Abstract
Poor growth is often observed in artificial young forests due to insufficient inorganic nitrogen in karst soils. However, little is known about the assimilatory demand of the whole plant for nitrate and the partitioning of nitrate assimilation in roots and leaves in woody plants grown in karst habitats. In this study, Broussonetia papyrifera (L.) Vent (B. papyrifera) seedlings were grown under nearly hydroponic conditions. The isotope mass balance approach was employed to quantify the δ15N values of the N assimilates in plant organs and in whole plants for B. papyrifera seedlings grown at different nitrate concentrations. The δ15N values of the N assimilates in the whole B. papyrifera seedlings showed a rising trend with increasing nitrate concentration. Increasing the supply of nitrate decreased the leaf–root difference in the δ15N values of the N assimilates for B. papyrifera seedlings. Quantifying the δ15N values of N assimilates in the whole B. papyrifera seedlings grown under different nitrate concentrations contributes to estimating the assimilatory demand of the B. papyrifera seedlings for nitrate. The leaf–root difference in the δ15N values of the N assimilates can be used to estimate the partitioning of nitrate assimilation in the roots and leaves.
1. Introduction
Vegetation restoration is the key to controlling karst rocky desertification. However, poor growth is often observed in artificial young forests due to insufficient inorganic nitrogen in karst soils [1,2]. Hence, it is necessary to effectively manage the supply of inorganic nitrogen in artificial forests. The major sources of inorganic nitrogen utilized by plants have been suggested to be nitrate and ammonium [3,4,5]. However, weakly alkaline soils (pH 7.8–8.4) are often observed in karst rocky desertification areas [6], and high pH values in soils usually lead to ammonia volatilization [7,8]. Hence, a high-nitrate and low-ammonium environment occurs in karst rocky desertification areas [8,9]. As a result, the supply of nitrate may be suitable for artificial young forest growth in such habitats. The uptake and assimilation of nitrate within plants depend on the internal demand and external supply [10,11]. Generally, a balance between internal nitrogen demand and external nitrogen supply is an ideal status for plants and contributes to preventing excess or limited nitrogen supply. However, little is known about the assimilatory demand of the whole plant for nitrate and the partitioning of nitrate assimilation in roots and leaves in woody plants grown in karst rocky desertification areas.
After uptake, nitrate may be assimilated in roots and/or leaves, and partially unassimilated nitrate returns to the medium [10,12]. The lighter N isotope (14N) is favored due to the kinetic process, and as a result, the heavier isotope (15N) is depleted in the product [10,13,14]. Accordingly, the effluxed unassimilated nitrate is enriched in 15N, and the whole-plant δ15N values are negative relative to the source [15]. The deviations in whole-plant δ15N values relative to source nitrogen δ15N values are referred to as the nitrogen isotope fractionation value [16]. Under the condition that nitrate is the sole nitrogen source, the nitrogen isotope discrimination of whole plants depends on nitrate reductase activity and the supply of reductants [17]. Generally, the influxed nitrate will be assimilated to as great a degree as possible with strong nitrate reductase activity and an adequate supply of reductant. Consequently, the amount of effluxed unassimilated nitrate will decrease, which will minimize the observed isotope fractionation for nitrate assimilation by plants. However, when plants experience nitrate reductase activity restriction and/or reductant restriction, the amount of effluxed unassimilated nitrate increases. Then, greater nitrogen isotope fractionation is observed. Hence, the nitrogen isotope fractionation value of whole plants is closely related to the assimilatory demand for nitrate.
Generally, there is preexisting nitrogen in young woody plants [18]. It is difficult to quantify the nitrogen isotope fractionation value of a whole woody plant grown at different nitrate concentrations owing to interference from the δ15N values of preexisting nitrogen. A simpler, more convenient approach is to quantify the nitrogen isotope fractionation value of N assimilates in whole woody plants grown at different nitrate concentrations to determine the assimilatory demand for nitrate over a greater time scale. Based on the isotope mass balance approach [19], the δ15N value of N assimilates in whole woody plants can be quantified for woody plants grown at different nitrate concentrations. Moreover, the δ15N values of N assimilates in plant organs can also be quantified using the isotope mass balance approach [19]. The δ15N values of newly acquired N assimilates in stems are derived from the mix of δ15N values of N assimilates in the roots and leaves. Consequently, the proportion of stem nitrogen obtained from the leaves (i.e., fleaf stem) can be estimated using a two end-member isotope mixing model [20,21]. Furthermore, the leaf–root difference in the δ15N values of N assimilates is closely linked with the partitioning of assimilatory activity between roots and leaves. Hence, quantifying the δ15N values of N assimilates in plant organs contributes to the estimation of the partitioning of nitrate assimilation in the roots and leaves.
There is increasing interest in using Broussonetia papyrifera (L.) Vent (B. papyrifera) for ecological reclamation [22]. B. papyrifera trees can rapidly colonize abandoned factories and are employed for mining rehabilitation. In addition, this species presents a wide array of potential uses, such as the utilization of its bark for paper production, leaves as a source of forage, roots and fruits for traditional Chinese medicinal purposes, and the entire plant as a bioethanol source. Its rapid growth, strong adaptability to adverse environments, and high economic value make B. papyrifera suitable for karst rocky desertification control [23,24,25,26]. In the present study, B. papyrifera seedlings were subjected to different nitrate regimes. The effects of different nitrate concentrations on the growth, photosynthesis, chlorophyll fluorescence, nitrate reductase activity in leaves and roots, nitrogen content of plant organs, and δ15N values of plant organs of B. papyrifera seedlings were investigated. The following were our main aims: (1) to estimate the assimilatory demand of whole B. papyrifera seedlings for nitrate over a greater time scale and (2) to estimate the partitioning of nitrate assimilation in the roots and leaves for B. papyrifera seedlings grown at different nitrate concentrations.
2. Materials and Methods
2.1. Plant Material and Experimental Treatments
Seeds of B. papyrifera were germinated in 12 drainage-hole-containing double-layer basin with a mixture of perlite and vermiculite (1:1 v/v) for 2 weeks at a temperature of 26/20 °C in the light/dark and 50–55% relative humidity. The lower basin contained a certain amount of water to keep the mixture moist. Seedlings of B. papyrifera were then incubated under a 12-h photoperiod, with 500 ± 20 μmol m−2 s−1 of photosynthetic photon flux density (PPFD). The lower basin contained adequate 1/8 strength Hoagland nutrient solution [27], and the solution was completely replaced every 3 days. After 6 weeks, vigorous seedlings were transplanted to pots (height of 8.5 cm, bottom diameter of 9 cm, and 12 holes at the bottom, which were 0.9 cm in diameter). Two layers of nylon mesh were placed inside the pot, and then a mixture of perlite and vermiculite (1:1 v/v) was added to the pot to fix the roots of the seedling. Each pot contained only one seedling. Six pots were placed in a tray that contained adequate 1/4 strength Hoagland nutrient solution [27]. The seedlings in the pots grew well without extra aeration. The tray (not including the pot) was covered with aluminum foil to prevent algal growth from light infiltration into the solution. The solution in the tray was completely replaced every 3 days, and the surface of the pot and the tray were cleaned to avoid algal contamination. After 3 weeks of growth, the nutrient solution was replaced by a modified Hoagland solution containing 1 mM MgSO4·7H2O, 0.125 mM KH2PO4, 2.5 mM KCl, 4 mM CaCl2, 0.1875 mM K2SO4, 50 μM Fe(Na)EDTA, 25 μM H3BO3, 2 μM MnSO4·1H2O, 2 μM ZnSO4·7H2O, 0.1 μM CuSO4, 0.04 μM CoCl2·6H2O, and 0.1 μM Na2MoO4·2H2O at a pH of 7.3 ± 0.1. NaNO3, with a δ15N of 22.35‰, was employed as the sole nitrogen source. The nitrate concentrations in the three treatments were set at 0.5 mM, 2 mM, and 8 mM. Each treatment contained three replicates, and each seedling was treated as a replicate. The seedlings in all treatments achieved the same growth status. Each pot was placed in a tray that contained 500 mL modified Hoagland solution. The small tray (not including the pot) was covered with aluminum foil to prevent algal growth from light infiltration into the solution. The modified Hoagland solution in the small tray was completely replaced every other day, and the surface of the pot and the tray were cleaned to avoid algal contamination. The treatments lasted for 20 days.
2.2. Measurements of Growth
After 20 days of culture, all B. papyrifera seedlings were harvested and divided into leaves, stems, and roots. The dry weights of the leaves, stems, and roots were determined after oven drying to constant mass at 80 °C. To obtain the dry weight of the leaves, stems, and roots of the B. papyrifera seedlings at the start of the experiment, three B. papyrifera seedlings with the same growth status were selected, and the corresponding dry weights were measured. The average dry weight of the leaves, stems, and roots of the three B. papyrifera seedlings at the start of the experiment was approximately equal to the initial dry weight of the leaves, stems, and roots of the B. papyrifera seedlings in this study [see Table A1]. Accordingly, the average nitrogen contents of the leaves, stems, and roots of the three B. papyrifera seedlings at the start of the experiment were approximately equal to the initial nitrogen contents of the leaves, stems, and roots of the B. papyrifera seedlings in this study [see Table A1]. In addition, the average δ15N values of the leaves, stems, and roots of the three B. papyrifera seedlings at the start of the experiment were approximately equal to the initial δ15N values of the leaves, stems, and roots of the B. papyrifera seedlings in this study [see Table A1].
2.3. Measurement of Chlorophyll Content and Gas Exchange
At the final harvest, the chlorophyll (Chl) content in the second fully expanded leaf was determined using the chlorophyll meter SPAD-502Plus (Konica Minolta, Tokyo, Japan). The gas exchange measurements were performed with a portable photosynthesis system LI-6800 (LI-COR, Lincoln, NE, USA). The photosynthetically available radiation, leaf temperature, relative humidity, and CO2 concentration during the measurements were 500 μmol m−2 s−1, 27.0 °C, 55%, and 400 μmol m−2 s−1, respectively. The net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) of the second fully expanded leaves were measured in the fluorescence leaf chamber using 6800-01A from 09:00 to 11:00.
2.4. Chlorophyll Fluorescence Measurements
Leaves were dark-adapted for 30 min to ensure complete relaxation of all reaction centers before the measurements. As mentioned earlier, the second fully expanded leaves were selected for Chl fluorescence measurements with a portable photosynthesis system LI-6800 (LI-COR, Lincoln, NE, USA). The initial (Fo) and maximum (Fm) Chl fluorescence were measured, and then, the maximum photochemical efficiency (Fv/Fm) was calculated. The maximum fluorescence (Fm′) in the light-adapted state, basic fluorescence after induction (Fo′), and fluorescence yield in the steady state (Fs) were simultaneously determined while determining the Pn. The Fv/Fm, the actual photochemical efficiency of PSII (Φp), the photochemical quenching coefficient (qP), the nonphotochemical quenching coefficient (qN), and the electron transport rate (ETR) were calculated according to the following formulae:
2.5. Analysis of Elements and Determination of δ15N in Plants
The nitrogen contents of the dried leaves, stems, and roots were determined using an elemental analyzer (vario MACRO cube, Langenselbold, Germany). The δ15N values of the leaves, stems, and roots were measured using a gas isotope ratio mass spectrometer (MAT-253, Thermo Fisher Scientific, Langenselbold, Germany). The δ15N values were calculated according to the following equation:
where Rsample refers to the nitrogen isotope ratio of the plant material and Rstandard refers to the isotope ratio of a known standard (N2 in air). IAEA N1, IAEA N2, and IAEA NO3 reference materials were used to calibrate the instrument to reach a precision of 0.2‰ [28].
2.6. Photosynthetic Nitrogen Use Efficiency
After determining the nitrogen contents and net photosynthetic rate of leaves, the photosynthetic nitrogen use efficiency (PNUE) was obtained by dividing the value of Pn by the N content of the leaf [29,30].
2.7. Nitrate Reductase Activity Determination
The nitrate reductase activity (NRA) in the leaves and roots was tested using a Micro Nitrate Reductase Assay Kit (Solarbio, Beijing, China). The NRA was expressed as U/g FW.
2.8. The δ15N of the Whole Plant
Using an isotope mass balance approach [19], the integrated δ15N values of the whole plant consisting of leaves, stems, and roots were calculated as follows:
where mleaf, mstem, and mroot are the total N accumulation (g) of the leaves, stems, and roots, respectively. δ15Nleaf, δ15Nstem, and δ15Nroot represent the δ15N values of the leaves, stems, and roots, respectively.
2.9. The δ15N of N Assimilates in the Whole Plant
Based on the isotope mass balance approach [19], we were able to calculate the δ15N of the N assimilates in the whole plant when the δ15N values of the initial and final whole plant (i.e., integrated δ15N values of the whole plant at the beginning and harvest stages) were calculated. The δ15N of the N assimilates in the whole plant (δ15Nassimilates) was calculated using the following equations:
where δ15Nwhole-plant1 and δ15Nwhole-plant0 represent the δ15N values of the final and initial whole plant, respectively. m and m0 are the total N accumulation (g) of the final and initial whole plant, respectively. δ15Nl, δ15Ns, and δ15Nr represent the δ15N values of the leaves, stems, and roots at the final harvest, respectively. ml, ms, and mr are the total N accumulation (g) of the leaves, stems, and roots at the final harvest, respectively. δ15Nl0, δ15Ns0, and δ15Nr0 represent the δ15N values of the leaves, stems, and roots at the start of the experiment, respectively. ml0, ms0, and mr0 are the total N accumulation (g) of the leaves, stems, and roots at the start of the experiment, respectively. The standard error (SE) of the δ15Nassimilates was achieved using the error propagation formula [31].
2.10. The δ15N of N Assimilates in Plant Organs
For woody plant species, we treat the plant as having three major organs. We assume that the major sites of nitrogen assimilation are only leaves and roots. The δ15N of the N assimilates in the stem can be predicted using a two end-member isotope mixing model using the δ15N of the N assimilates in the leaves and roots as end members depending on the source for that stem nitrogen [20,21]:
Equation (9) was rearranged to yield the fraction of stem N that is from the roots (froot stem) or the leaves (fleaf stem). For the fraction from the leaves, note that froot stem = 1 − fleaf stem:
where δ15Nleaf-assimilates, δ15Nstem-assimilates, and δ15Nroot-assimilates can be calculated using an isotope mass balance approach. The mass balance equations for δ15Nleaf-assimilates, δ15Nstem-assimilates, and δ15Nroot-assimilates are as follows:
where δ15Nleaf-assimilates and δ15Nroot-assimilates represent the δ15N values of N assimilates in leaves and roots, respectively. δ15Nstem-assimilates represents the δ15N values of newly acquired N assimilates in stems. The standard error (SE) of the fleaf stem, δ15Nleaf-assimilates, δ15Nstem-assimilates, and δ15Nroot-assimilates was determined using the error propagation formula [31].
2.11. Statistical Analysis
The data were subjected to analysis of variance (ANOVA). The means of the different groups were compared via Tukey’s test (p < 0.05). The data are shown as the mean ± standard deviation (SE). All analyses were conducted using Data Processing System (DPS) software 7.05 (Hangzhou Ruifeng Information Technology Co., Ltd., Hangzhou, China).
3. Results
3.1. Growth
The nitrate concentration had a significant effect on the growth of B. papyrifera seedlings. As shown in Table 1, the dry weight of the B. papyrifera seedlings increased significantly with increasing nitrate supply. However, compared to the dry weight of the leaves, stems, and roots at a 2 mM nitrate concentration, the excessive nitrate supply (8 mM) did not lead to a significant increase in dry biomass accumulation. In addition, the shoot length of B. papyrifera seedlings did not show a significant increase when the nitrate concentration increased from 2 to 8 mM. These results indicated that excessive nitrate supply did not significantly promote the growth of B. papyrifera seedlings.

Table 1.
The growth parameters of Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes.
3.2. Photosynthesis, SPAD, and Chl Fluorescence
We monitored the SPAD values and gas exchange parameters to determine the effects of different nitrate levels. With increasing nitrate supply, SPAD, Pn, Gs, and Tr showed a significant increasing trend (Table 2). The SPAD values and gas exchange parameters of B. papyrifera seedlings showed a positive response to nitrate concentration.

Table 2.
The photosynthetic parameters, chlorophyll fluorescence, and SPAD of Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes.
Chl fluorescence was further investigated to understand the internal causes of the effects of different nitrate levels on photosynthesis. The B. papyrifera seedlings grown at the lowest nitrate concentration had a significantly lower Fv/Fm, Φp, qP, and ETR, while the qN was the highest (Table 2). These results suggested that the activity of PSII was significantly affected by the supply of nitrate. Because of the N deficiency (0.5 mM nitrate), the B. papyrifera seedlings seemed to suffer from photoinhibition.
3.3. Nitrogen Content in the Leaves, Stems, and Roots of B. papyrifera Seedlings
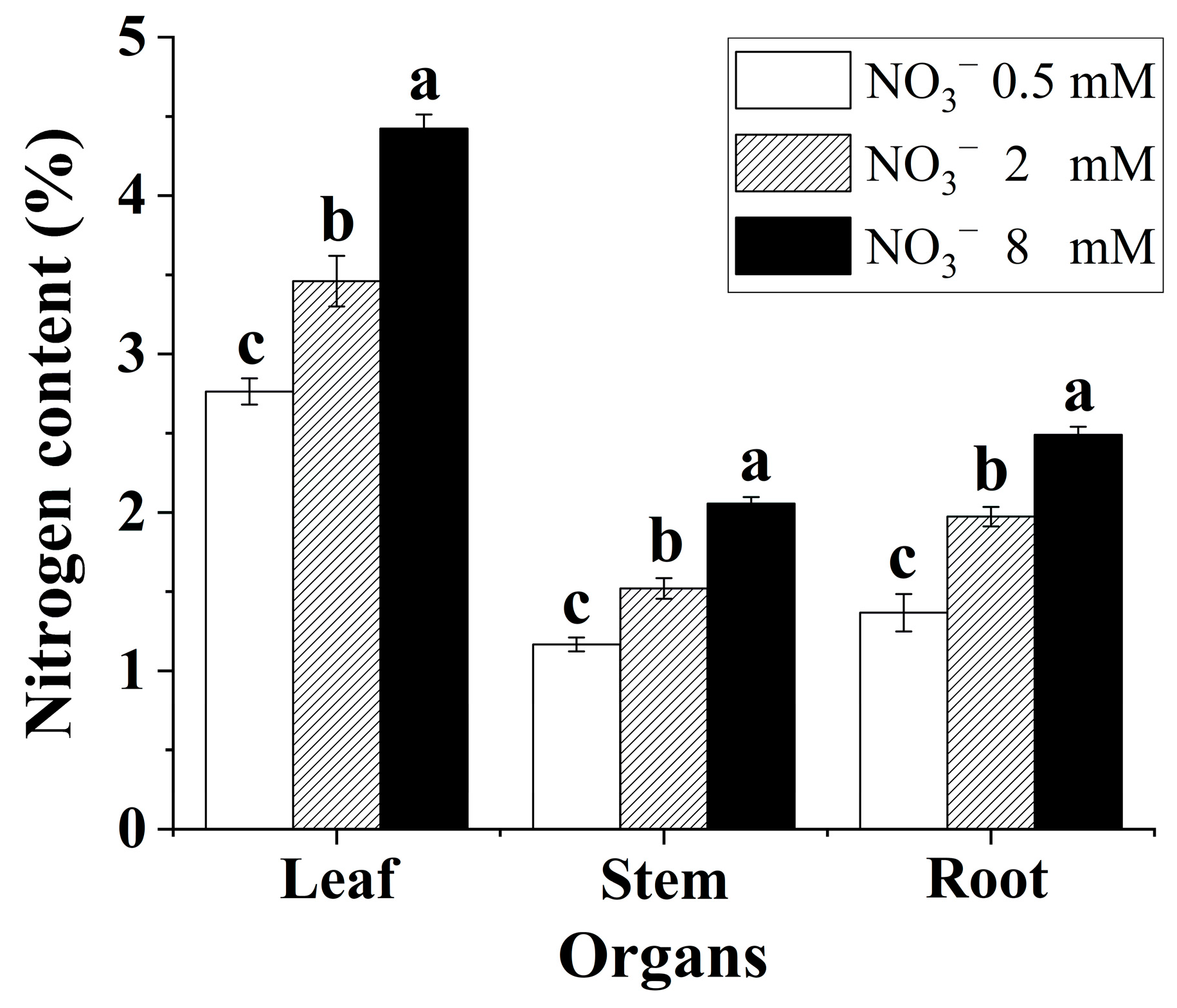
Increasing the nitrate supply significantly promoted nitrogen assimilation in the B. papyrifera seedlings. As shown in Figure 1, the nitrogen content in the leaves, stems, and roots showed a significant rising trend with increasing nitrate concentration. In addition, the nitrogen content of leaves was markedly higher than that of roots and stems in all treatments.
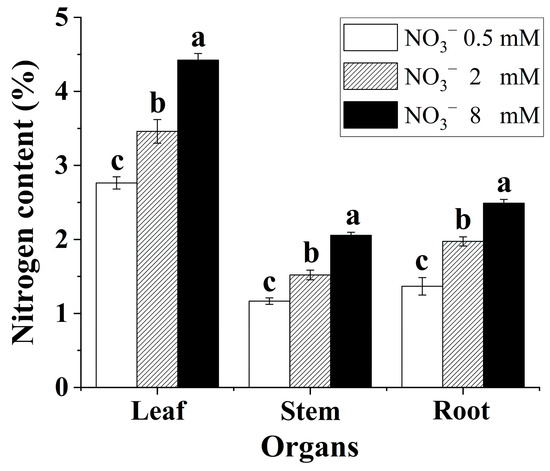
Figure 1.
Nitrogen content of the Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes. The mean ± SE (n = 3) followed by different letters in the same legend differ significantly (Tukey’s test, p < 0.05).
3.4. Photosynthetic Nitrogen-Use Efficiency (PNUE)
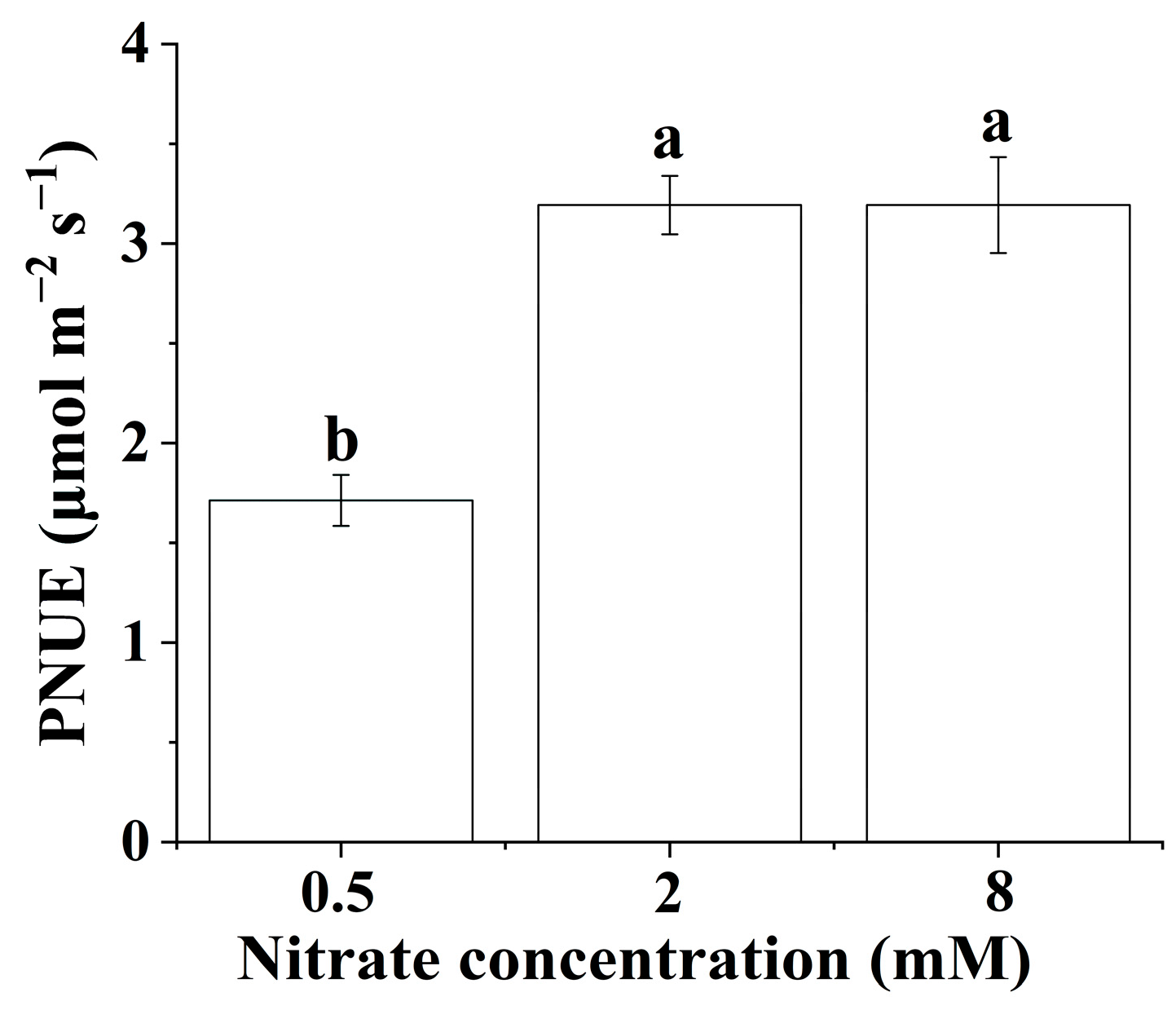
The PNUE of the B. papyrifera seedlings only showed significant differences at the lowest nitrate concentration. As shown in Figure 2, increasing the nitrate supply did not significantly affect the PNUE of the B. papyrifera seedlings when the nitrate concentration was in the range of 2 to 8 mM.
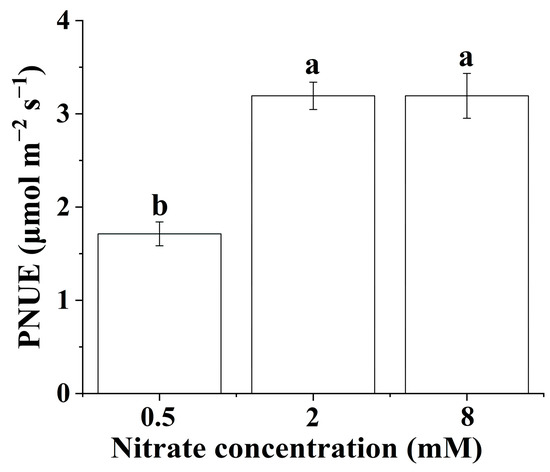
Figure 2.
Photosynthetic nitrogen-use efficiency of Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes. The mean ± SE (n = 3) followed by different letters in the same legend differ significantly (Tukey’s test, p < 0.05).
3.5. NRA in the Leaves and Roots
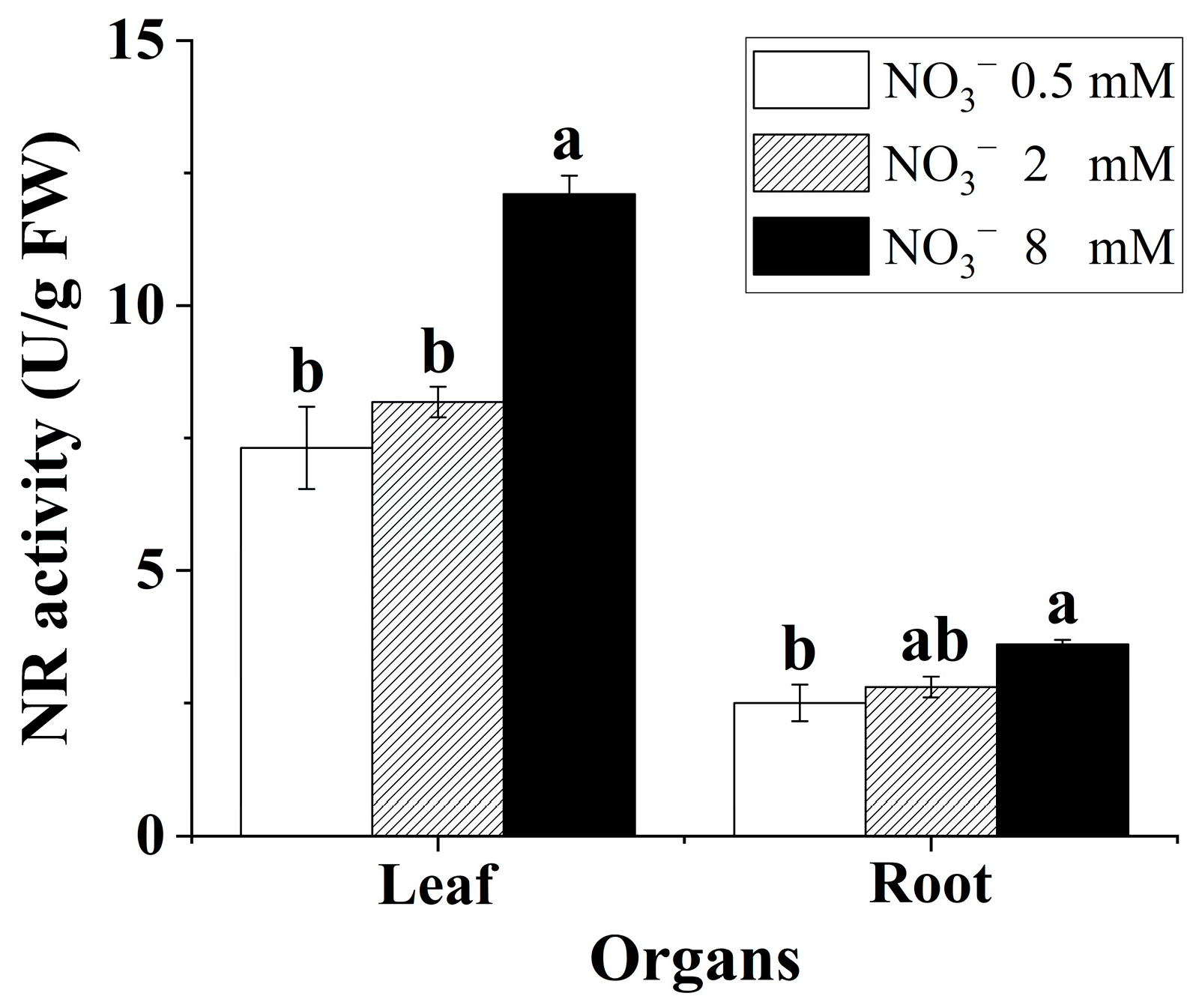
Nitrate supply had a significant effect on the NRA in the leaves and roots (Figure 3). Increasing the nitrate concentration contributed to enhancing the NRA in the leaves and roots. Generally, the NRA in the leaves was markedly higher than that in the roots for the B. papyrifera seedlings.
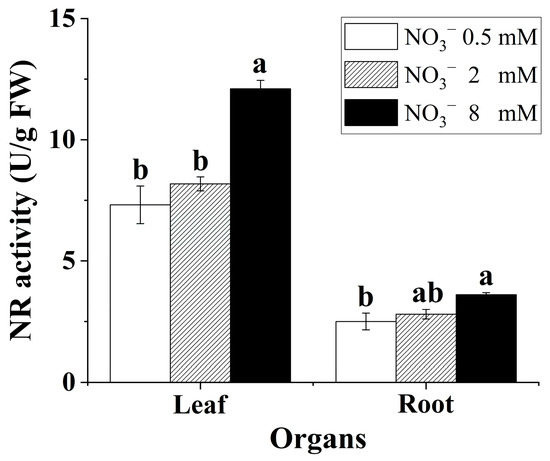
Figure 3.
NRA in the leaves and roots of Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes. The mean ± SE (n = 3) followed by different letters in the same legend differ significantly (Tukey’s test, p < 0.05).
3.6. Nitrogen Isotope Composition in the Leaves, Stems, and Roots of the B. papyrifera Seedlings
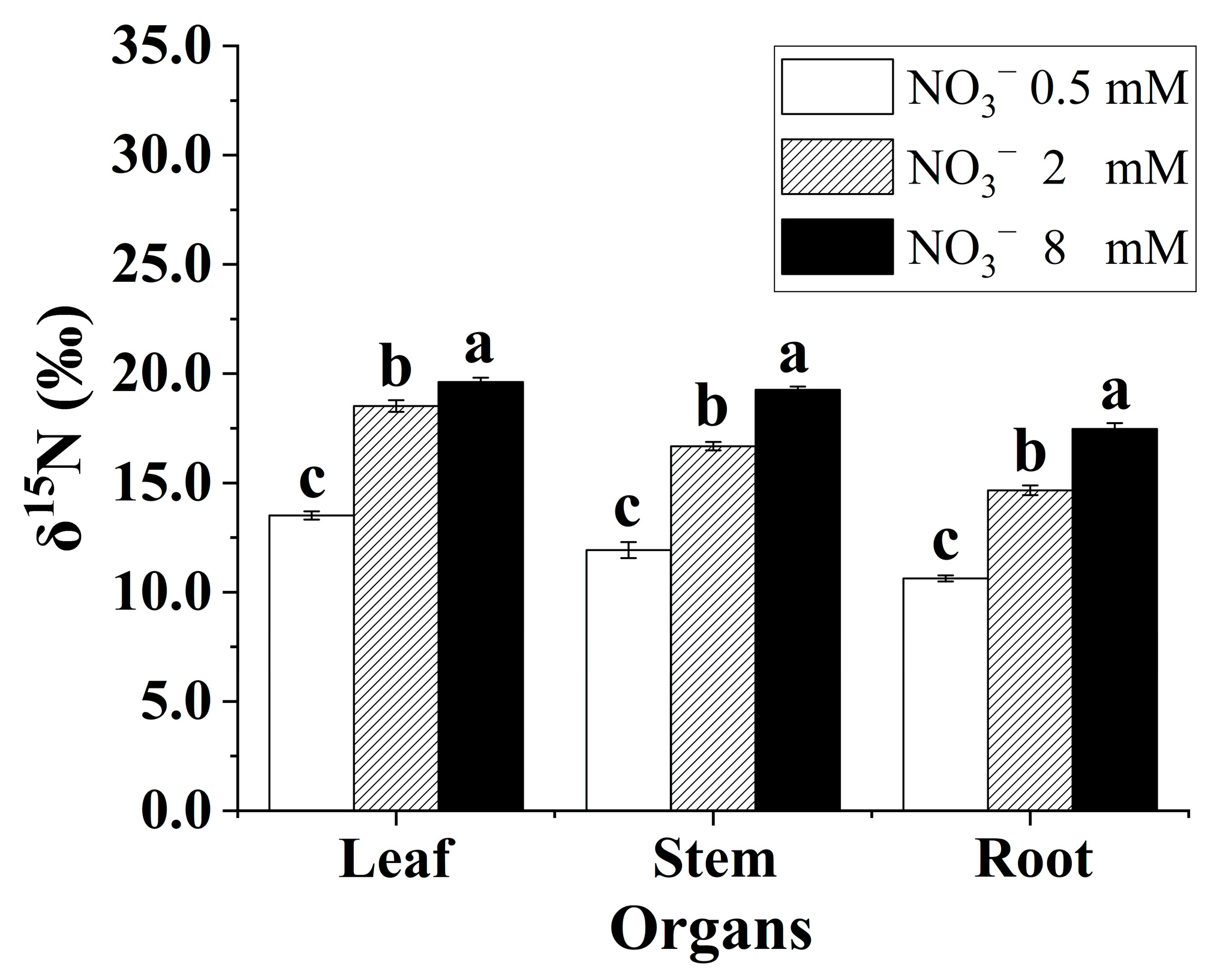
There were differences in organ-level δ15N for the B. papyrifera seedlings grown under the three nitrate regimes (Figure 4). Generally, the leaves were consistently enriched in 15N relative to the roots. In addition, the δ15N in the leaves, stems, and roots showed a significant rising trend with increasing nitrate concentration. Increasing the supply of nitrate contributed to enriching 15N in the B. papyrifera seedlings.
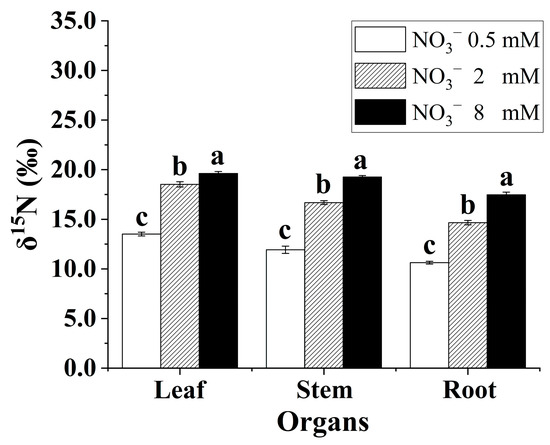
Figure 4.
The δ15N in leaves, stems, and roots of the Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes. The mean ± SE (n = 3) followed by different letters in the same legend differ significantly (Tukey’s test, p < 0.05).
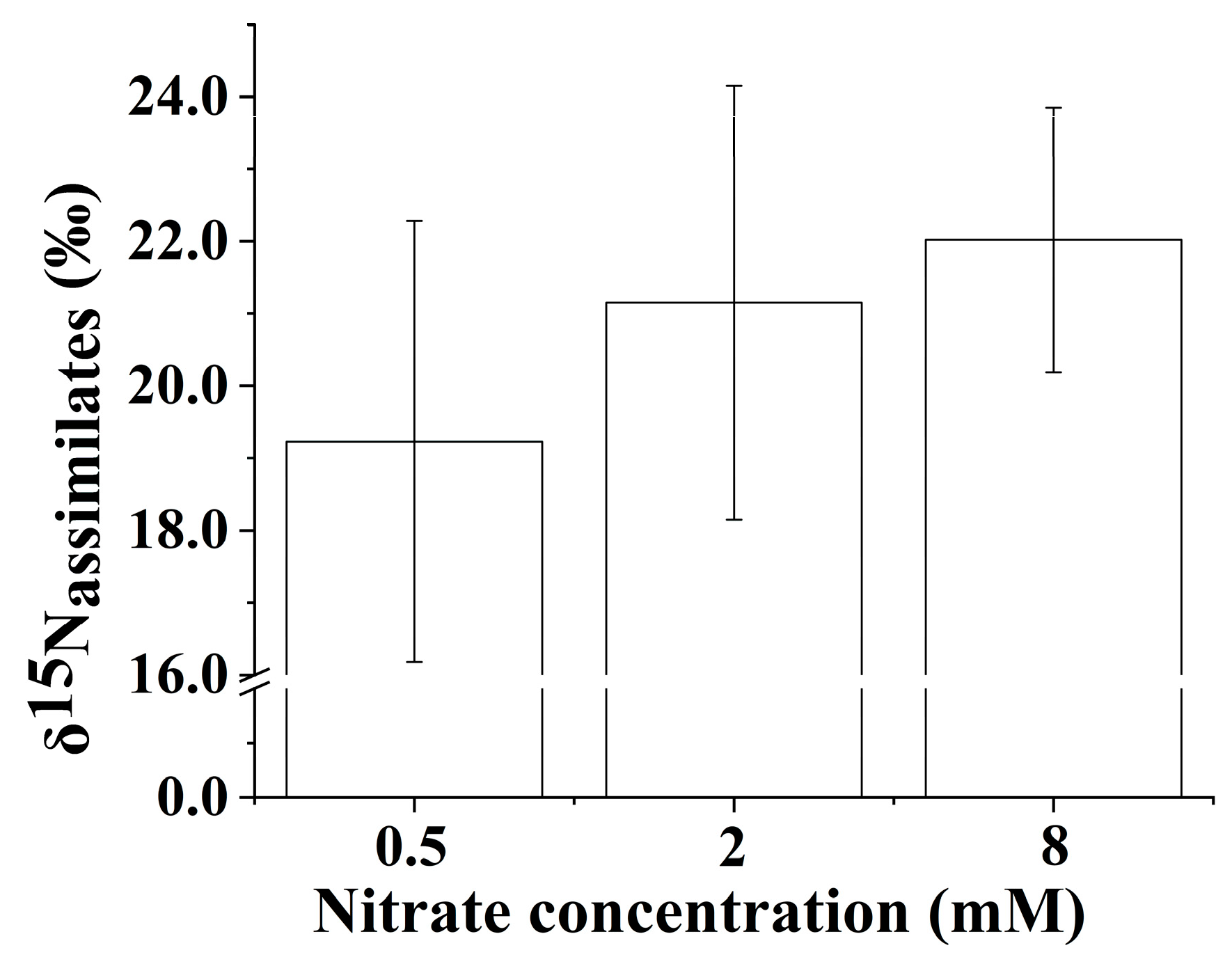
3.7. The δ15N Values of N Assimilates in the Whole Plant
The δ15N values of the N assimilates in the whole B. papyrifera seedlings were negative compared to the initial source δ15N under the three nitrate regimes (Figure 5), which suggested that isotope discrimination occurred during the process of nitrate assimilation. Increasing the nitrate concentration contributed to enriching 15N in the N assimilates in the whole B. papyrifera seedlings. The δ15N values of the N assimilates in the whole B. papyrifera seedlings at the lowest nitrate concentration were distinctly lower than that at other nitrate concentrations.
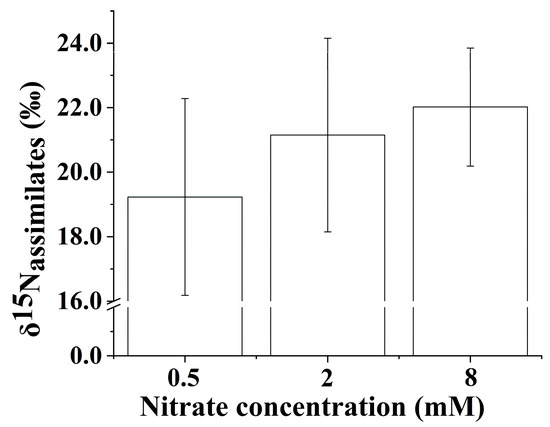
Figure 5.
The δ15N values of the N assimilates in the whole Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes. The error bars were calculated using the error propagation formula.
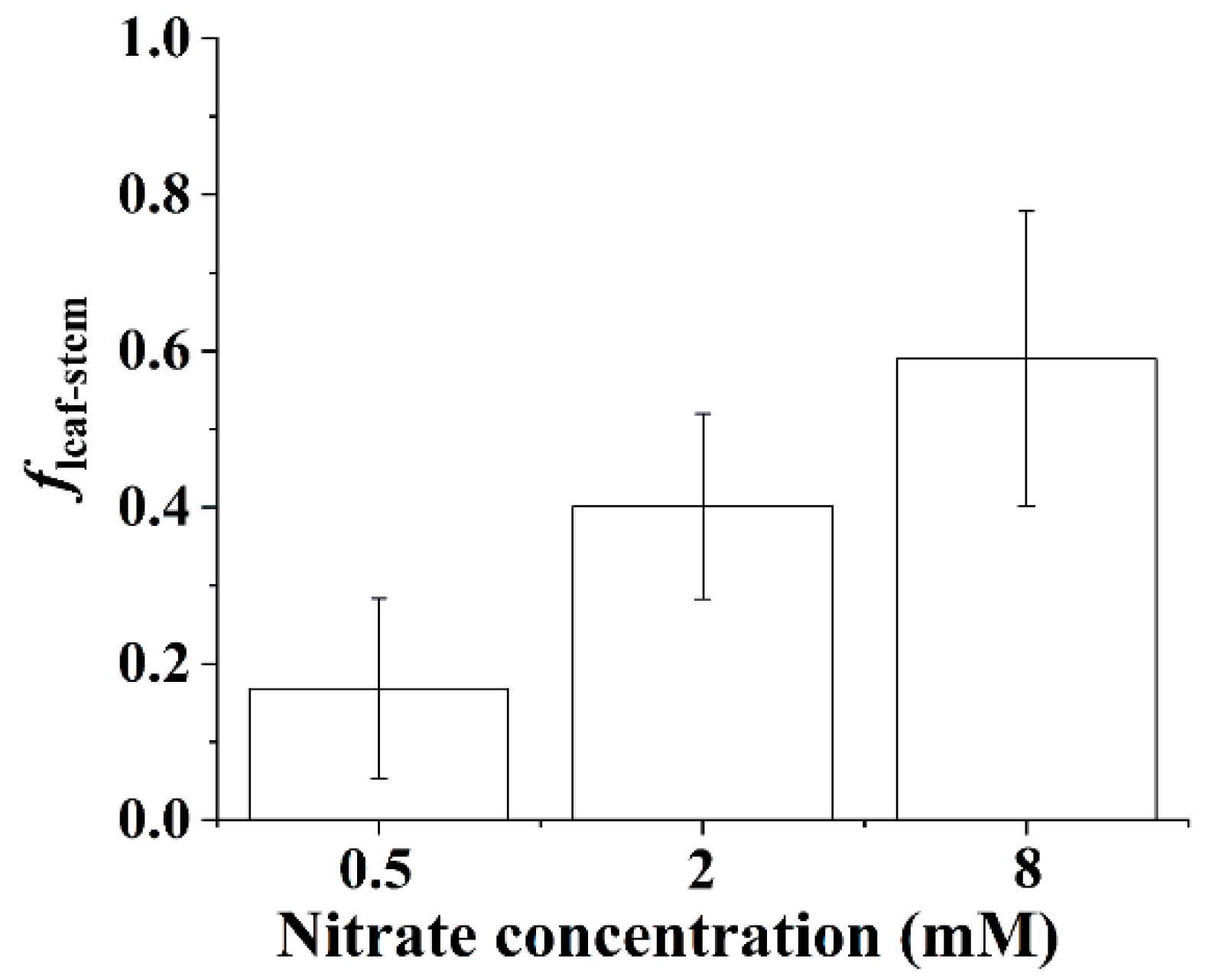
3.8. Proportion of Stem N Derived from the Leaves
The proportion of stem N derived from the leaves showed a linear increase with nitrate concentration for the B. papyrifera seedlings (Figure 6). As shown in Figure 6, increasing the supply of nitrate contributed to the translocation of the N assimilates from the leaves to the stems. We observed that approximately 60% of stem N was derived from the translocation of N assimilates from the leaves when the nitrate concentration reached 8 mM.
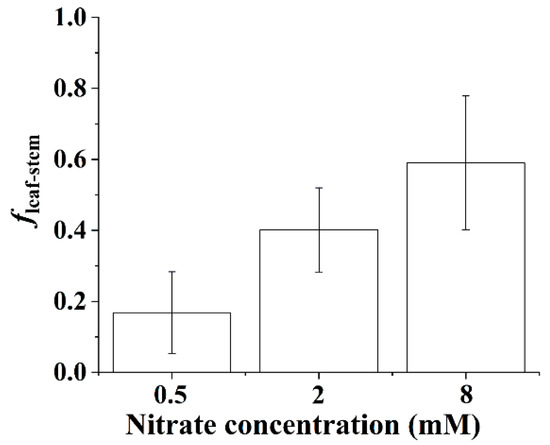
Figure 6.
Proportion of stem N derived from the leaves of the Broussonetia papyrifera (L.) Vent seedlings under the three nitrate regimes. The error bars were calculated using the error propagation formula.
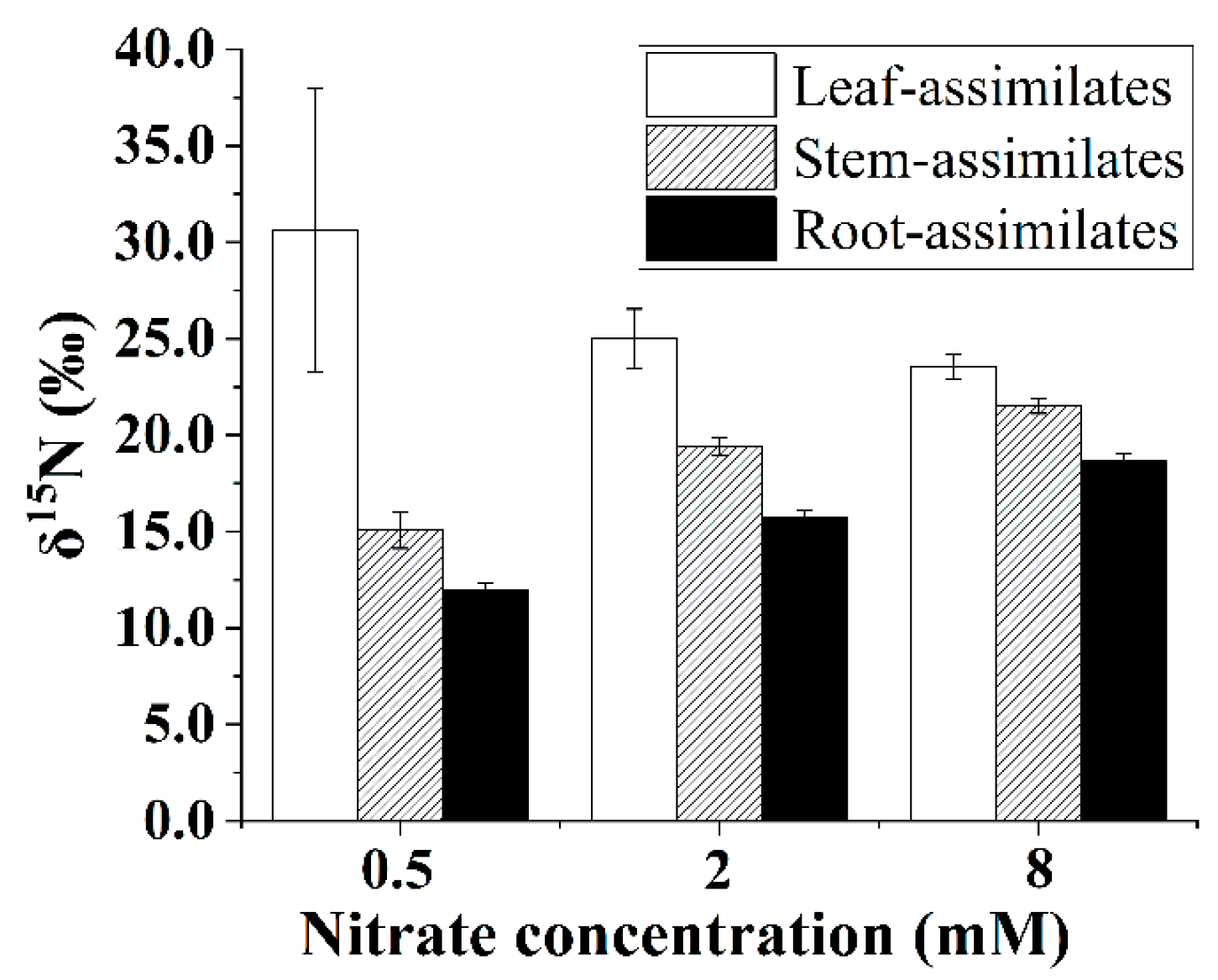
3.9. The δ15N of N Assimilates in Plant Organs
The δ15N values of the N assimilates in the leaves, stems, and roots of the B. papyrifera seedlings depended on the nitrate supply. As shown in Figure 7, the δ15N values of the N assimilates in the leaves showed a decreasing trend with increasing nitrate concentration. However, increasing the supply contributed to enriching 15N in the N assimilates of the stems and roots of the B. papyrifera seedlings. In addition, the difference between the δ15N values of the N assimilates in the leaves and roots decreased gradually with increasing nitrate concentration.
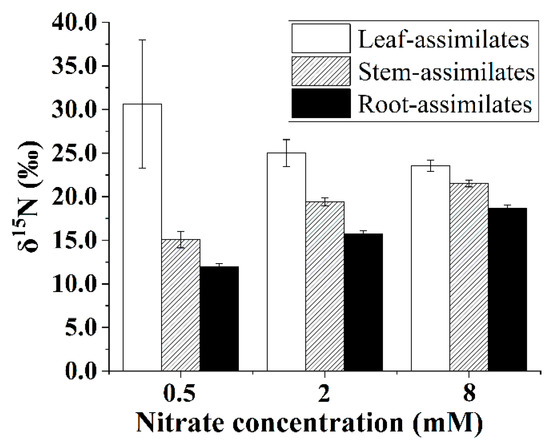
Figure 7.
The δ15N values of the N assimilates in the Broussonetia papyrifera (L.) Vent organs under the three nitrate regimes. The error bars were calculated using the error propagation formula.
4. Discussion
Plant δ15N is a physiological indicator of N demand and fractionation that reflects changes in metabolic N fluxes and/or environmental effects [10,11]. Generally, variation in N supply can affect organ-level nitrogen isotope composition [13,32]. As shown in Figure 4, there was variation in organ-level δ15N for the B. papyrifera seedlings grown under the three nitrate regimes. Enrichment of leaf δ15N relative to root δ15N for B. papyrifera grown at all nitrate concentrations indicated that some unassimilated inorganic nitrogen in the roots translocated to the shoot through the xylem [33]. Increasing the supply of nitrate enhanced the enrichment of 15N in the leaves, stems, and roots of the B. papyrifera seedlings, which might be attributed to an increased capacity to assimilate nitrate in the leaves and/or roots [33]. Increasing the supply of nitrate also led to a significant increase in the nitrogen content of the leaves, stems, and roots (Figure 1), which suggested that increasing the nitrate concentration contributed to improving the nitrogen assimilation ability of B. papyrifera seedlings. Hence, there might be a positive response between the enrichment of 15N and the nitrogen assimilation ability of B. papyrifera seedlings.
In the present study, the roots of B. papyrifera seedlings were always grown in the solution, which contributed to minimizing localized 15N enrichment of the solution around the roots. The uptake of nitrogen by B. papyrifera seedlings caused the nitrate concentration in the tray to decrease, which resulted in an 15N enrichment of the residual nitrate over time. Generally, the degree of 15N enrichment in the residual nitrate depended on the change in nitrate concentration in the solution; namely, the more the nitrate concentration in the solution decreased, the greater the residual nitrate enriched 15N [34]. After 20 days of culture, the B. papyrifera seedlings grown in three nitrate regimes (0.5 mM, 2 mM, and 8 mM) accumulated 0.0157 ± 0.0021 g N (n = 3, SE), 0.0510 ± 0.0056 g N (n = 3, SE), and 0.0807 ± 0.0048 g N (n = 3, SE), respectively. The total nitrogen supply of the three nitrate regimes (0.5 mM, 2 mM, and 8 mM) was 0.035 g N, 0.140 g N, and 0.560 g N, respectively. Hence, the δ15N value of the residual nitrate at low nitrate concentrations would be greater than that at high nitrate concentrations. Based on the isotope mass balance approach [19], the δ15N values of the N assimilates in the whole B. papyrifera seedlings grown at three nitrate regimes could be quantified. Because the actual δ15N value of the source (i.e., the residual nitrate in the solution) could not be known, it was very difficult to precisely calculate the nitrogen isotope discrimination for the N assimilates in the whole B. papyrifera seedlings. However, the δ15N values of the N assimilates in the whole B. papyrifera seedlings could still be used to indirectly indicate the degree of nitrogen isotope discrimination. The δ15N values of the N assimilates in the whole B. papyrifera seedlings gradually increased with increasing nitrate concentration (Figure 5). The actual δ15N value of the source (i.e., the residual nitrate in the solution) at the three nitrate concentrations was above 22.35‰ (the δ15N value of the initial source). Meanwhile, the actual δ15N value of the source (i.e., the residual nitrate in the solution) gradually decreased with increasing nitrate concentration. Hence, we concluded that increasing the nitrate concentration reduced the nitrogen isotope discrimination for B. papyrifera seedlings when the nitrate concentration was in the range of 0.5 to 8 mM [16].
In general, nitrogen isotope discrimination is dependent on the relationship between nitrogen supply and nitrogen demand [11]. Increased nitrogen isotope discrimination usually corresponds to a reduced assimilatory demand for nitrogen [35], while the enhanced assimilatory demand for nitrogen will reduce the efflux and result in decreased nitrogen isotope discrimination [34]. As a result, we speculated that the decrease in nitrogen isotope discrimination value (i.e., the increase in the δ15N value of the N assimilates in the whole B. papyrifera seedlings) was caused by enhanced assimilatory demand for nitrate in this study. Generally, increasing the supply of inorganic nitrogen leads to an increase in the nitrogen isotope discrimination value [13]. However, nitrogen isotope discrimination is not only dependent on the external inorganic nitrogen concentration. Nitrogen isotope discrimination was observed only when the demand for nitrogen was lower than the nitrogen supply [13]. Buschhaus [34] also found that increasing the supply of inorganic nitrogen resulted in a decrease in nitrogen isotope discrimination, which was attributed to the increased nitrogen demand resulting from stimulating growth. Hence, the assimilatory demand for nitrogen could be estimated using the δ15N value of the N assimilates in the whole plant. In this study, increasing the supply of nitrate increased the δ15N values of the N assimilates in the whole B. papyrifera seedlings when the nitrate concentration was in the range of 0.5 to 8 mM, which suggested that a relatively high external nitrate supply (8 mM) did not exceed the demand for nitrogen. In addition, the growth potential of the B. papyrifera seedlings also reflected that increasing the supply of nitrate contributed to enhancing the assimilatory demand for nitrate. As shown in Table 2, the Fv/Fm values of B. papyrifera seedlings were below 0.75 when the nitrate concentration was only 0.5 mM, which suggested that low N stress caused photoinhibition [36,37]. Photoinhibition usually damages the photosynthetic structure, thus significantly reducing the electron transport rate [37]. As a result, poor photosynthetic capacity was observed for B. papyrifera seedlings grown at 0.5 mM nitrate. Accordingly, the assimilatory demand for nitrate would be very low for B. papyrifera seedlings grown at 0.5 mM nitrate. As a whole, increasing the supply of nitrate could significantly enhance the photosynthetic capacity of B. papyrifera seedlings (Table 2). The enhanced photosynthetic capacity promoted the growth of B. papyrifera seedlings, which suggested that the demand for nitrate must have increased. Furthermore, increasing the nitrate concentration significantly elevated the nitrogen content of leaves, stems, and roots of B. papyrifera seedlings, which suggested that increasing the supply of nitrate enhanced the assimilatory demand for nitrate for B. papyrifera seedlings. Hence, quantifying the δ15N value of the N assimilates in the whole plant could estimate the assimilatory demand of the whole plant for nitrate over a greater time scale, which contributed to preventing the waste and insufficiency of the nitrate supply.
Given that effluxed nitrogen is enriched in 15N [15], the increased δ15N values of the N assimilates in the whole B. papyrifera seedlings indicated that the net efflux of nitrate in the roots decreased. Correspondingly, increasing the supply of nitrate enhanced the assimilation of nitrate in the B. papyrifera seedlings. Leaves and roots are thought to be the major sites of nitrogen assimilation [11]. Hence, the efflux of nitrate in the roots was closely related to the assimilation of nitrate in the leaves and roots. Generally, nitrate reductase is required for the assimilation of nitrate, NRA is inducible with available nitrate [38,39], and increasing the supply of nitrate contributes to enhancing the NRA [39,40]. In this study, the NRA in the leaves and roots showed a positive response to the nitrate concentration (Figure 3). However, the NRA was not the only factor that affected nitrogen isotopic fractionation. As shown in Figure 3, when the nitrate concentration was in the range of 0.5 to 2 mM, no significant difference was observed in the NRA in the roots, and the NRA in the leaves did not show a significant change. The δ15N values of the N assimilates in the whole B. papyrifera seedlings showed a marked increase when the nitrate concentration increased from 0.5 to 2 mM, which suggested that the increased assimilation of nitrate might benefit from an adequate supply of reductant [17]. As shown in Figure 2, the PNUE level was significantly higher in the 2 mM nitrate concentration than in the 0.5 mM nitrate concentration. The high PNUE level was accompanied by an adequate supply of reductant. The PNUE level did not show a significant increase when the nitrate concentration increased from 2 to 8 mM. Hence, the increased δ15N values of the N assimilates in the whole B. papyrifera seedlings might be attributed to the significantly enhanced NRA in the leaves when the nitrate concentration increased from 2 to 8 mM (Figure 3). Overall, the degree of nitrogen isotopic fractionation observed for nitrate assimilation depended on the NRA and the supply of reductant for the young, rapidly growing B. papyrifera seedlings.
Generally, the leaves had higher δ15N values than the roots [15,18,33], which was the expected pattern because residual unassimilated nitrate (enriched in 15N) is transported from roots to shoots. Accordingly, the δ15N values of the N assimilates in the leaves were higher than those in the roots for the B. papyrifera seedlings (Figure 7). As shown in Figure 7, increasing the nitrate concentration decreased the leaf–root difference in the δ15N values of the N assimilates, which might have been related to the partitioning of assimilatory activity between the roots and leaves. Although the NRA in the leaves was considerably greater than that in the roots (Figure 3), the location of nitrate assimilation appeared to depend on the internal demand of the B. papyrifera seedlings as well as the external nitrate concentration [33]. Under nonsaturating nitrate conditions, substantial nitrate will be assimilated by root nitrate reductase, and less nitrate may be assimilated in leaves [10]. A previous study also showed that assimilation is weighted more toward roots, particularly at concentrations below 1 mM nitrate [41]. Low assimilatory demand for nitrate was evidenced by the δ15N value of the N assimilates in the whole B. papyrifera seedlings grown at 0.5 mM nitrate. Hence, only a smaller proportion of nitrate was assimilated in the leaves of the B. papyrifera seedlings grown at 0.5 mM nitrate. As a result, distinct leaf–root differences in the δ15N values of the N assimilates were observed for the B. papyrifera seedlings grown at 0.5 mM nitrate (Figure 7). With increasing nitrate concentration, the assimilatory demand for nitrate in the roots reached saturation, and then, substantial nitrate was assimilated in the leaves, which led to a decrease in the δ15N values of the N assimilates in the leaves. Consequently, a gradually decreased leaf–root difference in the δ15N values of the N assimilates was observed for B. papyrifera seedlings when the nitrate concentration increased from 2 to 8 mM (Figure 7). Hence, the leaf–root difference in the δ15N values of the N assimilates could be used to estimate the partitioning of nitrate assimilation in the roots and leaves. The newly acquired organic nitrogen in the stems is translocated from the leaves and roots [11,15]. Hence, when the δ15N values of the N assimilates in the leaves, stems, and roots are quantified using the isotope mass balance approach [19], the proportion of stem N derived from the leaves (i.e., fleaf stem) can be calculated using Equation (10). As shown in Figure 6, the proportion of stem nitrogen obtained from the leaves showed an obviously rising trend with increasing nitrate concentration, which suggested that increasing the supply of nitrate promoted the translocation of the N assimilates from the leaves to the stems. The increased translocation of the N assimilates from the leaves to the stems might imply that the nitrate assimilation was weighted more toward leaves with increasing nitrate concentration. Hence, quantifying the δ15N values of the N assimilates in the plant organs not only contributes to estimating the partitioning of nitrate assimilation in roots and leaves but also provides an alternate way to calculate the proportion of stem N derived from the leaves.
5. Conclusions
Based on the isotope mass balance approach, the δ15N values of N assimilates in plant organs and in whole plants can be quantified for B. papyrifera seedlings grown at different nitrate concentrations. The δ15N values of N assimilates in whole B. papyrifera seedlings can be used to estimate the assimilatory demand of whole B. papyrifera seedlings for nitrate over a greater time scale. Increasing the supply of nitrate contributes to enhancing the assimilatory demand of the whole B. papyrifera seedlings for nitrate when the nitrate concentration was in the range of 0.5 to 8 mM. However, our results suggested that a concentration of 0.5 mM nitrate was insufficient to maintain the health of B. papyrifera seedlings. The optimal nitrate supply for B. papyrifera seedlings varies depending on the intended objective. To achieve ecological restoration, a 2 mM nitrate supply is recommended, whereas a nitrate supply of 8 mM is optimal for achieving a leaf forage with high protein content. Hence, quantifying the δ15N values of N assimilates in whole B. papyrifera seedlings grown under different nitrate concentrations contributes to preventing the waste and insufficiency of the nitrate supply, which provides a theoretical basis for effective inorganic nitrogen management in B. papyrifera seedlings grown in karst regions. The partitioning of nitrate assimilation in the roots and leaves can be estimated using the leaf–root difference in the δ15N values of the N assimilates for B. papyrifera seedlings grown under different nitrate concentrations. Increasing the nitrate supply contributes to increasing the partitioning of nitrate assimilation in leaves relative to roots.
Author Contributions
K.Z. and Y.W. conceived and designed the experiment. F.Z. performed most of the experiment. H.L. performed some of the experiment. K.Z. and F.Z. analyzed the data. K.Z. wrote the manuscript. Y.W. and Y.S. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Guizhou Provincial Science and Technology Foundation ([2020]1Y172) and the National Natural Science Foundation of China (32001101).
Data Availability Statement
All data generated or analyzed during this study are included in this published article and Table A1.
Acknowledgments
The authors would like to thank the technical staff at the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, for technical assistance during the measurement of the δ15N, in particular J.T.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The initial biomass, nitrogen content and δ15N of the Broussonetia papyrifera (L.) Vent seedlings at the start of the experiment.
Table A1.
The initial biomass, nitrogen content and δ15N of the Broussonetia papyrifera (L.) Vent seedlings at the start of the experiment.
| Parameters | Plant Organs | ||
|---|---|---|---|
| Leaves | Stems | Roots | |
| Dry weight (g) | 0.348 ± 0.031 | 0.075 ± 0.006 | 0.070 ± 0.011 |
| Nitrogen content (%) | 4.53 ± 0.02 | 2.81 ± 0.01 | 3.15 ± 0.01 |
| δ15N (‰) | 7.51 ± 0.09 | 6.97 ± 0.04 | 6.46 ± 0.02 |
Note: Each value represents the mean ± SE (n = 3).
References
- Garousi, F.; Shan, Z.J.; Ni, K.; Yang, H.; Shan, J.; Cao, J.H.; Jiang, Z.C.; Yang, J.L.; Zhu, T.B.; Müller, C. Decreased inorganic N supply capacity and turnover in calcareous soil under degraded rubber plantation in the tropical karst region. Geoderma 2021, 381, 114754. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Tian, J.; Li, X.Z.; Yao, M.J.; Wang, S.Q.; Kuzyakov, Y.; Dungait, J.A.J. Higher free-living N2 fixation at rock-soil interfaces than topsoils during vegetation recovery in karst soils. Soil Biol. Biochem. 2021, 159, 108286. [Google Scholar] [CrossRef]
- Cui, J.; Yu, C.; Qiao, N.; Xu, X.; Tian, Y.; Ouyang, H. Plant preference for NH4+ versus NO3− at different growth stages in an alpine agroecosystem. Field Crop. Res. 2017, 201, 192–199. [Google Scholar] [CrossRef]
- Tho, B.T.; Lambertini, C.; Eller, F.; Brix, H.; Sorrell, B.K. Ammonium and nitrate are both suitable inorganic nitrogen forms for the highly productive wetland grass Arundo donax, a candidate species for wetland paludiculture. Ecol. Eng. 2017, 105, 379–386. [Google Scholar] [CrossRef]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Abdelly, C.; Siddique, K.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hui, N.; Sun, N.X.; Du, H.M.; Umair, M.; Kang, H.Z.; Liu, X.X.; Romantschuk, M.; Liu, C.J. Karst rocky desertification does not erode ectomycorrhizal fungal species richness but alters microbial community structure. Plant Soil 2019, 445, 383–396. [Google Scholar] [CrossRef]
- Wu, Q.X.; Han, G.L.; Tao, F.X.; Yang, T. Chemical composition of rainwater in a karstic agricultural area, Southwest China: The impact of urbanization. Atmos. Res. 2012, 111, 71–78. [Google Scholar] [CrossRef]
- Xia, A.T.; Wu, Y.Y. Joint interactions of carbon and nitrogen metabolism dominated by bicarbonate and nitrogen in Orychophragmus violaceus and Brassica napus under simulated karst habitats. BMC Plant Biol. 2022, 22, 264. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wu, Y.S. The increase in the karstification-photosynthesis coupled carbon sink and its implication for carbon neutrality. Agronomy 2022, 12, 2147. [Google Scholar] [CrossRef]
- Kalcsits, L.A.; Guy, R.D. Whole-plant and organ-level nitrogen isotope discrimination indicates modification of partitioning of assimilation, fluxes and allocation of nitrogen in knockout lines of Arabidopsis thaliana. Physiol. Plant. 2013, 149, 249–259. [Google Scholar] [CrossRef]
- Kalcsits, L.A.; Buschhaus, H.A.; Guy, R.D. Nitrogen isotope discrimination as an integrated measure of nitrogen fluxes, assimilation and allocation in plants. Physiol. Plant. 2014, 151, 293–304. [Google Scholar] [CrossRef]
- Hu, Y.; Guy, R.D.; Soolanayakanahally, R.Y. Nitrogen isotope discrimination in open-pollinated and hybrid canola suggests indirect selection for enhanced ammonium utilization. Front. Plant Sci. 2022, 13, 1024080. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001, 6, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Hu, Y.; Guy, R.D. Isotopic composition and concentration of total nitrogen and nitrate in xylem sap under near steady-state hydroponics. Plant Cell Environ. 2020, 43, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D.; Bloom, A.J.; Sukrapanna, S.S.; Ehleringer, J.R. Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ. 1996, 19, 1317–1323. [Google Scholar] [CrossRef]
- Mariotti, A.; Mariotti, F.; Champigny, M.L.; Amarger, N.; Moyse, A. Nitrogen isotope fractionation associated with nitrate reductase activity and uptake of NO3- by pearl millet. Plant Physiol. 1982, 69, 880–884. [Google Scholar] [CrossRef]
- Kalcsits, L.A.; Guy, R.D. Quantifying remobilization of pre-existing nitrogen from cuttings to new growth of woody plants using 15N at natural abundance. Plant Methods 2013, 9, 27. [Google Scholar] [CrossRef]
- Hayes, J.M. An Introduction to Isotopic Calculations; Woods Hole Oceanographic Institution: Woods Hole, MA, USA, 2004; pp. 1–10. Available online: https://www.whoi.edu/cms/files/jhayes/2005/9/IsoCalcs30Sept04_5184.pdf (accessed on 25 May 2023).
- Zhang, K.Y.; Wu, Y.Y.; Hang, H.T. Differential contributions of NO3−/NH4+ to nitrogen use in response to a variable inorganic nitrogen supply in plantlets of two Brassicaceae species in vitro. Plant Methods 2019, 15, 86. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Wu, Y.Y.; Su, Y.; Li, H.T. Implication of quantifying nitrate utilization and CO2 assimilation of Brassica napus plantlets in vitro under variable ammonium/nitrate ratios. BMC Plant Biol. 2022, 22, 392. [Google Scholar] [CrossRef]
- Ni, J.W.; Su, S.; Li, H.; Geng, Y.H.; Zhou, H.J.; Feng, Y.Z.; Xu, X.Q. Distinct physiological and transcriptional responses of leaves of paper mulberry (Broussonetia kazinoki × B. papyrifera) under different nitrogen supply levels. Tree Physiol. 2020, 40, 667–682. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Li, H.; Wu, G. Overexpression of atnhx5 improves tolerance to both salt and drought stress in Broussonetia papyrifera (L.) vent. Tree Physiol. 2011, 31, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Wang, Y.; Du, G.; Liu, G.; Zhang, L.; Cheng, Y. Antioxidant lignans from the fruits of Broussonetia papyrifera. J. Nat. Prod. 2009, 72, 621. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yang, H.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Sykes, R.W. Adsorption of cellobiohydrolases I onto lignin fractions from dilute acid pretreated Broussonetia papyrifera. Bioresour. Technol. 2017, 244, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.J.; Liu, H.; Chen, P.L.; Tang, F.; Hu, Y.M.; Wang, F.F.; Pi, Z.; Zhao, M.L.; Chen, N.Z.; Chen, H.; et al. A chromosome-scale genome assembly of paper mulberry (Broussonetia papyrifera) provides new insights into its forage and papermaking usage. Mol. Plant 2019, 12, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=54aefd7ed4c118b6358b45db&assetKey=AS%3A273668901408776%401442259158553 (accessed on 25 May 2023).
- Yousfi, S.; Serret, M.D.; Araus, J.L. Comparative response of δ13C, δ18O and δ15N in durum wheat exposed to salinity at the vegetative and reproductive stages. Plant Cell. Environ. 2013, 36, 1214–1227. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef]
- Suárez, J.C.; Polanía, J.A.; Anzola, J.A.; Contreras, A.T.; Méndez, D.L.; Vanegas, J.I.; Noriega, J.E.; Rodríguez, L.; Urban, M.O.; Beebe, S.; et al. Influence of nitrogen supply on gas exchange, chlorophyll fluorescence and grain yield of breeding lines of common bean evaluated in the Amazon region of Colombia. Acta Physiol. Plant. 2021, 43, 66. [Google Scholar] [CrossRef]
- Taylor, J. Introduction to Error Analysis, the Study of Uncertainties in Physical Measurements; University Science Books: Sausalito, CA, USA, 1997; Available online: https://ui.adsabs.harvard.edu/abs/1997ieas.book.....T/abstract (accessed on 25 May 2023).
- Pritchard, E.S.; Guy, R.D. Nitrogen isotope discrimination in white spruce fed with low concentrations of ammonium and nitrate. Trees 2005, 19, 89–98. [Google Scholar] [CrossRef]
- Kalcsits, L.A.; Min, X.; Guy, R.D. Interspecific variation in leaf-root differences in δ15N among three tree species grown with either nitrate or ammonium. Trees 2015, 29, 1069–1078. [Google Scholar] [CrossRef]
- Buschhaus, H.A. 15N Discrimination as an Indicator of Nitrogen Dynamics in Populus Trichocarpa. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2007; p. 70. [Google Scholar] [CrossRef]
- Yousfi, S.; Serret, M.D.; Araus, J.L. Shoot δ15N gives a better indication than ion concentration or δ13C of genotypic differences in the response of durum wheat to salinity. Funct. Plant Biol. 2009, 36, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Ogaya, R.; Penuelas, J.; Asensio, D.; Llusià, J. Chlorophyll fluorescence responses to temperature and water availability in two co-dominant Mediterranean shrub and tree species in a long-term field experiment simulating climate change. Environ. Exp. Bot. 2011, 71, 123–127. [Google Scholar] [CrossRef]
- Wu, Y.W.; Li, R.; Chen, W.; Liu, X.L.; Kong, F.L.; Ke, Y.P.; Shi, H.C.; Yuan, J.C. Effect of low-nitrogen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different low-nitrogen tolerances. J. Integr. Agric. 2019, 18, 1246–1256. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.M.; Huber, S.C. Post-translational regulation of nitrate reductase: Mechanism, physiological relevance and environmental triggers. J. Exp. Bot. 2001, 52, 1981–1989. [Google Scholar] [CrossRef]
- Black, B.L.; Fuchigami, L.H.; Coleman, G.D. Partitioning of nitrate assimilation among leaves, stems and roots of poplar. Tree Physiol. 2002, 22, 717–724. [Google Scholar] [CrossRef]
- Andrews, M.; Morton, J.D.; Lieffering, M.; Bisset, L. The partitioning of nitrate assimilation between root and shoot of a range of temperate cereals and pasture grasses. Ann. Bot. 1992, 70, 271–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).