Investigation of the Detectability of Corn Smut Fungus (Ustilago maydis DC. Corda) Infection Based on UAV Multispectral Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
- Low concentration: 2500 spores/mL
- Medium concentration: 5000 spores/mL
- High concentration: 10,000 spores/mL
2.2. Experimental Devices and Image Acquisition Methods
3. Results
3.1. Results of the Sweet Maize Hybrid Dessert R 73
3.2. Results of the Sweet Maize Hybrid NOA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.ksh.hu/docs/hun/xftp/stattukor/vet/20210601/index.html (accessed on 25 January 2023).
- Széles, A.; Kovács, K.; Ferencsik, S. The effect of crop years and nitrogen basal and top dressing on the yield of different maize genotypes and marginal revenue. Időjárás/Q. J. Hung. Meteorol. Serv. 2019, 123, 265–278. [Google Scholar] [CrossRef]
- Széles, A.; Harsányi, E.; Kith, K.; Nagy, J. The effect of fertilisation and weather extremities caused by climate change on maize (Zea mays L.) yield in Hungary. J. Agric. Food Dev. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Széles, A.; Ragán, P.; Nagy, J. Abiotic stress impacts caused by weather and nutrient replenishment on the yield of maize (Zea mays L). Columella: J. Agric. Environ. Sci. 2017, 4, 39–44. [Google Scholar]
- Rácz, D.; Szőke, L.; Tóth, B.; Kovács, B.; Horváth, É.; Zagyi, P.; Duzs, L.; Széles, A. Examination of the Productivity and Physiological Responses of Maize (Zea mays L.) to Nitrapyrin and Foliar Fertilizer Treatments. Plants 2021, 10, 2426. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Christensen, J.J. Corn Smut Caused by Ustilago Maydis; Monographs; American Phytopathology Society: Worcester, MA, USA, 1963; Volume 2. [Google Scholar]
- Cao, X.; Luo, Y.; Zhou, Y.; Duan, X.; Cheng, D. Detection of powdery mildew in two winter wheat cultivars using canopy hyperspectral reflectance. Crop Prot. 2013, 45, 124–131. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Su, J.; Liu, C.; Hu, X.; Xu, X.; Guo, L.; Chen, W.H. Spatio-temporal monitoring of wheat yellow rust using UAV multispectral imagery. Comput. Electron. Agric. 2019, 167, 105035. [Google Scholar] [CrossRef]
- Su, J.; Liu, C.; Coombes, M.; Hu, X.; Wang, C.; Xu, X.; Chen, W.H. Wheat yellow rust monitoring by learning from multispectral UAV aerial imagery. Comput. Electron. Agric. 2018, 155, 157–166. [Google Scholar] [CrossRef]
- Calou, V.B.C.; dos Santos Teixeira, A.; Moreira, L.C.J.; Lima, C.S.; de Oliveira, J.B.; de Oliveira, M.R.R. The use of UAVs in monitoring yellow sigatoka in banana. Biosyst. Eng. 2020, 193, 115–125. [Google Scholar] [CrossRef]
- Ye, H.; Huang, W.; Huang, S.; Cui, B.; Dong, Y.; Guo, A.; Ren, Y.; Jin, Y. Recognition of banana fusarium wilt based on UAV remote sensing. Remote Sens. 2020, 12, 938. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Gómez Caro, S. Infection and Spread of Peronospora sparsa on Rosa sp. (Berk.). Ph.D. Thesis, Universitäts und Landesbibliothek Bonn, Bonn, Germany, 2014. [Google Scholar]
- Oerke, E.C.; Froehling, P.; Steiner, U. Thermographic assessment of scab disease on apple leaves. Precis. Agric. 2011, 12, 699–715. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of remote sensing in precision agriculture: A review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Polischuk, V.P.; Shadchina, T.M.; Kompanetz, T.I.; Bi, G.; Sozinov, A.L. Changes in reflectance spectrum characteristic of Nicotiana debneyi plant under the influence of viral infection. Arch. Phytopathol. Plant Prot. 1997, 31, 115–119. [Google Scholar] [CrossRef]
- Kuska, M.; Wahabzada, M.; Leucker, M.; Dehne, H.W.; Kersting, K.; Oerke, E.C.; Steiner, U.; Mahlein, A.K. Hyperspectral phenotyping on the microscopic scale: Towards automated characterization of plant-pathogen interactions. Plant Methods 2015, 11, 28. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Snetselaar, K.M.; Mims, C.W. Light and electron microscopy of Ustilago maydis hyphae in maize. Mycol. Res. 1994, 98, 347–355. [Google Scholar] [CrossRef]
- Frommer, D.; Veres, S.; Radócz, L. Susceptibility of stem infected sweet corn hybrids to common smut disease. Acta Agrar. Debr. 2018, 74, 55–57. [Google Scholar] [CrossRef]
- Morrison, E.N.; Emery, R.J.N.; Saville, B.J. Fungal derived cytokinins are necessary for normal Ustilago maydis infection of maize. Plant Pathol. 2017, 66, 726–742. [Google Scholar] [CrossRef]
- Mills, L.J.; Vanstaden, J. Extraction of cytokinins from maize, smut tumors of maize and Ustilago maydis cultures. Physiol. Plant Pathol. 1978, 13, 73–80. [Google Scholar] [CrossRef]
- Turian, G.; Hamilton, R.H. Chemical detection of 3-indolylacetic acid in Ustilago zeae tumors. Biochim. Biophy. Acta 1960, 41, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Szőke, L.; Moloi, M.J.; Kovács, G.E.; Biró, G.; Radócz, L.; Hájos, M.T.; Kovács, B.; Rácz, D.; Danter, M.; Tóth, B. The application of phytohormones as biostimulants in corn smut infected Hungarian sweet and fodder corn hybrids. Plants 2021, 10, 1822. [Google Scholar] [CrossRef]
- Moura, R.M.; Pedrosa, E.M.; Guimarães, L.M. A rare syndrome of corn smut. Fitopatol. Bras. 2001, 26, 782. [Google Scholar] [CrossRef]
- Király, G.; Rizzo, G.; Tóth, J. Transition to Organic Farming: A Case from Hungary. Agronomy 2022, 12, 2435. [Google Scholar] [CrossRef]
- Available online: https://www.dji.com/hu/p4-multispectral/specs (accessed on 25 January 2023).
- Available online: https://opendronemap.org/webodm (accessed on 25 January 2023).
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote sensing of chlorophyll concentration in higher plant leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T. Coincident detection of crop water stress, nitrogen status and canopy density using ground based multispectral data. In Proceedings of the 5th International Conference on Precision Agriculture and Other Resource Management, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Datt, B. A new reflectance index for remote sensing of chlorophyll content in higher plants: Tests using Eucalyptus leaves. J. Plant. Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Rasmussen, J.; Ntakos, G.; Nielsen, J.; Svensgaard, J.; Poulsen, R.N.; Christensen, S. Are vegetation indices derived from consumer-grade cameras mounted on UAVs sufficiently reliable for assessing experimental plots? Eur. J. Agron. 2016, 74, 75–92. [Google Scholar] [CrossRef]
- Guan, S.; Fukami, K.; Matsunaka, H.; Okami, M.; Tanaka, R.; Nakano, H.; Sakai, T.; Nakano, K.; Ohdan, H.; Takahashi, K. Assessing correlation of high-resolution NDVI with fertilizer application level and yield of rice and wheat crops using small UAVs. Remote Sens. 2019, 11, 112. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.R-project.org/ (accessed on 25 January 2023).
- RSTUDIO Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 25 January 2023).
- De Mendinburu, F.; Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1. 3-5. 2021. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 25 January 2023).
- Huzsvai, L.; Balogh, P. Lineáris Modellek az R-ben. Seneca Books, Debrecen. 109–124. 2015. Available online: http://seneca-books.hu/doc/Linearis_modellek.pdf (accessed on 25 January 2023).
- Garcia-Ruiz, F.; Sankaran, S.; Maja, J.M.; Lee, W.S.; Rasmussen, J.; Ehsani, R. Comparison of two aerial imaging platforms for identification of Huanglongbing-infected citrus trees. Comput. Electron. Agric. 2013, 91, 106–115. [Google Scholar] [CrossRef]
- De Castro, A.I.; Ehsani, R.; Ploetz, R.; Crane, J.H.; Abdulridha, J. Optimum spectral and geometric parameters for early detection of laurel wilt disease in avocado. Remote Sens. Environ. 2015, 171, 33–44. [Google Scholar] [CrossRef]
- Albetis, J.; Duthoit, S.; Guttler, F.; Jacquin, A.; Goulard, M.; Poilvé, H.; Féret, J.-B.; Dedieu, G. Detection of Flavescence dorée grapevine disease using unmanned aerial vehicle (UAV) multispectral imagery. Remote Sens. 2017, 9, 308. [Google Scholar] [CrossRef]
- Di Gennaro, S.F.; Battiston, E.; Di Marco, S.; Facini, O.; Matese, A.; Nocentini, M.; Palliotti, A.; Mugnai, L. Unmanned Aerial Vehicle (UAV)-based remote sensing to monitor grapevine leaf stripe disease within a vineyard affected by esca complex. Phytopathol. Mediterr. 2016, 55, 262–275. [Google Scholar]
- Abdulridha, J.; Ampatzidis, Y.; Kakarla, S.C.; Roberts, P. Detection of target spot and bacterial spot diseases in tomato using UAV-based and benchtop-based hyperspectral imaging techniques. Precis. Agric. 2020, 21, 955–978. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, B.; Singh, M.; Thind, S.K. Hyperspectral indices, correlation and regression models for estimating growth parameters of wheat genotypes. J. Indian Soc. Remote Sens. 2015, 43, 551–558. [Google Scholar] [CrossRef]











| Hybrid | Control | Low Dose | Medium Dose | High Dose |
|---|---|---|---|---|
| Dessert R 73 | 4 | 13 | 11 | 6 |
| NOA | 16 | 10 | 1 | 7 |
| Armagnac | 3 | 5 | 14 | 12 |
| P 9025 | 9 | 15 | 8 | 2 |
| Date | Overlap Front/Side | GSD | Number of Captured Channels | Size in Mb (Raw) | Size in Mb (Tiff) |
|---|---|---|---|---|---|
| 7 DAI | 80% | 1.4 cm/px | 6 | 1640 | 84.7 |
| 14 DAI | 80% | 1.4 cm/px | 6 | 1250 | 97.1 |
| 21 DAI | 80% | 1.4 cm/px | 6 | 1620 | 80.6 |
| Abbrev. | Formula | Reference |
|---|---|---|
| NDVI | (RNIR − RRed)/(RNIR + RRed) | [32] |
| GNDVI | (RNIR − RGreen)/(RNIR + RGreen) | [33] |
| NDRE | (RNIR − RRedEdge)/(RNIR + RRedEdge) | [34] |
| LCI | (RNIR − RRedEdge)/(RNIR + RRed) | [35,36] |
| ENDVI | (RNIR + RGreen – 2 × RBlue)/(RNIR + RGreen + 2 × RBlue) | [37] |
| Infection D73 | Df | Sum Sq | Mean Sq | F Value | Pr (>F) |
|---|---|---|---|---|---|
| LCI | 3 | 0.0030246 | 0.0010082 | 21,059 | 0.00138 ** |
| NDVI | 3 | 0.003874 | 0.0012913 | 7.927 | 0.0165 * |
| GNDVI | 3 | 0.010228 | 0.003409 | 15.61 | 0.00307 ** |
| Infection NOA | Df | Sum Sq | Mean Sq | F Value | Pr (>F) |
|---|---|---|---|---|---|
| ENDVI | 3 | 0.007891 | 0.002630 | 9.73 | 0.01167 * |
| GNDVI | 3 | 0.008164 | 0.002721 | 11.78 | 0.00631 ** |
| Infection D73 | Control | Low Dose | Medium Dose | High Dose | Pr (>F) |
| LCI ** | 0.110 a | 0.105 a | 0.083 b | 0.071 b | 0.00138 ** |
| NDVI * | 0.305 a | 0.290 a | 0.288 a | 0.256 b | 0.0165 * |
| GNDVI ** | 0.270 a | 0.265 a | 0.225 b | 0.208 b | 0.00307 ** |
| Infection NOA | Control | Low Dose | Medium Dose | High Dose | Pr (>F) |
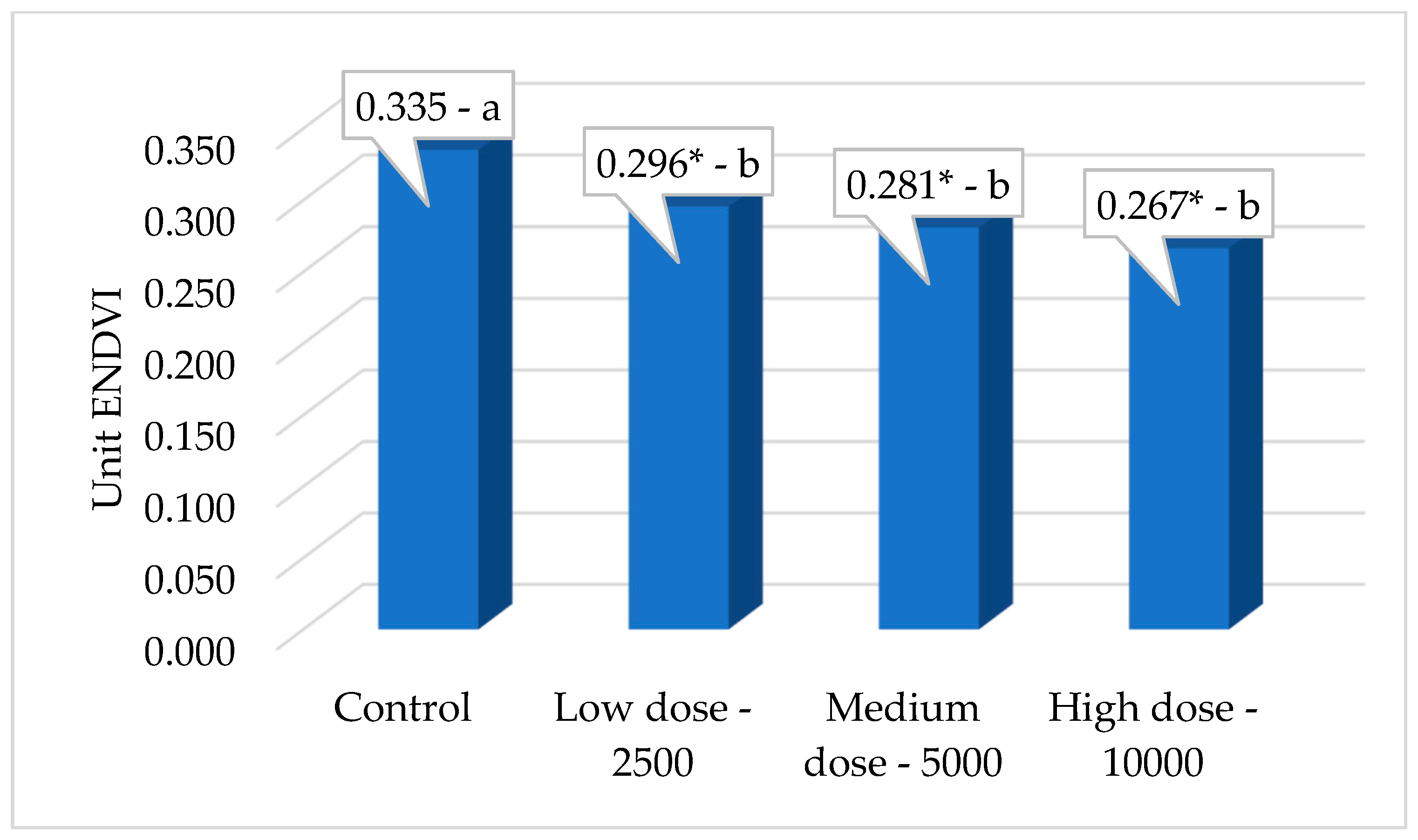
| ENDVI * | 0.335 a | 0.296 b | 0.281 b | 0.267 b | 0.01167 * |
| GNDVI ** | 0.258 a | 0.240 b | 0.209 b | 0.181 b | 0.00631 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radócz, L.; Szabó, A.; Tamás, A.; Illés, Á.; Bojtor, C.; Ragán, P.; Vad, A.; Széles, A.; Harsányi, E.; Radócz, L. Investigation of the Detectability of Corn Smut Fungus (Ustilago maydis DC. Corda) Infection Based on UAV Multispectral Technology. Agronomy 2023, 13, 1499. https://doi.org/10.3390/agronomy13061499
Radócz L, Szabó A, Tamás A, Illés Á, Bojtor C, Ragán P, Vad A, Széles A, Harsányi E, Radócz L. Investigation of the Detectability of Corn Smut Fungus (Ustilago maydis DC. Corda) Infection Based on UAV Multispectral Technology. Agronomy. 2023; 13(6):1499. https://doi.org/10.3390/agronomy13061499
Chicago/Turabian StyleRadócz, László, Atala Szabó, András Tamás, Árpád Illés, Csaba Bojtor, Péter Ragán, Attila Vad, Adrienn Széles, Endre Harsányi, and László Radócz. 2023. "Investigation of the Detectability of Corn Smut Fungus (Ustilago maydis DC. Corda) Infection Based on UAV Multispectral Technology" Agronomy 13, no. 6: 1499. https://doi.org/10.3390/agronomy13061499
APA StyleRadócz, L., Szabó, A., Tamás, A., Illés, Á., Bojtor, C., Ragán, P., Vad, A., Széles, A., Harsányi, E., & Radócz, L. (2023). Investigation of the Detectability of Corn Smut Fungus (Ustilago maydis DC. Corda) Infection Based on UAV Multispectral Technology. Agronomy, 13(6), 1499. https://doi.org/10.3390/agronomy13061499











