Multiple Insecticide Resistance and Associated Metabolic-Based Mechanisms in a Myzus Persicae (Sulzer) Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Pesticides, Synergists, and Other Chemicals
2.3. Bioassay Methods
2.4. Resistance Stability
2.5. Synergism Experiment
2.6. Detoxification Enzyme Activity Assays
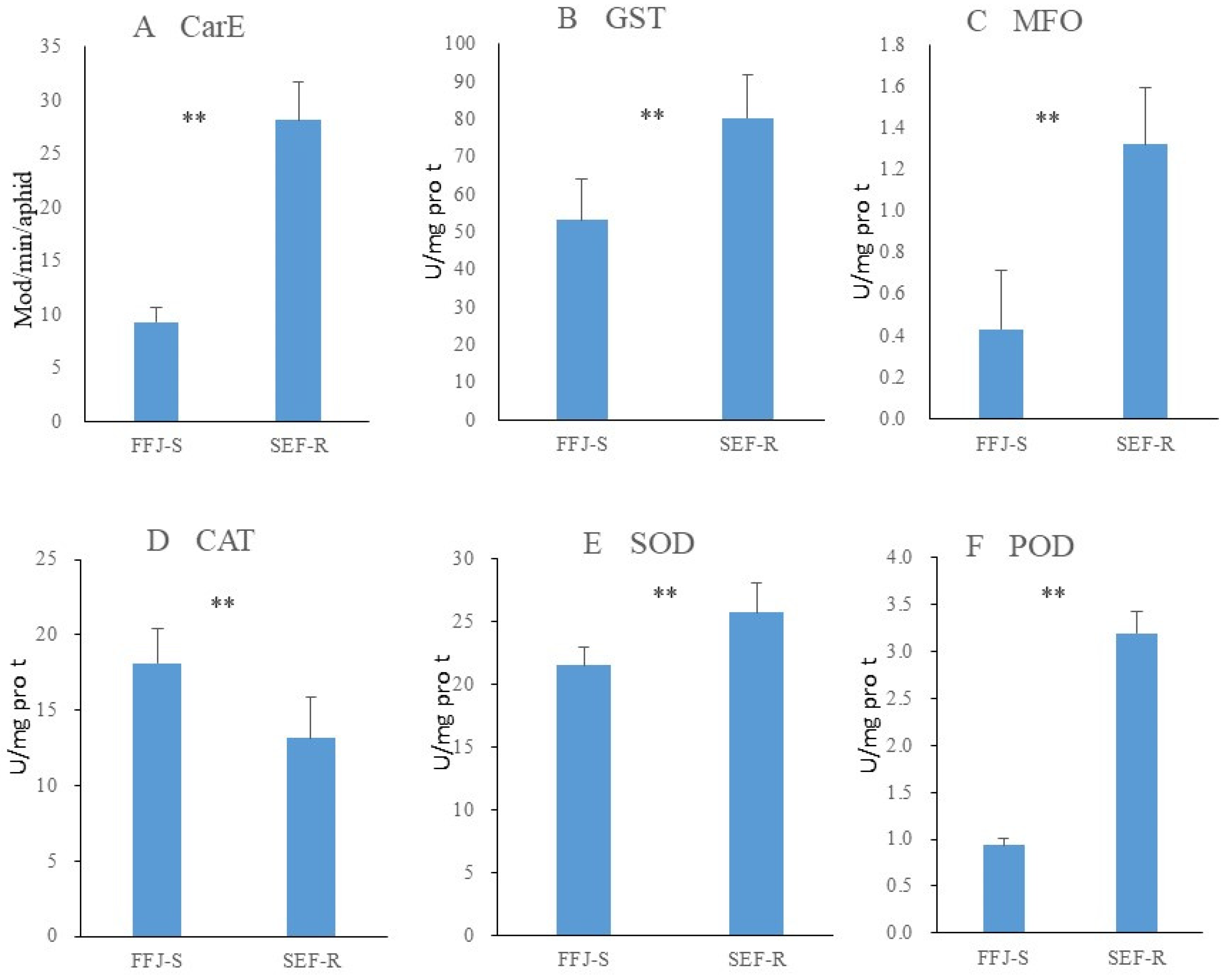
2.7. Determination of Protective Enzyme Activities in M. persicae
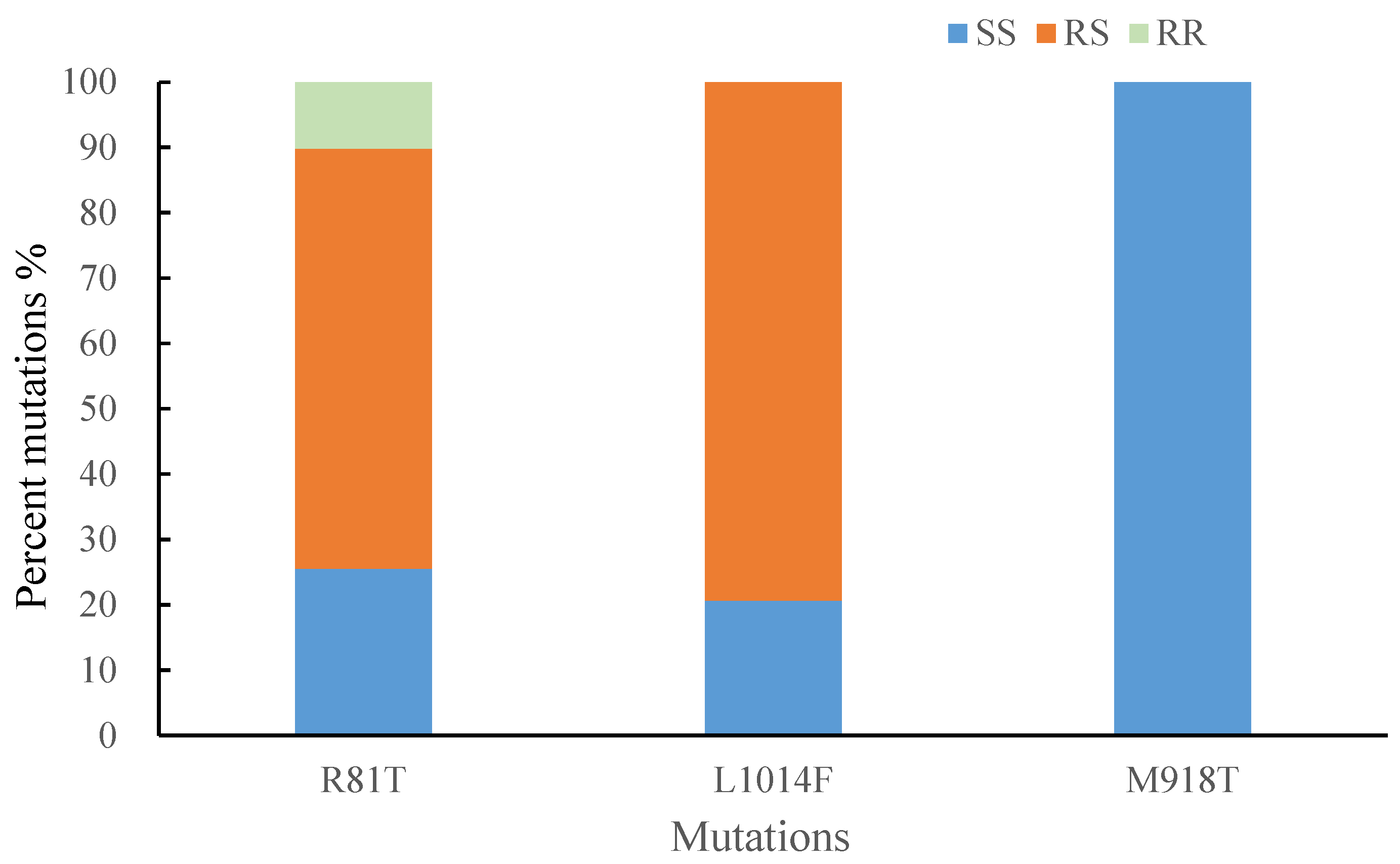
2.8. Detection of R81T, L1014F and M918T Mutations
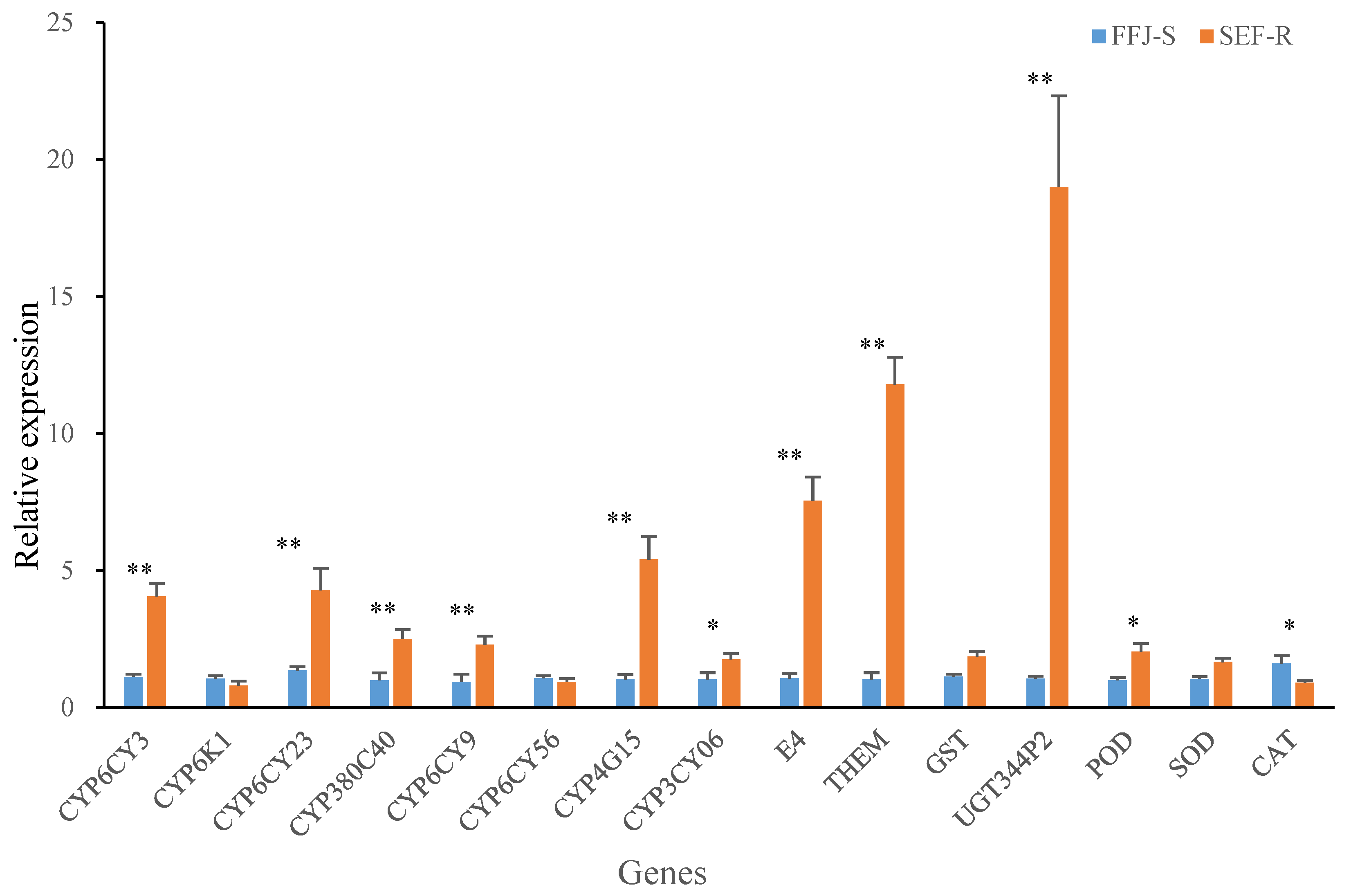
2.9. Gene Expression Analysis
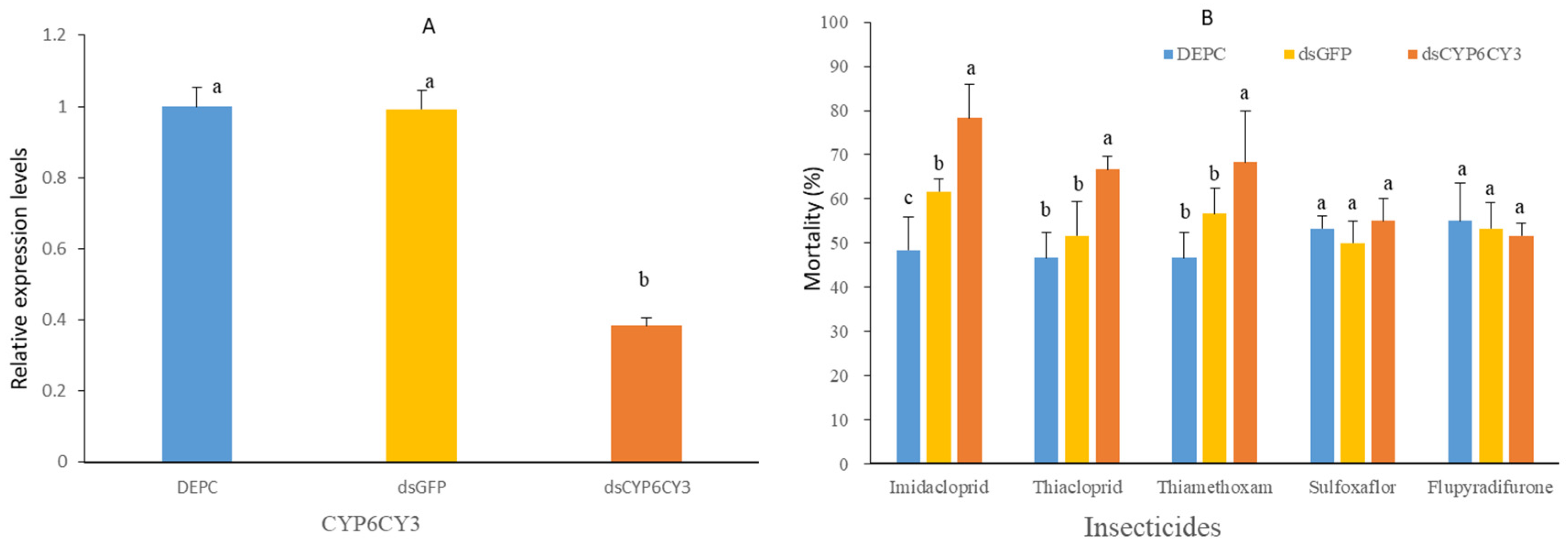
2.10. In Vivo RNAi of CYP6CY3 and Bioassays
2.11. Data Analysis
3. Results
3.1. Insecticide Resistance
3.2. Stability of Resistance to Selected Insecticides
3.3. Synergist Assessments and Enzyme Activity Levels
3.4. R81T, kdr and Super-kdr Resistance
3.5. Enzyme Genes Expression
3.6. Knockdown of CYP6CY3 Increases the Sensitivity of M. persicae to Group 4 Insecticides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Sood, A.K.; Ghongade, D.S. Assessment of losses inflicted by the aphid, Myzus persicae (Sulzer) to sweet pepper under protected environment in north western Indian Himalayan region. Phytoparasitica 2022, 50, 51–62. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Bosque-Perez, N.A.; Davis, T.S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef] [PubMed]
- van Emden, H.F. Plant resistance to Myzus persicae induced by a plant regulator and measured by aphid relative growth rate. Entomol. Exp. Appl. 1969, 12, 125–131. [Google Scholar] [CrossRef]
- IRAC. 2023. Available online: https://irac-online.org/mode-of-action/ (accessed on 7 April 2023).
- Gao, X.W.; Zheng, B.Z.; Cao, B.J. Resistance in Myzus persicae to organophosphorus and carbamate insecticides in China. Acta Phytophy Sinica 1992, 19, 365–371. [Google Scholar]
- ICAMA. Available online: http://www.chinapesticide.org.cn/zgnyxxw/kgls/index (accessed on 1 February 2023).
- Tang, Q.L.; Ma, K.S.; Hou, Y.M.; Gao, X.W. Monitoring insecticide resistance and diagnostics of resistance mechanisms in the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in China. Pestic. Biochem. Physiol. 2017, 143, 39–47. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Shi, L.; Shen, G.; He, L. Insecticide resistance monitoring and metabolic mechanism study of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), in Chongqing, China. Pestic. Biochem. Physiol. 2016, 132, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, M.M.; Santos, I.B.; Paula-Moraes, S.V. Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Fitness and Resistance Stability to Diamide and Pyrethroid Insecticides in the United States. Insects 2022, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Shad, S.A. Stability and Fitness Cost Associated with Spirotetramat Resistance in Oxycarenus Hyalinipennis Costa (Hemiptera: Lygaeidae). Pest Manag. Sci. 2021, 78, 572–578. [Google Scholar] [CrossRef]
- Abbas, N.; Khan, H.; Shad, S.A. Cross-resistance, stability, and fitness cost of resistance to imidacloprid in Musca domestica L., (Diptera: Muscidae). Parasitol. Res. 2015, 114, 247–255. [Google Scholar]
- Grossman, M.K.; Uc-Puc, V.; Rodriguez, J.; Cutler, D.J.; Morran, L.T.; Manrique-Saide, P.; Vazquez-Prokopec, G.M. Restoration of pyrethroid susceptibility in a highly resistant Aedes aegypti population. Biol. Lett. 2018, 14, 20180022. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Devonshire, A.L.; Field, L.M.; Foster, S.P.; Moores, G.D.; Williamson, M.S.; Blackman, R.L. The evolution of insecticide resistance in the peach–potato aphid, Myzus persicae. Phil. Trans. R. Soc. Lond. B 1998, 353, 1677–1684. [Google Scholar] [CrossRef]
- Lan, W.S.; Cong, J.; Jiang, H.; Jiang, S.R.; Qiao, C.L. Expression and characterization of carboxylesterase E4 gene from peach-potato aphid (Myzus persicae) for degradation of carbaryl and malathion. Biotechnol. Lett. 2005, 27, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef]
- Nakao, T.; Kawashima, M.; Banba, S. Differential metabolism of neonicotinoids by Myzus persicae CYP6CY3 stably expressed in Drosophila S2 cells. J. Pestic. Sci. 2019, 44, 177–180. [Google Scholar] [CrossRef]
- Kirkland, L.S.; Chirgwin, E.; Ward, S.E.; Congdon, B.S.; van Rooyen, A.; Umina, P.A. P450-mediated resistance in Myzus persicae (Sulzer) (Hemiptera: Aphididae) reduces the efficacy of neonicotinoid seed treatments in Brassica napus. Pest Manag. Sci. 2023, 79, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xu, P.; Zeng, X.; Liu, X.; Shang, Q. Characterization of UDP-glucuronosyltransferases and the potential contribution to nicotine tolerance in Myzus persicae. Int. J. Mol. Sci. 2019, 20, 3637. [Google Scholar] [CrossRef]
- Pym, A.; Umina, P.A.; Reidy-Crofts, J.; Troczka, B.J.; Matthews, A.; Gardner, J.; Hunt, B.J.; van Rooyen, A.R.; Edwards, O.R.; Bass, C. Overexpression of UDP-glucuronosyltransferase and cytochrome P450 enzymes confers resistance to sulfoxaflor in field populations of the aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2022, 143, 103743. [Google Scholar] [CrossRef]
- Kayser, H.; Palivan, C.G. Stable free radicals in insect cuticles: Electron spin resonance spectroscopy reveals differences between melanization and sclerotization. Arch. Biochem. Biophys. 2006, 453, 179–187. [Google Scholar] [CrossRef]
- Qin, D.; Liu, B.; Zhang, P.; Zheng, Q.; Luo, P.; Ye, C.; Zhao, W.; Zhang, Z. Treating green pea aphids, Myzus persicae, with azadirachtin affects the predatory ability and protective enzyme activity of harlequin ladybirds, Harmonia axyridis. Ecotoxicol. Environ. Saf. 2021, 212, 111984. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Jin, D. Protective and detoxifying enzyme activity and ABCG subfamily gene expression in Sogatella furcifera under insecticide stress. Front. Physiol. 2019, 9, 1890. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xin, T.; Wang, J.; Zou, Z.; Zhong, L.; Xia, B. Sublethal effects of bifenazate on biological traits and enzymatic properties in the Panonychus citri (Acari: Tetranychidae). Sci. Rep. 2021, 11, 20934. [Google Scholar] [CrossRef]
- Belmert, N.J.; Rund, S.S.; Ghazi, J.P.; Zhou, P.; Duffield, G.E. Time-of-day specific changes in metabolic detoxification and insecticide resistance in the malaria mosquito Anopheles gambiae. J. Insect Physiol. 2014, 64, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Brogdon, W.G. Chapter 5: Insecticide Resistance Monitoring; microplate enzyme activity assays. In Methods in Anopheles Research, 4th ed.; Benedict, M.Q., Ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014; pp. 240–247. [Google Scholar]
- de Little, S.C.; Umina, P.A. Susceptibility of Australian Myzus persicae (Hemiptera: Aphididae) to three recently registered insecticides: Spirotetramat, Cyantraniliprole, and Sulfoxaflor. J. Econ. Entomol. 2017, 110, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Umina, P.A.; Bass, C.; van Rooyen, A.; Chirgwin, E.; Arthur, A.L.; Pym, A.; Mackisack, J.; Mathews, A.; Kirkland, L. Spirotetramat resistance in Myzus persicae (Sulzer) (Hemiptera: Aphididae) and its association with the presence of the A2666V mutation. Pest Manag. Sci. 2022, 78, 4822–4831. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, F.; Folia, M.; Ilias, A.; Papapetrou, P.; Roditakis, E.; Bass, C.; Vontas, J.T.; Margaritopoulos, J. Flupyradifurone resistance in Myzus persicae populations from peach and tobacco in Greece. Pest Manag. Sci. 2022, 78, 304–312. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 1994, 39, 47–79. [Google Scholar] [CrossRef]
- Byrne, F.J.; Devonshire, A.L. Insensitive acetylcholinesterase and esterase polymorphism in susceptible and resistant populations of the Tobacco Whitefly Bemisia tabaci (Genn.). Pestic. Biochem. Physiol. 1993, 45, 34–42. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Shang, C.C.; Soderlund, D.M. Monooxygenase activity of tobacco budworm (Heliothis virescens) larvae: Tissue distribution and optimal assay conditions for the gut activity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1984, 79, 407–411. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Puinean, A.M.; Elias, J.; Slater, R.; Warren, A.; Field, L.M.; Williamson, M.S.; Bass, C. Development of a high-throughput real-time PCR assay for the detection of the R81T mutation in the nicotinic acetylcholine receptor of neonicotinoid-resistant Myzus persicae. Pest Manag. Sci. 2013, 69, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Anstead, J.A.; Williamson, M.S.; Eleftherianos, I.; Denholm, I. Highthroughput detection of knockdown resistance in Myzus persicae using allelic discriminating quantitative PCR. Insect Biochem. Mol. Biol. 2004, 34, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, J.L.; Thomas, D.S. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Peng, T.; Pan, Y.; Gao, X.; Xi, J.; Zhang, L.; Ma, K.; Wu, Y.; Zhang, J.; Shang, Q. Reduced abundance of the CYP6CY3-targeting let-7 and miR-100 miRNAs accounts for host adaptation of Myzus persicae nicotianae. Insect Biochem. Mol. Biol. 2016, 75, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Yu, X.R.; Shang, Q.L.; Shi, X.Y.; Gao, X.W. Oral delivery mediated RNA interference of a carboxylesterase gene results in reduced resistance to organophosphorus insecticides in the cotton aphid, aphis gossypii Glover. PLoS ONE 2014, 9, e102823. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- WHO. Status of Resistance in Houseflies, Musca Domestica, Document VBC/EC/80.7; World Health Organization: Geneva, Switzerland, 1980.
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA, 2023; Available online: https://www.pesticideresistance.org (accessed on 15 May 2023).
- Gu, C.; Wang, G.; Wang, K.Y.; Ma, H.; Guo, Q. Studies on the resistance level of Myzus persicae (Sulzer) in main tobacco regions of southwest. J. Plant Prot. 2006, 33, 77–80. [Google Scholar]
- Gong, Y.; Wang, Z.; Shi, B.; Kand, Z.; Zhu, L.; Guo, X.; Liu, J.; Wei, S. Resistance status of Myzus persicae (Sulzer) (Hemiptera: Aphididae) populations to pesticide in Beijing. Sci. Agric. Sin. 2011, 44, 4385–4394. [Google Scholar]
- Bo, J.; Zhou, X.; Fang, Y. Studies on monitoring of insecticide resistance of Myzus persicae. Agrochem. Res. Appl. 2009, 13, 27–29. [Google Scholar]
- Tang, Q. Insecticide resistance and genetic variation of field populations of Myzus pericae (Sulzer), in China. Ph.D. Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- Voudouris, C.C.; Williamson, M.S.; Kati, A.N.; Sahinoglou, A.J.; Margaritopoulos, J.T. Evolution of imidacloprid resistance in Myzus persicae in Greece and susceptibility data for spirotetramat. Pest Manag. Sci. 2017, 73, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Y. Resistance monitoring and biochemistry mechanism of thiamethoxam in Myzus persicae. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2014. [Google Scholar]
- Foster, S.P.; Cox, D.; Oliphant, L.; Mitchinson, S.; Denholm, I. Correlated responses to neonicotinoid insecticides in clones of the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae). Pest Manag. Sci. 2008, 64, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Cutler, P.; Slater, R.; Edmunds, A.J.; Maienfisch, P.; Hall, R.G.; Earley, F.G.; Pitterna, T.; Pal, S.; Paul, V.L.; Goodchild, J.; et al. Investigating the mode of action of sulfoxaflor: A fourth-generation neonicotinoid. Pest Manag. Sci. 2013, 69, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef]
- Mezei, I.; Valverde-Garcia, P.; Siebert, M.W.; Gomez, L.E.; Torne, M.; Watson, G.B.; Raquel, A.M.; Fereres, A.; Sparks, T.C. Impact of the nicotinic acetylcholine receptor mutation R81T on the response of European Myzus persicae populations to imidacloprid and sulfoxaflor in laboratory and in the field. Pestic. Biochem. Physiol. 2022, 187, 105187. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, B.; Cao, B. Resistance levels of Myzus persicae to pyrethroids in Beijing and Langfang of Hebei. Pesticides 1993, 32, 89. [Google Scholar]
- Flückiger, C.R.; Kristinsson, H.; Senn, R.; Rindlisbacher, A.; Buholzer, H.; Voss, G. CGA 215–944—A novel agent to control aphids and whiteflies. In Proceedings-Brighton Crop Protection Conference. Pests and Diseases; Ciba-Geigy Ltd., Plant protection div.: Basel, Switzerland, 1992; Volume 1, pp. 43–50. [Google Scholar]
- Morita, M.; Ueda, T.; Yoneda, T.; Koyanagi, T.; Haga, T. Flonicamid, a novel insecticide with a rapid inhibitory effect on aphid feeding. Pest. Manag. Sci. 2007, 63, 969–973. [Google Scholar] [CrossRef]
- Margaritopoulos, J.T.; Kati, A.N.; Voudouris, C.C.; Skouras, P.J.; Tsitsipis, J.A. Long-term studies on the evolution of resistance of Myzus persicae (Hemiptera: Aphididae) to insecticides in Greece. Bull. Entomol. Res. 2021, 111, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Margaritopoulos, J.T.; Tsamandani, K.; Kanavaki, O.M.; Katis, N.I.; Tsitsipis, J.A. Efficacy of pymetrozine against Myzus persicae and in reducing potato virus Y transmission on tobacco plants. J. Appl. Entomol. 2010, 134, 323–332. [Google Scholar] [CrossRef]
- Koo, H.; An, J.; Park, S.; Kim, J.; Kim, G. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Gorman, K.; Slater, R.; Blande, J.D.; Clarke, A.; Wren, J.; McCaffery, A.; Denholm, I. Cross-resistance relationships between neonicotinoids and pymetrozine in Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2010, 66, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cheng, S.; Chen, Z.; Guo, T.; Liang, P.; Zhen, C.; Wang, J.; Zhang, L.; Liang, P.; Gao, X. Establishment of toxicity and susceptibility baseline of broflanilide for Aphis gossypii Glove. Insects 2022, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Yokota, M.; Ihara, M.; Akamatsu, M.; Sattelle, D.B.; Matsuda, K. Role in the selectivity of neonicotinoids of insect-specific basic residues in loop D of the nicotinic acetylcholine receptor agonist binding site. Mol. Pharmacol. 2006, 70, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, Q.; Wang, X.; Wang, R.; Ullah, F.; Gao, X.; Song, D. V101I and R81T mutations in the nicotinic acetylcholine receptor β1 subunit are associated with neonicotinoid resistance in Myzus persicae. Pest Manag. Sci. 2022, 78, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Panini, M.; Dradi, D.; Marani, G.; Butturini, A.; Mazzoni, E. Detecting the presence of target-site resistance to neonicotinoids and pyrethroids in Italian populations of Myzus persicae. Pest. Manag. Sci. 2014, 70, 931–938. [Google Scholar] [CrossRef]
- Choi, B.R.; Lee, S.W.; Yoo, J.K. Resistance mechanisms of green peach aphid, Myzus persicae (Homoptera: Aphididae), to imidacloprid. Korean J. Appl. Entomol. 2001, 40, 265–271. (In Korean) [Google Scholar]
- Singh, K.S.; Troczka, B.J.; Duarte, A.; Balabanidou, V.; Trissi, N.; Carabajal Paladino, L.Z.; Nguyen, P.C.; Zimmer, T.; Papapostolou, K.M.; Randall, E.; et al. The genetic architecture of a host shift: An adaptive walk protected an aphid and its endosymbiont from plant chemical defenses. Sci. Adv. 2020, 6, eaba1070. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Origin and evolution of the CYP4G subfamily in insects, cytochrome P450 enzymes involved in cuticular hydrocarbon synthesis. Mol. Phylogenet Evol. 2020, 143, 106695. [Google Scholar] [CrossRef]
- Dulbecco, A.B.; Moriconi, D.E.; Calderón-Fernández, G.M.; Lynn, S.; McCarthy, A.; Roca-Acevedo, G.; Jhon, A.; Salamanca-Moreno; Juárez, M.P.; Pedrini, N. Integument CYP genes of the largest genome-wide cytochrome P450 expansions in triatomines participate in detoxification in deltamethrin-resistant Triatoma Infestans. Sci. Rep. 2018, 8, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Dulbecco, A.B.; Moriconi, D.E.; Pedrini, N. Knockdown of CYP4PR1, a cytochrome P450 gene highly expressed in the integument tissue of Triatoma infestans, increases susceptibility to deltamethrin in pyrethroid-resistant insects. Pestic. Biochem. Physiol. 2021, 173, 104781. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Zhang, L.; Liu, N. Overexpression of CYP4G19 associated with a pyrethroid-resistant strain of the German cockroach, Blattella Germanica (L.). Gene 2003, 314, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Mo, X.; Rizvi, S.A.H.; Li, C.; Zeng, X. Detection and biochemical characterization of insecticide resistance in field populations of Asian citrus psyllid in Guangdong of China. Sci. Rep. 2018, 8, 12587. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, C.M.D.; Nanson, J.D.; Patterson, E.I.; Forwood, J.K. Structure, function, and regulation of thioesterases. Prog. Lipid Res. 2020, 79, 101036. [Google Scholar] [CrossRef]
- Upasana, S.; Ioannis, E. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front. Immunol. 2017, 8, 759. [Google Scholar]

| Insecticides. | Group a | Strains | No. | Slope (SE) | LC50 (95% CI) mg·L−1 | χ2 | RF b |
|---|---|---|---|---|---|---|---|
| Thiacloprid | Group 4 | FFJ-S | 480 | 4 (0.33) | 1.89 (1.74–2.06) | 3.32 (df = 3) | |
| SEF-R | 560 | 1.57 (0.14) | 54.86 (45.64–66.52) | 3.04 (df = 4) | 29 | ||
| Imidacloprid | Group 4 | FFJ-S | 560 | 2.68 (0.21) | 1.09 (0.97–1.22) | 2.12 (df = 4) | |
| SEF-R | 480 | 1.83 (0.18) | 116.48 (97.95–138.88) | 2.45 (df = 3) | 106.9 | ||
| Thiamethoxam | Group 4 | FFJ-S | 560 | 4.71 (0.36) | 2.57 (2.4–3.74) | 5.5 (df = 4) | |
| SEF-R | 480 | 1.64 (0.18) | 33.2(27.37–40.49) | 2.61 (df = 3) | 12.9 | ||
| Sulfoxaflor | Group 4 | FFJ-S | 480 | 2.79 (0.25) | 1.59 (1.41–1.79) | 4.25 (df = 3) | |
| SEF-R | 480 | 2.31 (0.26) | 51.51 (42.81–61.19) | 1.6 (df = 3) | 32.4 | ||
| Flupyradifurone | Group 4 | FFJ-S | 480 | 2.68 (0.24) | 1.34 (1.17–1.51) | 2.61 (df = 3) | |
| SEF-R | 480 | 1.99 (0.19) | 12.67 (10.78–14.92) | 2.94 (df = 3) | 9.5 | ||
| Esfenvalerate | Group 3 | FFJ-S | 480 | 2.04 (0.19) | 9.66 (8.24–11.33) | 3.76 (df = 3) | |
| SEF-R | 480 | 1.77 (0.17) | 764.4 (639.34–915.07) | 3.09 (df = 3) | 79.1 | ||
| beta-Cypermethrin | Group 3 | FFJ-S | 560 | 1.8 (0.15) | 7.32 (6.17–8.69) | 3.15 (df = 4) | |
| SEF-R | 480 | 1.9 (0.18) | 2371.54 (2006.7–2823.87) | 0.64 (df = 3) | 324 | ||
| Flonicamid | Group 29 | FFJ-S | 560 | 2.02 (0.16) | 1.35 (1.15–1.57) | 2.47 (df = 4) | |
| SEF-R | 480 | 1.79 (0.18) | 7.85 (6.55–9.42) | 2.53 (df = 3) | 5.8 | ||
| Pymetrozine | Group 9B | FFJ-S | 560 | 1.84 (0.15) | 0.86 (0.73–1.01) | 3.54 (df = 4) | |
| SEF-R | 480 | 1.99 (0.19) | 29.89 (25.45–35.4) | 3.88 (df = 3) | 34.8 | ||
| Afidopyropen | Group 9D | FFJ-S | 480 | 2.03 (0.19) | 0.74 (0.62–0.87) | 2.38 (df = 3) | |
| SEF-R | 480 | 2.1 (0.2) | 2.92 (2.49–3.44) | 2.81 (df = 3) | 4 | ||
| Spirotetramat | Group 23 | FFJ-S | 480 | 2.09 (0.19) | 1.05 (0.9–1.23) | 2.51 (df = 3) | |
| SEF-R | 480 | 2.05 (0.19) | 8.45 (7.22–9.95) | 4.92 (df = 3) | 8.1 | ||
| Cyantraniliprole | Group 28 | FFJ-S | 480 | 1.95 (0.19) | 8.38 (7.07–9.87) | 3.55 (df = 3) | |
| SEF-R | 480 | 1.81 (0.18) | 28.81 (24.21–34.55) | 0.94 (df = 3) | 3.4 | ||
| Broflanilide | Group 30 | FFJ-S | 560 | 1.78 (0.15) | 0.98 (0.83–1.17) | 4.88 (df = 4) | |
| SEF-R | 480 | 1.82 (0.18) | 15.23 (12.81–18.27) | 0.43 (df = 3) | 15.5 |
| Treatment | No. | Slope ± SE | LC50 (95% CI) | χ2 | SR b | |
|---|---|---|---|---|---|---|
| FFJ-S | IMDP a | 560 | 2.68 (0.21) | 1.09 (0.97–1.22) | 2.12 (df = 4) | |
| IMDP + PBO | 480 | 3.18 (0.28) | 1.1 (0.99–1.22) | 4.07 (df = 3) | 0.99 | |
| IMDP + DEF | 480 | 3.3 (0.28) | 1.13 (1.02–1.25) | 5.08 (df = 3) | 0.96 | |
| IMDP + DEM | 480 | 2.7 (0.26) | 1.01 (0.9–1.14) | 5.31 (df = 3) | 1.08 | |
| SEF-R | IMDP | 480 | 1.83 (0.18) | 116.48 (97.95–138.88) | 2.45 (df = 3) | |
| IMDP + PBO | 560 | 1.56 (0.14) | 27.99 (23.23–33.81) | 6.37 (df = 4) | 4.16 | |
| IMDP + DEF | 560 | 1.63 (0.16) | 54.13 (45.19–64.94) | 6.42 (df = 4) | 2.15 | |
| IMDP + DEM | 560 | 1.55 (0.14) | 36.36 (30.14–43.91) | 5.43 (df = 4) | 3.2 |
| Insecticides | Treatment | No. | Slope ± SE | LC50 (95% CI) | χ2 | SR b |
|---|---|---|---|---|---|---|
| FFJ-S | CYPE a | 560 | 1.8 (0.15) | 7.32 (6.17–8.69) | 3.15 (df = 4) | |
| CYPE + PBO | 560 | 1.75 (0.14) | 7.09 (5.98–8.42) | 3.26 (df = 4) | 1.03 | |
| CYPE + DEF | 560 | 1.62 (0.14) | 7.1 (5.93–8.52) | 2.35 (df = 4) | 1.03 | |
| CYPE + DEM | 560 | 1.82 (0.15) | 7.61 (6.43–9.03) | 3.74 (df = 4) | 0.96 | |
| SEF-R | CYPE | 480 | 1.9 (0.18) | 2371.54 (2006.7–2823.87) | 0.64 (df = 3) | |
| CYPE + PBO | 480 | 1.81 (0.18) | 368.74(309.07–439.99) | 3.6 (df = 3) | 6.43 | |
| CYPE + DEF | 480 | 1.98 (0.18) | 617.18 (524.91–732.39) | 2.95 (df = 3) | 3.84 | |
| CYPE + DEM | 480 | 2.1 (0.21) | 1241.92 (1054.77–1468.27) | 1.2 (df = 3) | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Chen, F.; Wang, J.; Rao, W.; Lin, L.; Fan, G. Multiple Insecticide Resistance and Associated Metabolic-Based Mechanisms in a Myzus Persicae (Sulzer) Population. Agronomy 2023, 13, 2276. https://doi.org/10.3390/agronomy13092276
Hu J, Chen F, Wang J, Rao W, Lin L, Fan G. Multiple Insecticide Resistance and Associated Metabolic-Based Mechanisms in a Myzus Persicae (Sulzer) Population. Agronomy. 2023; 13(9):2276. https://doi.org/10.3390/agronomy13092276
Chicago/Turabian StyleHu, Jinfeng, Feng Chen, Jun Wang, Wenhua Rao, Lei Lin, and Guocheng Fan. 2023. "Multiple Insecticide Resistance and Associated Metabolic-Based Mechanisms in a Myzus Persicae (Sulzer) Population" Agronomy 13, no. 9: 2276. https://doi.org/10.3390/agronomy13092276
APA StyleHu, J., Chen, F., Wang, J., Rao, W., Lin, L., & Fan, G. (2023). Multiple Insecticide Resistance and Associated Metabolic-Based Mechanisms in a Myzus Persicae (Sulzer) Population. Agronomy, 13(9), 2276. https://doi.org/10.3390/agronomy13092276





