Untargeted Metabolite Profiling of Camellia tetracocca’s Response to an Empoasca onukii Attack Using GC-MS and LC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of C. tetracocca Leaves
2.2. GC/MS Analysis
2.3. LC/MS Analysis
2.4. Data Preprocessing and Annotation
2.5. Compound Identification
2.6. Data Analysis
3. Results
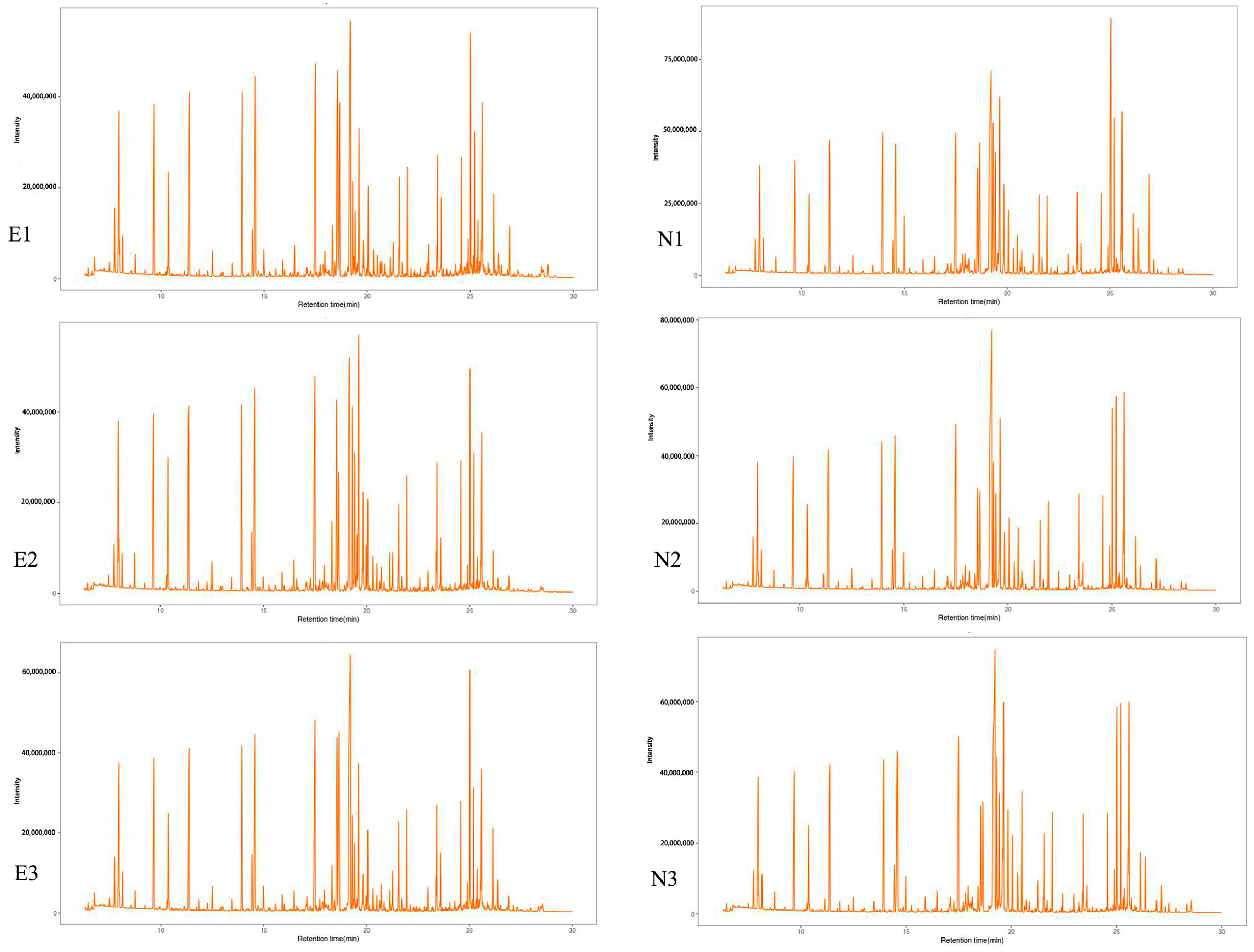
3.1. Metabolite Identification for Leaves of C. tetracocca
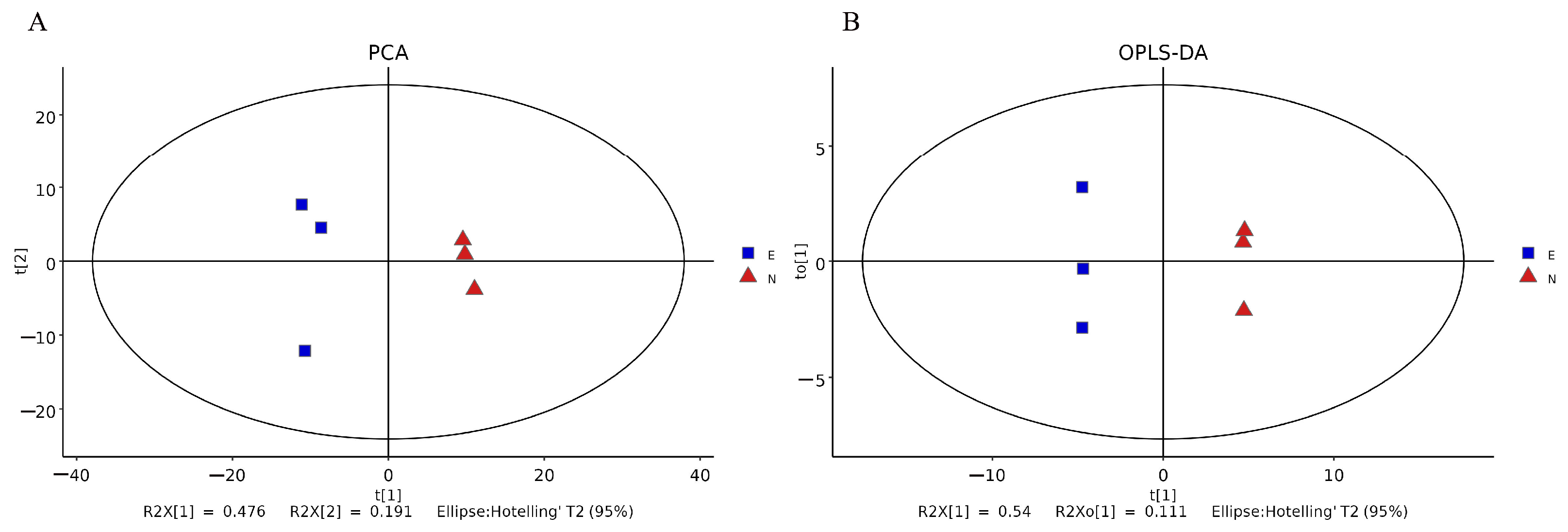
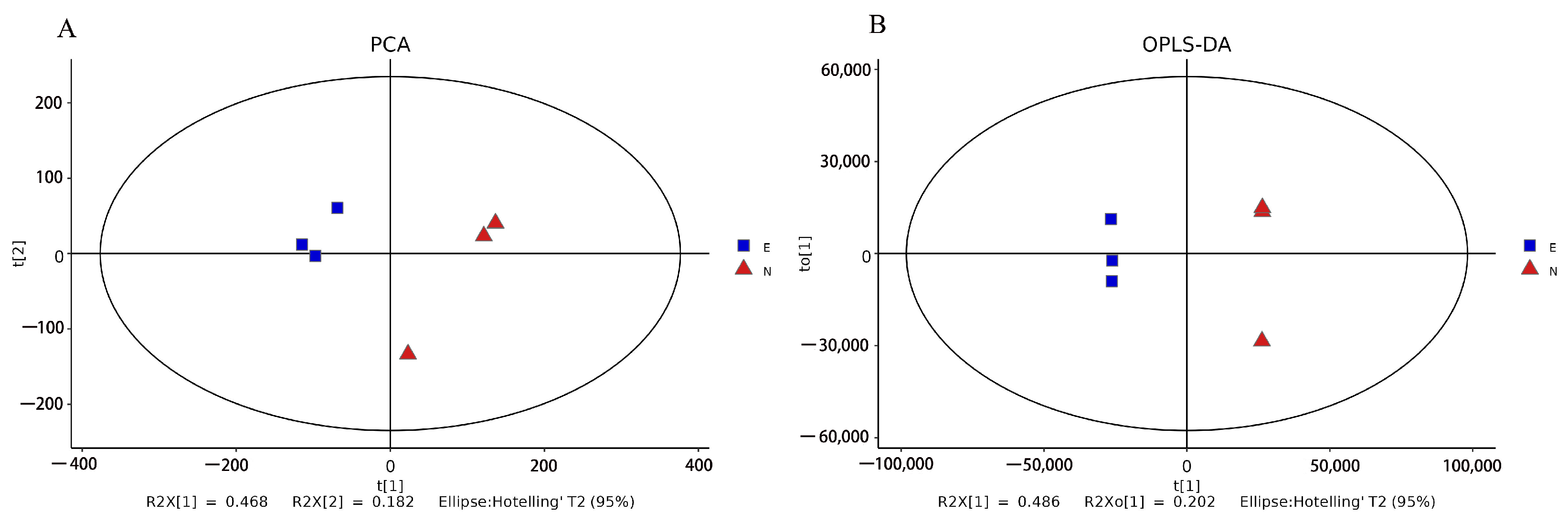
3.2. Principal Component Analysis (PCA) and Orthogonal Projections to Latent Structures-Discriminant Analysis (OPLS-DA)
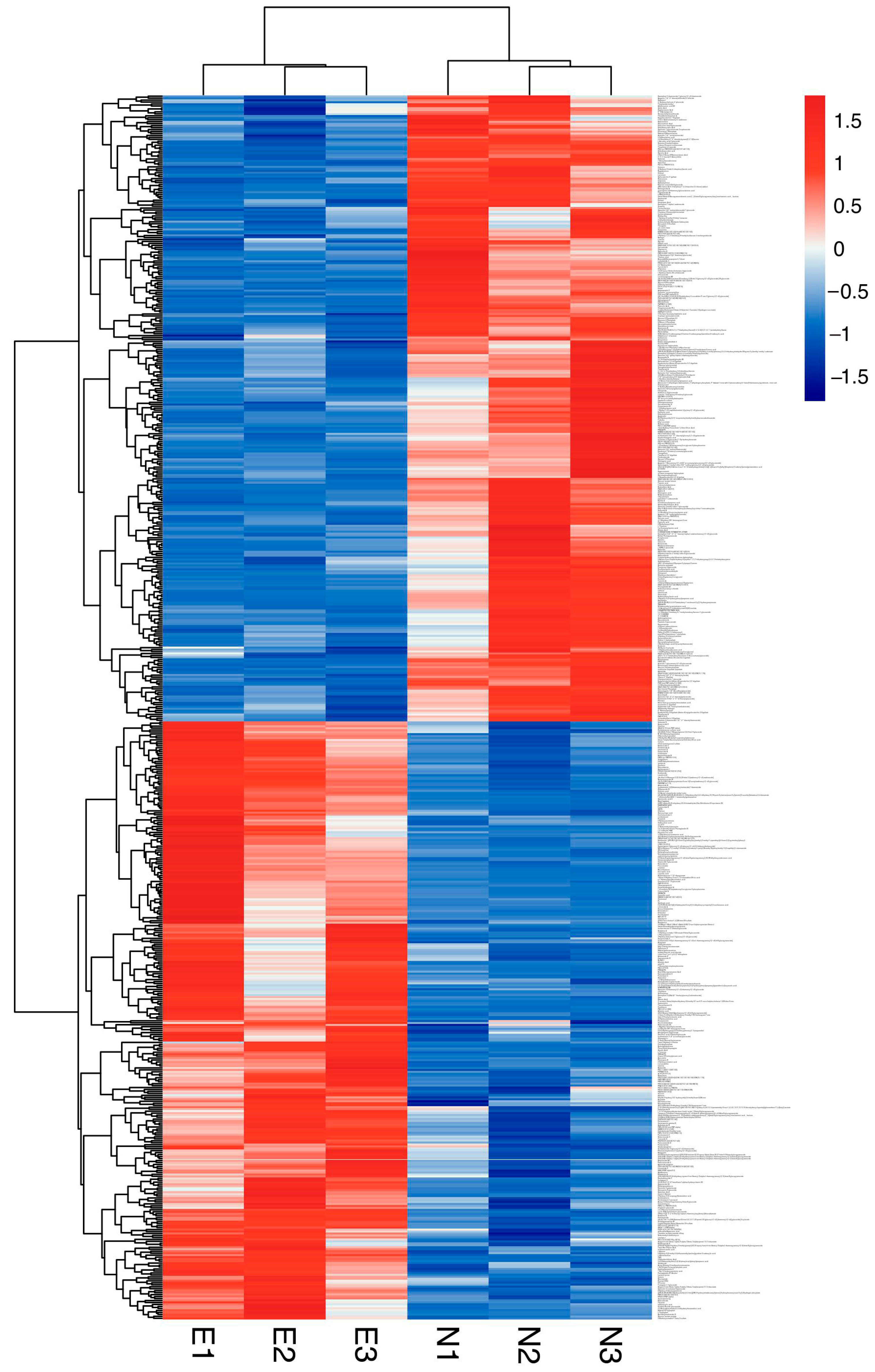
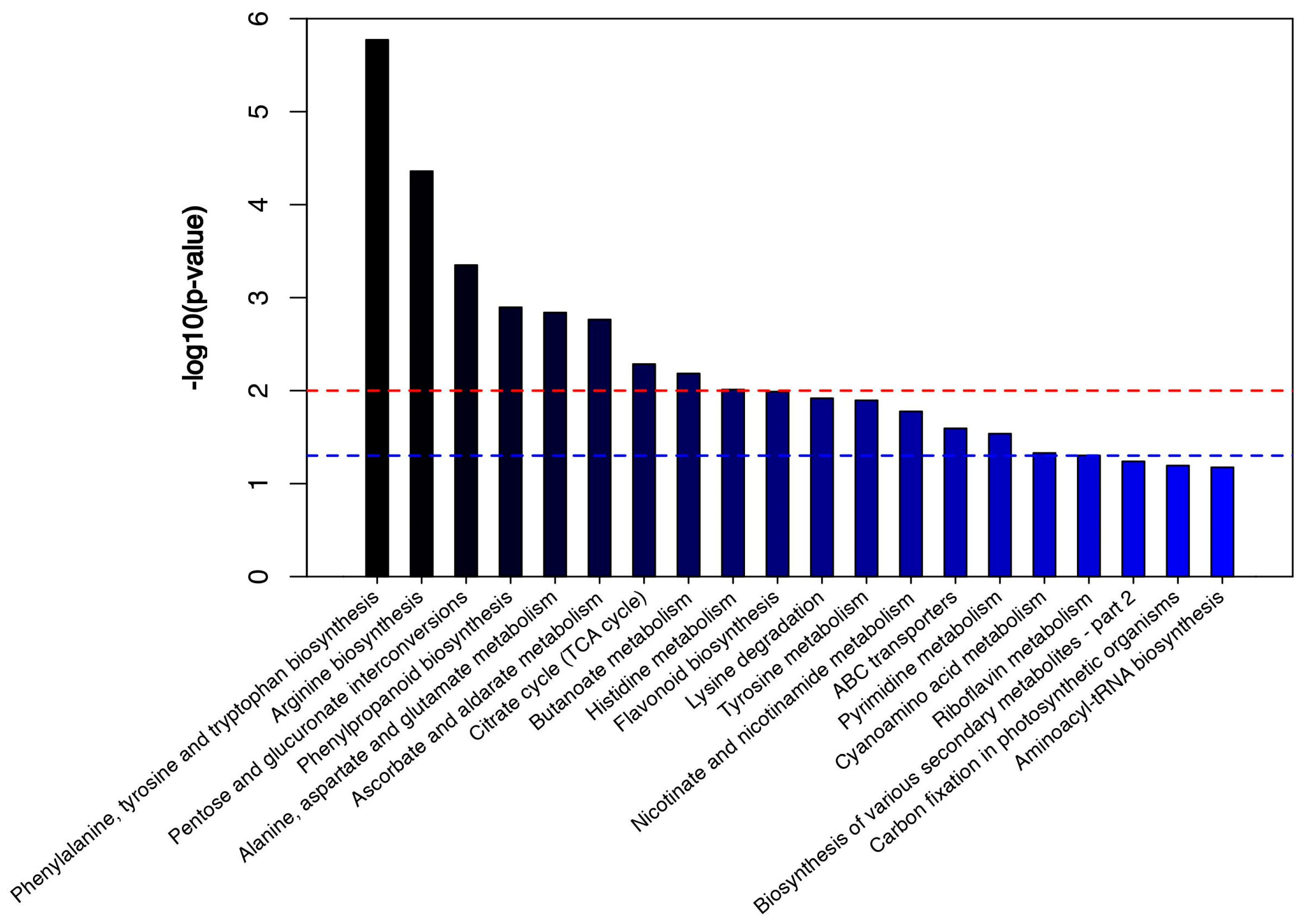
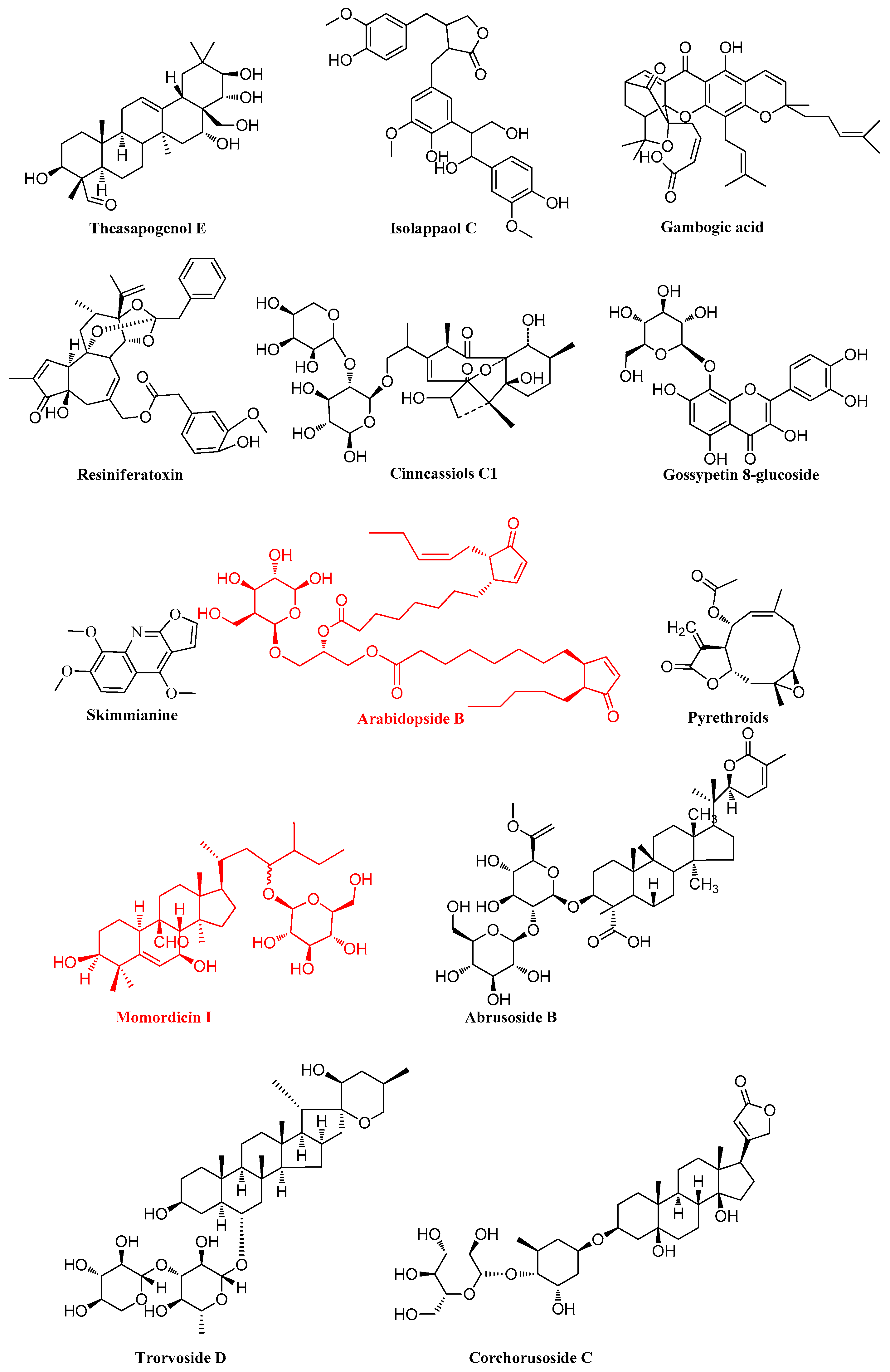
3.3. Differentially Abundant Metabolites and Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.D. Systematic classification of tea plants. Acta Sci. Nat. Univ. Sunyatseni 1981, 1, 87–99. [Google Scholar]
- Luo, W.; Chen, Y.Y.; Yin, S.H.; Wang, L.F.; Huang, X.X.; Geng, F.; Chen, X.M. Genetic diversity of Camellia tetracocca Zhang germplasm resources in Pu’an, Guizhou Province. Acta Agric. Univ. Jiangxiensis 2022, 44, 74–85. [Google Scholar]
- Yuan, M.Q.; Qian, C.J. Study on endemic plant Camellia tetracocca in Pu’an.an county, Guizhou. Guizhou Sci. 2009, 2, 80–85. [Google Scholar]
- Duan, X.Y.; Hu, H.J.; Wang, J.L.; Cao, Y.; Zhao, H.F. Present situation, protection and utilization of big tea tree Resources in southwest Guizhou. Agric. Tech. Serv. 2011, 28, 1736–1737. [Google Scholar]
- Li, Y.F.; Ouyang, S.H.; Chang, Y.Q.; Wang, T.M.; Li, W.X.; Tian, H.Y.; Cao, H.; Kurihara, H.; He, R.R. A comparative analysis of chemical compositions in Camellia sinensis var. Pu’anensis Kurihara, a novel Chinese tea, by HPLC and UFLC-Q-TOF-MS/MS. Food Chem. 2017, 216, 282–288. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhao, G.F.; Li, X.; Shen, Q.; Wu, Q.; Zhuang, J.H.; Zhang, X.Q.; Xia, E.H.; Zhang, Z.Z.; Qian, Y.M.; et al. Comparative analysis of phenolic compound metabolism among tea plants in the section Thea of the genus Camellia. Food Res. Int. 2020, 135, 109276. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Shi, L.Q.; Vasseur, L.; Zhao, Q.; Chen, J.; You, M.S.; You, S.J. Genetic analyses reveal regional structure and demographic expansion of the predominant tea pest Empoasca onukii (Hemiptera: Cicadellidae) in China. Pest Manag. Sci. 2022, 78, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Tan, R.; Gong, Z.; Kuang, S. Relationships between population dynamics of Empoasca vitis and meteorological factors in tea plantation. Hubei Agric. Sci. 2014, 53, 32. [Google Scholar]
- Chen, L.L.; Yuan, P.; Pozsgai, G.; Chen, P.; Zhu, H.P.; You, M.S. The impact of cover crops on the predatory mite Anystis baccarum (Acari, Anystidae) and the leafhopper pest Empoasca onukii (Hemiptera, Cicadellidae) in a tea plantation. Pest Manag. Sci. 2019, 75, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, L.; Xiao, Q.; Dietrich, C.; Matsumura, M. Clarification of the identity of the tea green leafhopper based on morphological comparison between Chinese and Japanese specimens. PLoS ONE 2015, 10, e0139202. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, M.Q.; Chen, Z.M. The relative preference of Empoasca onukii (Hemiptera: Cicadellidae) for oviposition on twenty-four tea cultivars. J. Econ. Entomol. 2022, 115, 1521–1530. [Google Scholar] [CrossRef]
- Kosugi, Y. Infuence of injury by tea green leafhopper, Empoasca onukii Matsuda on leaves in new shoots of tea plants. Tea Res. Bull. 2000, 88, 1–8. (In Japanese) [Google Scholar]
- Backus, E.A.; Serrano, M.S.; Ranger, C.M. Mechanisms of hopperburn: An overview of insect taxonomy, behavior, and physiology. Annu. Rev. Entomol. 2005, 50, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Muday, G.K.; Brown-Harding, H. Nervous system-like signaling in plant defense. Science 2018, 361, 1068–1069. [Google Scholar] [CrossRef]
- Bai, Y.C.; Yang, C.Q.; Halitschke, R.; Paetz, C.; Kessler, D.; Burkard, K.; Gaquerel, E.; Baldwin, L.T.; Li, D.P. Natural history—Guided omics reveals plant defensive chemistry against leafhopper pests. Science 2022, 375, eabm2948. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Zhao, S.Q.; Zhu, J.Y.; Li, F.D.; Tong, W.; Liu, S.R.; Wei, C.L. Transcriptomic analyses reveal a systemic defense role of the uninfested adjacent leaf in tea plant (Camellia sinensis) attacked by tea geometrids (Ectropis obliqua). Genomics 2020, 112, 3658–3667. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aroma. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Plant chemistry: Endless variety. In Insect-Plant Biology; Oxford University Press: Oxford, UK, 2005; pp. 3–82. [Google Scholar]
- Harborne, J.B.; Baxter, H. Phytochemical dictionary. In A Handbook of Bioactive Compounds from Plants; Taylor & Francis: London, UK, 1993. [Google Scholar]
- Harborne, J.B. Introduction to Ecological Biochemistry, 4th ed.; Academic Press: London, UK, 1993. [Google Scholar]
- Hrborne, J.B. Phenolics. In Natural Products: Their Chemistry and Biological Significance; Longman: Harlow, UK, 1998; pp. 362–388. [Google Scholar]
- Bahmani, M.; Golshahi, H.; Saki, K.; Rafieian-Kopaei, M.; Delfan, B.; Mohammadi, T. Medicinal plants and secondary metabolites for diabetes mellitus control. Asian Pac. J. Trop. Dis. 2014, 4, S687–S692. [Google Scholar] [CrossRef]
- Shi, J.H.; Liu, H.; Pham, T.C.; Hu, X.J.; Liu, L.; Wang, C.; Foba, C.F.; Wang, S.B.; Wang, M.Q. Volatiles and hormones mediated root-knot nematode induced wheat defense response to foliar herbivore aphid. Sci. Total Environ. 2022, 815, 152840. [Google Scholar] [CrossRef]
- Romero, B.; Dillon, F.M.; Zavala, J.A. Different soybean cultivars respond differentially to damage in a herbivore-specific manner and decreas herbivore performance. Arthropod-Plant Interact. 2019, 14, 89–99. [Google Scholar] [CrossRef]
- Ye, M.; Glauser, G.; Lou, Y.; Erb, M.; Hu, L. Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in Rice. Plant Cell 2019, 31, 687–698. [Google Scholar] [CrossRef]
- Uawisetwathana, U.; Jamboonsri, W.; Bamrungthai, J.; Jitthiang, P.; Nookaew, I.; Karoonuthaisiri, N. Metabolite profiles of brown planthopper-susceptible and resistant rice (Oryza sativa) varieties associated with infestation and mechanical stimuli. Phytochemistry 2022, 194, 113044. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, B.L.; Edrada-Ebel, R.; Costa, F.B.D. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for Plant Improvement: Status and Prospects. Front. Plant Sci. 2017, 8, 1302. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qiao, N.; Ning, F.J.; Huang, X.Y.; Luo, L.P. Identification and characterization of plant-derived biomarkers and physicochemical variations in the maturation process of Triadica cochinchinensis honey based on UPLC-QTOF-MS metabolomics analysis. Food Chem. 2022, 13, 13517. [Google Scholar] [CrossRef]
- Barrett, D.P.; Fowler, S.V.; Subbaraj, A.K.; Groenteman, R.; Clavijo-McCormick, A. Metabolomic analysis of host plant biochemistry could improve the effectiveness and safety of classical weed biocontrol. Biol. Control 2021, 160, 104663. [Google Scholar] [CrossRef]
- Hall, R.D.; D’Auria, J.C.; Ferreira, A.C.S.; Gibon, Y.; Kruszka, D.; Mishra, P.; Zedde, R.V.D. High-throughput plant phenotyping: A role for metabolomics? Trends Plant Sci. 2022, 27, 549–550. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant defense and insect adaptation with reference to secondary metabolites. In Co-Evolution of Secondary Metabolites, Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Uawisetwathana, U.; Graham, S.F.; Kamolsukyunyong, W.; Sukhaket, W.; Klanchui, A.; Toojinda, T.; Vanavichit, A.; Karoonuthaisiri, N.; Rlliott, C.T. Quantitative 1H NMR metabolome profiling of Thai Jasmine rice (Oryza sativa) reveals primary metabolic response during brown planthopper infestation. Metabolomics 2015, 11, 1640–1655. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Wang, H.Y.; Zhang, J.J.; Song, C.P.; Shuangguan, X.X.; Zhu, L.L.; He, G.C. Comparative metabolomics of the interaction between rice and the brown planthopper. Metabolomics 2016, 12, 132. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Lou, Y.R.; Tzin, V.; Jander, G. Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef]
- Coruzzi, G.M.; Last, R.L. Amino Acids. In Biochemistry and Molecular Biology of Plants; Buchanan, R.B., Gruissem, W., Jones, R., Eds.; Wiley Press: Rockville, MD, USA, 2000; pp. 358–410. [Google Scholar]
- Koyama, Y.; Yao, I.; Akimoto, S.Ä. Aphid galls accumulate high concentrations of amino acids: A support for the nutrition hypothesis for gall formation. Entomol. Exp. Appl. 2004, 113, 35–44. [Google Scholar] [CrossRef]
- Dorschner, K.W.; Ryan, J.D.; Johnson, R.C.; Eikenbary, R.D. Modification of host nitrogen levels by the greenbug (Homoptera: Aphididae): Its role in resistance of winter wheat to aphids. Environ. Entomol. 1987, 16, 1007–1011. [Google Scholar] [CrossRef]
- Sandström, J.; Telang, A.; Moran, N.A. Nutritional enhancement of host plants by aphids–A comparison of three aphid species on grasses. J. Insect Physiol. 2000, 46, 33–40. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, X.C.; Liao, Y.Y.; Yang, Z.Y. Roles of specialized metabolites in biological function and environmental adaptability of tea plant (Camellia sinensis) as a metabolite studying model. J. Adv. Res. 2019, 34, 159–171. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, J.; Lin, S.B.; Xing, Y.X.; Zhang, X.Z.; Meng, Y.; Chang, Y.L.; Guo, H.W.; Sun, X.L. (+)-Catechin, epicatechin and epigallocatechin gallate areimportant inducible defensive compounds against Ectropis grisescens in tea plants. Plant Cell Environ. 2021, 2, 496–511. [Google Scholar]
- Wan, X.C.; Xia, T. Secondary Metabolism of Tea Plant, 1st ed.; Science Press: Beijing, China, 2015. [Google Scholar]
- Zhao, X.M.; Chen, S.; Wang, S.S.; Shan, W.N.; Wang, X.X.; Lin, Y.Z.; Su, F.; Yang, Z.B.; Yu, X.M. Defensive Responses of Tea Plants (Camellia sinensis) Against Tea Green Leafhopper Attack: A Multi-Omics Study. Front. Plant Sci. 2020, 10, 1705. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Genva, M.; Akong, F.O.; Andersson, M.X.; Deleu, M.; Lins, L.; Fauconnier, M.L. New insights into the biosynthesis of esterified oxylipins and their involvement in plant defense and developmental mechanisms. Phytochem. Rev. 2019, 18, 343–358. [Google Scholar] [CrossRef]
- Ling, B.; Wang, G.C.; Ya, J.; Zhang, M.X.; Liang, G.W. Antifeedant activity and active ingredients against Plutella xylostella from Momordica charantia leaves. Agr. Sci. China 2008, 7, 1466–1473. [Google Scholar] [CrossRef]
- Böttcher, C. Studies on the Biology of Free and Esterified Phytodienoic Acids; Ruhr-University Bochum: Bochum, Germany, 2007. [Google Scholar]
- Böttcher, C.; Weiler, E.W. Cyclo-oxylipin-galactolipids in plants: Occurrence and dynamics. Planta 2007, 226, 629–637. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Tan, W.; Luo, G.; Pu, T.; Wang, J.; Zhang, X.; Song, Y. Untargeted Metabolite Profiling of Camellia tetracocca’s Response to an Empoasca onukii Attack Using GC-MS and LC-MS. Agronomy 2023, 13, 791. https://doi.org/10.3390/agronomy13030791
Zhang N, Tan W, Luo G, Pu T, Wang J, Zhang X, Song Y. Untargeted Metabolite Profiling of Camellia tetracocca’s Response to an Empoasca onukii Attack Using GC-MS and LC-MS. Agronomy. 2023; 13(3):791. https://doi.org/10.3390/agronomy13030791
Chicago/Turabian StyleZhang, Ni, Weiwen Tan, Guimei Luo, Tianyi Pu, Jinqiu Wang, Xianhu Zhang, and Yuehua Song. 2023. "Untargeted Metabolite Profiling of Camellia tetracocca’s Response to an Empoasca onukii Attack Using GC-MS and LC-MS" Agronomy 13, no. 3: 791. https://doi.org/10.3390/agronomy13030791
APA StyleZhang, N., Tan, W., Luo, G., Pu, T., Wang, J., Zhang, X., & Song, Y. (2023). Untargeted Metabolite Profiling of Camellia tetracocca’s Response to an Empoasca onukii Attack Using GC-MS and LC-MS. Agronomy, 13(3), 791. https://doi.org/10.3390/agronomy13030791




