. Duke Grown in Cuba. J. Essent. Oil Res. 2006, 18, 440–442. [Google Scholar] [CrossRef]
- Pereira, M.E.C.; Tieman, D.M.; Sargent, S.A.; Klee, H.J.; Huber, D.J. Volatile Profiles of Ripening West Indian and Guatemalan-West Indian Avocado Cultivars as Affected by Aqueous 1-Methylcyclopropene. Postharvest Biol. Technol. 2013, 80, 37–46. [Google Scholar] [CrossRef]
- Ehleringer, J.; Björkman, O.; Mooney, H.A. Leaf Pubescence: Effects on Absorptance and Photosynthesis in a Desert Shrub. Science 1976, 192, 376–377. [Google Scholar] [CrossRef]
- Konrad, W.; Burkhardt, J.; Ebner, M.; Roth-Nebelsick, A. Leaf Pubescence as a Possibility to Increase Water Use Efficiency by Promoting Condensation. Ecohydrology 2015, 8, 480–492. [Google Scholar] [CrossRef]
- Bost, J.B.; Smith, N.J.; Crane, J. History, Distribution and Uses. In The Avocado: Botany, Production and Uses; Schaffer, B., Wolstenholme, B., Whiley, A.W., Eds.; CAB International: Walliongford, UK, 2013; pp. 10–30. [Google Scholar]
- Espinosa-Alonso, L.G.; Paredes-López, O.; Valdez-Morales, M.; Oomah, B.D. Avocado Oil Characteristics of Mexican Creole Genotypes. Eur. J. Lipid Sci. Technol. 2017, 119, 1600406. [Google Scholar] [CrossRef]
- Ranjitha, V.; Chaitanya, H.; Ravi, C.; Shivakumar, B.; Naveen, N. Morphological Characterization of Avocado (Persea americana Mill.) Accessions Explored from Hill Zone Taluks of Chikkamagaluru District, Karnataka State. J. Pharmacogn. Phytochem. 2021, 10, 310–318. [Google Scholar]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, Flavor, Texture, and Nutritional Quality of Fresh-Cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and the Effects of Processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Shewfelt, R.L. Fruit and Vegetable Quality. In Fruit and Vegetable Quality: An Integrated View; Shewfelt, R.L., Bruckner, B., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 160–173. [Google Scholar]
- Allendorf, F.W.; Funk, W.C.; Aitken, S.N.; Byrne, M.; Luikart, G. Phenotypic Variation in Natural Populations. In Conservation and the Genomics of Populations; Allendorf, F.W., Funk, W.C., Aitken, S.N., Byrne, M., Luikart, G., Antunes, A., Eds.; Oxford University Press: New York, NY, USA, 2022; ISBN 9780198856566. [Google Scholar]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; de Freitas Munhoz, C. Microsatellite Markers: What They Mean and Why They Are So Useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Nodari, R.; Gepts, P. Genetic Diversity in Cultivated Common Bean: I. Allozymes. Crop Sci. 1991, 31, 19–23. [Google Scholar] [CrossRef]
- Alves, A.A.; Bhering, L.L.; Rosado, T.B.; Laviola, B.G.; Formighieri, E.F.; Cruz, C.D. Joint Analysis of Phenotypic and Molecular Diversity Provides New Insights on the Genetic Variability of the Brazilian Physic Nut Germplasm Bank. Genet. Mol. Biol. 2013, 36, 371–381. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An Introduction to Markers, Quantitative Trait Loci (QTL) Mapping and Marker-Assisted Selection for Crop Improvement: The Basic Concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Sunil, N.; Sujatha, M.; Kumar, V.; Vanaja, M.; Basha, S.D.; Varaprasad, K.S. Correlating the Phenotypic and Molecular Diversity in Jatropha curcas L. Biomass Bioenergy 2011, 35, 1085–1096. [Google Scholar] [CrossRef]
- Nkhoma, N.; Shimelis, H.; Laing, M.D.; Shayanowako, A.; Mathew, I. Assessing the Genetic Diversity of Cowpea [Vigna unguiculata (L.) Walp.] Germplasm Collections Using Phenotypic Traits and SNP Markers. BMC Genet. 2020, 21, 110. [Google Scholar] [CrossRef]
- Agre, P.; Asibe, F.; Darkwa, K.; Edemodu, A.; Bauchet, G.; Asiedu, R.; Adebola, P.; Asfaw, A. Phenotypic and Molecular Assessment of Genetic Structure and Diversity in a Panel of Winged Yam (Dioscorea alata) Clones and Cultivars. Sci. Rep. 2019, 9, 18221. [Google Scholar] [CrossRef]
- Guidoti, D.T.; Gonela, A.; Vidigal, M.C.G.; Conrado, T.V.; Romani, I. Interrelationship between Morphological, Agronomic and Molecular Characteristics in the Analysis of Common Bean Genetic Diversity. Acta Sci.—Agron. 2018, 40, 1–9. [Google Scholar] [CrossRef]
- Vinu, V.; Singh, N.; Vasudev, S.; Yadava, D.K.; Kumar, S.; Naresh, S.; Bhat, S.R.; Prabhu, K.V. Assessment of Genetic Diversity in Brassica juncea (Brassicaceae) Genotypes Using Phenotypic Differences and SSR Markers. Rev. Biol. Trop. 2013, 61, 1919–1934. [Google Scholar] [PubMed]
- Sartie, A.; Asiedu, R.; Franco, J. Genetic and Phenotypic Diversity in a Germplasm Working Collection of Cultivated Tropical Yams (Dioscorea Spp.). Genet. Resour. Crop Evol. 2012, 59, 1753–1765. [Google Scholar] [CrossRef]
- De Andrade, E.K.V.; de Andrade Júnior, V.C.; de Laia, M.L.; Fernandes, J.S.C.; Oliveira, A.J.M.; Azevedo, A.M. Genetic Dissimilarity among Sweet Potato Genotypes Using Morphological and Molecular Descriptors. Acta Sci.—Agron. 2017, 39, 447–455. [Google Scholar] [CrossRef]
- Thachuk, C.; Crossa, J.; Franco, J.; Dreisigacker, S.; Warburton, M.; Davenport, G.F. Core Hunter: An Algorithm for Sampling Genetic Resources Based on Multiple Genetic Measures. BMC Bioinform. 2009, 10, 243. [Google Scholar] [CrossRef]
- Franco, J.; Crossa, J.; Warburton, M.L.; Taba, S. Sampling Strategies for Conserving Maize Diversity When Forming Core Subsets Using Genetic Markers. Crop Sci. 2006, 46, 854–864. [Google Scholar] [CrossRef]
- Agrama, H.A.; Yan, W.; Lee, F.; Fjellstrom, R.; Chen, M.-H.; Jia, M.; McClung, A. Genetic Assessment of a Mini-Core Subset Developed from the USDA Rice Genebank. Crop Sci. 2009, 49, 1336–1346. [Google Scholar] [CrossRef]
- Nanjundan, J.; Aravind, J.; Radhamani, J.; Singh, K.H.; Kumar, A.; Thakur, A.K.; Singh, K.; Meena, K.N.; Tyagi, R.K.; Singh, D. Development of Indian Mustard [Brassica juncea (L.) Czern.] Core Collection Based on Agro-Morphological Traits. Genet. Resour. Crop Evol. 2022, 69, 145–162. [Google Scholar] [CrossRef]
- Ndjiondjop, M.N.; Gouda, A.C.; Eizenga, G.C.; Warburton, M.L.; Kpeki, S.B.; Wambugu, P.W.; Gnikoua, K.; Tia, D.D.; Bachabi, F. Genetic Variation and Population Structure of Oryza Sativa Accessions in the AfricaRice Collection and Development of the AfricaRice O. sativa Core Collection. Crop Sci. 2023, 63, 724–739. [Google Scholar] [CrossRef]
- Phogat, B.S.; Kumar, S.; Kumari, J.; Kumar, N.; Pandey, A.C.; Singh, T.P.; Kumar, S.; Tyagi, R.K.; Jacob, S.R.; Singh, A.K.; et al. Characterization of Wheat Germplasm Conserved in the Indian National Genebank and Establishment of a Composite Core Collection. Crop Sci. 2021, 61, 604–620. [Google Scholar] [CrossRef]
- Awachare, C.; Karunakaran, G.; Madhavi, M.; Sakthivel, T. Studies on Morphological Characterization of 72 Avocado (Persea americana Mill.) Accessions. Pharma Innov. J. 2023, 12, 1970–1975. [Google Scholar]
- Mahajan, R.; Bisht, I.; Dhillon, B. Establishment of a Core Collection of World Sesame (Sesamum indicum L.) Germplasm Accessions. SABRAO J. Breed. Genet. 2007, 39, 53–64. [Google Scholar]
- Reddy, L.J.; Upadhyaya, H.D.; Gowda, C.L.L.; Singh, S. Development of Core Collection in Pigeonpea [Cajanus cajan (L.) Millspaugh] Using Geographic and Qualitative Morphological Descriptors. Genet. Resour. Crop Evol. 2005, 52, 1049–1056. [Google Scholar] [CrossRef]


| Group | Size | Na | ar | Pa | H | λ | Ho | He | uHe | FIS | HWE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 67 | 16.25 | 13.49 | 2.50 | 4.19 | 0.98 | 0.59 | 0.81 | 0.81 | 0.28 | ** |
| Cluster 2 | 56 | 13.83 | 11.87 | 1.17 | 4.04 | 0.98 | 0.58 | 0.77 | 0.78 | 0.24 | ** |
| Cluster 3 | 66 | 18.83 | 15.08 | 5.08 | 4.19 | 0.98 | 0.53 | 0.81 | 0.82 | 0.35 | ** |
| mean | 63.00 | 16.30 | 13.48 | 2.92 | 4.14 | 0.98 | 0.56 | 0.80 | 0.80 | 0.288 |
| Variation | Sigma | % | Φ Statistics | p-Value |
|---|---|---|---|---|
| Among clusters | 0.46 | 7.74 | ΦCT = 0.18 | <0.01 |
| Among samples within clusters | 1.55 | 26.20 | ΦSC = 0.28 | <0.01 |
| Within samples | 3.90 | 66.06 | ΦST = 0.34 | <0.01 |
| Total | 5.91 | 100 |
| Trait | Cluster 1 | Cluster 2 | Cluster 3 | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | |
| FW | 336.11 a | 88.56 | 0.26 | 250.40 b | 95.44 | 0.38 | 341.61 a | 90.54 | 0.27 | 313.14 | 99.40 | 0.32 |
| FL | 13.79 a | 2.79 | 0.20 | 12.73 b | 1.74 | 0.14 | 11.72 b | 2.94 | 0.25 | 12.73 | 2.72 | 0.21 |
| SW | 92.78 a | 18.85 | 0.20 | 88.68 b | 15.18 | 0.17 | 89.74 b | 15.99 | 0.18 | 90.49 | 16.83 | 0.19 |
| LL | 22.52 b | 6.02 | 0.27 | 21.23 b | 6.17 | 0.29 | 37.39 a | 7.64 | 0.20 | 27.49 | 9.99 | 0.36 |
| LW | 13.06 | 3.53 | 0.27 | 12.38 | 3.41 | 0.28 | 12.89 | 3.77 | 0.29 | 12.80 | 3.57 | 0.28 |
| SL | 3.67 a | 0.66 | 0.18 | 3.65 a | 0.87 | 0.24 | 3.26 b | 0.85 | 0.26 | 3.52 | 0.81 | 0.23 |
| PL | 3.52 | 0.32 | 0.09 | 3.42 | 0.39 | 0.11 | 3.49 | 0.29 | 0.08 | 3.48 | 0.33 | 0.10 |
| TC | 107.12 a | 20.54 | 0.19 | 111.14 a | 19.60 | 0.18 | 96.23 b | 25.92 | 0.27 | 104.37 | 23.15 | 0.22 |
| Trait | Cluster 1 | Cluster 2 | Cluster 3 | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| λ | H | λ | H | λ | H | λ | H | χ2 | |
| TS | 0.66 | 1.58 | 0.66 | 1.57 | 0.65 | 1.56 | 0.67 | 1.58 | 3.672 ns |
| CYT | 0.79 | 2.30 | 0.79 | 2.29 | 0.77 | 2.21 | 0.79 | 2.28 | 5.32 ns |
| CML | 0.50 | 0.99 | 0.50 | 1.00 | 0.50 | 0.99 | 0.50 | 0.99 | 0.45 ns |
| LS | 0.85 | 2.97 | 0.86 | 2.99 | 0.86 | 2.99 | 0.87 | 3.06 | 20.58 ns |
| LAS | 0.47 | 0.96 | 0.49 | 0.98 | 0.39 | 0.83 | 0.48 | 0.97 | 12.91 *** |
| PP | 0.66 | 1.57 | 0.64 | 1.52 | 0.63 | 1.51 | 0.66 | 1.56 | 5.52 ns |
| PS | 0.67 | 1.58 | 0.66 | 1.58 | 0.58 | 1.40 | 0.65 | 1.56 | 10.34 * |
| FSS | 0.58 | 1.41 | 0.61 | 1.47 | 0.54 | 1.33 | 0.66 | 1.57 | 49.63 *** |
| MFSC | 0.73 | 2.33 | 0.86 | 2.80 | 0.85 | 2.79 | 0.83 | 2.71 | 21.50 * |
| FSh | 0.72 | 2.44 | 0.88 | 3.11 | 0.88 | 3.09 | 0.87 | 3.07 | 52.68 *** |
| FT | 0.73 | 1.94 | 0.51 | 1.41 | 0.75 | 1.99 | 0.71 | 1.90 | 25.10 *** |
| SS | 0.87 | 2.95 | 0.67 | 2.22 | 0.86 | 2.90 | 0.84 | 2.85 | 36.46 *** |
| CS | 0.56 | 1.38 | 0.53 | 1.29 | 0.47 | 1.18 | 0.66 | 1.56 | 79.20 *** |
| Criterion | CoreCollection | GeneticSubsetter | CC 01 | CC 02 | CC 03 | CC 04 | CC 05 |
|---|---|---|---|---|---|---|---|
| A-NE | 0.05 | 0.06 | 0.05 | 0.06 | 0.09 | 0.07 | 0.01 |
| E-NE | 0.22 | 0.26 | 0.24 | 0.23 | 0.24 | 0.23 | 0.22 |
| E-E | 0.12 | 0.13 | 0.13 | 0.13 | 0.12 | 0.12 | 0.13 |
| MD% | 46.34 | 55.26 | 37.56 | 25.13 | 22.95 | 37.69 | 22.37 |
| VD% | 75.34 | 63.93 | 82.40 | 63.04 | 95.56 | 77.67 | 91.04 |
| CR% | 84.92 | 72.06 | 78.76 | 69.51 | 92.06 | 89.45 | 85.05 |
| VR% | 104.05 | 115.52 | 93.98 | 109.24 | 108.71 | 117.36 | 101.06 |
| H′ | 1.04 | 0.99 | 0.92 | 1.45 | 1.33 | 1.46 | 1.31 |
| Mantel | 0.91 ** | 0.87 ** | 0.80 ** | 0.80 ** | 0.82 ** | 0.90 ** | 0.75 ** |
| Ho | 0.54 | 0.51 | 0.58 | 0.54 | 0.58 | 0.52 | 0.55 |
| Trait | Entire Germplasm | Core Collection | Comparative Statistics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SE | CV | IQR | Min | Max | Mean ± SE | CV | IQR | x̄a | x̄b | Vc | Fd | |
| FW | 44.39 | 584.16 | 313.14 ± 7.23 | 32.45 | 125.13 | 108.63 | 517.43 | 326.39 ± 14.08 | 34.61 | 141.13 | ns | ns | ns | ns |
| SW | 38.23 | 136.37 | 86.55 ± 1.31 | 21.89 | 25.88 | 48.55 | 136.37 | 90.09 ± 2.80 | 22.89 | 27.26 | ns | ns | ns | ns |
| FL | 3.46 | 18 | 11.54 ± 0.2 | 22.12 | 4.3 | 3.46 | 16.63 | 11.12 ± 0.44 | 26.13 | 3.73 | ns | ns | ns | ns |
| PL | 2.51 | 4.3 | 3.47 ± 0.02 | 10.53 | 0.44 | 2.76 | 4.27 | 3.45 ± 0.05 | 12.54 | 0.44 | ns | ns | ns | ns |
| LL | 5.22 | 36.91 | 22.66 ± 0.44 | 27.56 | 8.52 | 11.71 | 32.38 | 22.91 ± 0.86 | 26.97 | 7.99 | ns | ns | ns | ns |
| LW | 3.61 | 20.93 | 12.80 ± 0.26 | 28.78 | 4.96 | 5.68 | 20.24 | 12.81 ± 0.48 | 29.14 | 3.62 | ns | ns | ns | ns |
| SL | 1.16 | 5.21 | 3.51 ± 0.06 | 21.34 | 1.06 | 2.02 | 4.69 | 3.70 ± 0.09 | 25.56 | 0.60 | ns | ns | ** | ns |
| TC | 22.93 | 147.74 | 104.37 ± 1.68 | 22.89 | 27.93 | 49.22 | 142.42 | 103.47 ± 3.13 | 23.29 | 31.15 | ns | ns | ns | ns |
| Descriptor | Shannon–Weaver Diversity Index (H′) | H′ Max | Evenness | |||
|---|---|---|---|---|---|---|
| Entire Germplasm | Core Collection | Entire Germplasm | Core Collection | Entire Germplasm | Core Collection | |
| TS | 1 | 1.16 | 1.1 | 1.1 | 0.81 | 0.88 |
| CYT | 1.48 | 1.55 | 1.61 | 1.61 | 0.88 | 0.96 |
| CML | 0.61 | 0.69 | 0.69 | 0.69 | 0.95 | 0.99 |
| LS | 2.02 | 2.17 | 2.2 | 2.2 | 0.91 | 0.96 |
| LAS | 0.63 | 0.69 | 0.69 | 0.69 | 0.9 | 1 |
| PP | 0.93 | 1.1 | 1.1 | 1.1 | 0.93 | 1 |
| PS | 0.99 | 1.08 | 1.1 | 1.1 | 0.9 | 0.98 |
| FSS | 0.54 | 0.65 | 0.69 | 0.69 | 0.92 | 0.94 |
| MFSC | 1.75 | 1.94 | 1.95 | 1.95 | 0.96 | 1 |
| FSh | 2.18 | 2.17 | 2.2 | 2.2 | 0.99 | 0.97 |
| FT | 1.38 | 1.48 | 1.39 | 1.39 | 0.95 | 0.98 |
| SS | 2.06 | 2.03 | 2.08 | 2.08 | 0.99 | 0.97 |
| CS | 1.09 | 1.1 | 1.1 | 1.1 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Chután, J.A.; Kalousová, M.; Maňourová, A.; Degu, H.D.; Berdúo-Sandoval, J.E.; Villanueva-González, C.E.; Lojka, B. Core Collection Formation in Guatemalan Wild Avocado Germplasm with Phenotypic and SSR Data. Agronomy 2023, 13, 2385. https://doi.org/10.3390/agronomy13092385
Ruiz-Chután JA, Kalousová M, Maňourová A, Degu HD, Berdúo-Sandoval JE, Villanueva-González CE, Lojka B. Core Collection Formation in Guatemalan Wild Avocado Germplasm with Phenotypic and SSR Data. Agronomy. 2023; 13(9):2385. https://doi.org/10.3390/agronomy13092385
Chicago/Turabian StyleRuiz-Chután, José Alejandro, Marie Kalousová, Anna Maňourová, Hewan Demissie Degu, Julio Ernesto Berdúo-Sandoval, Carlos Enrique Villanueva-González, and Bohdan Lojka. 2023. "Core Collection Formation in Guatemalan Wild Avocado Germplasm with Phenotypic and SSR Data" Agronomy 13, no. 9: 2385. https://doi.org/10.3390/agronomy13092385
APA StyleRuiz-Chután, J. A., Kalousová, M., Maňourová, A., Degu, H. D., Berdúo-Sandoval, J. E., Villanueva-González, C. E., & Lojka, B. (2023). Core Collection Formation in Guatemalan Wild Avocado Germplasm with Phenotypic and SSR Data. Agronomy, 13(9), 2385. https://doi.org/10.3390/agronomy13092385










