Abstract
A factor that causes inconsistencies in rice yield receiving biochar reported in the literature has been identified as the length of time after biochar incorporation into the soil prior to planting. There is limited information on the effect of the varying lengths of time on soil properties and rice growth. This study aimed to determine the effects of the length of time of incorporation of rice husk biochar (RHB) into an acidic paddy soil before rice transplanting on soil properties and rice yield. A greenhouse experiment was conducted using a highly weathered paddy soil subjected to incorporation periods of RHB at various lengths, including 0, 15, 30, and 60 days before rice transplanting (DBT). The RHB incorporation was under a soil moisture content of 70% of the soil water holding capacity. At harvest time (98 days after incorporation), increases in the length of RHB incorporation led to significantly higher Mg, Mn, and Si concentrations, but lower Ca and Fe concentrations in rice whole shoots. Increasing the length of RHB incorporation to 15, 30, and 60 DBT significantly decreased the total rice grain yield to 61.4 g hill−1, 62.5 g hill−1, and 54.4 g hill−1, respectively, compared to 76.0 g hill−1 found at 0 DBT. The depression of rice grain yield with increasing RHB incorporation periods was due to the antagonistic effects of Mg on Ca and Si on Fe. Immediate rice transplanting without a prior RHB incorporation period is recommended for its use as a soil amendment in acidic paddy soils.
1. Introduction
The use of biochar as an amendment to improve the fertility of paddy soils has been under extensive investigation by researchers [1,2,3], while farmers have also been applying this material to their fields [4]. However, the effects of biochar on rice yields have been inconsistent, which has constrained the widespread adoption of this amendment.
Biochar made from rice husks was employed in this study. Rice husks are a byproduct of rice production that are available in large quantities, and are easily accessible in many Asian countries, including Thailand, where rice production is a primary agricultural activity that drives domestic consumption and export economies [5]. Transforming rice husks to biochar and using this biochar as a soil amendment meets the current policies of natural resource conservation through the recycling of resources and zero-waste undertaken by many countries worldwide [6]. The improvement of paddy soil fertility is crucial, as most paddy soils are infertile and continually degraded [7]. Some of these soils are acidic, with high Al, Fe, and Mn contents, which can be phytotoxic [8,9].
Biochar, including rice husk biochar (RHB), undergoing pyrolysis yields three major components, namely, fixed C, volatile matter, and ash, the contents of which vary depending upon pyrolysis conditions [10]. The volatile matter content of RHB decreases with increasing pyrolysis temperature, and the opposite is true for fixed C and ash [11]. Ash constituents can be beneficial or deleterious to soil fertility [12,13]. Ash consists primarily of oxides, hydroxides, and carbonates of cations, such as K, Na, Ca, and Si, which are formed during the pyrolysis of biomass [14]. Some of these inorganic ions are essential plant nutrients, e.g., P, K, Mg, Ca, and Si [12]. Pels et al. [15] reported that ash could improve soil fertility to a comparable degree to chemical fertilizer. It also plays a critical role in ameliorating soil acidity [12,13,16], alleviating the phytotoxicity of Al [12,17] and Mn [12], and decreasing P precipitation [18]. Nevertheless, the high ash content of biochar has adverse effects on plant growth by causing excessive pH increases and salinity stress [19]. Additionally, high-ash biochar causes a nutrient imbalance in plants due to high contents of micronutrients (i.e., Zn, Fe, and Cu), metals (i.e., Al and Pb) [20], and K. Plant K luxury consumption brought about antagonistic effects on Ca and Mg uptake [12].
Past studies have reported inconsistent effects of biochar on rice yields. Haefele et al. [21], Wang et al. [22], Zhang et al. [23], and Ghorbani et al. [24] reported that adding biochar to paddy fields increased soil physicochemical fertility and rice yield. However, some studies have shown no effect of biochar amendment on rice yields [2], while some have found adverse effects [1]. This is likely due to the different characteristics of soils, e.g., pH, texture, and available nutrients, and those of the biochar used in these studies. The length of time between biochar incorporation into soils and planting is likely a significant factor determining biochar benefits or detriments to plants. It is hypothesized that the length of time between biochar incorporation into soils and planting has an influence on soil properties, notably, soil pH and nutrient availability and balance affecting plant growth and yield. To our knowledge, no study has compared the effects of varying lengths of time between biochar incorporation and planting on soil and plant responses. Indeed, variable lengths of biochar incorporation before transplanting are found in the literature, for example, 7 days before rice transplanting (DBT) [22], 14 [1], 20 [3], and 44 DBT [25]. Meanwhile, others did not provide detailed biochar incorporation time lengths [2,21,23].
The length of time between biochar incorporation into the soil and planting has effects on the dissolubility of minerals derived from biochar ash which, in turn, has effects on plant nutrition. Ulery et al. [14], who studied wood ash composition after burning wildlands, reported two dissolution phases of mineral constituents of ash associated with pH increases. Both phases, occurring in sequence, were within 60 days since burning. An initial phase of soil pH increase occupied by readily soluble oxides, hydroxides, and carbonates of K and Na, and a later phase associated with less soluble calcite, CaCO3. For Si-rich rice husk and rice straw biochar, Wang et al. [26] also claimed two dissolution phases of Si, including the initial phase (45 days after RHB incorporation), which was the faster soluble phase of nonphytolith Si, and the slower dissolution phase (45–60 days) associated with phytolith Si. It has been consistently shown that longer incorporation periods result in greater soil Si contents [26].
Incorporating RHB into soil prior to planting induces higher dissolution of cation constituents of biochar ash, which is a way to bring about higher availability of these cations that are lacking in strongly acidic soils, which can, in turn, affect rice yield. In this paper, we investigate differences in the lengths of time between the incorporation of biochar into the soil (under approximate field capacity soil moisture content) and the planting of rice as a previously unrecognized factor causing yield inconsistencies. Therefore, the objective of the current study is to determine the effects of varying lengths of time between RHB incorporation into the soil and rice transplanting on the soil properties and rice yield.
2. Materials and Methods
2.1. Soil and Rice Husk Biochar
The Roi-Et soil series (isohyperthermic Aeric Kandiaquults) used in the current study is representative of Ultisol paddy soil, as it is the most widely used soil for rice production in Northeast Thailand. The Roi-Et soil series was identified in the location of its collection using a 1:25,000 soil map of Thailand developed by the Land Development Department [27]. The soil was collected at a depth of 0–15 cm from a paddy field in Muang district, Sakon Nakhon Province, Thailand (17°20′36.7″ N; 104°02′52.6″ E). The soil was air-dried and sieved to pass through a 2 mm mesh sieve. The initial physical and chemical properties of the studied Roi-Et soil are presented in Table 1.

Table 1.
Initial physical and chemical properties of the Roi-Et soil used in this study.
Rice husk biochar was produced in a kiln, which was modified from a 300-L metal cylindrical-shaped tank typically employed in biochar production for soil amendment in the provinces of Sakon Nakhon and Nakhon Phanom, Thailand. The biochar was pyrolyzed at approximately 450 °C for 2 h, after which it was left to cool for 6 h. Rice husk biochar was kept in a sealed polyethylene bag for further use in the experiment. The characteristics of the rice husk biochar are shown in Table 2.

Table 2.
Characteristics of the rice husk biochar used in this study.
2.2. Greenhouse Experiment
A pot experiment was conducted in a greenhouse equipped with an evaporative cooling system during the period from June to November 2019. The average air temperature over the experimental period was 30.8 °C. The experiment was arranged in a completely randomized design with three replications. Treatments involved mixing RHB with the Roi-Et soil, the mixture of which was incubated for various periods in the days before rice seedling transplanting. There were five treatments, including unamended without a soil incorporation period prior to rice transplant (no RHB, 0 DBT), and RHB amended and the mixture incubated for various periods prior to rice transplant, i.e., 0 (RHB, 0 DBT), 15 (RHB, 15 DBT), 30 (RHB, 30 DBT), and 60 (RHB, 60 DBT). The treatment (without RHB) was designed to act as the reference for the other treatments with RHB. An experimental unit consisted of an inverted truncated-pyramid shaped pot (h = 23.5 cm, top d = 19 cm, bottom d = 15 cm, v = 6823 cm3) filled with 6 kg of air-dried soil. Those units that received amended treatments were applied with two percent weight-by-weight (equivalent to 43.5 Mg ha−1) of RHB mixed thoroughly with soil in each pot [12]. The pots with their contents were supplemented with water to the amounts of 25.1% and 25.8% w/w for the RHB in the unamended and amended treatments, respectively. The amounts of added water were equivalent to 70% of the soil water-holding capacity. The experimental units were incubated for various periods until their predetermined rice transplanting time. Constant soil moisture contents were kept throughout the incorporation time length by weighing the pots and adding tap water to predetermined weights. At the rice transplanting time, water was added to each pot to a height of 30 mm above the soil surface [28]. Water application was discontinued 7 days before rice harvest, and the soil was not submerged at harvest [29].
Rice seedlings used for transplanting were the RD 22 rice variety that had been seeded in a nursery tray for 30 days. Two 30-day-old seedlings selected based on their high homogeneity and health were transplanted into the center of a pot under submerged conditions at a rate of 2 seedlings hill−1 and 1 hill pot−1 [30]. Fertilizers of commercial grades 46-0-0 (46% N of urea fertilizer), 18-46-0 (18% N and 46% P2O5 of diammonium phosphate fertilizer), and 0-0-60 (60% K2O of muriate of potash) were applied in solution form thrice at 3, 20, and 37 days after transplanting to achieve a total of 180 kg N ha−1, 60 kg P2O5 ha−1, and 90 kg K2O ha−1 [31]. Half of the fertilizers were applied during the first application, and the other half was split equally (25%) for the second and third applications [1].
Rice plants were harvested 98 days after transplanting. The plants were cut at the soil level, while fresh soil samples were immediately collected for mineral N determination. At harvest, tillering and panicle numbers were determined. Roots were extracted from the soil, after which the soil was air-dried. Grain, shoot, and root biomass were oven-dried at 65 °C to constant weights, and dry weights were determined. The oven-dried shoot was ground and sieved through a 1 mm mesh sieve for tissue nutrient analyses. The air-dried soil was ground and sieved to pass through a 2 mm sieve for further soil chemical analyses.
The numbers and weights of filled, unfilled, and total grain of each panicle were determined. The filled grains were weighed to evaluate the 1000-grain weight. The harvest index (HI) was calculated using the equation HI = [filled grain weight hill−1/(filled grain weight hill−1 + shoot dry weight hill−1)] × 100 [8].
2.3. Laboratory Analyses of Rice Husk Biochar, Soil, and Plant Tissue
2.3.1. Rice Husk Biochar
Proximate analysis parameters of RHB, i.e., fixed C, ash, and volatile matter, were determined following ASTM D7582-15 [32]. The carbon and N contents of RHB were determined on a TN analyzer (multi N/C® 2100S, Analytik, Jena, Germany). The phosphorus, K, Ca, and Mg of RHB were extracted using the nitric–perchloric digestion method [33]. Phosphorus was subsequently determined on a UV-Vis spectrophotometer (Specord250 plus, Analytik Jena, Germany), while K, Ca, and Mg were analyzed on an atomic absorption spectrophotometer (AAS) (novAA® 350, Analytik Jena, Germany). Silicon of the RHB was extracted using nitric acid–hydrofluoric acid [34], and measured on an inductively coupled plasma optical emission spectrometer (ICP-OES) (PlasmaQuant PQ9000, Analytik Jena, Germany).
2.3.2. Soil
The particle size distribution and texture of the soil were estimated by the pipette method. Soil bulk density was measured using the core method [35]. Soil pH and electrical conductivity (EC) were determined at soil-to-water ratios of 1:1 and 1:5, respectively. Soil total C analysis was performed using the Walkley and Black method [36], while total N analysis was performed by the micro-Kjeldahl method [37]. Soil mineral N (NH4+ and NO3−) was extracted using a 2 M KCl solution and determined by the distillation method [38]. Soil P was extracted by Bray2 solution (0.1 M HCl + 0.03 M NH4F) and colorimetrically determined on a spectrophotometer (Specord250 plus, Analytik Jena, Germany) at 820 nm [39]. For soil cation determination, K, Ca, and Mg extractions were performed with 1 M NH4OAc at pH 7.0, while Fe and Mn were extracted using diethylenetriaminepentaacetic acid. These cations were then determined on an AAS [35]. Soil Al was extracted with 1 M KCl and determined by the titrimetry method [40]. Soil Si was extracted by 0.5 M acetic acid and determined by ICP-OES [41].
2.3.3. Plant Tissue
The whole above-ground plant, excluding grains, was extracted for N, P, K, Ca, Mg, Fe, Mn, and Al by nitric–perchloric wet digestion [33], while Si was extracted by sodium hydroxide–hydrogen peroxide digestion. Tissue N was determined following the micro-Kjeldahl method [42]. Tissue P determination was performed on the spectrophotometer, while K, Ca, Mg, Fe, and Mn were on the AAS, and Al and Si were on the ICP-OES.
2.4. Statistical Analysis
The effects of time lengths of RHB incorporation before rice transplant on soil properties, tissue nutrient concentrations, and rice yield and yield components were evaluated using PROC ANOVA following SAS Institute Inc. [43]. Treatment mean comparisons were performed by Fisher’s least significant difference (LSD). Relationships among variables were determined by Pearson’s linear correlation (PROC CORR) procedure. The most important soil properties, rice tissue nutrient concentrations, and yield and yield components were analyzed by principal component analysis (PCA) using the PROC PRINCOMP model. The effects of soil properties and tissue nutrients, preselected based on the PCA results, on rice yield and yield components were performed by multiple linear regression using PROC ROBUSTREG. Significant differences were at p ≤ 0.05.
3. Results and Discussion
3.1. Elemental Concentrations of Soil and Plant Tissue in Relation to Soil pH as Affected by Various Lengths of Time of Rice Husk Biochar Incorporation
3.1.1. Elements of Macronutrients in Rice
The macronutrients studied included primary N, P, and K, and secondary Ca and Mg; Si is considered a beneficial macronutrient for rice [44]. The soil P, K, and Si concentrations increased with increasing length of RHB incorporation (p ≤ 0.01); however, this trend was not observed in TC, TN, mineral N (NH4+ and NO3−), Ca, and Mg (Table 3). Significant increases compared with the RHB found at 0 DBT treatment were shown in the RHB treatments from 15 to 60 DBT for P and Si, and in 15 and 60 DBT for K. Dissolution of the mineral component of ash in biochar played a crucial role in temporal increases in P, K, and Si of the soil [45]. The results were consistent with Yao et al. [46], who found increases in P and K concentrations in soil with the length of time of incorporation of biochar derived from sewage sludge. In addition, Li et al. [47] reported greater levels of Si release as the incorporation length of time of rice straw-derived biochar increased. Phosphorus, K, and, in particular Si, were major elements in the ash [48]. Silicon was 125.9 g kg−1, which was the second largest elemental content, next to C, in RHB, while the P and K contents were 3.68 and 5.05 g kg−1, respectively (Table 2). Our results of Si, P, and K contents were comparable to Costa et al. [48], who showed that the amounts of Si, P, and K in RHB were 350.2, 4.2, and 10.1 g kg−1, respectively. Si is a major element in various kinds of rice residue-derived biochar. Silicon in rice residue-derived biochar includes phytolith [SiOn/2(OH)4-n]m, hydrated amorphous Si (SiO2·nH2O), and crystalline Si, e.g., gonnardite (Na2CaAl4Si6O20∙7(H2O), cristobalite ((SiO2)n), tridymite ((SiO2)n), diopside MgCaSi2O6), kalsilite (KAlSiO4), albite (Na(AlSi3O8)), and quartz ((SiO2)n) [49,50]. The majority of these Si compounds, notably phytolith and amorphous Si, in rice residue-derived biochar existed in biochar pyrolyzed at approximately 500 °C [50], which was the same level of pyrolysis temperature in biochar production in this study. Both of these Si compounds in rice residue-derived biochar are easily dissoluble [47,50].

Table 3.
Soil physical and chemical properties of the Roi-Et soil at the rice harvest (98 days after transplanting) as influenced by the length of time of incorporation of rice husk biochar.
Pools of P in the biochar ash were the fast-release pool, e.g., monetite (Ca(HPO4)) and crandallite (CaAl3(PO4)2(OH)5∙(H2O)), and the slow-release pool, e.g., amorphous- and crystalline-P minerals [51]. Regarding K, the mineral form of K in ash was dominated by sylvite (KCl) [17]. The temporal dissolution of these ash-derived minerals contributed to increasing concentrations of Si, P, and K in soil subjected to increasing lengths of time of incorporation of RHB (Table 3). Greater soil Si and K concentrations (Table 3) corresponded to increasing concentrations in rice tissue (Table 4).

Table 4.
Nutrient concentrations of whole plant shoots at the rice harvest (98 days after transplanting) as influenced by the length of time of incorporation of rice husk biochar.
Soil pH was found to increase with RHB incorporation periods, and significant increases were shown in the longer periods of 30 and 60 DBT (Table 3). The increased soil pH was linked to the higher concentrations of Si and P in the soil. The relationships were confirmed by significant correlation coefficients of r = 0.635 * and 0.498 * for pH with Si and P, respectively. Dissolution of silicate [52] and phosphate [53] in ash consumed H+, increasing the soil pH.
The enhancement of certain soil elements, i.e., Si and K (Table 3), improved these plant nutrients, as demonstrated by the significant trends of increased tissue Si and K concentrations during the periods 15 to 60 DBT compared to 0 DBT (Table 4). Significantly higher tissue Mg concentrations at 30 and 60 DBT than those found at 0 DBT were due to rising soil pH leading to improved nutrient availability for plant uptake [9], as confirmed by the significant correlation of soil pH and tissue Mg (r = 0.581 *). Unchanged tissue P concentrations, despite increases in soil P, were likely due to P precipitation with Al in soil [9], as confirmed by their negative correlation (r = −0.604 *).
3.1.2. Elements of Micronutrients of Rice, Fe, and Mn, and Non-Nutrient Al
Many paddy soils have high contents of acidic elements, particularly Fe, Mn, and Al, which are harmful to rice plants [8]. The tissue Fe concentrations in this study were 0.20–0.44 g kg−1 (Table 4). This level was far from the phytotoxic range of 0.68–0.85 g kg−1 [8]. Incorporation of RHB for 15 to 60 DBT resulted in significantly lower tissue Fe concentrations than at 0 DBT (Table 4). The trend of decreasing Fe concentrations in the soil (Table 3) was likely the result of increased soil pH, which lowered Fe availability to plants [54]. The negative correlation between soil pH and tissue Fe in rice plants (r = −0.684 **) supported this argument. In addition, significantly decreased Fe in the rice plant may be regulated principally by the antagonistic effect of another cation, Si. The mechanism is presented in Section 3.2.
Manganese concentrations in soil (Table 3) and those concomitant in the plant (Table 4) were higher under RHB incorporation periods of 15 to 60 DBT than without time between incorporation and transplanting (0 DBT). Mn tissue concentrations ranged between 0.20 and 0.83 g kg−1, which were adequate contents. The critical deficiency of Mn in rice is 0.02 g kg−1, and the toxicity is 46 g kg−1 [8]. The increased concentrations of Mn in the soil and plants were due to the greater solubility of Mn with longer periods of RHB incorporation. Mechanisms proposed by Butnan et al. (2015) were such that organic molecules, such as acetic, citric, oxalic, tannic, and gallic acids, as well as catechol, which were produced during the biochar aging processes, enhanced the reduced conditions, causing higher solubility of soil Mn. These organic molecules then chelated with the soluble Mn, enabling greater plant uptake.
Active aluminum concentrations in soil decreased significantly at 60 DBT compared with all other treatments with shorter incorporation periods, except 30 DBT (Table 3), while those in rice shoots significantly decreased from 15 to 60 DBT relative to the 0 DBT treatment (Table 4). Aluminum is not a plant nutrient, unlike Fe and Mn. Moreover, its high contents are phytotoxic [9]. The critical concentration of Al reported to be toxic to rice plants is 0.3 g kg−1 [55]. The tissue Al concentrations found in this study were at phytotoxic levels, which ranged from 1.43–7.0 g kg−1 (Table 4). However, incorporation of RHB for a shorter period of 15 DBT could alleviate Al phytotoxicity. The amelioration of soil Al toxicity to rice plants was principally because of the liming effect of RHB, as shown by a negative correlation of soil Al with pH (p = −0.927 ***). Increases in soil pH resulted in the transformation of toxic Al3+ to less toxic Al hydroxides, e.g., AlOH2+, Al(OH)2+, Al(OH)30, Al(OH)4−, and Al(OH)52−, and finally to precipitated forms [56]. In addition, adsorption of Al to ash-derived minerals is another mechanism that results in mitigating Al toxicity to plants in soil [17]. Al3+ could be adsorbed onto particles of the ash-derived minerals by electrostatic force, leading to the formation of a complex structure via the H-bond between O atoms of AlOH2+, and Al(OH)2 with ─COOH and ─OH on the mineral surface through a ligand exchange reaction [45]. This reaction occurred on the particle surfaces of the mineral components of ash, particularly Si-containing minerals [49]. An additional role of Si-constituted minerals in decreasing soil Al concentrations was the coprecipitation between mineral components of ash and Al to form hydroxylaluminosilicate [57]. Principal ash minerals that react with soil Al to form precipitated compounds are diopside (MgCaSi2O6), kasilite (KAlSiO4), cristobalite ((SiO2)n), and albite (Na(AlSi3O8)) [17].
The precipitation reaction between RHB-derived phosphate and soil Al decreased the soil Al concentrations, as seen in the contrasting concentrations of soil Al and P, with increasing RHB incorporation periods (Table 3). This proposed mechanism was substantiated by a negative correlation between Al and P in soil (r = −0.604 *). The mechanism was consistent with that found by Hsu [58], who reported phosphate fixation by Al in acidic soils, leading to their conclusion that soil phosphate had a vital role in precipitating soil Al3+ to form Al6(OH)12(H2PO4)6. In addition, the precipitation of Al with Si was also reported [59], based on a negative correlation of Al and Si (r = −0.707 **) found in our study.
3.2. Rice Yield Decreases with Increasing Durations of Rice Husk Incorporation into Soil as Influenced by Soil and Tissue Elemental Concentrations
Significant decreases in root and shoot biomass (Table 5), tillering, panicle number, and filled and total grain yield (Table 6) were observed when RHB incorporation time lengths were 15 to 60 DBT compared to 0 DBT. Rice yield is regulated by its yield components [8]. In our study, decreases in rice grain yield with an increasing incorporation time length were linked to panicle number, which was, in turn, linked to tillering (Table 6).

Table 5.
Rice root and shoot biomass and shoot-to-root ratio at the rice harvest (98 days after transplanting) as influenced by the length of time of incorporation of rice husk biochar.

Table 6.
Rice yield and yield component at the rice harvest (98 days after transplanting) as influenced by the length of time of incorporation of rice husk biochar.
The results of the principal component analysis showed a strong association of concentrations of P, Si, and Mg in soil, and Fe, Si, and K in rice tissue with rice tillering. This was indicated by the higher values in absolute terms of the eigenvector of −0.262 (for soil P), −0.252 (soil Si), −0.232 (soil Mg), 0.272 (tissue Fe), −0.239 (tissue Si), and −0.230 (tissue K) than other elemental concentration parameters of soil and plants (Figure 1a). The longer incorporation times of RHB (15−60 DBT) had a positive association with soil P, K, Mg, Ca, Si, and pH, whereas negative associations were found for the 0 DBT and the unamended treatments (Figure 1b). The 15−60 DBT treatments also had positive associations with tissue P, Si, Mg, Mn, N, and Mn, while they had negative associations with tissue Fe, Ca, and Al (Figure 1b). A multiple linear regression analysis was further used to evaluate the effect of the aforementioned soil and plant elements on tillering, panicle number, and grain yield. The results showed that soil Si concentration had significant negative effects on such yield and yield components of rice, and the opposite was true for tissue Fe concentrations (Table 7). These results on the effects of soil Si and tissue Fe concentrations on the rice yield components prompted a further investigation of the interactions of Si and Fe in rice plants. Additionally, tissue K and soil Mg were also found to have significant negative effects on rice grain yield. These additional results also pointed out the interactions of K and Mg in rice plants. It was hypothesized that the antagonistic effect of cation nutrients occurred as affected by the prolonged length of incorporation time, which brought about the decrease in rice yield. To test this hypothesis, tissue nutrient ratios were calculated (Table 8). Calcium was included in the evaluation because our results showed that significant decreases in tissue Ca concentrations with increasing incorporation lengths occurred (Table 4). Calcium has been shown to regulate Mg and K uptake [9,12]. The tissue Ca/Mg ratio was significantly lower at 30 and 60 DBT, and a decreasing trend was found at 15 DBT compared to 0 DBT (Table 8). However, significant increases in Si/Fe ratios were found from 15 to 60 DBT compared to those found at 0 DBT. An increase in Mg availability due to higher soil pH may be a result of an antagonistic relationship between Mg and Ca. An excessive supply of Si from RHB (Table 2), and significant increases in Si in soil with longer incorporation time lengths (Table 3), led to a deficiency of Fe in the rice plant. Deficiency of Fe through a large amount of Si supply was likely attributed to the combined effect of several mechanisms. Wu et al. [60] revealed the interaction of Fe with Si to form iron silicate (Fe2SiO4), termed Fe plaque, which was deposited on rice root surfaces. The plaque behaved like a barrier, manifesting itself against Fe uptake. In addition, Pavlovic et al. [61] proposed that Si caused decreases in Fe concentrations in roots and shoots of rice by decreasing Fe uptake and translocation to the shoots. Silicon promoted the formation of a Casparian strip, which blocked the apoplastic pathway of Fe loading into the xylem vessel [62].
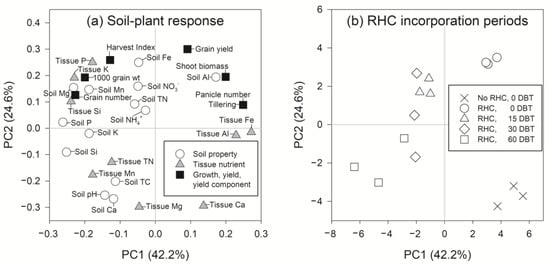
Figure 1.
Eigenvectors of principal component analysis of (a) soil–plant response parameters to (b) differing lengths of time of rice husk biochar (RHB) incorporation before rice transplanting (DBT). PC1 and PC2 are principal components 1 and 2.

Table 7.
Multiple linear regression analysis pertaining to the effects of nutrients in soil and in whole plant shoots on rice tillering, panicle number, and grain yield.

Table 8.
Selected rice tissue nutrient ratios at the rice harvest (98 days after transplanting) as influenced by the length of time of incorporation of rice husk biochar.
In our results, deficiencies of Ca and Fe due to prolonged RHB incorporation lengths of time were seen in their shoot tissue concentrations of 2.40–2.91 g kg−1 and 0.20–0.40 g kg−1, respectively (Table 4), which were within the range of the deficiency levels reported by Fageria [8], who found that the critical concentrations of tissue Ca and Fe in rice biomass were 2.5–4.0 g kg−1 and 0.07–0.3 g kg−1, respectively.
Although several studies, such as Chan et al. [63], Burrell et al. [64], and Hardy et al. [65], have highlighted the long-term benefits of biochar and the advantages of terra preta de indio, which remains fertile even after a millennium of application [66,67], the present study did not contradict these findings. Rather, differences in biochar properties and soil types, as well as their combined effects, contributed to divergent outcomes in soil and plant responses. It is important to note that this study did not compare the short-term and long-term effects of biochar, and instead focused on determining the optimal timing of rice husk biochar application before rice transplanting in a sandy-textured soil.
4. Conclusions
The results of this study support the hypothesis by clearly demonstrating the effects of lengths of incorporation of rice husk biochar (RHB) into the soil before transplanting rice. The effects were due mainly to the ash constituents of the RHB, which became soluble in higher amounts with time. The prolonged lengths of incorporation time brought about fertility amelioration of the highly weathered, acidic soil by increasing pH and decreasing Al, and increasing the contents of ash-derived nutrients for rice plants, including P, K, and Si. However, prolonged lengths of RHB incorporation created nutrient imbalances due to increases in Mg and Si concentrations in the rice plant, which brought about antagonistic effects of Mg to Ca and Si to Fe, leading to Ca and Fe deficiencies, which, in turn, negatively affected rice yield. To our knowledge, there appears to be no published results on the effect of varying lengths of time of biochar incorporation into soils on soil properties and rice growth. In agricultural practice, rice transplanting without a prior soil incorporation period of RHB is recommended for the effective use of RHB as a soil amendment in highly weathered, coarse-textured, acidic paddy soils.
Author Contributions
Conceptualization, S.B.; methodology, S.B.; formal analysis and investigation, S.B.; writing—original draft preparation, S.B.; writing—review and editing, P.V.; funding acquisition, S.B. and P.V.; resources, S.B. and P.V.; supervision, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Fund for Researchers from Revenue of Sakon Nakhon Rajabhat University (SNRU) FY 2019 (project no. 24/2562), the Research Career Development Fund FY 2022 (project no. 7/2565) from SNRU, and the Soil Organic Matter Management Research Group, Khon Kaen University FY 2020 and 2021.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We thank Janista Duangpukdee for coordinating data collection. A. Terry Rambo’s constructive comments on this manuscript are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ly, P.; Duong Vu, Q.; Jensen, L.S.; Pandey, A.; de Neergaard, A. Effects of rice straw, biochar and mineral fertiliser on methane (CH4) and nitrous oxide (N2O) emissions from rice (Oryza sativa L.) grown in a rain-fed lowland rice soil of Cambodia: A pot experiment. Paddy Water Environ. 2015, 13, 465–475. [Google Scholar] [CrossRef]
- Si, L.; Xie, Y.; Ma, Q.; Wu, L. The short-term effects of rice straw biochar, nitrogen and phosphorus fertilizer on rice yield and soil properties in a cold waterlogged paddy field. Sustainability 2018, 10, 537. [Google Scholar] [CrossRef]
- Kumputa, S.; Vityakon, P.; Saenjan, P.; Lawongsa, P. Carbonaceous greenhouse gases and microbial abundance in paddy soil under combined biochar and rice straw amendment. Agronomy 2019, 9, 228. [Google Scholar] [CrossRef]
- Latawiec, A.E.; Królczyk, J.B.; Kuboń, M.; Szwedziak, K.; Drosik, A.; Polańczyk, E.; Grotkiewicz, K.; Strassburg, B.B.N. Willingness to adopt biochar in agriculture: The producer’s perspective. Sustainability 2017, 9, 655. [Google Scholar] [CrossRef]
- Thambhitaks, K.; Kitchaicharoen, J. Valuation of external costs of wet-season lowland rice production systems in Northern Thailand. CMUJ Nat. Sci. 2021, 20, e2021057. [Google Scholar] [CrossRef]
- Zaman, A.U. A strategic framework for working toward zero waste societies based on perceptions surveys. Recycling 2017, 2, 1. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, C.; Sardans, J.; Vancov, T.; Fang, Y.; Wu, L.; Huang, X.; Gargallo-Garriga, A.; Peñuelas, J.; Wang, W. Effect of soil degradation on the carbon concentration and retention of nitrogen and phosphorus across Chinese rice paddy fields. CATENA 2022, 209, 105810. [Google Scholar] [CrossRef]
- Fageria, N.K. Mineral Nutrition of Rice; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 5th ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Antal, M.J.; Gronli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Phuong, H.T.; Uddin, M.A.; Kato, Y. Characterization of biochar from pyrolysis of rice husk and rice straw. J. Biobased Mater. Bioenergy 2015, 9, 439–446. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237–238, 105–116. [Google Scholar] [CrossRef]
- Deenik, J.L.; Diarra, A.; Uehara, G.; Campbell, S.; Sumiyoshi, Y.; Antal, M.J. Charcoal ash and volatile matter effects on soil properties and plant growth in an acid Ultisol. Soil Sci. 2011, 176, 336–345. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Amrhein, C. Wood–ash composition and soil pH following intense burning. Soil Sci. 1993, 156, 358–364. [Google Scholar] [CrossRef]
- Pels, J.R.; Nie, D.S.; Kiel, J.H.A. Utilization of ashes from biomass combustion and gasification. In Proceedings of the 13th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Berek, A.K.; Hue, N.V. Characterization of biochars and their use as an amendment to acid soils. Soil Sci. 2016, 181, 412–426. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Dual role of biochars as adsorbents for aluminum: The effects of oxygen–containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 2013, 47, 8759–8768. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Buss, W.; Jansson, S.; Mašek, O. Unexplored potential of novel biochar–ash composites for use as organo–mineral fertilizers. J. Clean. Prod. 2019, 208, 960–967. [Google Scholar] [CrossRef]
- Bieser, J.M.H.; Thomas, S.C. Biochar and high–carbon wood ash effects on soil and vegetation in a boreal clearcut. Can. J. For. Res. 2019, 49, 1124–1134. [Google Scholar] [CrossRef]
- Haefele, S.M.; Konboon, Y.; Wongboon, W.; Amarante, S.; Maarifat, A.A.; Pfeiffer, E.M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice-based systems. Field Crops Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Thammasom, N.; Vityakon, P.; Lawongsa, P.; Saenjan, P. Biochar and rice straw have different effects on soil productivity, greenhouse gas emission and carbon sequestration in Northeast Thailand paddy soil. Agric. Nat. Resour. 2016, 50, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Chen, B. Biochar impacts on soil silicon dissolution kinetics and their interaction mechanisms. Sci. Rep. 2018, 8, 8040. [Google Scholar] [CrossRef] [PubMed]
- Land Development Department. Thailand Soil Map Scale 1:25000. Available online: http://eis.ldd.go.th/lddeis/SoilView.aspx (accessed on 15 May 2019).
- Pratiwi, E.P.A.; Shinogi, Y. Rice husk biochar application to paddy soil and its effects on soil physical properties, plant growth, and methane emission. Paddy Water Environ. 2016, 14, 521–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Peng, S.; Xing, D.; Qin, J.; Laza, R.C.; Punzalan, B.R. Water use efficiency and physiological response of rice cultivars under alternate wetting and drying conditions. Sci. World J. 2012, 2012, 287907. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, M.C.S.; Kropff, M.J.; Maligaya, A.R.; Tuong, T.P. Drought–stress responses of two lowland rice cultivars to soil water status. Field Crops Res. 1996, 46, 21–39. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Powlson, D.; Min, J.; Shi, W. Rice production, nitrous oxide emission and ammonia volatilization as impacted by the nitrification inhibitor 2–chloro–6–(trichloromethyl)–pyridine. Field Crops Res. 2015, 173, 1–7. [Google Scholar] [CrossRef]
- American Standard of Testing Material. Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis; American Standard of Testing Material International: West Conshohocken, PA, USA, 2015.
- Miller, R.O. Nitric–perchloric acid wet digestion in an open vessel. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 57–61. [Google Scholar]
- Novozamsky, I.; van Eck, R.; Houba, V.J.G.; van der Lee, J.J. Solubilization of plant tissue with nitric acid–hydrofluoric acid–hydrogen peroxide in a closed–system microwave digestor. Commun. Soil Sci. Plant Anal. 1996, 27, 867–875. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Propterties; Spark, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen–Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Propterties; Spark, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Stevenson, F.J. Nitrogen–Inorganic forms. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Propterties; Spark, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Fixen, P.E.; Grove, J.H. Testing soils for phosphorus. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 141–181. [Google Scholar]
- Bertsch, P.M.; Bloom, P.R. Aluminum. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1996; pp. 517–550. [Google Scholar]
- Korndörfer, G.H.; Snyder, G.H.; Ulloa, M.; Powell, G.; Datnoff, L.E. Calibration of soil and plant silicon analysis for rice production. J. Plant Nutr. 2001, 24, 1071–1084. [Google Scholar] [CrossRef]
- Horneck, D.A.; Miller, R. Determination of total nitrogen in plant tissue. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 75–83. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.1: User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 17–39. [Google Scholar]
- Qian, L.; Chen, B. Interactions of aluminum with biochars and oxidized biochars: Implications for the biochar aging process. J. Agric. Food Chem. 2013, 62, 373–380. [Google Scholar] [CrossRef]
- Yao, F.X.; Arbestain, M.C.; Virgel, S.; Blanco, F.; Arostegui, J.; Maciá-Agulló, J.A.; Macías, F. Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere 2010, 80, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Unzué-Belmonte, D.; Cornelis, J.-T.; Linden, C.V.; Struyf, E.; Ronsse, F.; Delvaux, B. Effects of phytolithic rice–straw biochar, soil buffering capacity and pH on silicon bioavailability. Plant Soil 2019, 438, 187–203. [Google Scholar] [CrossRef]
- Costa, H.M.d.; Visconte, L.L.Y.; Nunes, R.C.R.; Furtado, C.R.G. Rice husk ash filled natural rubber. III. Role of metal oxides in kinetics of sulfur vulcanization. J. Appl. Polym. Sci. 2003, 90, 1519–1531. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Chen, M. Novel alleviation mechanisms of aluminum phytotoxicity via released biosilicon from rice straw–derived biochars. Sci. Rep. 2016, 6, 29346. [Google Scholar] [CrossRef]
- Li, Z.; Delvaux, B. Phytolith–rich biochar: A potential Si fertilizer in desilicated soils. GCB Bioenergy 2019, 11, 1264–1282. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M. The fate of phosphorus of ash–rich biochars in a soil–plant system. Plant Soil 2014, 375, 61–74. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the mechanism of the dissolution of quartz and silica in aqueous solutions. J. Am. Chem. Soc. 2017, 2, 1116–1127. [Google Scholar] [CrossRef]
- Semerci, N.; Ahadi, S.; Coşgun, S. Comparison of dried sludge and sludge ash for phosphorus recovery with acidic and alkaline leaching. Water Environ. J. 2021, 35, 359–370. [Google Scholar] [CrossRef]
- Lindsay, W.L. Soil and plant relationships associated with iron deficiency with emphasis on nutrient interactions. J. Plant Nutr. 1984, 7, 489–500. [Google Scholar] [CrossRef]
- Tanaka, A.; Navasero, S.A. Aluminum toxicity of the rice plant under water culture conditions. Soil Sci. Plant Nutr. 1966, 12, 9–14. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry; Academic Press: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Jugdaohsingh, R.; Brown, A.; Dietzel, M.; Powell, J.J. High–aluminum–affinity silica is a nanoparticle that seeds secondary aluminosilicate formation. PLoS ONE 2013, 8, e84397. [Google Scholar] [CrossRef] [PubMed]
- Hsu, O.H. Fixation of phosphate by aluminum and iron in acidic soils. Soil Sci. 1965, 99, 398–402. [Google Scholar] [CrossRef]
- Tubaña, B.S.; Heckman, J.R. Silicon in soils and plants. In Silicon and Plant Diseases; Rodrigues, F.A., Datnoff, L.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 7–51. [Google Scholar]
- Wu, C.; Zou, Q.; Xue, S.-G.; Pan, W.-S.; Huang, L.; Hartley, W.; Mo, J.-Y.; Wong, M.-H. The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss (ROL). Environ. Pollut. 2016, 212, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.-T. The long-term effect of biochar on soil microbial abundance, activity and community structure is overwritten by land management. Front. Environ. Sci. 2019, 7, 110. [Google Scholar] [CrossRef]
- Sombroek, W.G. Amazon Soils: A Reconnaissance of the Soils of the Brazilian Amazon Region; Centre for Agricultural Publications and Documentation: Wageningen, The Netherlands, 1966. [Google Scholar]
- Glaser, B.; Guggenberger, L.H.G.; Zech, W. The ‘Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Sci. Nat. 2001, 88, 37–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).