Abstract
Belowground root systems under pasture intercropping exhibit complex interactions, and the root interactions of different intercropping combinations are still poorly understood. Therefore, in this work, two perennial and annual herbages were intercropped in pairs and evaluated at a ratio of 1:1. The root morphology and topological structure differed significantly with intercropping combinations. (1) Compared with other cropping patterns, the mean root diameter (RD) of intercropped alfalfa (Medicago sativa L.) and common vetch (Vicia sativa L.) increased notably. The root surface area (RSA), root volume (RV), and mean RD increased significantly when oat (Avena sativa L.) was intercropped with alfalfa. Similarly, the RSA and RV increased in intercropped oat, intercropping relative to monocropping. (2) The forage topological index of the intercropping system was close to one, which was close to that of the herringbone branching. Additionally, the intercropping system had a lower intensity of underground root competition. The root system of the different forage intercropping combinations tended to transition to dichotomous branching. (3) The correlations between root parameters differed according to forage species. Therefore, different intercropping combinations had different belowground root levels of competitiveness and interactions, thereby changing the resource competition environment.
1. Introduction
Roots play an important role in the plant ecosystem. Plant roots are important organs for transporting water and nutrients and anchoring plants [1]. The root structure is the spatial distribution of the whole root system in the soil and is primarily determined by the topological structure [2]. Fitter et al. described two types of root topological structures, namely, herringbone and dichotomous branching, whereby the topological index (TI) represents the branching pattern of the root system [3]. For example, the root system topology of saltcedar (Tamarix chinensis Lour.), which grows in the Yellow River Delta of China, tends to be dichotomous when the groundwater level is high, but exhibits a herringbone branching pattern when the groundwater level is medium or low; the root architecture of saltcedar changed from dichotomous to a herringbone branching pattern with increased water salinity, indicating that saltcedar adapts to salt stress by reducing its root branching. Thus, differences in the root topological structure show the plant’s adaptive strategies to environmental stresses [4,5]. The characteristic parameters of root geometry mainly include root length (RL), diameter, surface area, and volume, among others. These root traits were shown to have some phenotypic plasticity. For example, the root system of tall fescue (Festuca arundinacea Schreb.) produces physiological changes through the perception of external salinity pressure, which is closely related to the aboveground parts, and may be the reason plants adapt to the environment and maintain their stability [6,7]. The fractal dimension (FD) of the root system can be used to analyze the spatial distribution and branching status of the roots; thus, FD is closely related to relative competition between plant species [8]. Functional forage intercropping mainly depends on niche complementarity between species [9], and forage-interspecific coexistence could be enhanced by promoting root depth and growth phenology [10].
The use of perennial forage grass to build an efficient and high-quality artificial grassland through bean and grain intercropping is an important way of solving the imbalance in integrated crop–livestock systems and restoring naturally degraded grasslands [11]. In agricultural headlands where the soil structure has been destroyed, planting perennial pastures increases the nitrogen and carbon contents of the soil and also affects the soil structure, which increases the yield and quality of the subsequent crop spring wheat (Triticum aestivum L.) without affecting its root density [12]. Competition and complementarity are the main factors involved in legume and grain intercropping. When silage corn (Zea mays L.) is intercropped with different annual forages, the yield and quality of the forages vary, indicating that different forages have varying levels of competitiveness with silage corn intercropping [13]. Grains are generally more competitive, and the total nitrogen uptake can be increased through niche complementarity between leguminous and gramineous plants [14]. The water use efficiency and RL density of crops were improved in the intercropping system that involved corn and soybean (Glycine max (L.) Merrill) [15]. The interspecific interaction between underground roots in the intercropping system is complex, and some crops increase their root distribution in soil by enhancing their ability to expand [16]. In the soybean and triticale intercropping system, the elongation of soybean roots increased the nitrogen and phosphorus absorption efficiency of triticale, but reduced its root biomass [17]. Some plants promoted soil nitrogen absorption by increasing the number of fine roots [18].
Leguminosae and Gramineae are often combined in intercropping systems because of their niche complementarity [14] ensured through the nodulation and nitrogen fixation of the Leguminosae, which enhances nitrogen utilization [19]. This is a key factor that affects crop yield and quality [20]. Studies on the structure of underground root systems are mostly concentrated in cereals and edible legumes. However, there are plasticity changes in the underground root systems of perennial forages that are intercropped. Studies have shown that there is an interaction between the seeds of two leguminous crops during germination [21], particularly the geometric shape, topological structure, and typing characteristics, which are affected by the competitive environment. However, the full nature of these interactions still remains unclear. Therefore, this study used perennial herbages, including alfalfa and smooth brome, and annual herbages, including common vetch (Vicia sativa L.) and oat (Avena sativa L.), for pairwise intercropping to provide a theoretical basis for the establishment, stability, and sustainable utilization of perennial bean–grass intercropping grasslands.
2. Materials and Methods
2.1. Experimental Location
This experiment was conducted in the Jiaozhou Science and Technology Demonstration Park (36°26′22″ N, 120°04′43″ E) at the Qingdao Agricultural University in Shandong province, China. The experimental site has a temperate monsoon climate, which is affected by the southeast monsoon and ocean current, with typical marine climate characteristics. The annual average temperature is 12 °C, with an annual precipitation of 698.1 mm. The soil type is brown loam with uniform soil fertility and a pH of 7.9.
2.2. Experimental Design
The plants in the investigation were alfalfa (Medicago sativa L.), common vetch, smooth brome (Bromus inermis Leyss.), oat, and sainfoin (Onobrychis viciaefolia Scop.). The experimental field was sown in March 2021. Three legumes and two gramineous grass types were intercropped in pairs, and only two gramineous grass types were intercropped with sainfoin. There were six intercropping systems and four sole-sowing systems. Alfalfa, common vetch, smooth brome, and oat were planted as controls. A randomized complete block design was adopted for the study with the following 12 treatments:
- Sole Medicago sativa (M)
- Sole Vicia sativa (V)
- Sole Bromus inermis (B)
- Sole Avenia sativa (A)
- Sole Onobrychis viciaefolia (O)
- M-V
- M/V-B (M-B or V-B)
- M/V-A (M-A or V-A)
- B-A
- B/A-M (B-M or A-M)
- B/A-V (B-V or A-V)
- B/A-O (B-O or A-O)
Each treatment had three replicates. The experiment had 12 plots with each measuring 24 m2 (4 m × 6 m). There were 20 rows in each plot, and the intercropping treatment had 5:5 rows of intercropping, with 10 rows of each of the two plants intercropped, and 5 rows planted at intervals with a row spacing of 20 cm. The sowing rates (kg/ha) were 15 for alfalfa, 75 for vetch, 80 for smooth brome, 80 for oat, and 60 for sainfoin. Diammonium phosphate (225 kg/ha) and potassium sulfate (120 kg/ha) were applied before sowing.
2.3. Measurement of the Root Parameters
The sampling time was 10 September 2021. Four points were selected per plot and two points per each plant. A 40 cm long soil block was dug 40 cm wide and 40 cm deep from the horizontal plane centered on the plant. The soil block was placed in a net bag, soaked in water for 30 min, and then rinsed with running water. Tweezers were used to clean the fine impurities in the roots while they were submerged in water and the root system was evenly combed before being placed in the water, so that the roots unfolded naturally. The roots were then scanned with a ScanMaker i800 Plus (MICROTEK, Shanghai, China) and the scanned root morphology was saved in a graphic file format. The total RL, root surface area (RSA), root volume (RV), root diameter (RD), root tip number, and cross number were obtained with the LA-S root analytical software.
The TI reflects the branching patterns of different plant roots [22,23]; the TI was calculated as TI = lg(A)/lg(M) (where M is the sum of all the exterior links of the root system and A is the sum of the interior links of the longest path of the root system, Figure 1). The branches of plant roots were between the herringbone branching pattern and the dichotomous branching pattern. When TI was close to 1, it was a herringbone branching pattern, and when TI was close to 0.5, it was a dichotomous branching pattern.
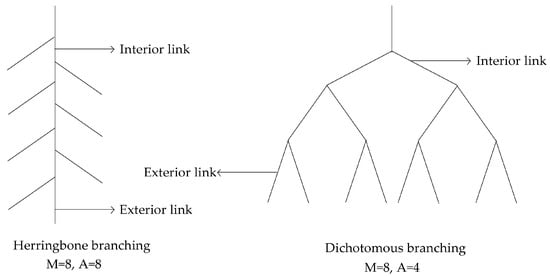
Figure 1.
Root topology diagram. A is the number of links of the greater path length, which is the interior link of the root system. M is the number of the tip roots, which is the exterior link.
The intermediate transition form of the root branching status was determined using the modified topological parameters proposed by Oppelt [24]. The correction value of the herringbone branching was qa = qb = 1, while that of the dichotomous branching was qa = qb = 0, where a is the same topological length as A in the TI equation, b is the average topological length of the root system, and v0 is the same as M in the TI equation. The relevant calculation formulae were as follows:
The root FD was directly analyzed with the LA-S root analytical software.
2.4. Data Processing
Microsoft Excel 2016 (Redmond, WA, USA) and SPSS 22.0 (IBM, Inc., Armonk, NY, USA) were used to analyze the data, and Sigma Plot 12.5 (Systat Software, San Jose, CA, USA) was used to generate the graphs.
3. Results
3.1. Comparison of Morphological Parameters of Forage Roots under Different Intercropping Modes
As shown in Figure 2, the morphology of the forage roots differed significantly under different intercropping modes. The RSA, RL, and RV of the perennial leguminous forage alfalfa intercropping increased by 57%, 62%, and 70%, respectively, compared with the monoculture system. Compared with the other treatments, the number of root tips and crosses increased by approximately 60~70%, while the number of root connections increased by approximately 75%. When alfalfa was intercropped with the annual leguminous pasture, vetch, the average diameter of its root system increased by approximately 56%. In addition, the RSA, RV, and root average diameter of the intercropped oat and alfalfa changed significantly. The RSA and volume increased significantly by 80% and 85%, respectively, when oat was intercropped with alfalfa compared with monocropping. The average RD of intercropping with smooth brome was significantly higher than that of other treatments by approximately 43%. In general, intercropping alfalfa with different types of forage significantly changed the root morphology. Similarly, intercropping with different types of forage resulted in different root morphological parameters. The root morphology of smooth brome and the annual leguminous common vetch had no significant differences across intercropping modes.
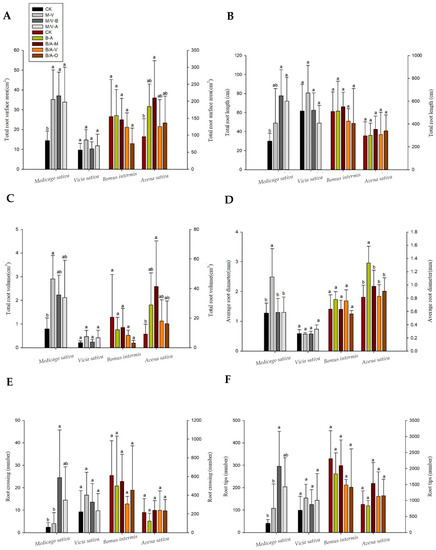
Figure 2.
CK: monocropping of each herbage; M. Medicago sativa; V. Vicia sativa; B. Bromus inermis; A, Avena sativa; O, Onobrychis viciaefolia Scop. (M/V-B is M-B or V-B, same below.) (A) Significant differences in the root surface area of each pasture under different intercropping patterns. (B) Root total length. (C) Total root volume. (D) Average root diameter. (E) Root crossing. (F) Root tips. Differences in the geometric parameters of plant roots under different intercropping patterns. Different lowercase letters (a, b) indicate that the root system of the grass species was significantly different among planting patterns (p < 0.05).
3.2. Comparison of the Topological Structure of the Forage Root System under Different Intercropping Modes
As shown in Figure 3, the root TI of the various forages under different intercropping combinations was close to one, indicating that their root configuration was close to that of the herringbone branching pattern. A one-way analysis of variance (ANOVA) showed no significant differences in the root TI between forage intercropping and monoculture systems (p > 0.05). When alfalfa and common vetch were intercropped, the alfalfa roots had the lowest average TI (TI ≈ 0.86), with a modified topological parameter (qa) ≈ 0.5, and there was a trend of transition to dichotomous branching. The results of the one-way ANOVA showed that the link number (Pe) of alfalfa intercropping was significantly higher than that of the monoculture (CK) system. Moreover, the FD of the intercropped alfalfa was significantly higher than that of its monoculture and other intercropping combinations, with an average FD value of 1.65. When oat was intercropped with smooth brome, the FD of the two forages was higher. However, the lowest FD of oat intercropped with annual leguminous forage was close to that of monocropping, which was 1.63. The average TI and qa of intercropped oat and alfalfa were the smallest (TI = 0.95, qa = 0.69), and there was a trend of transition to dichotomous branching. The TI and qa of intercropped smooth brome were higher than that of the monoculture (CK) system, and the average TI and qa of intercropping smooth brome and sainfoin were the highest.
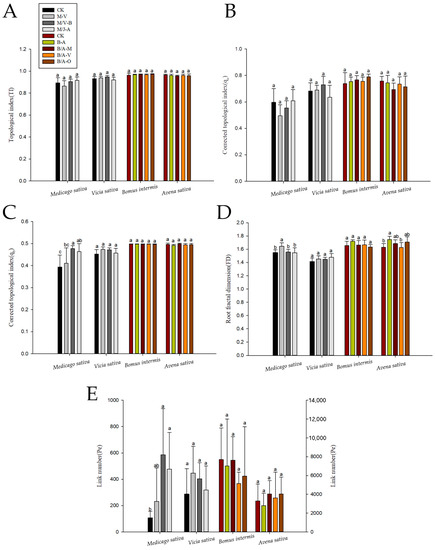
Figure 3.
CK: monocrop for each herbage; M, Medicago sativa; V, Vicia sativa; B, Bromus inermis; A, Avena sativa; O, Onobrychis viciaefolia Scop. (M/V-B is M-B or V-B, same below.) (A) Significant differences in the topological index of each pasture under different intercropping patterns. (B) Corrected topological index (qa). (C) Corrected topological index (qb). (D) Root fractal dimension. (E) Link number. Different lowercase letters (a, b, and c) indicate that the root system of the grass species was significantly different among planting patterns (p < 0.05).
3.3. The Relationship between Root Morphological Parameters and Root Topological Structure under Different Intercropping Modes
To study the correlation between root configuration characteristics and root morphological parameters, a Pearson correlation analysis was used to analyze the geometric parameters, topological structure, and FD of the forage root system. As shown in Figure 4, the results showed no significant correlation between the RL and volume of common vetch and smooth brome, but there was a very significant positive correlation between the RL and volume of alfalfa and oat. A significant positive correlation also existed between the RL and FD of oat and between RL, TI, and Pe of several types of forages. There was no significant correlation between the RSA and the average RD of alfalfa and common vetch, but there was a very significant positive correlation between the RSA and the average RD of the gramineous forages. Furthermore, there were significant positive correlations between the RSA and the root tip and cross number in several forages except for smooth brome. There was no significant correlation between the RSA and TI in the four forages. There were no significant correlations between the RV, root tip number, and Pe, but there was a significant correlation between the RV in alfalfa, vetch, and oat, except for the cross number and TI. The average RD of alfalfa significantly positively correlated with the FD and TI, but that of the other three forages had a significant positive correlation with the FD. In addition, the average RD of smooth brome had significant negative correlations with the number of root tips, crossing numbers, and Pe. The root tip number of alfalfa positively correlated with the TI and qb, while that of common vetch negatively correlated with qa. Moreover, the root tip number of oat positively correlated with the FD. Except for common vetch, the crossover number of the other three forages had significant positive correlation with the TI, while that of oat had a significant negative correlation with the FD. The smooth brome FD positively correlated with TI and qa, while the oat FD positively correlated with Pe. There was no significant correlation between TI and Pe in oat and common vetch.
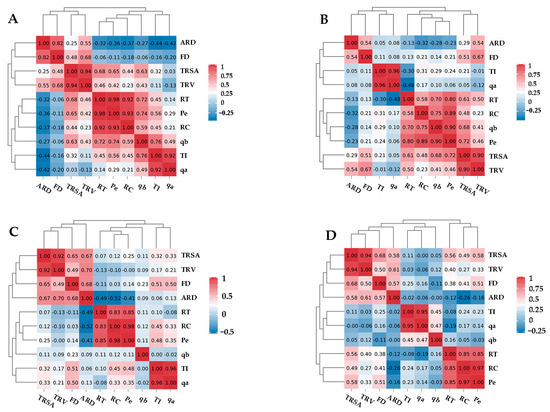
Figure 4.
Correlation analysis of the root parameters. Alfalfa (A), common vetch (B), smooth brome (C), and oat (D). TRL: total root length; TRSA: total root surface area; TRV: total root volume; ARD: average root diameter; RT: root tips; RC: root crossing; FD: fractal dimension; TI: topological index; qa, qb: corrected topological index; Pe: link number.
4. Discussion
4.1. Effects of Different Intercropping Patterns on Root Geometry
The forage species and the competitive abilities of crops are important factors that affect the stability of intercropping grasslands. The RD, RL, RSA, RV, cross number, and root tip number represent the distribution attributes of root systems in soil. The RD shows the thickness of the root system, which results from the adaptation of plants to different environments [25]. The RSA is an important index for measuring the water and nutrient absorption potential of roots. The total RL shows the growth and elongation ability of root systems, which is related to the acquisition of nutrients by plants [26]. The main factors that affect the root structure of several natural grass vegetations in the lower reaches of the Yellow River of China are the average RD, RSA, and RV [27]. In this study, the root morphology of leguminous and gramineous herbage changed significantly in the six intercropping systems, and there was no significant difference in the root geometry between smooth brome and common vetch. However, the root morphology of intercropped alfalfa was different from that of the monoculture system. When alfalfa was intercropped with common vetch, its RD increased significantly. This could be because common vetch has shallow roots that are mainly distributed in the 0~30 cm layer of soil, while alfalfa has deep roots with a complementary niche and little interspecific competition, which promotes their growth. The main reason for the difference in alfalfa root morphology is that the niche overlap of different forages varies. The root morphology changes observed when alfalfa was intercropped with gramineous forages might be related to the nitrogen fixation of alfalfa nodules; when intercropping between alfalfa and gramineous grasses, gramineous grasses absorb more nitrogen, which promotes the ability of alfalfa root nodules to fix nitrogen. The competition of gramineous plants for nitrogen in the environment indirectly affects the change of alfalfa root morphology; for example, in this paper, alfalfa intercropped with grass had a longer RL and smaller RD. Furthermore, the RV and RSA of intercropped oat and alfalfa were significantly higher than those of the monoculture system, while the RV and RSA of alfalfa were not significantly different from those of monoculture crops. The effect of root interaction on root morphology among species results from competition for underground nutrients [28]. The plant root system is a dynamic structure that produces different morphological structures under the influence of biological and abiotic factors. When there is little competition, plants exhibit larger RDs and longer roots, and when there is high competition, plants exhibit thinner RDs and shorter roots [29]. In this study, the root morphological parameters of the different intercropping combinations of forage grass changed significantly, indicating that the differences in plant root morphology may have resulted from plant competition.
4.2. Effects of Different Intercropping Patterns on the Root Architecture
The architecture of the root surface area determines the ability of plants to obtain water and nutrients, and can objectively reflect the plasticity of roots to adapt to the environment [30]. The root system structure is usually described by its topological structure and FD [31]. The topological structure reflects the spatial distribution of roots in the soil. Previous studies have shown that root resources utilize herringbone branching the most, because it can reduce the intraspecific competition of the root system and requires a higher energy cost from plants; however, dichotomous branching has higher intraspecific competition, smaller RDs, and a lower plant cost [22,32]. For example, under water shortage and rehydration conditions, the root system of alfalfa tends to be dichotomous, and its FD increases, which results in more branches [33]. In this study, the TI of several forages under different intercropping systems was greater than one, with qa ≈ 1, and their root systems had herringbone branches. The minimum TI was observed when alfalfa was mixed with common vetch, with a transition tendency to bifurcation branches, compared with the other planting patterns. The Pe of mixing alfalfa with common vetch increased compared with that of other types of intercropping, and there was less competition between alfalfa and common vetch. This increased the RV, RSA, and average RD of alfalfa. The intercropping between alfalfa and two gramineous grasses had a higher TI than the monoculture system and had a significantly higher Pe than the other combinations, indicating that gramineous herbage had a stronger competitive ability. The TI of the intercropped smooth brome was higher than that of the monoculture system, indicating that intercropping promoted the water and nutrient acquisition efficiency of smooth brome without a canopy and there was more competition. However, the TI of intercropped oat was lower than that of the monoculture system, indicating that intercropping reduced the root competition of oat.
Plant roots have fractal structures [34], and the FD of the roots reflects the branching degree and ability of the roots [27]. Therefore, the FD is an index that reflects the growth of plant roots, and it differs among the roots of different species. In this study, the FD of the alfalfa root system increased significantly when alfalfa was intercropped with common vetch, indicating that the fine roots of alfalfa increased. This also indicated that the intercropping of alfalfa with vetch promoted root growth. However, the FD of alfalfa intercropping with Gramineae decreased, indicating high competition for water and nutrients. Oat intercropped with smooth brome had the largest root FD, and that of intercropped alfalfa was larger than that of the monoculture system, indicating that oat could improve the utilization efficiency of water and nutrients by increasing the number of fine roots. Studies have shown that the FD of the saltcedar root system positively correlates with the soil water content [5], and its root system branching is reduced in areas of poor-quality soil. Some studies reported that drought reduces the FD. For example, the FD of soybean and Adzuki bean (Vigna angularis (Willd.) Ohwi and Ohashi) roots increased in parallel with the soil water content, and alfalfa had a greater FD when rehydrated after severe dehydration [33,35,36]. Thus, alfalfa plants that had more developed roots had a larger FD. In this study, the RD of several forages positively correlated with the FD. The FD reflects the relationship between the taproot and lateral roots and quantifies root space filling and complexity, which can show the adaptability of plants to environmental changes [37]. The FD of the intercropping root system of Chinese elm (Castanea mollissima Blume)/tea (Camellia sinensis L.) is subject to interspecific competition, and stronger competition results in a smaller FD [38]. A larger FD value results in the wider distribution of plant roots in the soil and a higher ability to obtain resources.
Therefore, in this study, the changes in the root FD of plants in different planting combinations indicated different plant competitiveness, and the interspecific competition for water and nutrients caused changes in the rooting strategies of the herbages to improve resource acquisition efficiency. The structure of plant roots depends on the external environment, not only the intercropping species but also the soil [39], and it also affects the soil [40]. In our study, we observed significant differences in root morphology and topological structure among different intercropping combinations of perennial and annual herbages. These findings suggest that intercropping can influence root development and architecture, which can, in turn, have implications for soil health. The increased RSA, RV, and mean RD observed in specific intercropping combinations, such as alfalfa and oat intercropping, indicated potential benefits for soil health. A larger root system can enhance nutrient uptake, improve soil structure, and promote soil microbial activity. These effects can contribute to an increase in the accumulation of organic matter, nutrient cycling, and overall soil fertility.
In addition to the influence of the external environment, the root structure of plants is also regulated genetically. For example, drought-tolerant varieties of wheat have longer roots [41]. Breeding programs that consider the response of plant roots to intercropping conditions could lead to the development of cultivars with optimized root systems for specific intercropping combinations. By selecting traits, such as enhanced root branching, exploration capacity, and resource acquisition efficiency, in mixed cropping environments, it may be possible to further improve soil health and optimize the performance of intercropping systems.
5. Conclusions
The different intercropping combinations resulted in different root morphologies. Moreover, the intensity of interspecific competition varied with intercropping patterns, and the different competitive environments influenced the root morphology, topological structure, and fractal dimension of forages. For example, there was little interspecific competition when alfalfa was intercropped with common vetch. This resulted in an increase in the alfalfa RD and fractal dimension and a reduction in the TI to promote water and nutrient acquisition. Gramineae exhibited strong competitiveness when intercropped with alfalfa. Alfalfa adapted to the strong competitive environment by increasing its RL, RSA, and TI and reducing the fractal dimension. In the other intercropping systems, the root morphology of smooth brome and common vetch had no significant changes, but the oat roots adapted to the competitive environment by increasing the number of fine roots, fractal dimension, RV, RSA, and RD. Thus, different forages adapt to different competitive environments by comprehensively adjusting the plasticity of root geometry, root TI, and root fractal dimension, which is important for intercropping grassland stability and high yields.
Author Contributions
Conceptualization and methodology, X.L. and F.M.; writing, X.L.; resources, Q.Z. and F.M.; data curation, X.L., Y.J., X.Y. and X.Z.; review and editing, F.M., S.L., W.T., C.Y. and L.M.; funding, F.M., G.Y. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Shandong province (ZR2020QC189), the Doctoral Scientific Research Startup of Qingdao Agricultural University (6631116024), the China Agriculture Research System (CARS-34), the Shandong Forage Research System (SDAIT-23-01), the First-Class Grassland Science Discipline Program of Shandong province (1619002), China, and the National Natural Science Foundation of China (31802133).
Acknowledgments
We sincerely thank Ran Xue for their technical support and help in revising and improving the paper. We thank Zhenyi Li and Hongying Zhang for their assistance in conducting these studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colombi, T.; Torres, L.C.; Walter, A.; Keller, T. Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth—A vicious circle. Sci. Total Environ. 2018, 626, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H. An architectural approach to the comparative ecology of plant root systems. N. Phytol. 1987, 106, 61–77. [Google Scholar] [CrossRef]
- Fitter, A.H.; Stickland, T.R.; Harvey, M.L.; Wilson, G.W. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. N. Phytol. 1991, 118, 375–382. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, X.; Fang, Y.; Xu, W.; Gao, F.; Zhao, W.; Fu, Q.; Xia, J. Root growth and architecture of Tamarix chinensis in response to the groundwater level in the Yellow River Delta. Mar. Pollut. Bull. 2022, 179, 113717. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, X.; Fang, Y.; Gao, F.; Wu, C.; Xia, J. Effects of water and salt for groundwater-soil systems on root growth and architecture of Tamarix chinensis in the Yellow River Delta, China. J. For. Res. 2022, 34, 441–452. [Google Scholar] [CrossRef]
- Salim, M.; Chen, Y.; Ye, H.; Nguyen, H.T.; Solaiman, Z.M.; Siddique, K.H.M. Screening of Soybean Genotypes Based on Root Morphology and Shoot Traits Using the Semi-Hydroponic Phenotyping Platform and Rhizobox Technique. Agronomy 2021, 12, 56. [Google Scholar] [CrossRef]
- Amombo, E.; Li, H.; Fu, J. Research Advances on Tall Fescue Salt Tolerance: From Root Signaling to Molecular and Metabolic Adjustment. J. Amer. Soc. Hort. Sci. 2017, 142, 337–345. [Google Scholar] [CrossRef]
- Walk, T.C.; Van Erp, E.; Lynch, J.P. Modelling Applicability of Fractal Analysis to Efficiency of Soil Exploration by Roots. Ann. Bot. 2004, 94, 119–128. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.L.; Thomson, K.T. Plant diversity and ecosystem productivity: Theoretical considerations. Proc. Natl. Acad. Sci. USA 1997, 94, 1857–1861. [Google Scholar] [CrossRef]
- Fargione, J.; Tilman, D. Niche differences in phenology and rooting depth promote coexistence with a dominant C4 bunchgrass. Oecologia 2005, 143, 598–606. [Google Scholar] [CrossRef]
- Reynolds, J.; Bell, M.M.; Grace, J.; Gratton, C.; Jackson, R.D.; Keeley, K.O.; Mayerfeld, D. An agroecological vision of perennial agriculture. Agroecol. Sustain. Food Syst. 2021, 45, 1470–1479. [Google Scholar] [CrossRef]
- Kautz, T.; Stumm, C.; Kösters, R.; Köpke, U. Effects of perennial fodder crops on soil structure in agricultural headlands. J. Plant Nutr. Soil Sci. 2010, 173, 490–501. [Google Scholar] [CrossRef]
- La Guardia Nave, R.; Corbin, M.D. Forage Warm-Season Legumes and Grasses Intercropped with Corn as an Alternative for Corn Silage Production. Agronomy 2018, 8, 199. [Google Scholar] [CrossRef]
- Justes, E.; Bedoussac, L.; Dordas, C.; Frak, E.; Louarn, G.; Boudsocq, S.; Journet, E.-P.; Lithourgidis, A.; Pankou, C.; Zhang, C.J.F.o.A.S.; et al. The 4C approach as a way to understand species interactions determining intercropping productivity. Front. Agric. Sci. Eng. 2021, 8, 3. [Google Scholar]
- Ren, Y.Y.; Wang, X.L.; Zhang, S.Q.; Palta, J.A.; Chen, Y.L. Influence of spatial arrangement in maize-soybean intercropping on root growth and water use efficiency. Plant Soil 2016, 415, 131–144. [Google Scholar] [CrossRef]
- Schenk, H.J. Root competition: Beyond resource depletion. J. Ecol. 2006, 94, 725–739. [Google Scholar] [CrossRef]
- Esnarriaga, D.N.; Mariotti, M.; Cardelli, R.; Arduini, I. The Importance of Root Interactions in Field Bean/Triticale Intercrops. Plants 2020, 9, 1474. [Google Scholar] [CrossRef]
- Lu, B.; Qian, J.; Hu, J.; Wang, P.; Jin, W.; Tang, S.; He, Y.; Zhang, C. The role of fine root morphology in nitrogen uptake by riparian plants. Plant Soil 2022, 472, 527–542. [Google Scholar] [CrossRef]
- Olasantan, F.O. Effects of preceding maize (Zea mays) and cowpea (Vigna unguiculata) in sole cropping and intercropping on growth, yield and nitrogen requirement of okra (Abelmoschus esculentus). J. Agric. Sci. 1998, 131, 293–298. [Google Scholar] [CrossRef]
- Nilahyane, A.; Islam, M.A.; Mesbah, A.O.; Garcia y Garcia, A. Effect of Irrigation and Nitrogen Fertilization Strategies on Silage Corn Grown in Semi-Arid Conditions. Agronomy 2018, 8, 208. [Google Scholar] [CrossRef]
- Elsalahy, H.; Bellingrath-Kimura, S.; Kautz, T.; Döring, T. Effects of mixing two legume species at seedling stage under different environmental conditions. PeerJ 2021, 9, e10615. [Google Scholar] [CrossRef] [PubMed]
- Bouma, T.J.; Nielsen, K.L.; Van Hal, J.; Koutstaal, B. Root system topology and diameter distribution of species from habitats differing in inundation frequency. Funct. Ecol. 2001, 15, 360–369. [Google Scholar] [CrossRef]
- Fitter, A.H. Architecture and Biomass Allocation as Components of the Plastic Response of Root Systems to Soil Heterogeneity. In Exploitation of Environmental Heterogeneity by Plants; Caldwell, M.M., Pearcy, R.W., Eds.; Academic Press: Boston, MA, USA, 1994; pp. 305–323. [Google Scholar] [CrossRef]
- Oppelt, A.L.; Kurth, W.; Jentschke, G.; Godbold, D.L. Contrasting rooting patterns of some arid-zone fruit tree species from Botswana—I. Fine root distribution. Agrofor. Syst. 2005, 64, 1–11. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M.; Nadelhoffer, K.J.; McClaugherty, C.A.; Pastor, J. Fine root turnover in forest ecosystems in relation to quantity and form of nitrogen availability: A comparison of two methods. Oecologia 1985, 66, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.; Gu, H.; Zhang, R.; Yang, X. Root architecture characteristics of five natural homogeneous grasslands in riparian buffers from lower reaches of Yellow River. Grassl. Sci. 2022, 68, 301–309. [Google Scholar] [CrossRef]
- Fransen, B.; Blijjenberg, J.; de Kroon, H. Root morphological and physiological plasticity of perennial grass species and the exploitation of spatial and temporal heterogeneous nutrient patches. Plant Soil 1999, 211, 179–189. [Google Scholar] [CrossRef]
- Belkacem El, A. Exploring the importance of root architecture plasticity in plant adaptation to environmental constraints. Plant Species Biol. 2023. [Google Scholar] [CrossRef]
- Zhiyong, Z.; Baomin, F.; Chao, S.; Xiaoxian, Z.; Qingwen, Z.; Bing, Y. Advances in Root System Architecture: Functionality, Plasticity, and ResearchMethods. J. Resour. Ecol. 2023, 14, 15–24. [Google Scholar] [CrossRef]
- Balduzzi, M.; Binder, B.M.; Bucksch, A.; Chang, C.; Hong, L.; Iyer-Pascuzzi, A.S.; Pradal, C.; Sparks, E.E. Reshaping Plant Biology: Qualitative and Quantitative Descriptors for Plant Morphology. Front. Plant Sci. 2017, 8, 117. [Google Scholar] [CrossRef]
- Taub, D.R.; Goldberg, D. Root System Topology of Plants from Habitats Differing in Soil Resource Availability. Funct. Ecol. 1996, 10, 258. [Google Scholar] [CrossRef]
- Li, S.; Wan, L.; Nie, Z.; Li, X. Fractal and Topological Analyses and Antioxidant Defense Systems of Alfalfa (Medicago sativa L.) Root System under Drought and Rehydration Regimes. Agronomy 2020, 10, 805–826. [Google Scholar] [CrossRef]
- Tatsumi, J.; Yamauchi, A.; Kono, Y. Fractal Analysis of Plant Root Systems. Ann. Bot. 1989, 64, 499–503. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, J.; Ni, H.; Wang, H.; Zhou, B. Three Subtropical Species Adapt to Drought by Reallocating Biomass and Adjusting Root Architecture. Forests 2023, 14, 806. [Google Scholar] [CrossRef]
- Chun, H.C.; Lee, S.; Choi, Y.D.; Gong, D.H.; Jung, K.Y. Effects of drought stress on root morphology and spatial distribution of soybean and adzuki bean. J. Integr. Agric. 2021, 20, 2639–2651. [Google Scholar] [CrossRef]
- Eshel, A. On the fractal dimensions of a root system. Plant Cell Environ. 1998, 21, 247–251. [Google Scholar] [CrossRef]
- Izumi, Y.; Iijima, M. Fractal and Multifractal Analysis of Cassava Root System Grown by the Root-Box Method. Plant Prod. Sci. 2002, 5, 146–151. [Google Scholar] [CrossRef]
- Liu, Y.; Bortier, M.F.; De Boeck, H.J.; Nijs, I. Root distribution responses to three-dimensional soil heterogeneity in experimental mesocosms. Plant Soil 2017, 421, 353–366. [Google Scholar] [CrossRef]
- Adams, C.B.; Reyes-Cabrera, J.; Nielsen, J.; Erickson, J.E. Root system architecture in genetically diverse populations of grain sorghum compared with shallow and steeply rooted monocultures. Crop Sci. 2020, 60, 2709–2719. [Google Scholar] [CrossRef]
- Alaguero-Cordovilla, A.; Gran-Gómez, F.; Tormos-Moltó, S.; Pérez-Pérez, J. Morphological Characterization of Root System Architecture in Diverse Tomato Genotypes during Early Growth. Int. J. Mol. Sci. 2018, 19, 3888. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).