Integration of Remote Sensing and Field Observations in Evaluating DSSAT Model for Estimating Maize and Soybean Growth and Yield in Maryland, USA
Abstract
1. Introduction
2. Materials and Methods
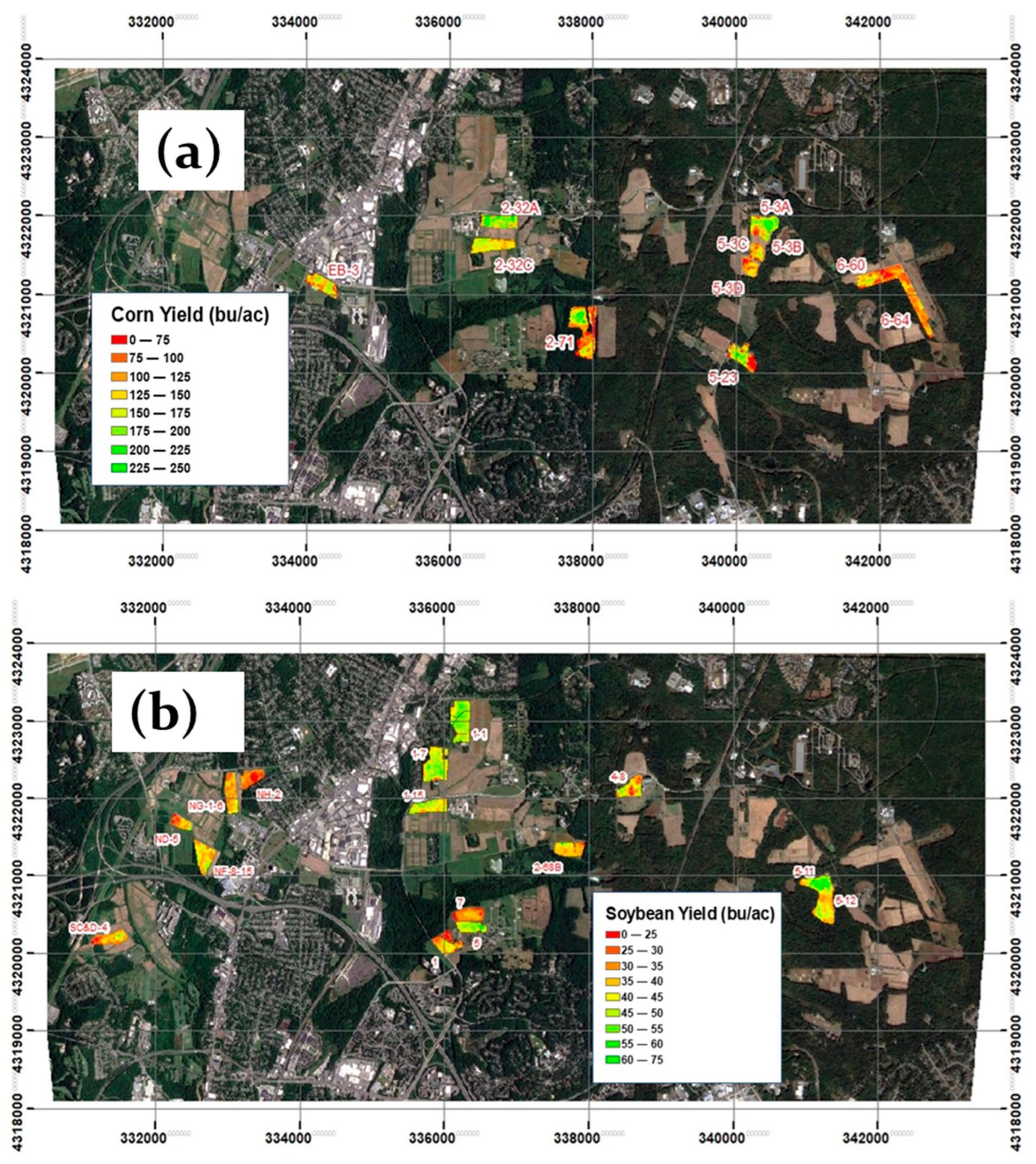
2.1. Description of the Study and Experimental Area
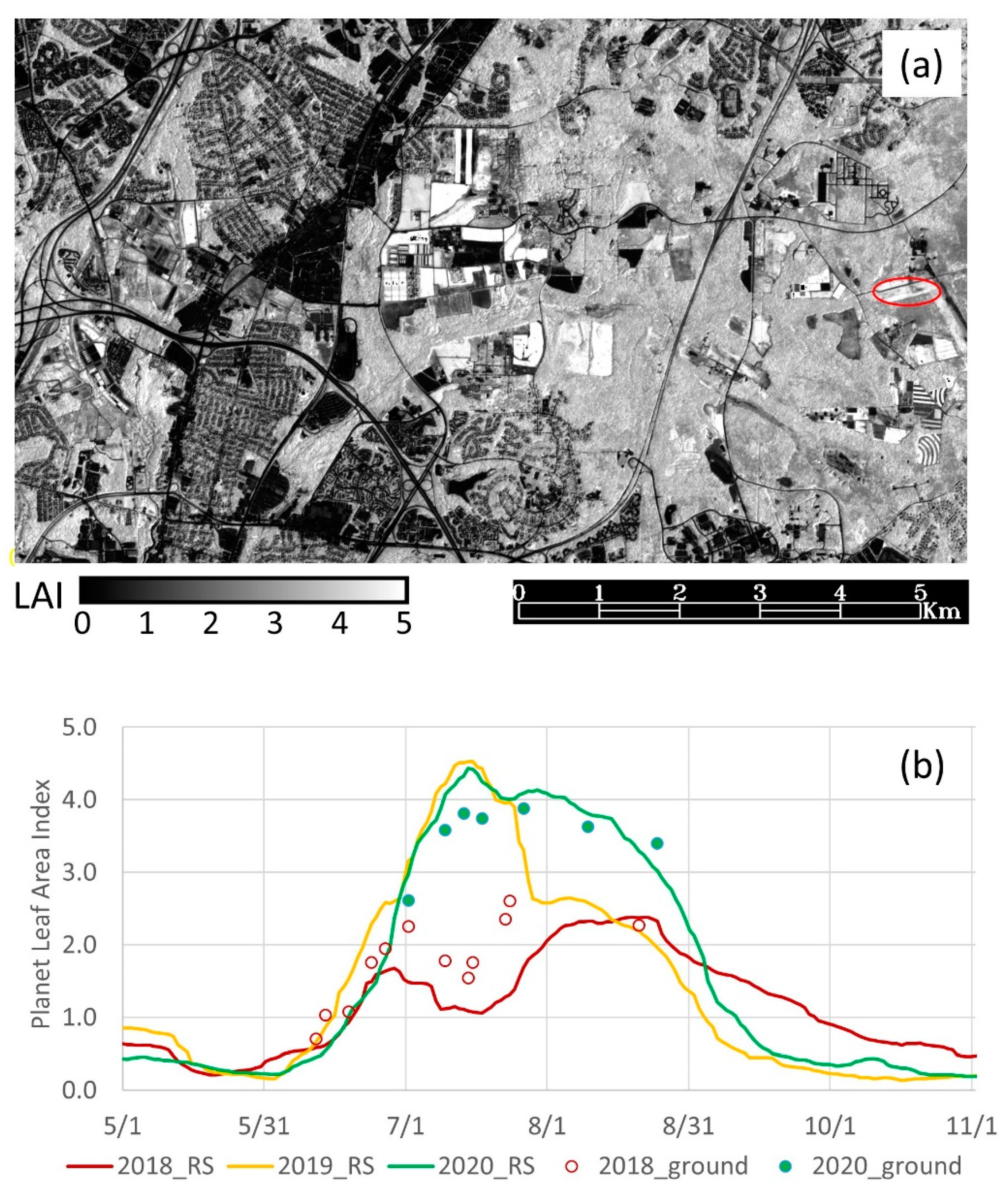
2.2. Remote-Sensing-Derived LAI and Phenology
2.2.1. Leaf Area Index
2.2.2. Crop Growth Stages
2.3. DSSAT Model Description
2.4. Model Calibration
2.5. Model Evaluation
3. Results and Discussion
3.1. Remote Sensing LAI and Phenology Results
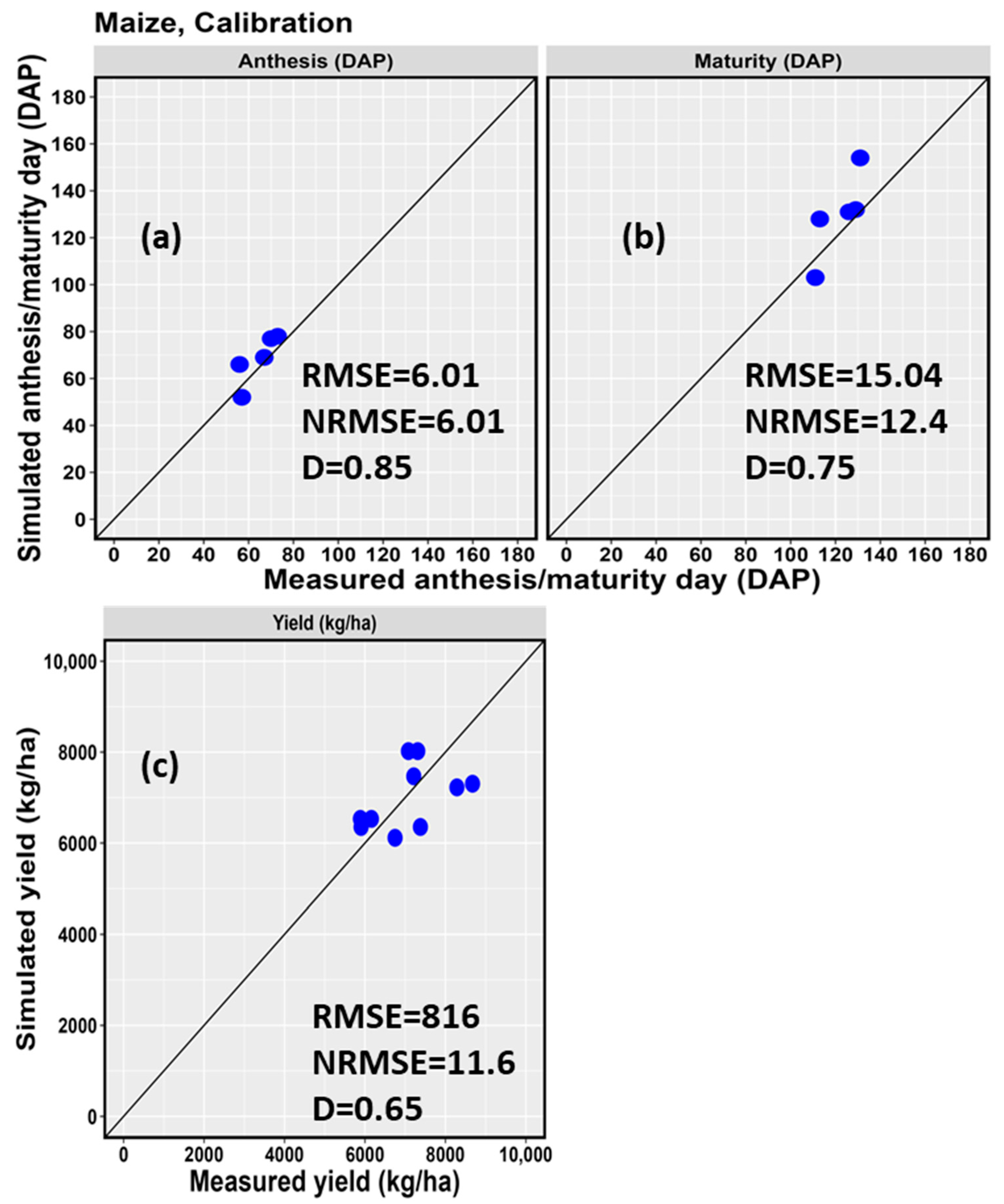
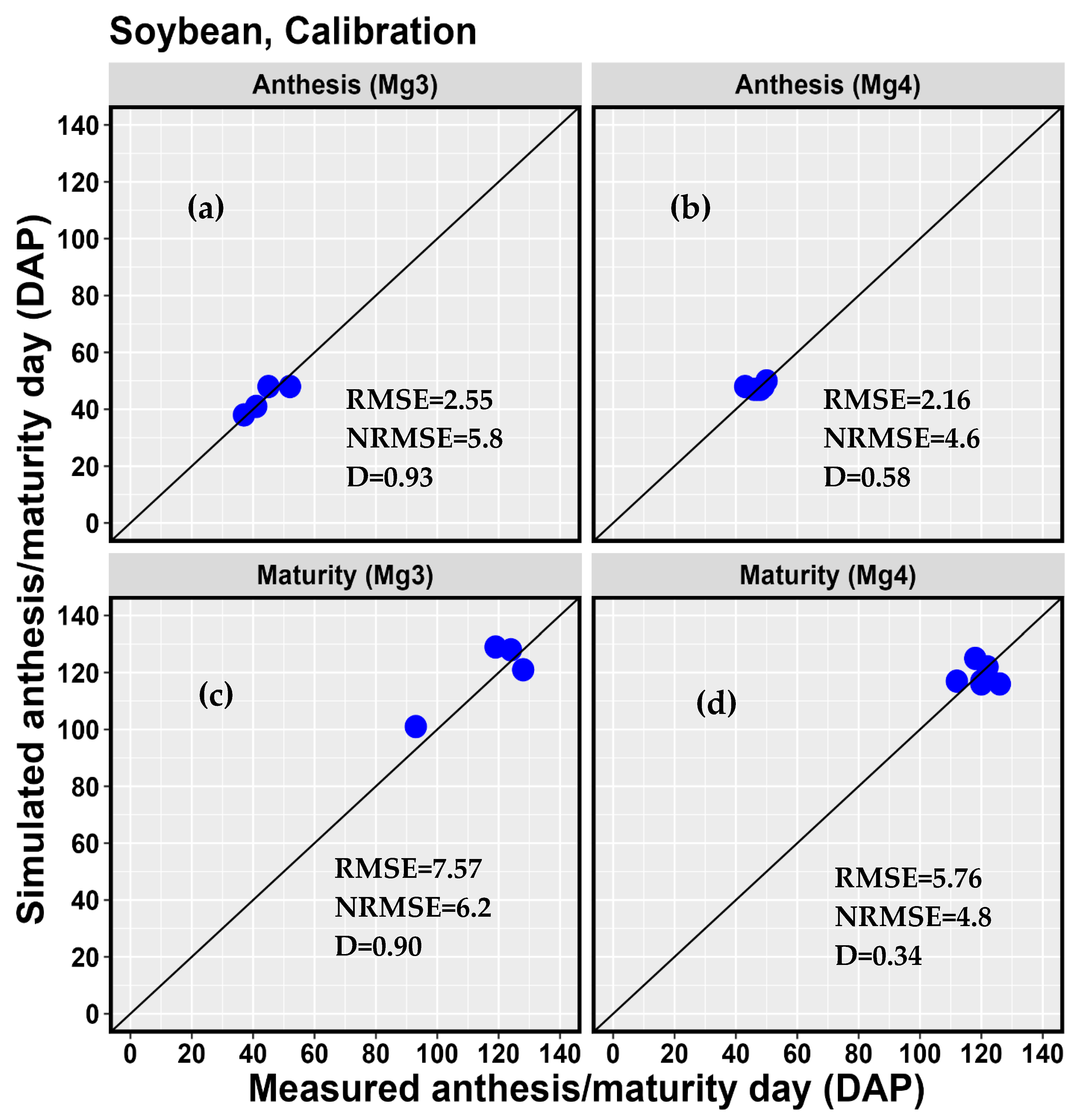
3.2. Model Calibration Results
3.3. Model Evaluation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutikani, L. Soybean Exports Power U.S. Economy to Best Performance in Two Years; Reuters: London, UK, 2016. [Google Scholar]
- US Department of Agriculture, Economic Research Service (USDA-ERS). Ag and Food Statistics: Charting the Essentials; Administrative Publication: Beijing, China, 2018.
- Campos, H.; Cooper, M.; Habben, J.; Edmeades, G.; Schussler, J. Improving drought tolerance in maize: A view from industry. Field Crop. Res. 2004, 90, 19–34. [Google Scholar] [CrossRef]
- Hatfield, J.; Takle, G.; Groyjahn, R.; Holden, P.; Izaurralde, R.C.; Mader, T.; Marshall, E.; Liverman, D. Chapter 6: Agriculture. In Climate Change Impacts in the United States: The Third National Climate Assessment; Melillo, J.M., Terese, T.C.R., Yohe, G.W., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2014; pp. 150–174. [Google Scholar]
- Dhakal, K.; Kakani, V.G.; Linde, E. Climate Change impact on wheat production in Southern Great Plains of the US using downscaled climate data. Atmos. Clim. Sci. 2018, 8, 143–162. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report Onclimate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosytems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Grigg, N.S. The 2011–2012 drought in the United States: New lessons from a record event. Int. J. Water Resour. Dev. 2014, 30, 183–199. [Google Scholar] [CrossRef]
- Gowda, P.; Steiner, J.L.; Olson, C.; Boggess, M.; Farrigan, T.; Grusak, M.A. Agriculture and rural communities. In Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II; U.S. Global Change Research Program: Washington, DC, USA, 2018; pp. 391–437. [Google Scholar]
- IPCC. Summary for policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.C.; Zolin, C.A.; Sentelhas, P.C.; Hain, C.R.; Semmens, K.; Yilmaz, M.T.; Gao, F.; Otkin, J.A.; Tetrault, R. The Evaporative Stress Index as an indicator of agricultural drought in Brazil: An assessment based on crop yield impacts. Remote Sens. Environ. 2016, 174, 82–99. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture-Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Huang, J.; Gomez-Dans, J.L.; Huang, H.; Ma, H.; Wu, Q.; Lewis, P.E.; Liang, S.; Chen, Z.; Xue, J.H.; Wu, Y.; et al. Assimilation of remote sensing into crop growth models: Current status and perspective. Agric. For. Meteorol. 2019, 276, 107609. [Google Scholar] [CrossRef]
- Chipanshi, A.; Ripley, E.; Lawford, R. Large-scale simulation of wheat yields in a semi-arid environment using a crop-growth model. Agric. Syst. 1999, 59, 57–66. [Google Scholar] [CrossRef]
- Moulin, S.; Guerif, M. Impacts of model parameter uncertainties on crop reflectance estimates: A regional case study on wheat. Int. J. Remote Sens. 1999, 20, 213–218. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Lu, L.; Fang, F. Estimating near future regional corn yields by integrating multi-source observations into a crop growth model. Eur. J. Agron. 2013, 49, 126–140. [Google Scholar] [CrossRef]
- Kasampalis, D.A.; Alexandridis, T.K.; Deva, C.; Challinor, A.; Moshou, D.; Zalidis, G. Contribution of Remote Sensing on Crop Models: A Review. J. Imaging 2018, 4, 52. [Google Scholar] [CrossRef]
- Li, Z.H.; Jin, X.L.; Zhao, C.J.; Wang, J.H.; Xu, X.G.; Wang Li, C.J.; Shen, J.X. Estimating wheat yield and quality by coupling the DSSAT-CERES model and proximal remote sensing. Eur. J. Agron. 2015, 71, 53–62. [Google Scholar] [CrossRef]
- Levitan, N.; Kang, Y.; Ozdogan, M.; Maglillo, V.; Castillo, P.; Moshary, F.; Gross, B. Evaluation of the uncertainty in Satellite-Based crop state variable retrievals due to site and growth stage specific factors and their potential in coupling with crop growth models. Remote Sens. 2019, 11, 1928. [Google Scholar] [CrossRef]
- Morel, J.; Begue, A.; Todoroff, P.; Martine, J.F.; Lebourgeois, V.; Petiti, M. Coupling a sugarcane crop model with the remotely sensed time series of FIPAR to optimize the yield estimation. Eur. J. Agron. 2014, 61, 60–68. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Zhou, J.; Zhang, G.; Chen, C.; Wang, J. Assimilating remote sensing information into a coupled hydrology-crop growth model to estimate regional maize yield in arid regions. Ecol. Model. 2014, 291, 15–27. [Google Scholar] [CrossRef]
- Dente, L.; Satalino, G.; Mattia, F.; Rinaldi, M. Assimilation of leaf area index derived from ASAR and MERIS data into CERES-Wheat model to map wheat yield. Remote Sens. Environ. 2008, 112, 1395–1407. [Google Scholar] [CrossRef]
- Liang, S.; Qin, J. Data Assimilation Methods for Land Surface Variable Estimation, Advances in Land Remote Sensing, System, Modeling, Inversion and Applications; Liang, S., Ed.; Springer Science Business Media, BV: Berlin/Heidelberg, Germany, 2008; pp. 319–339. [Google Scholar]
- Fang, H.; Liang, S.; Hoogenboom, G. Integration of MODIS LAI and vegetationindex products with the CSM-CERES-MAIZE model for corn yield estimation. Int. J. Remote Sens. 2011, 32, 1039–1065. [Google Scholar] [CrossRef]
- de Wit, A.; Duveiller, G.; Defourny, P. Estimating regional winter wheat yield with WOFOST through the assimilation of green area index retrieved from MODIS observations. Agric. For. Meteorol. 2012, 164, 39–52. [Google Scholar] [CrossRef]
- Ma, J.; Qin, S. Recent advances and developments of data assimilationalgorithms. Adv. Earth Sci. 2012, 27, 747–757. [Google Scholar]
- Huang, J.; Tian, L.; Liang, S.; Ma, H.; Becker-Reshef, I.; Huang, Y.; Su, W.; Zhang, X.; Zhu, D.; Wu, W. Improving winter wheat yield estimation by assimilation of the leaf area index from Landsat TM and MODIS data into the WOFOST mode. Agric. For. Meteorolo. 2015, 204, 106–121. [Google Scholar] [CrossRef]
- Wardlow, B.D.; Anderson, M.C.; Verdin, J.P. (Eds.) Remote Sensing for Drought: Innovative Monitoring Approaches; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2012. [Google Scholar]
- Basso, B.; Cammarano, D.; Carfagna, E. Review of crop yield forecasting methods and early warning systems. In Proceedings of the First Meeting of the Scientific Advisory Committee of the Global Strategy to Improve Agricultural and Rural Statistics; FAO: Rome, Italy, 2013. [Google Scholar]
- Rembold, F.; Atzberger, C.; Savin, I.; Rojas, O. Using low resolution satellite imagery for yield prediction and yield anomaly detection. Remote Sens. 2013, 5, 1704–1733. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Yu, Y.; Gao, F.; Yang, Z. Real-Time Monitoring of Crop Phenology in the Midwestern United States Using VIIRS Observations. Remote Sens. 2018, 10, 1540. [Google Scholar] [CrossRef]
- Olson, D.; Chatterjee, A.; Franzen, D.W.; Day, S.S. Relation of drone-based vegetation indices with corn and sugarbeet yields. Agron. J. 2019, 111, 2545–2557. [Google Scholar] [CrossRef]
- Diao, C. Remote sensing phenological monitoring framework to characterize corn and soybean physiological growing stages. Remote Sens. Environ. 2020, 248, 111960. [Google Scholar] [CrossRef]
- Gao, F.; Anderson, M.C.; Johnson, D.M.; Seffrin, R.; Wardlow, B.; Suyker, A.; Diao, C.; Browning, D.M. Towards Routine Mapping of Crop Emergence within the Season Using the Harmonized Landsat and Sentinel-2 Dataset. Remote Sens. 2021, 13, 5074. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, H.; Huang, J. Regional Crop Yield Assessment by Combination of a Crop Growth Model and Phenology Information Derived from MODIS. Sens. Lett. 2011, 9, 981–989. [Google Scholar] [CrossRef]
- Mishra, V.; Cruise, J.F.; Mecikalski, J.R. Assimilation of coupled microwave/thermal infrared soil moisture profiles into a crop model for robust maize yield estimates over Southeast United States. Eur. J. Agron. 2021, 123, 126–208. [Google Scholar] [CrossRef]
- Shanahan, J.F.; Schepers, J.S.; Francis, D.D.; Varvel, G.E.; Wilhelm, W.W.; Tringe, J.M.; Schlemmer, M.R.; Major, D.J. Use of Remote-Sensing Imagery to Estimate Corn Grain Yield. Agron. J. 2001, 93, 583–589. [Google Scholar] [CrossRef]
- Launay, M.; Guerif, M. Assimilating remote sensing data into a crop model to improve predictive performance for spatial applications. Agric. Ecosyst. Environ. 2005, 111, 321–339. [Google Scholar] [CrossRef]
- Nearing, G.S.; Crow, W.; Thorp, K.; Moran, M.S.; Reichle, R.; Gupta, H. Assimilating remote sensing observations of leaf area index and soil moisture for wheat yield estimates: An observing system simulation experiment. Water Resour. Res. 2012, 48, W05525. [Google Scholar] [CrossRef]
- LACIE. Proceedings of Plenary Session: The LACIE Symposium, NASA-JSC 14557; US Gov. Public Use Permitted: Houston, TX, USA, 1978.
- Delécolle, R.; Maas, S.; Guérif, M.; Baret, F. Remote sensing and crop production models: Present trends. ISPRS J. Photogramm. Remote Sens. 1992, 47, 145–161. [Google Scholar] [CrossRef]
- Bouman, B.A.M. Linking physical remote-sensing models with crop growth simulation-models, applied for sugar-beet. Int. J. Remote Sens. 1992, 13, 2565–2581. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral imaging: A review on UAV-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogram. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Gao, F.; Anderson, M.; Daughtry, C.; Karnieli, A.; Hively, D.; Kustas, W. A within-season approach for detecting early growth stages in corn and soybean using high temporal and spatial resolution imagery. Remote Sens. Environ. 2020, 242, 111752. [Google Scholar] [CrossRef]
- Dulaney, W.; Anderson, M.C.; Gao, F.; Daughtry, C.S.T.; Akumaga, U. Development of a Gridded Data Archive for Farm Management and Research at the USDA Beltsville Agricultural Research Center. Agrosyst. Geosci. Environ. 2023; in review process. [Google Scholar]
- Gao, F.; Anderson, M.C.; Kustas, W.P.; Wang, Y. Simple method for retrieving leaf area index from Landsat using MODIS leaf area index products as reference. J. Appl. Remote Sens. 2012, 6, 063554. [Google Scholar] [CrossRef]
- European Space Agency (ESA). Sentinel-2 User Handbook. 2015. Available online: https://sentinels.copernicus.eu/documents/247904/685211/Sentinel-2_User_Handbook (accessed on 13 May 2023).
- Houborg, M.F. Daily Retrieval of NDVI and LAI at 3 m Resolution via the Fusion of CubeSat, Landsat, and MODIS data. Remote Sens. 2018, 10, 890. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. S2ToolBox Level 2 Products: LAI, FAPAR, FCOVER, 1.1. ed.; Institut National de la Recherche Agronomique: Avignon, France, 2016. [Google Scholar]
- Djamai, N.; Fernandes, R.; Weiss, M.; McNairn, H.; Goïta, K. Validation of the Sentinel Simplified Level 2 Product Prototype Processor (SL2P) for mapping cropland biophysical variables using Sentinel-2/MSI and Landsat-8/OLI data. Remote Sens. Environ. 2019, 225, 416–430. [Google Scholar] [CrossRef]
- Planet Labs, Planet Fusion Monitoring Technical Specification, Version 1.0.0-beta.3, 2021. Available online: https://assets.planet.com/docs/Planet_fusion_specification_March_2021.pdf (accessed on 13 May 2023).
- Houborg, M.F. A Cubesat Enabled Spatio-Temporal Enhancement Method (CESTEM) utilizing Planet, Landsat and MODIS data. Remote Sens. Environ. 2018, 209, 211–226. [Google Scholar] [CrossRef]
- Frantz, D. FORCE – Landsat + Sentinel-2 analysis ready data and beyond. Remote Sens. 2019, 11, 1124. [Google Scholar] [CrossRef]
- Sakoe, H.; Chiba, S. Dynamic programming algorithm optimization for spoken word recognition. IEEE Trans. Acoust. Speech Signal Process. 1978, 26, 43–49. [Google Scholar] [CrossRef]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Hoogenbom, G.; Jones, J.W.; Porter, C.H.; Wilkens, P.W.; Boote, K.J.; Hunt, L.A.; Tsuji, G.Y. (Eds.) Decision Support System for Agrotechnology Transfer Version 4.5; University of Hawaii: Honolulu, HI, USA, 2010; Volume 1. [Google Scholar]
- Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Shelia, V.; Singh, U.; Wilkens, P.W.; White Pavan, W.; Oliveira, F.A.A.; Moreno-Cadena, L.P.; Lizaso, J.I.; et al. Decision Support System for Agrotechnology Transfer (DSSAT) Version 4.8 (DSSAT.net); DSSAT Foundation: Gainsville, FL, USA, 2021. [Google Scholar]
- Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Shelia, V.; Wilkens, P.W.; Singh, U.; White, J.W.; Asseng, S.; Lizaso, J.I.; Moreno, L.P.; et al. The DSSAT crop modeling ecosystem. In Advances in Crop Modelling for a Sustainable Agriculture; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 173–216. [Google Scholar]
- Hoogenboom, G.; Jones, J.W.; Traore, P.C.; Boote, K.J. Experiments and Data for Model Evaluation and Application., Improving Soil Fertility Recommendations in Africa Using the Decision Support System for Agrotechnology Transfer (DSSAT); Springer: Dordrecht, The Netherlands, 2012; pp. 9–18. [Google Scholar]
- He, J.; Jones, J.W.; Graham, W.D.; Dukes, M.D. Influence of likelihood function choice for estimating crop model parameters using the generalized likelihood uncertainty estimation method. Agric. Syst. 2010, 103, 256–264. [Google Scholar] [CrossRef]
- Willmott, C.J. Some comments on the evaluation of model performance. Bull. Am. Meteorol. Soc. 1982, 63, 1309–1313. [Google Scholar] [CrossRef]
- Akumaga, U.; Tarhule, A.; Yusuf, A.A. Validation and testing of the FAO AquaCrop model under different levels of nitrogen fertilizer on rainfed maize in Nigeria, West Africa. Agric. For. Meteorol. 2017, 232, 225–234. [Google Scholar] [CrossRef]
- Jamieson, P.D.; Porter, J.R.; Wilson, D.R. A test of the computer simulation model ARC-WHEAT1 on wheat crops grown in New Zealand. Field Crop. Res. 1991, 27, 337–350. [Google Scholar] [CrossRef]
- Singh, J.; Knapp, H.V.; Demissie, M. Hydrologic Modeling of the Iroquois River Watershed Using HSPF and SWAT; ISWS CR 2004-08; Illinois State Water Survey: Champaign, IL, USA, 2004. [Google Scholar]
- Araya, A.; Kisekka, I.; Gowda, P.H.; Prasad, P.V. Evaluation of water-limited cropping systems in a semi-arid climate using DSSAT-CSM. Agric. Syst. 2017, 150, 86–98. [Google Scholar] [CrossRef]
- Kim, S.; Daughtry, C.; Russ, A.; Pedrera-Parrilla, A.; Pachepsky, Y. Analysis of Spatiotemporal Variability of Corn Yields Using Empirical Orthogonal Functions. Water 2020, 12, 3339. [Google Scholar] [CrossRef]
- Araya, A.; Prasad, P.V.V.; Gowda, P.H.; Afewerk, A.; Abadi, B.; Foster, A.J. Modeling irrigation and nitrogen management of wheat in northern Ethiopia. Agric. Water Manag. 2019, 216, 264–272. [Google Scholar] [CrossRef]


| Corn Genetic Coefficient | Description | Default Cultivar Value | Calibrated Value |
|---|---|---|---|
| P1 | Thermal time from seedling emergence to end of juvenile phase (expressed in degree days above a base temp of 8 deg.C) during which the plant is not responsive to changes in the photoperiod. | 200 (5–450) * | 154.1 |
| P2 | The extent to which development is delayed for each hour increase in the photoperiod above the longest photoperiod at which development proceeds at max. rate (which is 12.5 h). | 0.300 (0–2) * | 0.001 |
| P5 | Thermal time from silking to physiological maturity (expressed in degree days above a base temp of 8 deg.C) | 800 (580–999) * | 592.7 |
| G2 | Maximum number of kernels per plant | 700 (248–990) * | 381.8 |
| G3 | Kernel filling rate during the linear grain filling state and under optimum condition (mg/day); | 8.5 (5–16.5) * | 15.31 |
| PHINT | Phylochron interval; the interval in thermal time (degree days) between successive leaf tip appearances. | 38.90 (38–49) * | 49.00 |
| Soybean Genetic Coefficient | Description | Default Value * | MG3 | MG4 |
|---|---|---|---|---|
| CSDL | Critical short day length below which reproductive development progresses with no daylength effect (for shortday plants) (hour) | 13.09 | 12.00 | 12.98 |
| PPSEN | Slope of the relative response of development to photoperiod with time (positive for shortday plants) (1/hour) | 0.302 | 0.233 | 0.234 |
| EM—FL | Time between plant emergence and flower appearance (R1) (photothermal days) | 19.4 | 13.37 | 23.65 |
| FL—SH | Time between first flower and first pod (R3) (photothermal days) | 7.0 | 6.00 | 7.0 |
| FL—SD | Time between first flower and first seed (R5) (photothermal days) | 15.0 | 17.19 | 19.45 |
| SD—PM | Time between first seed (R5) and physiological maturity (R7) (photothermal days) | 34.00 | 37.60 | 37.67 |
| FL—LF | Time between first flower (R1) and end of leaf expansion (photothermal days) | 26.00 | 26.00 | 26.00 |
| LFMAX | Maximum leaf photosynthesis rate at 30 C, 350 vpm CO2, and high light (mg CO2/m2/s) | 1.030 | 1.038 | 1.006 |
| SLAVR | Specific leaf area of cultivar under standard growth conditions (cm2/g) | 375 | 340.7 | 356.8 |
| SIZLF | Maximum size of full leaf (three leaflets) (cm2) | 180.0 | 138.3 | 144.8 |
| XFRT | Maximum fraction of daily growth that is partitioned to seed + shell | 1.00 | 1.00 | 1.00 |
| WTPSD | Maximum weight per seed (g) | 0.19 | 0.161 | 0.165 |
| SFDUR | Seed filling duration for pod cohort at standard growth conditions (photothermal days) | 23.0 | 25.07 | 23.16 |
| SDPDV | Average seed per pod under standard growing conditions (#/pod) | 2.20 | 2.093 | 1.7401 |
| PODUR | Time required for cultivar to reach final pod load under optimal conditions (photothermal days) | 10.0 | 10.00 | 10.0 |
| THRSH | Threshing percentage. The maximum ratio of (seed/(seed + shell)) at maturity. Causes seeds to stop growing as their dry weight increases until shells are filled in a cohort. | 77.0 | 77.00 | 77.0 |
| SDPRO | Fraction protein in seeds (g(protein)/g(seed)) | 0.405 | 0.405 | 0.405 |
| SDLIP | Fraction oil in seeds (g(oil)/g(seed)) | 0.205 | 0.205 | 0.205 |
| Field Info | Days to Flowering (R1) | Days to Maturity (R6) | Yield (kg/ha) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Field | Year | Obs | Sim | dev (%) | Obs | Sim | dev (%) | Obs | Sim | dev (%) |
| 5-3B | 2017 | 57 | 52 | −8.8 | 111 | 103 | −7.2 | 6160 | 6531 | 6.0 |
| 5-3B | 2019 | 56 | 66 | 17.9 | 113 | 128 | 13.3 | 7378 | 6353 | −13.9 |
| 5-3B | 2020 | 67 | 69 | 3.0 | 131 | 154 | 17.6 | 7312 | 8021 | 9.7 |
| 5-3C | 2017 | 57 | 52 | −8.8 | 111 | 103 | −7.2 | 6748 | 6115 | −9.4 |
| 5-3C | 2020 | 67 | 69 | 3.0 | 131 | 154 | 17.6 | 8674 | 7304 | −15.8 |
| 5-3D | 2017 | 57 | 52 | −8.8 | 111 | 103 | −7.2 | 5886 | 6531 | 11.0 |
| 5-3D | 2019 | 56 | 66 | 17.9 | 113 | 128 | 13.3 | 5903 | 6353 | 7.6 |
| 5-3D | 2020 | 67 | 69 | 3.0 | 131 | 154 | 17.6 | 7080 | 8021 | 13.3 |
| 2-71 | 2019 | 73 | 78 | 6.9 | 129 | 132 | 2.3 | 7213 | 7467 | 3.5 |
| 5-23 | 2019 | 70 | 77 | 10.0 | 126 | 131 | 4.0 | 8286 | 7224 | −12.8 |
| d | 0.85 | 0.75 | 0.65 | |||||||
| RMSE | 6.01 | 15.04 | 816 | |||||||
| NRMSE (%) | 9.6 | 12.4 | 11.6 | |||||||
| Field Info | Days to Flowering (R1) | Days to Maturity (R6) | Yield (kg/ha) * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Field | Year | Obs | Sim | dev (%) | Obs | Sim | dev (%) | Obs | Sim | dev (%) |
| 5-3a | 2018 | 41 | 41 | 0.0 | 128 | 121 | −5.79 | 2937 | 3145 | 6.6 |
| 1-11 | 2019 | 37 | 38 | 2.6 | 93 | 101 | 7.92 | * | ||
| 1-34 | 2019 | 52 | 48 | −8.3 | 124 | 128 | 3.13 | * | ||
| 2-51 | 2019 | 45 | 48 | 6.3 | 119 | 129 | 7.75 | * | ||
| d | 0.93 | 0.90 | ||||||||
| RMSE | 2.55 | 7.57 | ||||||||
| NRMSE (%) | 5.8 | 6.5 | ||||||||
| Field Info | Days to Flowering (R1) | Days to Maturity (R6) | Yield (kg/ha) * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Field | Year | Obs | Sim | dev (%) | Obs | Sim | dev (%) | Obs | Sim | dev (%) |
| 8-15 | 2018 | 49 | 48 | −2.04 | 120 | 117 | −2.5 | 3230 | 3251 | 0.7 |
| 1-6 | 2018 | 47 | 47 | 0.00 | 126 | 116 | −7.9 | 3955 | 3501 | −11.5 |
| 1-6 | 2020 | 50 | 50 | 0.00 | 118 | 125 | 5.9 | 3366 | 3300 | −2.0 |
| NH-2 | 2018 | 48 | 47 | −2.08 | 120 | 116 | −3.3 | 3196 | 3320 | 3.9 |
| ND-5 | 2018 | 46 | 47 | 2.17 | 112 | 117 | 4.5 | 3022 | 3380 | 11.8 |
| ND-5 | 2020 | 43 | 48 | 11.63 | 122 | 122 | 0.0 | 3138 | 3818 | 21.7 |
| d | 0.58 | 0.34 | 0.38 | |||||||
| RMSE | 2.16 | 5.76 | 368.97 | |||||||
| NRMSE (%) | 4.6 | 4.8 | 11.1 | |||||||
| Field Info | Days to Flowering (R1) | Days to Maturity (R6) | Yield (kg/ha) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Field | Year | Obs | Sim | dev (%) | Obs | Sim | dev (%) | Obs | Sim | dev (%) |
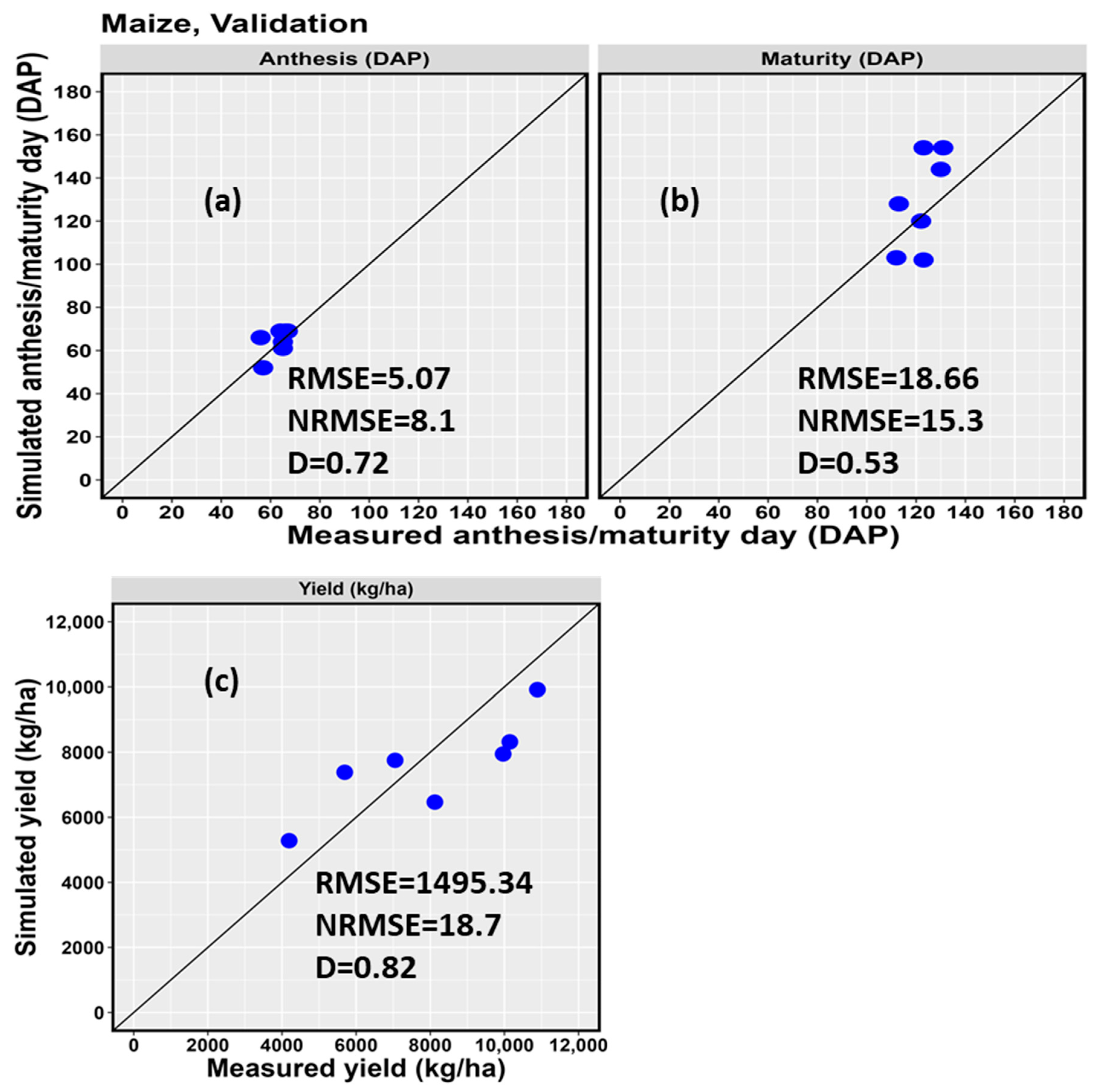
| 5-3A | 2017 | 57 | 52 | −9.6 | 112 | 103 | −8.7 | 8124 | 6466 | −25.6 |
| 5-3A | 2020 | 67 | 69 | 2.9 | 131 | 154 | 14.9 | 10,146 | 8318 | −22.0 |
| 5-3C | 2019 | 56 | 66 | 15.2 | 113 | 128 | 11.7 | 7051 | 7752 | 9.0 |
| 6-60 | 2018 | 65 | 61 | −6.6 | 123 | 102 | −20.6 | 4193 | 5281 | 20.6 |
| 6-60 | 2019 | 65 | 64 | −1.6 | 122 | 120 | −1.7 | 5692 | 7383 | 22.9 |
| 6-60 | 2020 | 66 | 69 | 4.3 | 123 | 154 | 20.1 | 9966 | 7946 | −25.4 |
| 2-71 | 2020 | 64 | 69 | 7.2 | 130 | 144 | 9.7 | 10,890 | 6353 | −9.8 |
| D | 0.72 | 0.53 | 0.82 | |||||||
| RMSE | 5.07 | 18.66 | 1495.34 | |||||||
| NRMSE (%) | 8.1 | 15.3 | 18.7 | |||||||
| Field Info | Days to Flowering (R1) | Days to Maturity (R6) | Yield (kg/ha) * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Field | Year | Obs | Sim | dev (%) | Obs | Sim | dev (%) | Obs | Sim | dev (%) |
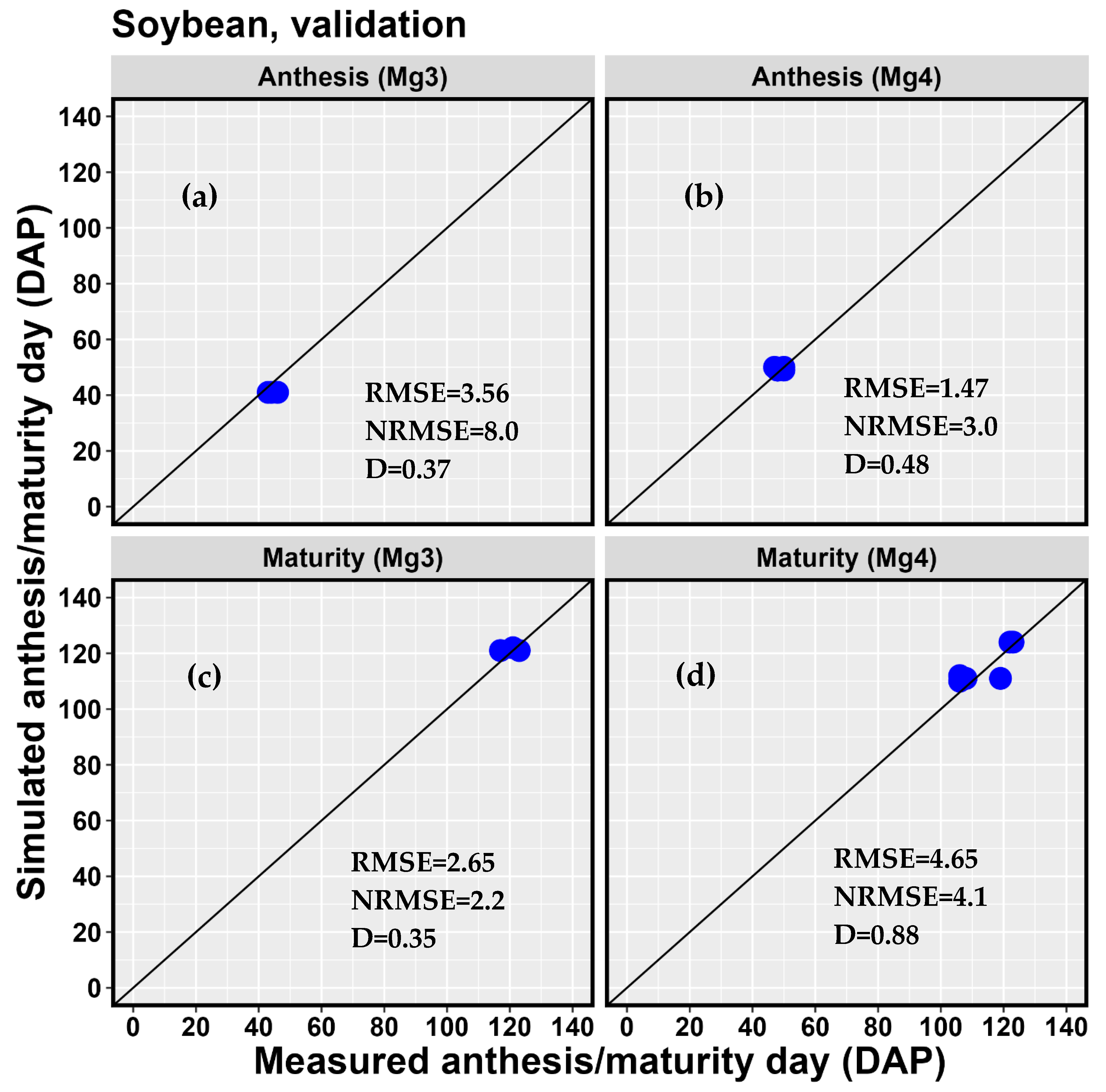
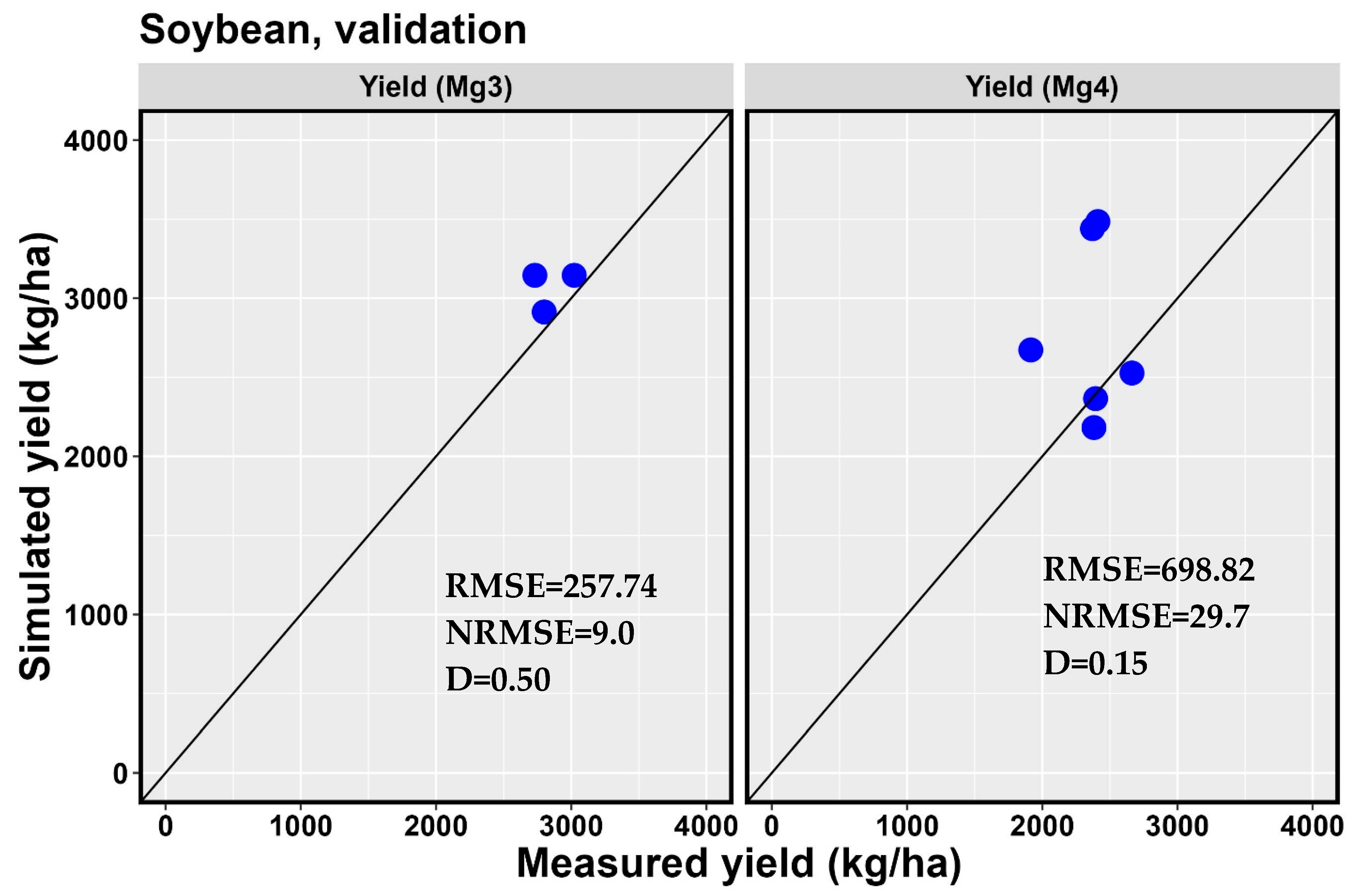
| 5-3B | 2018 | 44 | 41 | −6.8 | 123 | 121 | −1.6 | 2731 | 3145 | 15.2 |
| 5-3C | 2018 | 46 | 41 | −10.9 | 121 | 122 | 0.8 | 2800 | 2913 | 4.0 |
| 5-3D | 2018 | 43 | 41 | −4.7 | 117 | 121 | 3.4 | 3022 | 3145 | 4.1 |
| d | 0.37 | 0.35 | 0.50 | |||||||
| RMSE | 3.56 | 2.65 | 257.74 | |||||||
| NRMSE (%) | 8.0 | 2.2 | 9.0 | |||||||
| Field Info | Days to Flowering (R1) | Days to Maturity (R6) | Yield (kg/ha) * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Field | Year | Obs | Sim | dev (%) | Obs | Sim | dev (%) | Obs | Sim | dev (%) |
| 8-15 | 2019 | 48 | 49 | 2.1 | 108 | 111 | 2.8 | 2663 | 2527 | −5.1 |
| 8-15 | 2020 | 47 | 50 | 6.4 | 123 | 124 | 0.8 | 2371 | 3440 | 45.1 |
| 1-6 | 2019 | 50 | 49 | −2.0 | 119 | 111 | −6.7 | 2382 | 2182 | −8.4 |
| NH-2 | 2019 | 48 | 49 | 2.1 | 106 | 112 | 5.7 | 1915 | 2673 | 39.6 |
| NH-2 | 2020 | 50 | 50 | 0.0 | 122 | 124 | 1.6 | 2411 | 3485 | 44.5 |
| ND-5 | 2019 | 48 | 49 | 2.1 | 106 | 110 | 3.8 | 2394 | 2365 | −1.2 |
| d | 0.48 | 0.88 | 0.15 | |||||||
| RMSE | 1.47 | 4.65 | 698.87 | |||||||
| NRMSE (%) | 3.0 | 4.1 | 29.7 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akumaga, U.; Gao, F.; Anderson, M.; Dulaney, W.P.; Houborg, R.; Russ, A.; Hively, W.D. Integration of Remote Sensing and Field Observations in Evaluating DSSAT Model for Estimating Maize and Soybean Growth and Yield in Maryland, USA. Agronomy 2023, 13, 1540. https://doi.org/10.3390/agronomy13061540
Akumaga U, Gao F, Anderson M, Dulaney WP, Houborg R, Russ A, Hively WD. Integration of Remote Sensing and Field Observations in Evaluating DSSAT Model for Estimating Maize and Soybean Growth and Yield in Maryland, USA. Agronomy. 2023; 13(6):1540. https://doi.org/10.3390/agronomy13061540
Chicago/Turabian StyleAkumaga, Uvirkaa, Feng Gao, Martha Anderson, Wayne P. Dulaney, Rasmus Houborg, Andrew Russ, and W. Dean Hively. 2023. "Integration of Remote Sensing and Field Observations in Evaluating DSSAT Model for Estimating Maize and Soybean Growth and Yield in Maryland, USA" Agronomy 13, no. 6: 1540. https://doi.org/10.3390/agronomy13061540
APA StyleAkumaga, U., Gao, F., Anderson, M., Dulaney, W. P., Houborg, R., Russ, A., & Hively, W. D. (2023). Integration of Remote Sensing and Field Observations in Evaluating DSSAT Model for Estimating Maize and Soybean Growth and Yield in Maryland, USA. Agronomy, 13(6), 1540. https://doi.org/10.3390/agronomy13061540






