Diniconazole Promotes the Yield of Female Hemp (Cannabis sativa) Inflorescence and Cannabinoids in a Vertical Farming System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation Conditions
2.2. DIN Treatment
2.3. Measurement of Plant Growth Parameters
2.4. Measurement and Analyses of Cannabinoid Content
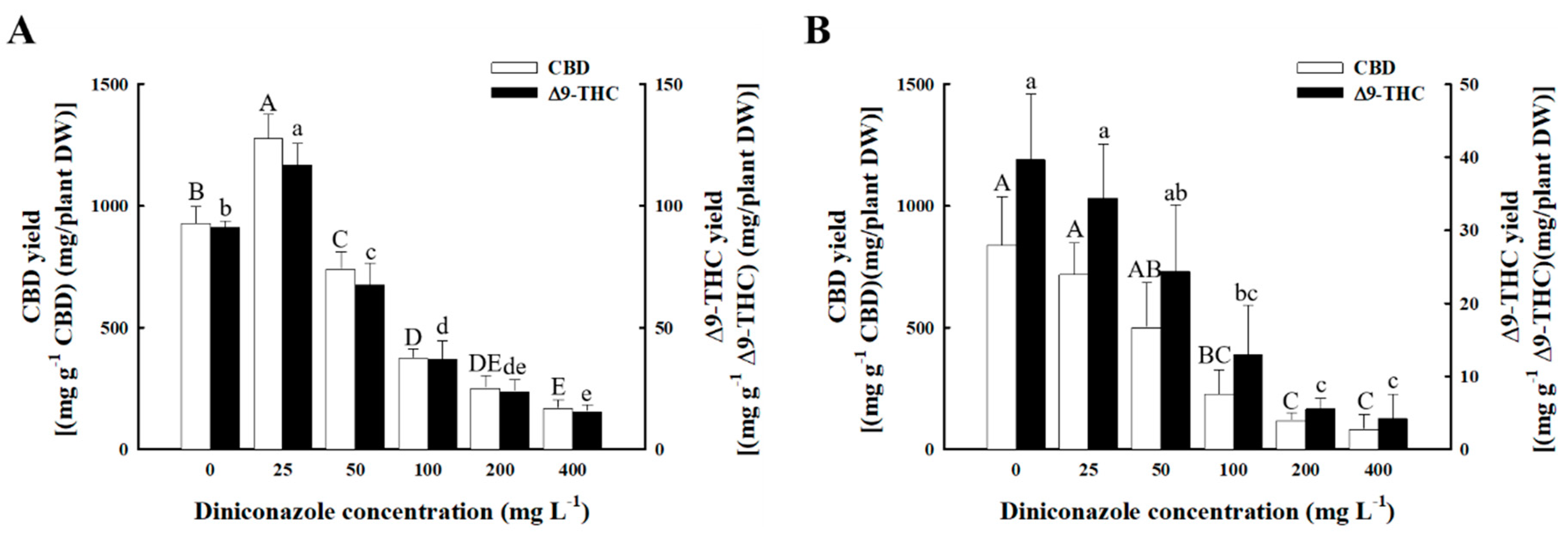
2.5. Total Yield of Major Cannabinoids
2.6. Statistical Analyses
3. Results
3.1. Analyses of Female Hemp Growth Parameters According to DIN Concentration
3.2. Analysis of Female Hemp Cannabinoids Content According to DIN Concentration
3.3. DIN Treatment Contributes to the Contents of Major Cannabinoids in Female Hemp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, E.B.; Jiang, H.-E.; Li, X.; Sutton, A.; Carboni, A.; Del Bianco, F.; Mandolino, G.; Potter, D.J.; Zhao, Y.-X.; Bera, S. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J. Exp. Bot. 2008, 59, 4171–4182. [Google Scholar] [CrossRef]
- Hussain, T.; Jeena, G.; Pitakbut, T.; Vasilev, N.; Kayser, O. Cannabis sativa research trends, challenges, and new-age perspectives. iScience 2021, 24, 103391. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Delogu, G.; Cabras, A.; Marceddu, S.; Bullitta, S. Differentiation between fiber and drug types of hemp (Cannabis sativa L.) from a collection of wild and domesticated accessions. Genet. Resour. Crop Evol. 2013, 60, 2331–2342. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2022, 62, 108074. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New insight into ornamental applications of cannabis: Perspectives and challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef]
- Karche, T. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Krüger, M.; van Eeden, T.; Beswa, D. Cannabis sativa Cannabinoids as Functional Ingredients in Snack Foods—Historical and Developmental Aspects. Plants 2022, 11, 3330. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-cannabinoid metabolites of Cannabis sativa L. with therapeutic potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef]
- Pacifico, D.; Miselli, F.; Carboni, A.; Moschella, A.; Mandolino, G. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 2008, 160, 231–240. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef]
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Taura, F.; Tanaka, Y.; Ishikawa, Y.; Morimoto, S.; Shoyama, Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 2005, 46, 1578–1582. [Google Scholar] [CrossRef]
- Happyana, N.; Kayser, O. Monitoring metabolite profiles of Cannabis sativa L. trichomes during flowering period using 1H NMR-based metabolomics and real-time PCR. Planta Med. 2016, 82, 1217–1223. [Google Scholar]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of cannabinoids from Cannabis sativa L.(Hemp). Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Capano, A.; Weaver, R.; Burkman, E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: A prospective cohort study. Postgrad. Med. 2020, 132, 56–61. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Machado Bergamaschi, M.; Helena Costa Queiroz, R.; Waldo Zuardi, A.; Crippa, A.S. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Shannon, S.; Opila-Lehman, J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: A case report. Perm. J. 2016, 20, 16-005. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chu, R.-M.; Wang, C.-C.; Lee, C.-Y.; Lin, S.-H.; Jan, T.-R. Cannabidiol-induced apoptosis in primary lymphocytes is associated with oxidative stress-dependent activation of caspase-8. Toxicol. Appl. Pharmacol. 2008, 226, 260–270. [Google Scholar] [CrossRef]
- Moher, M.; Llewellyn, D.; Jones, M.; Zheng, Y. Light intensity can be used to modify the growth and morphological characteristics of cannabis during the vegetative stage of indoor production. Ind. Crop. Prod. 2022, 183, 114909. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Despommier, D. Farming up the city: The rise of urban vertical farms. Trends Biotechnol. 2013, 31, 388–389. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the lights for leafy greens in indoor vertical farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Heo, H.K.; Lee, E. Types of vertical smart farms and awareness of their use in Korean cities types and feasibility analysis of vertical smart farms in Korean cities. J. People Plants Environ. 2021, 24, 257–266. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products: Manual for Use by National Drug Testing Laboratories; United Nations Publications: New York, NY, USA, 2009. [Google Scholar]
- Mańkowski, J.; Kołodziej, J.; Pudełko, K.; Kozłowski, R.M. Bast fibres: The role of hemp (Cannabis sativa L.) in remediation of degraded lands. In Handbook of Natural Fibres; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–417. [Google Scholar]
- Ranalli, P. Current status and future scenarios of hemp breeding. Euphytica 2004, 140, 121–131. [Google Scholar] [CrossRef]
- Padilla, I.M.; Fernández-García, N.; Olmos, E.; Burgos, L.; Piqueras, A. Effects of growth retardants on sprouting and development of apricot (Prunus armeniaca L.) and neem (Azarchta indica A. Juss.) nodal buds. Plant Cell Tissue Organ Cult. 2015, 122, 285–297. [Google Scholar] [CrossRef]
- Jarret, R. Effects of chemical growth retardants on growth and development of sweetpotato (Ipomoea batatas (L.) Lam.) in vitro. J. Plant Growth Regul. 1997, 16, 227–231. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Rademacher, W. Chemical regulators of gibberellin status and their application in plant production. In Annual Plant Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; Volume 49, pp. 359–404. [Google Scholar] [CrossRef]
- Teng, C.; Gu, Y.; Wang, Y.; Wang, Z.; Zhao, H.; Qi, P.; Guo, C.; Xu, H.; Di, S.; Wang, X. Enantioselective dissipation, residue, and risk assessment of diniconazole enantiomers in four kinds of fruits. J. Agric. Food Chem. 2021, 69, 15512–15520. [Google Scholar] [CrossRef]
- Choi, S.-H.; Kang, J.-S.; Choi, Y.-W.; Lee, Y.-J.; Park, Y.-H.; Kim, M.-R.; Son, B.-G.; Kim, H.-K.; Kim, H.-Y.; Oh, W. Effect of diniconazole on growth and flowering of Vinca rocea and Salvia splendis. J. Life Sci. 2011, 21, 1004–1008. [Google Scholar] [CrossRef]
- Gilbertz, D.A. Chrysanthemum response to timing of paclobutrazol and uniconazole sprays. HortScience 1992, 27, 322–323. [Google Scholar] [CrossRef]
- Lam, V.P.; Anh, V.K.; Loi, D.N.; Park, J. Minimizing plant height and optimizing bioactive compound accumulation of Agastache rugosa (Fisch. & CA Mey.) kuntze by spraying or soaking with diniconazole in a plant factory. Plant Growth Regul. 2023, 1–13. [Google Scholar] [CrossRef]
- Anderson, S.L.; Pearson, B.; Kjelgren, R.; Brym, Z. Response of essential oil hemp (Cannabis sativa L.) growth, biomass, and cannabinoid profiles to varying fertigation rates. PLoS ONE 2021, 16, e0252985. [Google Scholar] [CrossRef]
- Hädener, M.; König, S.; Weinmann, W. Quantitative determination of CBD and THC and their acid precursors in confiscated cannabis samples by HPLC-DAD. Forensic Sci. Int. 2019, 299, 142–150. [Google Scholar] [CrossRef]
- Kvien, C.; Csinos, A.; Ross, L.; Conkerton, E.; Styer, C. Diniconazole’s effect on peanut (Arachis hypogaea L.) growth and development. J. Plant Growth Regul. 1987, 6, 233–244. [Google Scholar] [CrossRef]
- Jang, D.C.; Xu, C.; Kim, S.H.; Kim, D.H.; Kim, J.K.; Heo, J.Y.; Vu, N.T.; Choi, K.Y.; Kim, I.S. Effects of Different Application Approaches with Diniconazole on the Inhibition of Stem Elongation and the Stimulation of Root Development of Cylindrical Paper Pot Seedling. J. Bio-Environ. Control 2020, 29, 365–372. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, D.; Gao, C.; Wan, S.; Lei, C.; Wang, J.; Zuo, X.; Dong, F.; Li, Y.; Shah, K. Mediation of flower induction by gibberellin and its inhibitor paclobutrazol: mRNA and miRNA integration comprises complex regulatory cross-talk in apple. Plant Cell Physiol. 2018, 59, 2288–2307. [Google Scholar] [CrossRef]
- Goldberg-Moeller, R.; Shalom, L.; Shlizerman, L.; Samuels, S.; Zur, N.; Ophir, R.; Blumwald, E.; Sadka, A. Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering-control genes in Citrus buds. Plant Sci. 2013, 198, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fambuena, N.; Mesejo, C.; González-Mas, M.C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Gibberellic acid reduces flowering intensity in sweet orange [Citrus sinensis (L.) Osbeck] by repressing CiFT gene expression. J. Plant Growth Regul. 2012, 31, 529–536. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Winter, C.M.; Wu, M.-F.; Kanno, Y.; Yamaguchi, A.; Seo, M.; Wagner, D. Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science 2014, 344, 638–641. [Google Scholar] [CrossRef]
- Proietti, S.; Scariot, V.; De Pascale, S.; Paradiso, R. Flowering mechanisms and environmental stimuli for flower transition: Bases for production scheduling in greenhouse floriculture. Plants 2022, 11, 432. [Google Scholar] [CrossRef]
- Apicella, P.V.; Sands, L.B.; Ma, Y.; Berkowitz, G.A. Delineating genetic regulation of cannabinoid biosynthesis during female flower development in Cannabis sativa. Plant Direct 2022, 6, e412. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Mansouri, H.; Asrar, Z.; Mehrabani, M. Effects of gibberellic acid on primary terpenoids and Δ9-tetrahydrocannabinol in Cannabis sativa at flowering stage. J. Integr. Plant Biol. 2009, 51, 553–561. [Google Scholar] [CrossRef]
- Mansouri, H.; Asrar, Z.; Amarowicz, R. The response of terpenoids to exogenous gibberellic acid in Cannabis sativa L. at vegetative stage. Acta Physiol. Plant. 2011, 33, 1085–1091. [Google Scholar] [CrossRef]
- Beacham, A.M.; Vickers, L.H.; Monaghan, J.M. Vertical farming: A summary of approaches to growing skywards. J. Hortic. Sci. Biotechnol. 2019, 94, 277–283. [Google Scholar] [CrossRef]
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef]
- Kwon, C.-T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Folta, K. Breeding new varieties for controlled environments. Plant Biol. 2019, 21, 6–12. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F. Vertical farming: Moving from genetic to environmental modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Ohtaka, K.; Yoshida, A.; Kakei, Y.; Fukui, K.; Kojima, M.; Takebayashi, Y.; Yano, K.; Imanishi, S.; Sakakibara, H. Difference between day and night temperatures affects stem elongation in tomato (Solanum lycopersicum) seedlings via regulation of gibberellin and auxin synthesis. Front. Plant Sci. 2020, 11, 577235. [Google Scholar] [CrossRef]
- Qian, M.; Rosenqvist, E.; Flygare, A.-M.; Kalbina, I.; Teng, Y.; Jansen, M.A.; Strid, Å. UV-A light induces a robust and dwarfed phenotype in cucumber plants (Cucumis sativus L.) without affecting fruit yield. Sci. Hortic. 2020, 263, 109110. [Google Scholar] [CrossRef]
- Wang, M.; Dong, C.; Fu, Y.; Liu, H. Growth, morphological and photosynthetic characteristics, antioxidant capacity, biomass yield and water use efficiency of Gynura bicolor DC exposed to super-elevated CO2. Acta Astronaut. 2015, 114, 138–146. [Google Scholar] [CrossRef]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New insights into gibberellin signaling in regulating plant growth–metabolic coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef] [PubMed]



| Environmental Conditions of Cutting Growth Chamber | |
|---|---|
| Light Quality | Blue |
| Light intensity (μmol·m−2·s−1) | 100 |
| Temperature (°C) | 25 |
| Humidity (%) | 90 |
| EC (ds m−1) | 2.0 |
| Nutrient solution | Hoagland |
| Diniconazole Concentration (mg·L−1) | Plant Height (cm) | No. of Nodes | Stem Diameter (mm) | No. of Leaves | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | wSFW (g/plant) | LFW (g/plant) | FFW (g/plant) | SDW (g/plant) | LDW (g/plant) | FDW (g/plant) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 103.9 ± 0.6 a | 25.0 ± 0.1 a | 9.7 ± 0.6 a | 791.0 ± 10.0 a | 14.3 ± 0.1 a | 15.0 ± 1.2 a | 11580.5 ± 1269.1 a | 183.4 ± 13.2 a | 244.9 ± 12.0 a | 98.9 ± 4.3 a | 29.2 ± 2.8 a | 45.9 ± 4.2 a | 18.3 ± 0.2 b |
| 25 | 68.0 ± 1.6 b | 22.6 ± 0.3 b | 6.6 ± 0.4 ab | 651.6 ± 32.9 b | 11.9 ± 0.2 ab | 11.6 ± 0.6 ab | 7544.0 ± 405.6 b | 108.1 ± 4.4 b | 162.7 ± 6.5 b | 123.1 ± 7.9 a | 18.6 ± 1.1 b | 35.0 ± 1.6 ab | 22.7 ± 1.6 a |
| 50 | 54.4 ± 2.8 c | 23.3 ± 0.3 b | 5.7 ± 0.3 b | 529.3 ± 53.4 b | 10.4 ± 0.7 b | 10.4 ± 0.7 bc | 5455.5 ± 913.2 b | 57.7 ± 11.4 c | 110.5 ± 20.3 c | 58.5 ± 12.8 b | 9.9 ± 2.3 c | 23.0 ± 4.0 bc | 13.2 ± 0.3 c |
| 100 | 28.2 ± 2.5 d | 22.0 ± 0.5 bc | 3.9 ± 0.2 b | 300.0 ± 18.5 c | 7.6 ± 0.5 c | 7.6 ± 0.2 cd | 2007.2 ± 43.37 c | 12.4 ± 3.0 d | 48.6 ± 3.0 d | 34.2 ± 7.4 bc | 1.9 ± 0.5 d | 10.2 ± 2.2 cd | 7.2 ± 0.6 d |
| 200 | 22.1 ± 0.8 de | 21.0 ± 0.1 cd | 4.3 ± 0.0 b | 164.0 ± 16.0 cd | 5.9 ± 0.8 c | 5.9 ± 1.0 d | 656.2 ± 133.16 c | 4.5 ± 0.6 d | 19.2 ± 3.7 d | 23.4 ± 2.9 c | 0.8 ± 0.0 d | 5.4 ± 0.7 d | 4.6 ± 0.4 de |
| 400 | 17.8 ± 1.4 e | 20.3 ± 0.3 d | 6.9 ± 1.6 ab | 74.0 ± 19.0 d | 6.9 ± 0.1 c | 6.9 ± 0.1 d | 298.7 ± 72.1 c | 2.1 ± 0.4 d | 8.4 ± 2.7 d | 13.2 ± 1.2 c | 0.5 ± 0.1 d | 3.8 ± 1.2 d | 3.1 ± 0.3 e |
| Significancex | *** | *** | ** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Ly | *** | *** | * | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Qz | *** | *** | NS | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Diniconazole Concentration (mg·L−1) | wCBDA (mg·g−1) | CBD | Δ9-THC | THCA | Total CBD | Total Δ9-THC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Inflorescence | Leaf | Inflorescence | Leaf | Inflorescence | Leaf | Inflorescence | Leaf | Inflorescence | Leaf | Inflorescence | |
| Control | 20.54 ± 1.08 | 56.80 ± 2.24 | 0.12 ± 0.022 | 0.76 ± 0.08 c | 0.0124 ± 0.0009 ab | 0.09 ± 0.0007 | 0.96 ± 0.045 | 5.56 ± 0.027 | 18.14 ± 0.9781 | 50.57 ± 2.057 | 0.85 ± 0.041 | 4.97 ± 0.024 |
| 25 | 23.20 ± 1.61 | 62.73 ± 1.16 | 0.13 ± 0.019 | 1.32 ± 0.07 a | 0.0107 ± 0.0003b | 0.11 ± 0.0056 | 1.10 ± 0.107 | 5.75 ± 0.216 | 20.48 ± 1.4305 | 56.34 ± 1.484 | 0.97 ± 0.094 | 5.15 ± 0.193 |
| 50 | 24.40 ± 1.12 | 62.45 ± 1.90 | 0.14 ± 0.025 | 1.34 ± 0.07 a | 0.0106 ± 0.0013b | 0.10 ± 0.0087 | 1.17 ± 0.065 | 5.71 ± 0.278 | 21.54 ± 1.0001 | 56.12 ± 1.694 | 1.04 ± 0.057 | 5.11 ± 0.252 |
| 100 | 25.33 ± 0.62 | 58.61 ± 2.68 | 0.14 ± 0.027 | 1.33 ± 0.02 a | 0.0132 ± 0.0013 ab | 0.09 ± 0.0008 | 1.39 ± 0.153 | 5.77 ± 0.081 | 22.36 ± 0.5554 | 52.73 ± 2.335 | 1.24 ± 0.134 | 5.15 ± 0.072 |
| 200 | 25.35 ± 0.32 | 61.47 ± 0.92 | 0.07 ± 0.001 | 0.85 ± 0.04 bc | 0.0171 ± 0.0001 a | 0.09 ± 0.0001 | 1.16 ± 0.010 | 5.74 ± 0.118 | 22.31 ± 0.2901 | 54.76 ± 0.767 | 1.03 ± 0.009 | 5.14 ± 0.104 |
| 400 | 24.43 ± 1.37 | 60.98 ± 0.09 | 0.07 ± 0.024 | 1.08 ± 0.01 ab | 0.0169 ± 0.0013 a | 0.10 ± 0.0030 | 1.21 ± 0.110 | 5.64 ± 0.102 | 21.49 ± 1.2308 | 54.56 ± 0.065 | 1.08 ± 0.098 | 5.05 ± 0.087 |
| Significancex | NS | NS | NS | *** | ** | NS | NS | NS | NS | NS | NS | NS |
| Ly | * | NS | NS | NS | *** | NS | NS | NS | * | NS | NS | NS |
| Qz | ** | NS | * | * | *** | NS | * | NS | ** | NS | * | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahm, S.; Lee, B.; Bok, G.; Kim, S.; Park, J. Diniconazole Promotes the Yield of Female Hemp (Cannabis sativa) Inflorescence and Cannabinoids in a Vertical Farming System. Agronomy 2023, 13, 1497. https://doi.org/10.3390/agronomy13061497
Hahm S, Lee B, Bok G, Kim S, Park J. Diniconazole Promotes the Yield of Female Hemp (Cannabis sativa) Inflorescence and Cannabinoids in a Vertical Farming System. Agronomy. 2023; 13(6):1497. https://doi.org/10.3390/agronomy13061497
Chicago/Turabian StyleHahm, Seungyong, Beomseon Lee, Gwonjeong Bok, Sungjin Kim, and Jongseok Park. 2023. "Diniconazole Promotes the Yield of Female Hemp (Cannabis sativa) Inflorescence and Cannabinoids in a Vertical Farming System" Agronomy 13, no. 6: 1497. https://doi.org/10.3390/agronomy13061497
APA StyleHahm, S., Lee, B., Bok, G., Kim, S., & Park, J. (2023). Diniconazole Promotes the Yield of Female Hemp (Cannabis sativa) Inflorescence and Cannabinoids in a Vertical Farming System. Agronomy, 13(6), 1497. https://doi.org/10.3390/agronomy13061497





