Abstract
Seeds stored for a prolonged period are subject to aging and a reduction in germination potential (GP), which will negatively affect seed sales. Rare-earth elements have a synergistic effect on the improvement of seed GP. In this study, we examined the effects of neodymium on biochemical components, the antioxidant protective system, and metabolism-related enzymes during germination of naturally and artificially aged seeds of three wheat cultivars. Seed germination indices, biochemical substance contents, and enzyme activities decreased after seed aging. Soaking seeds in a neodymium nitrate solution revived aged wheat seeds at an optimal concentration of 20 µmol/L for 8 h. Soaking in a neodymium nitrate solution increased the GP4 (by 2.25–60.9%), germination index (by 1.69–29.2%), and vigor index (by 3.36–18.7%) of aged seeds. Compared with non-soaked seeds, soaking significantly changed the contents of biochemical substances, and the activities of antioxidant protective enzymes and metabolic enzymes in seedlings were increased. Soaking with neodymium may revive aged seeds by regulating the synthesis of soluble sugars, soluble proteins, chlorophyll, and carotenoids and decomposing malondialdehyde in the germinating seed. Root dehydrogenase and amylase showed different responses to the aging modes. The differential responses of root dehydrogenase and amylase may reflect differences in the resistance of enzymes to long-term mild seed aging and short-term severe environmental aging.
1. Introduction
Wheat (Triticum aestivum L.) is among the most important food crops worldwide and plays an important role in human nutrition. In China, more than 220 million ha of winter wheat are sown annually, and the winter wheat sowing range is 150–375 kg/hm2 [1]. A substantial market exists for the production and storage of wheat seeds. However, similar to other organisms, wheat seeds age and lose viability during storage [2]. Under normal storage conditions, post-harvest wheat seeds consume stored nutrients to provide energy, thereby maintaining metabolism [3]. Seeds are subject to a variety of biochemical and metabolic alterations during storage, and some of the metabolic intermediates generated cause lipid peroxidation, enzyme inactivation, and disruption of cellular membranes [4]. In agricultural production, aged seeds result in commercial and genetic losses. Storage environments that adversely affect seeds accelerate seed deterioration and reduce seed germinability and vitality [5]. Seed vitality is fundamental for seed germination and the early development of seedlings. Seed aging can reduce seed quality, viability, and seedling vigor [6].
Delouche and Baskin proposed a model for seed aging and deterioration. According to this model, partial degradation of membranes occurs first during seed deterioration, which leads to loss of membrane permeability and leakage of cell components and electrolytes, thereby affecting the viability of the seed [7]. It has been reported that aged wheat seeds show many changes, including in hardness [8], quality deterioration [9], enzyme activities [10], integrity of genetic material [11,12], scutellum nuclear content [13], and patterns of simple sequence repeat variation [14]. Thus, changes in genetic material and physiological activity are crucial effects of seed aging.
Seed aging results in the accumulation of superoxide free radicals (O2•−) and hydrogen peroxide (H2O2). The accumulation of reactive oxygen species causes changes in the internal seed environment, leading to the transformation of biomacromolecules within the seed [15,16,17]. Both stress treatment and aging could increase the concentration of O2•− and H2O2. Some pretreatments are capable of partially restoring wheat seed vigor after stress. For example, 5-aminolevulinic acid pretreatment of seeds alleviates damage caused by drought and high temperatures in wheat seedlings [18]. Ultrasonic treatment of wheat seeds increases the content of γ-aminobutyric acid (GABA) and thus increases seed germinability and seedling vigor [19]. Soaking in lanthanum affects the antioxidant protective pathways in wheat seeds [20]. Soaking wheat seeds in sodium selenate may increase the seedling growth rate but has no effect on percentage germination [21]. Soaking in polyamine increases the germination percentage of wheat seeds under drought stress [22]. Exogenous melatonin may increase the germination percentage of wheat seeds by increasing the activity of antioxidant protective enzymes [23]. Some experiments indicate that treatment with rare-earth elements increases the germination percentage and vigor [20,24,25,26,27,28]. Lanthanum and cerium are currently mainly used in agricultural production. Both elements can significantly increase the percentage germination of seeds at appropriate concentrations [24,29,30,31]. Previous studies have mainly focused on lanthanum and cerium, whereas the effect of neodymium on seed germination has not been reported. In terms of chemical structure, lanthanum has no 4f electron layer and a non-commutative character, whereas neodymium has a 4f electron layer but also has a non-commutative character [32,33,34]. Neodymium may play an important role in accelerating the germination of naturally or artificially aged wheat seeds.
Reviving aged seeds is an important strategy for seed companies to reduce the cost of seed production. However, aged seeds often fail to meet the standards for seed marketing because of low germinability and vitality. Therefore, it is important to understand the mechanism of aging-induced damage to seeds and to explore approaches to repair the damage to aged seeds. Seed treatments are among the most promising techniques for the revival of aged seeds because they may stimulate primary metabolic processes during seed germination, strengthen the antioxidant system, and increase the seed germination percentage [35]. Most previous studies of aged seeds have focused on germination rather than seedling emergence and indicate that different mechanisms may promote the germination of aged seeds. The effects of neodymium on aged wheat seeds have not been reported previously. Therefore, the aim of the present study was to investigate the effects of seed aging treatment and soaking of seeds in neodymium nitrate on germination indices, biochemical contents, and antioxidant enzyme activities of wheat seeds and seedlings. The overall objective is to establish an effective treatment to revive aged wheat seeds.
2. Materials and Methods
2.1. Plant Materials and Experimental Design
The wheat cultivars ‘AK58’, ‘BN4199’, and ‘BN207’ were used. Two samples of seeds were treated in the experiment. First, seeds of the three cultivars were harvested in 2019 and stored for 3 years in an indoor sample cabinet (Xinxiang, China; east longitude 113°23′–115°01′, north latitude 34°53′–35°50′). The study region has four distinct seasons with annual average high and low temperatures of 22 °C and 10 °C, respectively. July and January are the months with the highest temperatures (average high of 35 °C) and lowest temperatures (average low of −5 °C), respectively. This sample was designated the ‘natural aging test’ (NAT), and such seeds soaked in neodymium nitrate were labeled as NATS. The second sample (the current-year seeds) comprised seeds of the three cultivars harvested in 2022 with an initial germination percentage of 99.2% (AK58), 99.5% (BN4199), and 99.3% (BN207). The current-year seeds were surface-sterilized for 15 min with a 0.1% mercuric chloride solution, then washed with distilled water. The surface-sterilized seeds were dried and then artificially aged. Seed artificial aging was based on the International Rules for Seed Testing regulations [36] with some modifications. The current-year seeds were placed in a seed aging cabinet (LH-150, Zhejiang Topu Yunnong Technology Co., LTD., Hangzhou City, China) and were artificially aged under 100% relative humidity at 41 °C for 96 h. The artificially aged seeds were designated the ‘accelerated aging test’ (AAT), and such seeds soaked in neodymium nitrate were labeled as AATS. After treatment, the seeds were dried and restored to their original moisture content, and then stored at 4 °C until the following experiments were performed.
2.2. Determination of the Optimal Neodymium Nitrate Treatment Concentration
Current-year seeds of BN4199 were used to determine the optimal concentration for neodymium nitrate treatment. Full grains of uniform size were selected after artificial-aging treatment. The selected seeds were divided into nine subsamples of 200 grains each and soaked at 25 °C for 8 h in a solution of 0 (control), 5, 15, 20, 30, 60, 100, 150, or 200 µM neodymium nitrate. After soaking, germination tests were performed at 25 °C with four replicates of 50 grains per solution. The soaked seeds were placed in petri dishes with the crease facing downward, and 5 mL of double-distilled water was added to each petri dish. This volume of water kept the filter papers uniformly moist without flooding. The petri dishes were covered and incubated in a germination cabinet at 25 °C in the dark for 7 days. Seeds were considered to have germinated when the radicle was visible and were recorded each day. After 7 days, the final germination indices were calculated based on the number of normally developed seedlings using the following formulas: germination index (GI) = Σ(Gt/Tt), where Gt is the number of germinated seeds on day t and Tt is the number of days from the start of the test; germination potential (GP; %) = the number of seeds germinated at the germination peak/the number of seeds tested × 100; and seed vigor index (VI) = GI × average weight of seedlings [37].
2.3. Seedling Growth and Biochemical Analyses
Based on the results of the preceding experiment (Section 2.2), 20 µM neodymium nitrate was selected as the optimal concentration. After seed germination for 7 days, the leaves, roots, and whole seedlings were collected. After weighing and recording the fresh weight, the samples were frozen in liquid nitrogen for 2 min and stored at −80 °C for later use.
To measure the photosynthetic pigment contents, approximately 0.2 g of fresh leaf tissue was homogenized in 95% ethanol at 25 °C and stored in the dark until the leaf tissue turned white. The homogenate was mixed and centrifuged, and the fluorescence of the supernatant was measured at 664, 649, and 470 nm with a UV-VIS spectrophotometer (Lambda 365, Perkin Elmer, Shelton, CT, USA). Leaf chlorophyll contents were calculated in accordance with the formula provided by Sumanta [38,39]. The formulas used for quantification of the chlorophyll a, chlorophyll b, and total carotenoids contents were as follows:
Ca = 13.36Abs664 − 5.19Abs649,
Cb = 27.43Abs649 − 8.12Abs664,
Cc = (1000Abs470 − 2.13Ca − 97.63Cb)/209.
Total soluble sugars were assayed using the phenol-sulfuric acid method [40]. The reaction mixture contained 0.5 mL of 5% phenol, 2.5 mL of concentrated sulfuric acid, and 1 mL of extract. The reaction mixture was incubated for 10 min for color development, and the absorbance was measured at 490 nm with a spectrophotometer. Standard curves were prepared with glucose.
The total soluble protein content was determined using the method of Lowry et al. [41]. The reaction mixture contained 0.9 mL of 7 mM potassium-sodium tartrate, 0.81 M sodium carbonate, 0.5 N sodium hydroxide, and 1 mL of the extract. The reaction mixture was incubated in a water bath at 50 °C for 10 min. Next, 0.1 mL of a solution containing 70 mM potassium-sodium tartrate, 40 mM copper sulfate, and 3 mL of Folin-Ciocalteu reagent was added to the reaction mixture and incubated in a water bath at 50 °C for 10 min. After cooling, the absorbance was determined at 650 nm. Bovine serum albumin was used to prepare the standard curve.
The malondialdehyde (MDA) content was determined. First, wheat seedlings were weighed and homogenized in 5.0 mL of 10% (w/v) trichloroacetic acid. The homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C. Subsequently, 4.0 mL of the supernatant and 4.0 mL of 0.67% (w/v) thiobarbituric acid were mixed and incubated at 95 °C for 30 min. After 5 min in an ice bath, the mixture was centrifuged at 10,000 rpm for 5 min at 25 °C. The absorbance of the extracted sample was measured at 450, 532, and 600 nm with a spectrophotometer. The MDA content was calculated using the following formula [42]:
where Vt is the total volume of extracted liquid, Vs is the volume of the extracted liquid used for the experiment, W is the seedling weight (mg), and Abs450, Abs532, and Abs600 are the absorbances of the extracted liquid at 450, 532, and 600 nm, respectively.
MDA (μmol/mg) = [6.45 × (Abs532 − Abs600) − 0.56Abs450] × Vt/(W × Vs) × 1000,
2.4. Enzyme Activity Assays
For enzyme extraction, approximately 2 g of whole seedling samples were homogenized in 10 mL of ice-cold extraction buffer (100 mM sodium phosphate, pH 6.4) containing 0.5 g of polyvinyl polypyrrolidone. The homogenate was centrifuged at 5000 rpm for 15 min at 4 °C. The resulting supernatant was filtered and used directly for superoxide dismutase (SOD; EC 1.15.1.1), peroxidase (POD; EC 1.11.1.11), catalase (CAT; EC 1.11.1.6), and polyphenol oxidase (PPO; EC 1.10.3.1) assays.
The SOD activity was measured following the method described by C. Beauchamp [43]. Briefly, the reaction mixture (3 mL) contained 50 mM potassium phosphate buffer (pH 7.8), 3 mM EDTA, 2.25 mM nitro blue tetrazolium chloride (NBT), 200 mM methionine, 50 µL enzyme extract, and 60 µM riboflavin. The reaction mixture was placed under 40-watt fluorescent lamps for 15 min, and the reaction was stopped by switching off the lamp. The development of purple coloration indicated the photoreduction of NBT, and the absorbance was measured at 560 nm. Tubes without the enzyme extract served as a blank control. One unit of SOD activity was defined as the amount of enzyme that inhibited NBT reduction by 50%. Enzyme activity was expressed as units per milligram of protein.
The POD activity was measured as the H2O2-dependent oxidation of ascorbic acid. The reaction mixture contained 25 mM potassium phosphate buffer (pH 7), 0.1 mM EDTA, 1 mM H2O2, 0.25 mM ascorbate, and 50 μL enzyme extract. The enzyme activity was determined using an extinction coefficient of 2800 M/cm. The activity was measured as the decrease in absorbance at 290 nm for 1 min. One unit of POD activity was defined as the amount of enzyme needed for the oxidation of 1 μmol of ascorbate per minute, and the specific activity was expressed as units per milligram of protein [44].
The CAT activity was determined by measuring the decrease in absorbance at 240 nm as a result of the degradation of H2O2. The reaction mixture (3 mL) contained 50 mM potassium phosphate buffer (pH 7.0), 15 mM H2O2, and 50 μL enzyme extract. One unit of CAT activity was defined as the amount of enzyme that catalyzed the decomposition of 1 μmol of H2O2 per minute. The specific activity was calculated using the extinction coefficient of 39,400 M/cm, and the specific activity was expressed as units per milligram of protein [45].
The PPO activity was assayed spectrophotometrically following the procedure of Baik with slight modifications [46]. The reaction solution (3.0 mL) comprised 1 mL of 0.1 M catechol and 2 mL of enzyme extract. The tubes were placed in a 37 °C water bath for 10 min. The reaction was stopped by cooling in an ice bath and the addition of 2 mL of 20% trichloroacetic acid. As the control, 2 mL of phosphoric acid buffer (pH 7.8), 1 mL of 0.1 M catechol, and 2 mL of 20% trichloroacetic acid were used. The enzyme activity was defined as ΔAbs420 per gram FW of sample per minute. One unit of enzyme activity was expressed as the change in absorbance per minute.
Root dehydrogenase activity was determined using the triphenyl tetrazolium chloride (TTC) method [47]. Fresh root tissue (0.2 ± 0.05 g) was immersed in 10 mL of 0.067 M phosphate buffer solution containing 0.4% (w/v) TTC and incubated in the dark for 3 h at 37 °C. Next, 2 mL of 1 M H2SO4 was added. The roots were then dried and extracted with ethyl acetate. The volume of the extract was increased to 5 mL by the addition of ethyl acetate. The absorbance of the extract was measured at 485 nm. Root dehydrogenase activity was calculated using the following equation:
The amylase activity was measured using the 3,5-dinitrosalicylic acid method [48]. Three seedlings were weighed and placed in a mortar containing 5 mL of phosphate buffer solution (pH 7.5) and ground to a homogenate in an ice bath. The homogenate was collected in a centrifuge tube and centrifuged for 30 min at 10,000 rpm [49]. The supernatant represented the total amylase solution. The absorbance was measured at 540 nm. A standard curve was prepared using maltose. With regard to amylase activity, one unit of enzyme activity was defined as the amount of enzyme that produced 1 μmol of reduced sugar per minute under these reaction conditions.
The glutamate decarboxylase (GAD; EC 4.1.1.15) activity was assayed spectrophotometrically using the procedure of Xu with slight modification [50]. The substrate solution comprised 0.2 M phosphate buffer (pH 5.8) containing 0.4 mM pyridoxal phosphate and 10 mM L-monosodium glutamate. The reaction mixture consisted of 200 µL of substrate solution, 100 µL of distilled water, and 100 µL of enzyme extract prepared from seedlings 6 days after seed germination and was incubated in a water bath at 37 °C for 30 min. The enzymatic reaction was terminated by immersion in ice-cold water and the addition of 200 µL of 0.2 M borate buffer (pH 9.0), then 0.2 mL of a 6% phenol solution, and 200 µL of sodium hypochlorite. Color development was conducted in boiling water for 5 min, then stopped by immediate transfer to an ice-cold water bath for 5 min. When blue-green coloration appeared in the solution, 2 mL of 60% ethanol was added. The optical density was read at 645 nm. The amount of GABA produced was calculated using a standard curve. In terms of GAD enzyme activity, one unit of enzyme activity was defined as the amount of enzyme that produced 1 μmol of γ-aminobutyric acid per minute under these reaction conditions.
2.5. Statistical Analysis
The germination experiments were performed in four replications, with each replication comprising 50 seeds. The biochemical analyses and enzyme assays were conducted with three replications. The results were analyzed using IBM SPSS Statistics for Windows (version 19). The data presented in the figures are the means of three replications. The statistical significance of differences between means was determined by performing a one-way analysis of variance followed by Duncan’s multiple range test at the p = 0.05 significance level.
3. Results
3.1. Neodymium Nitrate Concentration Affects Vigor Recovery in Aged Seeds
Soaking seeds in neodymium nitrate solution significantly affected seed germination, especially at concentrations of 20–60 µM (Figure 1). The effects of neodymium nitrate treatment on seed germination were observed after treatment for 4 days. In comparison with the 0 µM treatment, the GP4 of wheat seeds soaked in 20, 30, and 60 µM solutions was respectively increased by 45.3%, 41.8%, and 37.1%; the GI was respectively increased by 33.16, 30.17, and 26.02; and the seed VI was respectively increased by 9.88, 4.96, and 4.61 after treatment for 7 days. A significant difference was observed between the soaked seeds and the controls (0 µM vs. 20, 30, and 60 µM) (p = 0.05). However, the effects of neodymium nitrate at 20, 30, and 60 µM also differed. It should be noted that the GP4, GI, and VI of the seeds soaked in 20 µM neodymium nitrate were superior to the remainder of the treatments. Taken together, 20 µM neodymium nitrate was selected as the optimal treatment for subsequent experiments.

Figure 1.
Effect of neodymium nitrate on the germination of wheat seeds. The horizontal axis is the concentration of neodymium nitrate solution, and the vertical axis is the germination potential (GP4; %), germination index (GI), or vigor index (VI) of wheat seeds soaked in neodymium nitrate solutions of different concentrations (0, 5, 15, 20, 30, 60, 100, 150, or 200 μM) for 8 h. Neodymium was supplied as Nd (NO3)3∙6H2O. The GP was determined on the fourth day after the start of the experiment, whereas the GI and VI were determined on the seventh day. The bars and error bars indicate the mean ± SD of four independent replicates, with each replicate comprising 50 seeds. Bars with different lowercase letters indicate a statistical difference among treatments (Duncan’s multiple range test, p = 0.05).
3.2. Effects of Neodymium on Seed Germination and Seedling Biochemical Components
The 20 µM neodymium nitrate soaking treatment significantly affected the GP4, GI, and VI of seeds from three wheat cultivars (Table 1). With regard to the seed germination parameters of the control, no significant difference in GP4 was observed among the three cultivars, and no significant difference in GI was detected between AK58 and BN207, but BN4199 differed significantly from the other cultivars. The VI differed significantly among the three cultivars, of which BN207 was the cultivar with the highest seed vigor. With consideration of differences between the soaked seeds and non-soaked seeds for the seed sample types, the GP4, GI, and VI of the control were significantly higher than those of NAT and AAT seeds, indicating that seed aging resulted in a decrease in seed GP4, GI, and VI. Compared with the control, the GP4 of AK58, BN4199, and BN207 decreased by 44.83–47.95%, 32.55–66.27%, and 11.54–83.27%, respectively; the GI decreased by 42.19–52.87%, 13.37–62.38%, and 14.38–83.74, respectively; and the VI decreased by 31.90–43.88%, 28.15–62.88%, and 15.61–84.70%, respectively. In the comparison between the NAT- and AAT-treated seeds, the germination of AAT seeds was more strongly affected. Soaking the seeds in a 20 µM neodymium nitrate solution for 8 h had a notable effect on seed germination. The GP4 of AK58, BN4199, and BN207 increased by 2.25–8.48%, 17.7–23.3%, and 14.6–60.9%, respectively. The GI of AK58, BN4199, and BN207 was increased by 1.69–5.95%, 11.8–29.2%, and 7.85–25.5%, respectively. The VI of AK58, BN4199, and BN207 was increased by 3.36–9.59%, 16.7–18.7%, and 10.6–14.5%, respectively.

Table 1.
Effects of seed treatments on germination percentage (GP4), germination index (GI), vigor index (VI), and the contents of chlorophyll a (Ca), chlorophyll b (Cb), and total carotenoids (Cc).
After AAT treatment, the effects of soaking in neodymium nitrate solution on chlorophyll and carotenoid contents, soluble sugar contents, soluble protein contents, and MDA contents were assessed on the seventh day of seed germination. The chlorophyll and carotenoid contents in the leaves of the three cultivars were increased in both NATS and AATS seeds. However, a significant difference in the increase in chlorophyll and carotenoid contents between NATS and AATS seeds was detected. The increase in chlorophyll a content of AK58, BN4199, and BN207 seeds soaked in neodymium nitrate solution was 13.64–14.99%, 3.78–5.36%, and 3.92–4.14%, respectively; the increase in chlorophyll b content of AK58, BN4199, and BN207 was 9.87–26.92%, 0.29–5.50%, and 7.54–9.22%, respectively; and the increase in carotenoid content of AK58, BN4199, and BN207 was 9.98–22.13%, 2.10–10.40%, and 1.89–10.97%, respectively.
Both NAT and AAT had significant effects on the contents of soluble sugars in seedlings emerging from aged seeds. No significant difference in soluble sugar content was observed among the controls of the three cultivars (Table 1). However, for a single material, the soluble sugar contents during seed germination of NAT and AAT seeds were significantly lower than those of the controls. With regard to NATS and AATS seeds, soaking in a 20 µM neodymium nitrate solution increased the soluble sugar contents of seedlings, but the degree of increase differed among the cultivars. The increase in soluble sugar content of AK58, BN4199, and BN207 after soaking was 82.16–93.47%, 7.19–55.11%, and 27.33–71.69%, respectively. With respect to soluble proteins, the trends were similar to those observed for soluble sugars among cultivars and within cultivars. Among NATS and AATS seeds, 20 µM neodymium nitrate soaking increased the soluble protein content in seedlings, but the degree of increases differed among the cultivars. The increases in soluble protein contents of AK58, BN4199, and BN207 after soaking were 8.21–32.57%, 5.19–37.50%, and 5.14–30.01%, respectively.
To further evaluate the effects of soaking on aging-related damage, the change in MDA content was evaluated. No significant difference in MDA content was detected among the cultivar controls. For an individual cultivar, aging caused an increase in MDA content. The content of MDA differed significantly between BN4199 and BN207. The contents of MDA were significantly reduced by soaking the seeds in 20 µM of neodymium nitrate solution. After soaking, the MDA contents of AK58, BN4199, and BN207 decreased by 19.43–28.50%, 26.63–31.71%, and 29.07–32.13%, respectively.
The SOD activity of seeds soaked in neodymium nitrate solution was measured (Figure 2A). The SOD activity of AK58 seeds was significantly different from that of the other cultivars, but no significant difference between BN4199 and BN207 was detected. For a single cultivar, seed aging resulted in a decrease in SOD activity. Compared with that of the control, the SOD activity of AK58, BN4199, and BN207 decreased to 45.84–48.83%, 12.75–29.53%, and 15.06–33.15%, respectively. Soaking the seeds significantly increased the SOD activity compared with that of the non-soaked seeds. The increase in SOD activity in soaked seeds of AK58, BN4199, and BN207 was 16.65–39.90%, 5.75–20.50%, and 10.54–11.67%, respectively.
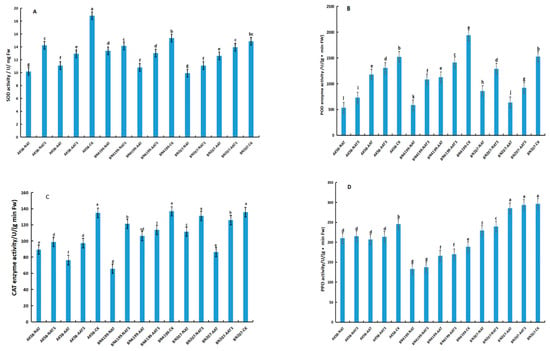

Figure 2.
Effect of neodymium nitrate on the enzyme activity of wheat seedlings. (A): superoxide dismutase (SOD) activity; (B): peroxidase (POD) activity; (C): catalase (CAT) activity; (D): polyphenol oxidase (PPO) activity; (E): root activity; (F): amylase activity; (G): glutamate decarboxylase (GAD) activity. Bars and error bars indicate the mean ± SD. Different lowercase letters above bars indicate a significant difference. The difference between means was compared using one-way analysis of variance and Duncan’s test (p = 0.05).
The POD activity of seeds soaked in neodymium nitrate solution was measured (Figure 2B). Significant differences in POD activity were observed among the controls. The POD activity of BN4199 was higher than that of AK58 or BN207. For a single cultivar, seed aging resulted in a decrease in POD activity. Compared with the control, the POD activity of AK58, BN4199, and BN207 decreased to 22.64–64.98%, 41.94–69.73%, and 43.76–58.29%, respectively. The POD activity of soaked seeds was significantly higher than that of non-soaked seeds. The increase in POD activity in soaked seeds of AK58, BN4199, and BN207 was 10.88–36.77%, 25.6–84.67%, and 44.65–50.14%, respectively.
The CAT activity of the seedlings that emerged from NAT and AAT wheat seeds was measured (Figure 2C). No significant difference in CAT activity was detected among the controls. For a single cultivar, both NAT and AAT treatments resulted in a decrease in CAT activity. Soaking the aged seeds in a neodymium nitrate solution significantly affected CAT activity. Compared with the controls, the CAT activities after soaking seeds of AK58, BN4199, and BN207 decreased to 33.76–43.52%, 22.30–51.96%, and 11.79–36.37%, respectively, but neodymium nitrate soaking caused an increase in CAT activity in the 7-day-old seedlings. The degree of increase in CAT activity after seed soaking differed among the cultivars. The increase in CAT activity of AK58, BN4199, and BN207 after seed soaking was 10.71–27.79%, 7.04–84.42%, and 17.53–45.70%, respectively.
The PPO activity of seeds soaked in neodymium nitrate solution was measured (Figure 2D). The PPO activity differed significantly among cultivars. The PPO activity of BN207 was the highest and was 1.57 times that of BN4199 and 1.2 times that of AK58. For a single cultivar, significant differences between the aged and control seeds were detected. Compared with the control, the PPO activity of AK58, BN4199, and BN207 decreased to 15.80–45.84%, 11.86–29.57%, and 3.78–22.54%, respectively. Although soaked seeds showed an increase in PPO activity, no significant difference between the soaked and non-soaked seeds was detected. The increase in PPO activity of AK58, BN4199, and BN207 after seed soaking was 2.38–3.40%, 2.41–3.78%, and 2.81–4.37%, respectively.
The effects of neodymium nitrate soaking on root dehydrogenase activity (as measured by TTC-reducing capacity) of the different materials were analyzed (Figure 2E). The root dehydrogenase activity varied significantly (p = 0.05) among the soaked seed materials. Significant differences in root dehydrogenase activity were observed among the controls, whereas no significant difference was detected between AK58 and BN207, but a significant difference was observed between AK58 and BN4199. Seed aging led to a decrease in root dehydrogenase activity in seedlings. In all three cultivars, the decrease in enzyme activity in seedlings emerging from NAT seeds was greater than that in seedlings emerging from AAT seeds. For a single cultivar, when soaked seeds were compared with the control, the NAT root dehydrogenase activity of AK58, BN4199, and BN207 decreased by 63.12%, 54.80%, and 64.87%, respectively, and the AAT root dehydrogenase activity of AK58, BN4199, and BN207 decreased by 38.98%, 5.89%, and 16.65%, respectively. For a single cultivar, the root dehydrogenase activity of seedlings from both NATS and AATS seeds was significantly improved after soaking the seeds. The increase in root dehydrogenase activity of AK58, BN4199, and BN207 in seedlings emerging from soaked seeds was 14.48–27.62%, 9.64–51.88%, and 40.67–102.4%, respectively.
The changes in amylase activity during wheat seed germination after soaking in a neodymium nitrate solution are shown in Figure 2F. No difference in amylase activity was observed among the controls. For a single cultivar, both NAT and AAT seeds showed a decrease in amylase activity during germination. Compared with the control, the amylase activities of AK58, BN4199, and BN207 decreased to 10.24–36.22%, 3.34–47.98%, and 4.13–46.86%, respectively. Significant differences in amylase activity were detected between the soaked and non-soaked seeds. For all three cultivars, the decrease in amylase activity induced by NAT was less than that caused by AAT. Compared with the control, the NAT amylase activities of AK58, BN4199, and BN207 decreased by 10.25%, 3.34%, and 4.12%, respectively; the AAT amylase activities of AK58, BN4199, and BN207 decreased by 36.22%, 48.68%, and 46.86%, respectively. The enzyme activities of AK58 and BN4199 were significantly higher than those of the control with NATS. The activity of amylase was increased in aged seeds after soaking in a neodymium nitrate solution. The increases in amylase activity in the soaked seeds of AK58, BN4199, and BN207 were 16.07–36.02%, 21.31–58.90%, and 4.12–6.76%, respectively.
The changes in GAD activity during the germination of seeds soaked in a neodymium nitrate solution are shown in Figure 2G. The GAD activity differed significantly among the three controls. Compared with the control, seed aging led to a decrease in GAD activity. For a single cultivar, the GAD activities of AK58, BN4199, and BN207 decreased to 30.66–33.12%, 31.57–45.02%, and 10.84–16.74%, respectively. Comparison of the soaked and non-soaked seeds showed that neodymium nitrate soaking significantly increased GAD activity. The increases in GAD activity in soaked seeds of AK58, BN4199, and BN207 were 13.79–32.30%, 6.72–64.00%, and 10.87–17.46%, respectively.
4. Discussion
Seed germination is the initial stage of plant growth and is readily affected by diverse factors [51]. During seed storage, high humidity and temperature increase the rate of seed aging and deterioration, resulting in a loss of seed vigor and vitality, which limits the utility of the seeds in agricultural production [52]. The aging of seeds not only leads to a reduction in the mobilization of substances during seed germination but also may enhance oxidative stress, resulting in the production of superoxide anion free radicals in the cells, which directly cause an imbalance in the intracellular environment [53,54]. However, exogenous biological and abiotic factors may improve the germination rate of seeds [55]. Therefore, seed treatments may be useful to enhance seed germination after aging and may help to regulate changes in reserve and enzyme metabolism in seeds during germination [56].
At low doses, neodymium can have a stimulatory effect on plant development [57,58]. In the present study, we assessed the effects of neodymium nitrate on the germination of aged seeds of three wheat cultivars that had been stored naturally for 3 years or artificially aged for 4 days. The current-year-harvested seeds served as the control. Rare earth elements can be absorbed by soaking seeds and thus affect seed germination [36]. The present results revealed that suitable concentrations of neodymium stimulated the germination of aged wheat seeds and enhanced the GP4 of the seeds. However, high concentrations of neodymium were toxic to seed germination and resulted in a reduction in GP4, GI, and VI (Figure 1). Compared with the control, soaking aged wheat seeds in a 20 µM neodymium nitrate solution for 8 h at room temperature optimally increased the seed GP4, GI, and VI.
In an earlier study conducted to evaluate ecological risk, neodymium at a relatively low dose (100 mg/kg) was non-toxic to plants (Brassica chinensis L. and Helianthus annuus L.) [58]. In the current study, we used a considerably lower dose (20 µmol/L) that was not toxic to plants. According to a previous investigation, neodymium concentrations in non-contaminated soils range from 5.8 to 53 mg/kg [59]. The concentration of an individual rare earth element released into the environment should not exceed 10 mg/L [GB26451-2011 Emission Standards for Rare Earth Industry Pollutants (China)] [60]. The recommended neodymium nitrate dose for soaking wheat seeds is 20 µmol/L (8.76 mg/L), which is within the normal range of rare earth element concentrations in non-polluted regions and lower than the industrial pollution emission standards. Therefore, the neodymium nitrate dose used in the present study was insufficient to pollute the environment.
Seed aging is a complex process that involves changes in biochemical components and antioxidant systems to limit lipid peroxidation, remove reactive oxygen species, and repair disrupted mechanisms. Aging results in a decrease in the GP and vigor of seeds [52,61]. In the present study, germination was significantly inhibited in both naturally aged and artificially aged wheat seeds. Regardless of the seed aging treatment, the seed germination indices for the three wheat cultivars decreased significantly compared with those of the controls. In contrast, irrespective of whether soaked seeds were naturally or artificially aged, the seed germination indices were increased by soaking in a 20 µM neodymium nitrate solution. Among the three cultivars, no significant difference in the increase in the three seed germination indices was observed between the soaked and non-soaked seeds of AK58. For BN4199, the GI of NAT and NATS seeds and the VI of AAT and AATS seeds differed significantly between the soaked and the non-soaked seeds. With regard to BN207, the VI compared between NAT and NATS seeds and between AAT and AATS seeds showed no significant difference, whereas the GP4 and GI differed significantly between the soaked and non-soaked seeds. Considering the AATS seeds of the three cultivars, BN207 showed the smallest GP4, GI, and VI for both the soaked and non-soaked seeds, which indicated that the GP4, GI, and VI of BN207 seeds decreased more severely under high temperature and high humidity, and thus that BN207 seeds suffered greater damage and weaker recovery. Comparing the three cultivars, the degree of increase in GP4, GI, and VI of soaked seeds differed among the cultivars; the cultivars were ranked in decreasing order as follows: BN4199 > BN207 > AK58. These results indicated that the cultivars differed in the anti-aging capability of the seeds. Although BN207 seeds showed favorable resistance to natural aging, this cultivar showed the worst resistance to artificial aging. Thus, BN207 seeds could withstand mild natural aging but not severe short-term high temperatures and high humidity. In contrast, BN4199 seeds showed the best capability for aging-damage recovery in the present experiment.
Changes in the biochemical components in seeds of many crop species, such as soluble sugars, fatty acids, tocopherol, H2O2, and MDA contents, coincident with seed aging have been reported [52,53]. Previous experiments directly measured the changes in biochemical parameters of aged seeds but did not measure the corresponding changes in seedlings that germinated from aged seeds. In the present experiment, biochemical changes were observed in 7-day-old seedlings that developed from aged seeds. We observed the effects of neodymium nitrate soaking on the soluble sugar, soluble protein, and MDA contents of the seedlings and analyzed the effects on the chlorophyll and carotenoid contents of the 7-day-old seedlings. Previous results have suggested that soluble sugars and soluble proteins in seeds can increase the stability of the internal environment of the seed, prolong seed longevity, and improve the percentage of seed germination [62,63]. The present results showed that seed aging led to decreases in soluble sugar and soluble protein contents and increases in MDA content in seedlings during seed germination. These findings indicated that aging caused disruption of the internal environmental balance of the seed, and these changes persisted during seed germination. Soaking aged seeds in a 20 µM neodymium nitrate solution effectively increased the contents of soluble sugars and soluble proteins and decreased the MDA content in 7-day-old seedlings (Table 1), which indicated that soaking improved the internal environmental stability of the seedlings.
Singh reported that seed aging leads to a decline in chlorophyll accumulation in soybean cotyledons [64]. In the present experiment, the photosynthetic pigment content of 7-day-old seedlings emerging from aged seeds decreased slightly compared with that of the control, but the difference was not significant. This conclusion was consistent with that of Singh. Further analysis of the effects of soaking wheat seeds in a 20 µM neodymium nitrate solution showed that soaking increased the chlorophyll and carotenoid contents in the leaves of 7-day-old seedlings. Although the degree of increase in response to soaking differed among the aged materials, the trend to increase was consistent.
Previous studies have suggested that the chlorophyll content in seeds enhances seed oxidative stress induced by abiotic stress factors. Carotenoids are antioxidants that protect seeds from oxidative stress [65,66]. After the aged wheat seeds were soaked in neodymium nitrate solution, the contents of chlorophyll and carotenoids in the 7-day-old seedlings that developed from the soaked seeds increased to different degrees compared with those of seedlings derived from non-soaked seeds. These results indicated that neodymium nitrate-impregnated seeds showed improved ability to resist oxidative stress by increasing the contents of photosynthetic pigments.
It has been reported that the mitochondrial ascorbic acid-glutathione cycle activity of seeds is weakened with aging [67], leading to the accumulation of reactive oxygen species, which in turn leads to changes in the internal environment of the seed. As a result, enzymes in the seeds are deactivated, and the germinability of the seed is gradually lost. Seed aging reduces the activities of enzymes associated with seed germination, such as SOD, CAT, and POD, thereby hindering germination [68]. The present experiment revealed that SOD, CAT, and POD activities decreased in aged wheat seeds. Regardless of whether aging was natural or artificial, we were especially interested in whether the activities of these antioxidant-protective enzymes could be restored. Among the three cultivars, SOD, CAT, and POD activities were increased by soaking in neodymium nitrate solution in the ranges of 5.75–39.90%, 7.04–84.42%, and 10.88–84.67%, respectively (Figure 2). In wheat seeds, PPO is an important enzyme that mainly functions in relation to disease resistance but also influences flour color [69,70]. The role of PPO in wheat seed germination has not been reported previously. We evaluated the changes in PPO activity during the germination of aged wheat seeds. The results showed that the PPO activity differed significantly among the three cultivars, which were ranked in decreasing order as follows: BN207 > BN4199 > AK58. The PPO activity decreased with seed aging, but the recovery of activity after soaking in a neodymium nitrate solution was small and not significantly improved.
Wheat seeds are rich in starch, and starch absorption and reuse during seed germination are associated with amylase activity. The aging of wheat seeds decreases the activity of amylase, and amylase activities are directly related to seed germination rate [71,72,73]. In the present experiment, aging led to the decline of amylase activity, and the decline under natural aging was significantly less than that under artificial aging, which indicated that the amylase decline was associated with the severity of aging. Soaking the seeds in a neodymium nitrate solution had a restorative effect on amylase activity. Amylase activity decreased to a lesser extent and recovered to a greater degree in the NAT and NATS materials, whereas amylase activity was more severely damaged under AAT (Figure 2F). The effect of soaking AK58 and BN4199 seeds on amylase activity exceeded that of the control, whereas the activity in soaked BN207 seeds was similar to that of the control.
The activity of root dehydrogenase reflects the vigor of the roots. Seed dehydrogenase has been used as an index to evaluate seed viability [74]. In the current experiment, for the first time, the activity of root dehydrogenase in 7-day-old seedlings was used to evaluate the viability of aged wheat seeds. Compared with the control, the root dehydrogenase activity of the 7-day-old seedlings decreased significantly, indicating that seed aging also resulted in a decrease in seedling dehydrogenase activity. For a single cultivar, the decline in root dehydrogenase activity under NAT was greater than that under AAT, which indicated that longer-term NAT had a more severe effect on seedling root vitality than short-term AAT. Neodymium nitrate soaking had a significant effect on the improvement of root dehydrogenase activity in the three cultivars; in particular, the activity of BN4199 and BN207 under AATS was higher than that of the controls, indicating that neodymium nitrate soaking had a strong effect on stimulating enzyme activity.
The activity reduction data for several enzymes under the two aging modes were analyzed. The data showed that, compared with that under AAT, dehydrogenase activity was more severely decreased under the NAT mode, whereas amylase activity showed the opposite response. The difference in the responses of dehydrogenase and amylase to the two aging modes may reflect the differential resistance of enzymes to long-term mild seed aging and short-term severe environmental aging.
Al-Quraan (2019) reported that GABA is accumulated in response to biotic and abiotic stresses, including senescence [75]. Recent studies of the GABA shunt pathway have shown that its function is required for proper growth in response to abiotic stresses [76]. GABA is the product of glutamate decarboxylase, and thus its abundance reflects the activity of GAD [77]. Xu reported that GABA metabolism is a novel mechanism to improve plant stress resistance [78]. The present experimental results showed that the GAD activity of 7-day-old seedlings decreased compared with that of the control. Significant differences in GAD activity were observed among the three cultivars, indicating that GAD showed cultivar specificity. Both NAT and AAT resulted in a significant decline in GAD activity (Figure 2G). Neodymium nitrate soaking had a strongly stimulatory effect, which resulted in a significant increase in GAD activity. However, the recovery of GAD activity was not greater than that of the control.
5. Conclusions
Both natural and artificial aging resulted in a decrease in the GP, GI, and VI of wheat seeds. Artificial aging exerted a stronger effect than natural aging. Neodymium nitrate soaking promoted the germination of aged wheat seeds, but a high concentration inhibited seed germination. The GP, GI, and VI were improved by a suitable concentration of neodymium nitrate. The results of the present experiment showed that 20 µM neodymium nitrate was the optimal concentration for germination of aged wheat seeds.
The present experimental results showed that, during the germination of aged wheat seeds, soaking in a 20 µM neodymium nitrate solution not only affected the contents of biochemical substances associated with stress resistance in the seedlings but also affected the activity of important enzymes involved in seed germination. Seed aging resulted in decreased soluble sugar, soluble protein, chlorophyll, and carotenoid contents and increased MDA content in 7-day-old seedlings. Seeds soaked in a 20 µM neodymium nitrate solution showed increased contents of soluble sugars, soluble proteins, chlorophyll, and carotenoids and decreased MDA content, enhancing the recovery of the vitality of aged seeds. Of the two aging methods, artificial aging resulted in more severe damage to the seeds than natural aging with regard to changes in biochemical substance contents. With regard to the degree of recovery after soaking in neodymium nitrate solution, the changes in soluble sugar, soluble protein, and MDA contents were more highly significant after the soaking of artificially aged seeds.
The enzyme activity required for germination decreased significantly after seed aging. In the present study, we not only reported the activities of SOD, CAT, and POD in aged wheat seeds but also measured PPO, root dehydrogenase, amylase, and GAD activities for the first time. The effect of natural aging on root dehydrogenase activity was more significant, whereas artificial aging had a greater effect on amylase activity. Other enzyme activities monitored showed no obvious pattern between the two aging methods. Neodymium nitrate soaking had a synergistic effect on the activities of several enzymes measured. Among antioxidant protective enzymes, the effects on CAT and POD were significant, whereas the effect on PPO was the weakest observed. Root dehydrogenase, amylase, and GAD are important metabolic enzymes. Seed aging results in decreased activity of these enzymes. Root dehydrogenase and amylase were affected differentially under the two aging modes, indicating that short-term artificial aging and longer-term natural aging have different effects. The soaking of seeds in neodymium nitrate solution had significant effects on root dehydrogenase, amylase, and GAD activities in 7-day-old seedlings, especially on root dehydrogenase and amylase. The novelty of the present study is that the effects of neodymium nitrate treatment on the germination of aged wheat seeds are explored for the first time. The effects of neodymium nitrate on biochemical indicators and enzyme activities in aged seeds and on chlorophyll and carotenoid contents and PPO, amylase, and GAD activities were analyzed for the first time.
As a potential seed treatment, neodymium nitrate applied at an optimal concentration is capable of reviving aged seeds by increasing the contents of soluble sugars, soluble proteins, and photosynthetic pigments; reducing the content of MDA; and increasing the activities of antioxidant protective enzymes and selected metabolic enzymes (root dehydrogenase, amylase, and GAD).
Author Contributions
Conceptualization and methodology, G.H. and X.Z.; software, G.H.; validation, G.H. and X.Z.; investigation, M.C.; resources, Q.Z.; data curation, Y.F.; writing—original draft preparation, G.H.; writing—review and editing, M.C.; visualization, Y.F.; supervision, H.H. and M.C.; project administration, H.H.; funding acquisition, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Major Science and Technology Project of Henan Province, Funder: Henan Provincial Department of Science and Technology. No. 221100110700.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Robert McKenzie for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yin, X.; Lv, G.; Mou, Q.; Mi, Y.; Yin, F.; Li, N.; Qian, Z.; Wu, K. Effects of Different Sowing amounts on yield and dry matter production and transport of ‘Xinmai 296’. Chin. Agric. Sci. Bull. 2022, 38, 1–7. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Melov, S. On the Programmed/Non-Programmed Nature of Ageing within the Life History. Curr. Biol. 2011, 21, R701–R707. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.; Noli, E.; Demir, I.; Khajeh-Hosseini, M.; Wagner, M.-H. Evaluation of seed quality: From physiology to international standardization. Seed Sci. Res. 2012, 22, S69–S73. [Google Scholar] [CrossRef]
- Hu, D.; Ma, G.; Wang, Q.; Yao, J.; Wang, Y.; Pritchard, H.W.; Wang, X. Spatial and temporal nature of reactive oxygen species production and programmed cell death in elm (Ulmus pumila L.) seeds during controlled deterioration. Plant Cell Environ. 2012, 35, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Murthy, U.M.N.; Kumar, P.P.; Sun, W.Q. Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) Wilczek: Lipid peroxidation, sugar hydrolysis, Maillard reactions and their relationship to glass state transition. J. Exp. Bot. 2003, 54, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Siadat, S.A.; Moosavi, A.; Zadeh, M.S. Effects of Seed Priming on Antioxidant Activity and Germination Characteristics of Maize Seeds under Different Ageing Treatment. Res. J. Seed Sci. 2012, 5, 51–62. [Google Scholar] [CrossRef]
- Delouche, J.C.; Baskin, C.C. Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci. Technol. 1973, 1, 427–452. [Google Scholar]
- Zhang, S.-B.; Lv, Y.-Y.; Wang, Y.-L.; Jia, F.; Wang, J.-S.; Hu, Y.-S. Physiochemical changes in wheat of different hardnesses during storage. J. Stored Prod. Res. 2017, 72, 161–165. [Google Scholar] [CrossRef]
- Tian, P.-P.; Lv, Y.-Y.; Yuan, W.-J.; Zhang, S.-B.; Hu, Y.-S. Effect of artificial aging on wheat quality deterioration during storage. J. Stored Prod. Res. 2019, 80, 50–56. [Google Scholar] [CrossRef]
- Calucci, L.; Capocchi, A.; Galleschi, L.; Ghiringhelli, S.; Pinzino, C.; Saviozzi, F.; Zandomeneghi, M. Antioxidants, Free Radicals, Storage Proteins, Puroindolines, and Proteolytic Activities in Bread Wheat (Triticum aestivum) Seeds during Accelerated Aging. J. Agric. Food Chem. 2004, 52, 4274–4281. [Google Scholar] [CrossRef]
- Mira, S.; Pirredda, M.; Martín-Sánchez, M.; Marchessi, J.E.; Martín, C. DNA methylation and integrity in aged seeds and regenerated plants. Seed Sci. Res. 2020, 30, 92–100. [Google Scholar] [CrossRef]
- Kranner, I.; Chen, H.; Pritchard, H.W.; Pearce, S.R.; Birtić, S. Inter-nucleosomal DNA fragmentation and loss of RNA integrity during seed ageing. Plant Growth Regul. 2011, 63, 63–72. [Google Scholar] [CrossRef]
- Ahmed, Z.; Yang, H.; Fu, Y.-B. The Associative Changes in Scutellum Nuclear Content and Morphology with Viability Loss of Naturally Aged and Accelerated Aging Wheat (Triticum aestivum) Seeds. Front. Plant Sci. 2016, 7, 1474. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-B.; Yang, M.-H.; Horbach, C.; Kessler, D.; Diederichsen, A.; You, F.M.; Wang, H. Patterns of SSR variation in bread wheat (Triticum aestivum L.) seeds under ex situ genebank storage and accelerated ageing. Genet. Resour. Crop Evol. 2017, 64, 277–290. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Bellani, L.M.; Salvini, L.; Dell’aquila, A.; Scialabba, A. Reactive oxygen species release, vitamin E, fatty acid and phytosterol contents of artificially aged radish (Raphanus sativus L.) seeds during germination. Acta Physiol. Plant. 2012, 34, 1789–1799. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf-Bouteau, H.; Kranner, I.; Bogatek, R.; Corbineau, F.; Bailly, C. The Mechanisms Involved in Seed Dormancy Alleviation by Hydrogen Cyanide Unravel the Role of Reactive Oxygen Species as Key Factors of Cellular Signaling during Germination. Plant Physiol. 2009, 150, 494–505. [Google Scholar] [CrossRef]
- Suliman, M.S.E.; Elradi, S.B.M.; Zhou, G.; Nimir, N.E.A.; Zhu, G.; Ali, A.Y.A. Seeds primed with 5-aminolevulinic acid mitigated temperature and drought stresses of wheat at germination and early seedling growth. Chil. J. Agric. Res. 2022, 82, 111–123. [Google Scholar] [CrossRef]
- Samarah, N.H.; Al-Quraan, N.A.; Al-Wraikat, B.S. Ultrasonic treatment to enhance seed germination and vigour of wheat (Triticum durum) in association with γ-aminobutyric acid (GABA) shunt pathway. Funct. Plant Biol. 2023, 50, 277–293. [Google Scholar] [CrossRef]
- D’aquino, L.; de Pinto, M.C.; Nardi, L.; Morgana, M.; Tommasi, F. Effect of some light rare earth elements on seed germination, seedling growth and antioxidant metabolism in Triticum durum. Chemosphere 2009, 75, 900–905. [Google Scholar] [CrossRef]
- Rocha, L.; Silva, E.; Pavia, I.; Ferreira, H.; Matos, C.; Osca, J.M.; Moutinho-Pereira, J.; Lima-Brito, J. Seed Soaking with Sodium Selenate as a Biofortification Approach in Bread Wheat: Effects on Germination, Seedling Emergence, Biomass and Responses to Water Deficit. Agronomy 2022, 12, 1975. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Wen, X.-X.; Liao, Y.-C. Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J. Integr. Agric. 2016, 15, 2759–2774. [Google Scholar] [CrossRef]
- Wang, J.; Lv, P.; Yan, D.; Zhang, Z.; Xu, X.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Exogenous Melatonin Improves Seed Germination of Wheat (Triticum aestivum L.) under Salt Stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef] [PubMed]
- Fashui, H.; Ling, W.; Chao, L. Study of Lanthanum on Seed Germination and Growth of Rice. Biol. Trace Elem. Res. 2003, 94, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Fashui, H.; Lei, Z. Effects of rare earth elements on vigor enhancement of aged spinach seeds. J. Rare Earth 2004, 22, 547–551. [Google Scholar] [CrossRef]
- Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; García-Morales, S.; Pérez-Sato, J.A.; Gómez-Merino, F.C. Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS ONE 2018, 13, e0194691. [Google Scholar] [CrossRef] [PubMed]
- Sobarzo-Bernal, O.; Gómez-Merino, F.C.; Alcántar-González, G.; Saucedo-Veloz, C.; Trejo-Téllez, L.I. Biostimulant Effects of Cerium on Seed Germination and Initial Growth of Tomato Seedlings. Agronomy 2021, 11, 1525. [Google Scholar] [CrossRef]
- Song, K.; Gao, J.; Li, S.; Sun, Y.; Sun, H.; An, B.; Hu, T.; He, X. Experimental and Theoretical Study of the Effects of Rare Earth Elements on Growth and Chlorophyll of Alfalfa (Medicago sativa L.) Seedling. Front. Plant Sci. 2021, 12, 731838. [Google Scholar] [CrossRef]
- Fashui, H. Study on the Mechanism of Cerium Nitrate Effects on Germination of Aged Rice Seed. Biol. Trace Elem. Res. 2002, 87, 191–200. [Google Scholar] [CrossRef]
- Gudasi, K.B.; Shenoy, R.V.; Vadavi, R.S.; Patil, M.S.; Patil, S.A.; Hanchinal, R.R.; Desai, S.A.; Lohithaswa, H. Lanthanide(III) and Yttrium(III) Complexes of Benzimidazole-2-Acetic Acid: Synthesis, Characterisation and Effect of La(III) Complex on Germination of Wheat. Bioinorg. Chem. Appl. 2006, 2006, 075612. [Google Scholar] [CrossRef]
- Lu, C.H.; Niu, H.J.; Qi, F.; Liu, M.L.; Sun, H. Effects of cerium on physiological Parameters of wheat seed germination under salt stress. Agric. Sci. Equip. 2014, 8–9, 11. [Google Scholar] [CrossRef]
- Guo, B.S.; Zhu, W.M.; Xiong, B.K. Rare Earths in Agriculture; China Agricultural Science and Technology Press: Beijing, China, 1988; pp. 1–22, 45–202. (In Chinese) [Google Scholar]
- Ni, J.Z. Bioinorganic Chemistry of Rare Earth Elements; Science Press: Beijing, China, 1995; pp. 13–37. (In Chinese) [Google Scholar]
- Ning, J.B. Application of Rare Earths in Agriculture; Hunan Science and Technology Press: Changsha, China, 1988; pp. 94–98. (In Chinese) [Google Scholar]
- Akbari, G.A.; Heshmati, S.; Soltani, E.; Dehaghi, M.A. Influence of Seed Priming on Seed Yield, Oil Content and Fatty Acid Composition of Safflower (Carthamus tinctorius L.) Grown Under Water Deficit. Int. J. Plant Prod. 2020, 14, 245–258. [Google Scholar] [CrossRef]
- Matthews, S.; Powell, A.A.; Perry, D.A.; Hampton, J.G.; Tekrony, D.M.; Tekrony, D.; Powellrichards, A.; Matheus, S.; Carvalho, N.M. Handbook for Vigour Test Methods, 3rd ed.; The ISTA Vigour Test Committee: Wallisellen, Switzerland, 1995. [Google Scholar]
- Wang, J.Q.; Yang, Z.R.; Zhang, F.L.; Zhang, X.Y.; Zhang, D.; Song, Y.J. Effects of different treatments of Rare Earth Elements on the vigor and physiological and biochemical characteristics of allium mongolicum regel seeds. North. Hortic. 2021, 20, 11–17. [Google Scholar]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Sumanta, N.; Choudhury, I.; Haque Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxi-dation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Baik, B.-K.; Czuchajowska, Z.; Pomeranz, Y. Comparison of Polyphenol Oxidase Activities in Wheats and Flours from Australian and U.S. Cultivars. J. Cereal Sci. 1994, 19, 291–296. [Google Scholar] [CrossRef]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Ben Elarbi, M.; Khemiri, H.; Jridi, T.; Ben Hamida, J. Purification and characterization of α-amylase from safflower (Carthamus tinctorius L.) germinating seeds. Comptes Rendus Biol. 2009, 332, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Hu, Q.-P. Effect of Bacillus subtilis QM3 on β-amylase Isoenzyme in Early Germination of Wheat Seed. South Asian J. Res. Microbiol. 2020, 6, 24–32. [Google Scholar] [CrossRef]
- Xu, J.-J.; Jiang, B.; Xu, S.-Y. Rapid determination of glutamate decarboxylase activity from lactic acid bacteria by spectrometric method and its application. Bull. Microbiol. 2004, 31, 66–71. [Google Scholar] [CrossRef]
- Rifna, E.; Ramanan, K.R.; Mahendran, R. Emerging technology applications for improving seed germination. Trends Food Sci. Technol. 2019, 86, 95–108. [Google Scholar] [CrossRef]
- Probert, R.; Adams, J.; Coneybeer, J.; Crawford, A.; Hay, F. Seed quality for conservation is critically affected by pre-storage factors. Aust. J. Bot. 2007, 55, 326–335. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Zhao, S.; Yang, C.; Meng, Y.; Shuai, H.; Luo, X.; Dai, Y.; Yin, H.; Du, J.; et al. DA-6 promotes germination and seedling establishment from aged soybean seeds by mediating fatty acid metabolism and glycometabolism. J. Exp. Bot. 2019, 70, 101–114. [Google Scholar] [CrossRef]
- Xia, F.; Cheng, H.; Chen, L.; Zhu, H.; Mao, P.; Wang, M. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef]
- Sheng, Y.; Xiao, H.; Guo, C.; Wu, H.; Wang, X. Effects of exogenous gamma-aminobutyric acid on α-amylase activity in the aleurone of barley seeds. Plant Physiol. Biochem. 2018, 127, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.A.; Laware, S.L. Seed Priming A Critical Review. Int. J. Sci. Res. Biol. Sci. 2018, 5, 94–101. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J.; Wang, Y. Changes in endogenous hormone levels and redox status during enhanced adventitious rooting by rare earth element neodymium of Dendrobium densiflorum shoot cuttings. J. Rare Earths 2008, 26, 869–874. [Google Scholar] [CrossRef]
- Rezaee, A.; Hale, B.; Santos, R.M.; Chiang, Y.W. Accumulation and toxicity of lanthanum and neodymium in horticultural plants (Brassica chinensis L. and Helianthus annuus L.). Can. J. Chem. Eng. 2018, 96, 2263–2272. [Google Scholar] [CrossRef]
- Wang, L.; Christakos, G.; Wu, C.; Wu, J. Spatial variability assessment of La and Nd concentrations in coastal China soils following 1000 years of land reclamation. J. Soils Sediments 2020, 20, 1651–1661. [Google Scholar] [CrossRef]
- The Ministry of Environmental Protection and the State Administration of Quality Supervision and Inspection. GB26451-2011; Emission Standards for Rare Earth Industry Pollutants. China Environmental Science Press: Beijing, China, 2011; pp. 4–9.
- Tonguç, M.; Güler, M.; Önder, S. Germination, reserve metabolism and antioxidant enzyme activities in safflower as affected by seed treatments after accelerated aging. S. Afr. J. Bot. 2023, 153, 209–218. [Google Scholar] [CrossRef]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E Is Essential for Seed Longevity and for Preventing Lipid Peroxidation during Germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moumen, A.B.; Masouri, F.; Richard, G.; Fauconnier, M.L.; Sindic, M.; Nabloussi, A.; Elamrani, A.; Caid, H.S. Variations in the phytosterol and tocopherol compositions and the oxidative stability in seed oils from four safflower (Carthamus tinctorius L.) varieties grown in north-eastern Morocco. Int. J. Food Sci. Technol. 2015, 50, 2264–2270. [Google Scholar] [CrossRef]
- Singh, B.; Amritphale, D. Effect of seed aging on chlorophyll(ide) accumulation and Hill activity in greening soybean seedling cotyledons. Photosynthetica 1992, 26, 455–459. [Google Scholar]
- Smolikova, G.N.; Laman, N.A.; Boriskevich, O.V. Role of chlorophylls and carotenoids in seed tolerance to abiotic stressors. Russ. J. Plant Physiol. 2011, 58, 965–973. [Google Scholar] [CrossRef]
- Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Tian, Q.; Yin, G.; Chen, X.; Zhang, J.; Ng, S.; Lu, X. Reduced mitochondrial and ascorbate–glutathione activity after artificial ageing in soybean seed. J. Plant Physiol. 2014, 171, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yalamalle, V.; Ithape, D.; Kumar, A.; Bhagat, K.; Ghosh, S.; Singh, M. Seed treatment with 5-azacytidine reduces ageing-induced damage in onion seeds. Seed Sci. Technol. 2020, 48, 407–412. [Google Scholar] [CrossRef]
- Cabas-Lühmann, P.A.; Manthey, F.A.; Elias, E.M. Variations of colour, polyphenol oxidase and peroxidase activities during the production of low temperature dried pasta in various durum wheat genotypes. Int. J. Food Sci. Technol. 2021, 56, 4700–4709. [Google Scholar] [CrossRef]
- Raj, S.N.; Sarosh, B.R.; Shetty, H.S. Induction and accumulation of polyphenol oxidase activities as implicated in development of resistance against pearl millet downy mildew disease. Funct. Plant Biol. 2006, 33, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, S.; Sen-Mandi, S. Effects of ageing on amylase activity and scutellar cell structure during imbibition in wheat seed. Ann. Bot. 1993, 71, 411–416. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Deswal, D.P.; Dahiya, O.S.; Punia, R.C. Change in storage enzymes activities in natural and accelerated aged seed of wheat (Triticum aestivum). Indian J. Agric. Sci. 2011, 81, 1037–1040. [Google Scholar]
- Hu, Q.-P.; Guo, J.; Liu, J.-J. Wheat seed germination based on α-amylase activity to study promoting mechanism of Bacillus subtilis QM3. J. Seed Sci. 2022, 44, e202244039. [Google Scholar] [CrossRef]
- Sharma, S.; Punia, R.C.; Singh, V.; Mor, V.; Tanwar, H. Genetic Divergence Analysis Based on Seed Vigour Parameters in Wheat. Seed Res. 2017, 45, 105–110. [Google Scholar]
- AL-Quraan, N.; Al-Ajlouni, Z.; Obedat, D.I. The GABA shunt pathway in germinating seeds of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) under salt stress. Seed Sci. Res. 2019, 29, 250–260. [Google Scholar] [CrossRef]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging Roles of γ Aminobutyric Acid (GABA) Gated Channels in Plant Stress Tolerance. Plants 2021, 10, 2178. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Samarah, N.H.; Tanash, A.A. Effect of drought stress on wheat (Triticum durum) growth and metabolism: Insight from GABA shunt, reactive oxygen species and dehydrin genes expression. Funct. Plant Biol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).